Abstract
Excellent reviews on central N-methyl-D-Aspartate receptor (NMDAR) signaling and function in cardiovascular regulating neuronal pools have been reported. However, much less attention has been given to NMDAR function in peripheral tissues, particularly the heart and vasculature, although a very recent review discusses such function in the kidney. In this short review, we discuss the NMDAR expression and complexity of its function in cardiovascular tissues. In conscious (contrary to anesthetized) rats, activation of the peripheral NMDAR triggers cardiovascular oxidative stress via the PI3K-ERK1/2-NO signaling pathway, which ultimately leads to elevation in blood pressure. Evidence also implicates Ca2+ release, in the peripheral NMDAR-mediated pressor response. Despite evidence of circulating potent ligands (e.g. D- and L-aspartate, L-homocysteic acid and quinolonic acid) as well as their co-agonist (e.g. glycine or D-serine), the physiological role of peripheral cardiovascular NMDAR remains elusive. Nonetheless, the cardiovascular relevance of the peripheral NMDAR might become apparent when its signaling is altered by drugs, like alcohol, which interact with the NMDAR or its downstream signaling mechanisms.
Keywords: peripheral NMDA receptor, phosphoinositide 3-kinase (PI3K)-Akt signaling, nitric oxide, reactive oxygen species, blood pressure, ethanol
Introduction
L-Glutamate receptors are divided into metabotropic (mGluRs) and ionotropic (iGluRs) receptors, which regulate glutamate signaling within the central nervous system (CNS) and extraneuronal tissues. The mGluRs are one family of G-protein coupled receptors (GPCRs), which are divided into three groups: mGluR1 and mGluR5 (group I), mGluR2 and mGluR3 (group II) and mGluR4, mGluR6, mGluR7 and mGluR8 (group III). The mGluRs act via second messenger systems to modulate excitability and synaptic transmission. Group I mGluRs activate phospholipase C by coupling to Gq/G11, which leads to generation of inositol 1, 4, 5-trisphosphate and diacylglycerol.1 Groups II and III mGluRs are coupled to Gi/Go, which leads to adenylyl cyclase inhibition. In addition, these receptors are coupled to the mitogen-activated protein kinase (MAPK) and phosphatidylinositol-3-kinase (PI3K) signaling pathways.2 Group I mGluR activation produces cell depolarization or hyperpolarization and neuronal excitability depending upon the neuronal population.3 Groups II and III mGluR activation plays a role in neurotransmitter release inhibition.4
The four subtypes of iGluRs are: 1) N-methyl-D-aspartate (NMDA); 2) DL-α-amino-3-hydroxy-5-methylisoxasole-4-propionate (AMPA); 3) kainate (KA); and 4) receptors. These receptors are generally named based on the agonist that binds them. AMPA receptors are located within the excitatory glutamate synapses and involved in long term potentiation and long term depression.5 Kainate receptors, which are expressed in the hippocampus (pyramidal neurons, neocortex and gyrus) and cortical interneurons, modulate synaptic release of GABA and glutamate, postsynaptic depolarization and neural circuit development.6 Localized predominantly in the cerebellar Purkinje cells, glutamate receptors of the delta (δ) family are essential for cerebellum function and promotion of synapse formation.7, 8
Central NMDA receptor (NMDAR) mediates diverse physiological processes including excitatory synaptic transmission and synaptic plasticity. These receptors are implicated in various diseases such as Huntington’s disease, Parkinson’s disease, Alzheimer’s and psychological disorders such as bipolar disorder and schizophrenia.9 However, recent findings indicate that these receptors can also be found in peripheral tissues and continue to gain attention. In this review, we discuss the current knowledge and knowledge gaps in our understanding of the peripheral NMDAR role in the regulation of blood pressure and heart rate.
Structure
The NMDAR is a heterodimer composed of multiple subunits. The subunits are NR1, NR2 (A-D) and NR3 (A and B). The NR1 subunit is the ubiquitously expressed subunit of the receptor. The NR2 subunits (A, B, C and D) are involved in modulatory processes and not essential for function. The NR3 subunit was recently discovered within the last decade and binds glycine.10 The NR2 and NR3 subunits assemble with the obligatory NR1 subunit to form various combinations. Each heterometric complex requires two NR1 subunits that bind glycine and two NR2 subunits that bind glutamate or a combination of NR2 and NR3.11
The NMDAR is considered a nonselective cation channel, which leads to sodium and calcium ion influx and potassium ion efflux. It also has high calcium permeability when compared to the AMPA and kainate glutamate receptors and can undergo blockade by magnesium in a voltage-dependent manner. The NMDAR contains several domains: 1) an amino-terminal domain; 2) three transmembrane domains (M1, M3 and M4) with a pore channel-forming re-entrant loop; 3) a pore-channel agonist-binding domain; and 4) cytoplasmic terminal domain.12 The NMDAR contains numerous binding and modulatory sites for glutamate or NMDA, glycine, magnesium, cysteine sulfhydryl group that reacts with nitric oxide, zinc (Zn2+), polyamines (i.e. spermidine) and a proton-sensitive site.13
NMDAR Agonist and Antagonist Pharmacology
The NMDAR, unlike other glutamate receptors, requires both glutamate and glycine (co-agonist) for activation.14 Endogenous agonists, L-glutamate and L-aspartate activate the NMDAR via binding at the glutamate site of the receptor.12 The glycine binding site can bind either glycine or D-serine.15 Partial agonists, D-cycloserine, 1-aminocyclopropane carboxylic acid and 1-aminocyclobutane carboxylic acid bind at the NR1 subunit of the NMDAR and produce domain closure similar to agonists such as glycine and D-serine.16
NMDAR antagonists are divided into competitive, uncompetitive, and noncompetitive antagonists. Competitive antagonists, 2-amino-5-phosphonopentanoic acid (AP-5) and 2-amino-7-phosphonoheptanoic acid (AP-7) are phospho derivatives of short chain amino acids that bind at the glutamate site of the NMDAR.17 R-(+)-3-amino-1-hydroxypyrrolid-2-one (R-(+)-HA-966) and 5,7-dichlorokynurenic acid (DCKA) bind at the glycine sensitive site of the NMDAR and can also act as competitive antagonists.18 These antagonists are capable of blocking normal and pathophysiological functions.
Uncompetitive antagonists of the NMDAR act as channel blockers only when the channel is in an active conformational state. Dizocilpine (MK-801), phenylcyclidine, amantadine, ketamine and memantine are uncompetitive antagonists that induce behavioral changes and cardiovascular alterations.19–21 Ethanol, at intoxicating levels, is a noncompetitive antagonist of the NMDAR. Prior reports suggest acute ethanol exposure inhibits NMDAR function, whereas chronic ethanol exposure upregulates NMDAR activity.22 Another noncompetitive antagonist, ifenprodil acts at the NR1 subunit (proton-sensor) and potentially, the NR2 subunit. However, ifenprodil discriminates between NR2 subtypes with a lower IC50 for NR2B compared to the other NR2 subtypes (A, C and D).23 Ifenprodil has been used in combination with L-DOPA, tricyclic antidepressants or selective serotonin reuptake inhibitors to potentially treat Parkinson’s disease or depression.24, 25 Nitrous oxide (N2O, laughing gas) is widely used as an analgesic agent and has NMDAR antagonist properties,26 but the mechanism of action is not clear. In recent years, newer NMDAR antagonists have demonstrated therapeutic potential in treating neuropathic pain, depression and Parkinson’s disease.27
Peripheral NMDAR
Previously thought to exist only within the CNS, recent findings indicate the NMDAR is localized within peripheral tissues. These studies suggest that the NMDAR can be found in multiple cells and tissues including pancreatic islet cells,28 bone cells,29–31 epithelial cells,32 human keratinocytes,33 taste buds,34 lung,35 rat medulla, cortex and renal pelvis of the kidney,13, 36 the male urogenital tract,37 and as detailed in a very recent review.38 Within the cardiovascular system, the NMDAR is expressed in the heart and vasculature.39,40 The NR1 subunit is expressed within different components of the heart (atrium and ventricle), and the vasculature (pulmonary artery and aorta) as well as within H9c2 cultured rat myoblasts.40 Interestingly, the NR2B subunit is expressed within the developing rat heart during embryonic day 14 until postnatal day 21 disappearing at 10 weeks after birth.41 The NR1 subunit is also present in rat mesenteric and carotid arteries.42, 43 Although several investigations have confirmed its expression in the heart and vasculature, the exact functional role of the peripheral NMDAR in these tissues is not fully elucidated.
Peripheral NMDAR-Mediated Cardiovascular Responses
There are a limited number of studies on the role of the peripheral NMDAR in the cardiovascular system. Sitniewska et al. showed that the middle dose of NMDA (25, 50 and 1000 μg/kg i.v.) produced slight hypotension and no change in heart rate in anesthetized rats.44 In the presence of chlorisondamine, a nicotinic receptor antagonist, the observed hypotension declined suggesting a central origin. Realizing the potential confounding effects of anesthesia on cardiovascular responses, McGee et al. evaluated peripheral NMDAR activation in conscious rats.42 Following systemic NMDA administration (125, 250, 500 and 1000 μg/kg, i.v.), a dose-dependent pressor, along with dose-independent tachycardic, responses were observed. The persistence of the pressor response after systemic ganglionic blockade with a blocker that does not cross the blood brain barrier (hexamethonium) supported a role for peripheral NMDAR in the observed responses. This conclusion was further supported by NMDA-evoked increase in contractile force in rat aorta rings.42
In addition to alterations in blood pressure and heart rate, acute and chronic NMDAR activation has been linked to increased susceptibility of arrhythmias. Chronic NMDAR activation induced increased heart rate and prolonged repolarization (action potential duration and beat-to-beat variability of repolarization). Furthermore, electrical instability, reduced expression of potassium (K+) channel proteins, and mild myocardial fibrosis were noted. However, these changes were abolished following co-treatment with MK-801.45 Both myocardial ischemia and ischemia-reperfusion induce ventricular fibrillation, ventricular tachycardia and mortality. These events induced by reperfusion were drastically reduced by pretreatment (5 min) with the NMDAR antagonists, MK-801 (0.3 mg/kg i.v.), CNQX (6-cyano-7-nitroquinoxaline-2, 3-dione, 1 mg/kg i.v.), ketamine (10 mg/kg) or memantine (1.5 mg/kg) indicating NMDAR involvement.46
NMDAR Signaling
Numerous investigations have been conducted to assess NMDAR-mediated signaling processes. These signaling processes have been well established within the CNS, but not within the peripheral tissues. Specifically, we will focus on signaling molecules involved in peripheral NMDAR-mediated cardiovascular responses. However, given that little is known about the peripheral NMDAR, we will provide a broad overview of the potentially common signaling networks that regulate or are triggered by peripheral and central NMDAR activation, and how they modulate NMDAR activity and physiological processes.
Protein Kinase C and NMDAR
Phosphorylation of the NMDAR occurs via protein kinase C (PKC), protein kinase A, calcium/calmodulin-dependent protein kinase II (CaMKII) and tyrosine kinases, Fyn and Src kinases. Here, we will focus on PKC, which is a ubiquitous family of serine/threonine protein kinases and activated via G-protein coupled receptors. This kinase has been extensively studied in the regulation of NMDAR via phosphorylation of the NR1 and NR2 subunits of the NMDAR.
Activation of PKC can lead to a wide range of alterations within the NMDAR: 1) potentiation or desensitization,47 2) Mg+ block sensitivity,48 and 3) receptor trafficking.49 An electrophysiological study demonstrated a potentiation of NMDAR currents due to the activation of PKC by 4β-phorbol-12-myristate-13-acetate (4β-PMA). This potentiation was dependent upon receptor composition, specifically NR2 subunits. In the presence of PKC inhibitors such as chelerythrine or calphostin, NMDAR-mediated responses were attenuated.50 Stimulation of PKC causes NMDAR desensitization in HEK-293 cells expressing NR1/NR2A and NR1/NR2B. NMDAR desensitization resulted in a decreased steady-state current, which was abolished following administration of bisindolylmaleimide derivatives, Go 6850 and Go 6983.47 With regards to Mg2+ block sensitivity, PKC is essential for phosphorylation at mechanosensitive domains of the NR1/NR2B subunits restoring mechanical stretch of the NMDAR following a potential Mg2+ block.48,51 Furthermore, PKC mediates the phosphorylation (activation) of nitric oxide synthase (NOS) leading to the generation of nitric oxide (NO).52 Consistent with this view is the finding that inhibition of PKC by chelerythrine attenuated glutamate-induced increases in NO in cerebellar granule cells.53
Calcium Signaling
NMDAR activation promotes Ca2+ influx, which regulates physiological processes such as synaptic plasticity, gene transcription, and NOS activity.54, 55 The magnitude of Ca2+ influx influences both neuronal survival and death because elevated Ca2+ levels can trigger excitotoxicity under abnormal conditions within neuronal cells. Synaptic NMDAR-mediated Ca2+ influx also activates two signaling pathways, a Ras–ERK1/2 pathway and the nuclear Ca2+-calmodulin (CaM) kinase pathway.56
Within the cardiovascular system, Ca2+ determines the excitation-contraction coupling. The transient reversible increase and the overload in intracellular Ca2+ caused by lower and higher NMDA concentrations, respectively are consistent with earlier findings on NMDAR-mediated increase in intracellular Ca2+ oscillation frequency.57,58 The MK-801-induced abrogation of NMDA-evoked Ca2+ influx supports the involvement of the NMDAR in this cellular response and highlights the functional importance of such responses. The latter conclusion gains credence from recent findings that the Ca2+ entry blocker nifedipine caused dose-dependent attenuation of the pressor response caused by peripheral NMDAR activation in conscious rats.59
Nitric Oxide
NO is synthesized from L-arginine via Ca2+-dependent activation on eNOS , iNOS and nNOS in different tissues.60 As discussed above, stimulation of NMDAR causes Ca2+ and PKC mediated activation of NOS leading to NO as well as reactive oxygen species (ROS) generation.61,62,53 The coupling of nNOS to the NMDAR via scaffolding protein, postsynaptic density protein (PSD-95) generates NO and exerts its effects via the activation of cGMP and S-nitroslyation of proteins.63,64 Within the CNS, the debate continues about the role of NO because both excitatory and inhibitory effects have been reported in numerous investigations including ours.65,66 Moreover, these opposing responses can be attributed to, but are not limited to, the following factors: anatomical location in the brain, models, sources of NO and the use of anesthesia when using animal models. Interestingly, the use of either NO (NG-methyl-L-arginine, NG-nitro-L-arginine methyl ester) or NMDA (MK-801 and 6,7-dinitroquinoxaline-2,3-dione) inhibitors reversed the effects caused by NMDAR stimulation in both in vivo and in vitro studies suggesting the reciprocity between NO and NMDA.65,67 NO reaction with ROS (both generated by NMDAR activation) results in the formation of peroxynitrite,68 which causes mitochondrial dysfunction and cytotoxicity.69
Within the periphery, eNOS and nNOS isoforms participate in peripheral cardiovascular regulation via the NO-cGMP-dependent vasodilation.70 Therefore, their stimulation by NMDAR activation is expected to generate NO and ultimately vasodilation. While not directly investigated, this view might explain the hypotensive response elicited by systemic NMDA administration in anesthetized rats.44 Importantly, however, the NMDAR signaling and its interaction with NOS might have been confounded by the use of anesthesia in the latter study. In support of this notion are our findings that similar systemic doses of NMDA produced dose-dependent pressor responses in conscious rats along with NO and ROS generation in tissues collected from these rats.42 It is also possible that eNOS and nNOS uncoupling, which generates ROS,71,72 might be facilitated by NMDAR activation. Overall, there are only a few studies that have dealt with these signaling molecules in the peripheral NMDAR-NOS signaling pathway and their impact on blood pressure and heart rate.
Our laboratory previously conducted studies examining NO and ROS as molecular mediators in the peripheral NMDAR-mediated pressor response42 with a premise based on the ability of central NMDAR-mediated NO generation to increase ROS production.73 Subsequently, the use of nonspecific (NG-Nitro-L-arginine methyl ester: L-NAME) and specific eNOS (N5-(1-Iminoethyl)-L-ornithine dihydrochloride: L-NIO) and nNOS (N-propyl-l-arginine: NPLA) inhibitors highlighted a causal role for the nNOS isoform in the peripheral NMDAR-mediated pressor response, which contradicts the conventional vasodilating action of NO. Therefore, it is likely that nNOS-derived NO reaction with NADPH oxidase (NOX)-derived reactive oxygen species (see below), which results in peroxynitrite formation within the vasculature, is a driving force for the pressor response elicited by peripheral NMDAR activation.
Reactive Oxygen Species (ROS)
Superoxide, hydroxyl radical and hydrogen peroxide generation occurs during cellular responses and mitochondrial metabolism. NMDAR activation can lead to the production of ROS throughout the CNS.74,75 Sustained NMDAR activation yields abnormal levels of ROS, which ultimately contribute to cell death.76,77 There is evidence suggesting the NOX pathway and the conversion of NO to peroxynitrite constitute potential sources of ROS in neurons.78,79 Brennan et al examined the role of the NOX in NMDAR-mediated superoxide production in cultured neurons and those within the mouse hippocampus.80 Superoxide production was increased following the administration of NMDA and was inhibited when apocynin, a NOX inhibitor and neurons lacking the p47phox, an essential subunit of the NOX, were used confirming the NMDAR-NOX signaling pathway.80
The NOX2 family is the prototypical NOX and the most widely distributed NOX isoform. NMDAR activation increases NOX2 and NO, which synergistically contribute to ROS generation in CNS,81 at least partly, via ERK1 activation. This conclusion is supported by the ability of NMDAR antagonists (APV or MK-801), antioxidants such as N-acetyl-L-cysteine (L-NAC), superoxide dismutase (SOD), Mn(III) tetrakis (4-benzoic acid) porphyrin (MnTBAP), catalase or ebselen to reduce or abolish ERK activation caused by NMDA within hippocampal slices.82 Further, NOX inhibitors abolished NMDAR-mediated ROS production.81,83
Similar to findings within the CNS, peripheral NMDAR activation leads to ROS generation in cardiovascular tissues.42, 53, 59,57 Despite the widespread distribution of the different NOX isoforms in cardiovascular tissues,84 the possible contribution of NOX to peripheral NMDAR-mediated ROS and its functional relevance remained unknown until very recently. Data generated in our laboratory showed that pretreatment with the nonspecific NOX inhibitor, apocynin, attenuated the ROS production in vasculature, and pressor response caused by peripheral NMDAR activation.59 Nevertheless, the exact NOS isoform(s) implicated in this phenomenon remain(s) to be elucidated.
Phosphoinositide 3-Kinase (PI3K)-Akt Signaling
The phosphoinositide 3-kinase-Akt (PI3K-Akt) signaling pathway plays an important function in the NMDAR-mediated induction of neuronal responses and excitotoxicity,85–87 at least partly, via NOX activation/ROS generation.80 More specifically, NMDAR induction of Ca2+ influx activates PI3K, and its downstream effector, PKCζ, and the latter phosphorylates the NOX2 isoform.88
Given that PI3K-Akt-NOX signaling occurs as a result of central NMDAR activation, it is plausible to consider the ability of peripheral NMDAR to mediate similar signaling events in cardiovascular tissues. In the latter, PI3K-Akt signaling contributes to Ca2+ influx 89,90 and NO generation via eNOS or nNOS,91,92 which highlight the importance of this signaling network in cardiovascular pathophysiology.93,94 However, evidence for implicating this signaling pathway in peripheral NMDAR-mediated cardiovascular responses has only been recently investigated. In vivo and biochemical findings have shown that the PI3K inhibitor, wortmannin significantly attenuated the pressor response, and the associated increases in NO, ROS and NOX activity caused by peripheral NMDAR activation.59
MAP Kinase Signaling
Ca2+ influx, which occurs following NMDAR activation, can activate the MAPK signaling pathway.95,96 This signaling pathway consists of different MAPKs. To date, there are three major families of MAPKs: 1) extracellular-signal-regulated protein kinases (ERKs), 2) Jun kinases/SAPK (JNKs) and 3) p38.97 The activation of the MAPKs (ERK, JNK and p38) following NMDAR activation98 can stimulate the synthesis of Wnt proteins, which play a role in neuronal development and plasticity.99
Numerous studies have documented rapid ERK phosphorylation following NMDAR activation.96,100,101 Further, a potential mechanism for ERK activation by NMDAR may depend upon the PI3K102 as discussed earlier. Similar to the JNK or stress activated protein kinases, the p38 MAPK family plays a key role in NMDAR signaling.103 Outside of the CNS, the MAPKs promote ROS production in cardiovascular tissues.104,105 For example, ERK1/2 phosphorylation is implicated in angiotensin II-evoked oxidative stress and hypertension,106 and in vascular smooth muscle cell growth and ROS generation.107
Both JNK and p38 act as stress-activated kinases and are key mediators of cardiovascular diseases. The MAPK, JNK, which is expressed in the heart and vasculature,108 is implicated in cardiac hypertrophy, ischemia-reperfusion and cardiac remodeling. However, the exact roles in these pathologies are unclear because both protective and detrimental effects depending upon the stimulus have been reported.109 Within the vasculature, JNK2+3 deletion enhanced contractility in carotid arteries lending support for therapeutic use of JNK inhibitors.110 As discussed above, p38 plays a pivotal role in hypertension111,112 perhaps via NOX-generated ROS.113 While a vast number of publications have focused on the role of MAPKs in central NMDAR signaling, very few investigations have dealt with the role of these MAPKs in peripheral NMDAR signaling and function. We present the first evidence of enhanced phosphorylation of Akt, ERK1, JNK and p38 in the vasculature (aortas) collected from male Sprague-Dawley rats during the pressor response elicited by systemic NMDA injections.59 While no pharmacologic inhibitors were used to target each MAPK and to prove causal role for this molecular event in the peripheral NMDAR-mediated cardiovascular responses, these biochemical findings provide a foundation for future in vivo and mechanistic studies. Nevertheless, based on the cardiovascular biology of these kinases, discussed above, it is highly likely that they are implicated in the oxidative stress and subsequent pressor response elicited by peripheral NMDAR activation in conscious rats in our recent studies.
Peripheral NMDAR Signaling Modulates the Blood Pressure Effect of Ethanol
The NMDAR has been suggested as one of the prominent molecular targets for both acute and chronic effects of ethanol.114 Notably, acute ethanol acts as a noncompetitive NMDAR antagonist whereas chronic ethanol upregulates the NMDAR.115,116 Various studies have dealt with central NMDAR-ethanol interaction including effects on cardiovascular responses.117–120 In contrast, no previous investigations have examined the peripheral NMDAR-ethanol interaction within the cardiovascular system until recently. We recently reported the first investigation on the impact of this interaction on cardiovascular responses such as blood pressure and heart rate and molecular mediators such as NO and ROS in conscious rats.121
The findings of this recent investigation on peripheral NMDAR-ethanol interaction showed that: 1) acute ethanol attenuates peripheral pressor and bradycardic responses elicited by NMDA infusion, 2) an increase in vascular oxidative stress contributes to the dampening, by ethanol, of the systemic NMDA-evoked oxidative stress and pressor responses, and 3) NMDAR blockade uncovers ethanol-evoked hypotension. Importantly, the attenuation of peripheral NMDAR-mediated cardiovascular responses was consistent with previous reports on similar findings within cardiovascular regulating brain stem nuclei.117,118,122 In particular, one prior report compared excitatory receptor agonists, l-glutamate, NMDA, kainic acid or α-amino-2, 3-dihydro-5-methyl-3-oxo-4-isoxazolepropanoic acid in the presence of ethanol and its effect on blood pressure, heart rate and baroreflex sensitivity in urethane-anesthetized Sprague-Dawley rats.117 Interestingly, systemic (1 g/kg) or intra-NTS (10 μg) ethanol attenuated the cardiovascular and baroreflex responses elicited by central NMDAR activation. In a subsequent study in conscious rats, ethanol blockade of NMDAR in the rostral ventrolateral medulla (RVLM), which also controls central cardiovascular function, was suggested as a mechanism for the ethanol-evoked impairment of baroreflexes.118 Collectively, the recent investigation, and the previous reports provide new insight into the peripheral NMDA-ethanol interaction.
Future Clinically Relevant Studies
Future studies are warranted to elucidate the role of peripheral NMDAR in chronic ethanol-evoked hypertension and in models of human hypertension in the absence and presence of ethanol. First, while central NMDAR signaling might be altered in the SHR, there are currently no studies on vascular or cardiac NMDAR signaling in the SHR, particularly following chronic NMDAR activation or blockade. Based on the findings discussed above, we predict that a heightened vascular NMDAR signaling might contribute to the elevated vascular oxidative stress and vascular resistance in SHRs.53,59 Second, since the original observation by Lian in 1915,123 many studies including ours have shown that chronic heavy ethanol consumption causes sympathetically-mediated hypertension in rodents,124,125 which agree with the J-shaped blood pressure effect of chronic ethanol in humans.126 It is intriguing to note that behavioral studies revealed that chronic ethanol administration in rodents upregulates central NMDAR.116,127,128 It will be important to determine if chronic ethanol administration causes a similar NMDAR upregulation in cardiovascular regulating nuclei. Notably, our recent study implicated RVLM NMDAR in neuronal oxidative stress and sympathoexcitation.129 Therefore, it is possible that NMDAR-dependent sympathoexcitation contributes to chronic ethanol-evoked hypertension. Future studies also need to determine if a similar NMDAR up-regulation occurs in the vasculature and the heart of chronic ethanol-treated rats, and contributes to the development of hypertension. Interestingly, chronic ethanol-evoked hypertension in rats is associated with vascular endothelial oxidative stress and dysfunction.130 If these predictions are confirmed in models of human essential and ethanol-induced hypertension, selective peripheral NMDAR blockade might be considered a viable antihypertensive agent modality in general and for the treatment of ethanol-induced hypertension in particular. Finally, given the rapidly growing interest in studying sex as a biological variable, it would be important to elucidate peripheral NMDAR signaling in female rats in general as well as under the aforementioned conditions.
Conclusion
Numerous studies have been reported over the past three decades on the role of the NMDAR in neural control of a number of biological functions and neurological disorders. Furthermore, emphasis has also been placed on the central NMDAR interaction with ethanol as the basis for a number of deleterious behavioral as well as cardiovascular effects of ethanol. In recent years, studies have begun to examine the role of NMDAR in various peripheral tissues with scarce reports focusing on the cardiovascular tissues. The presence of the peripheral NMDAR has been confirmed in both the heart and the vasculature; yet, its physiological function remains unknown. This review provides insight into the signaling pathway(s) triggered by peripheral NMDAR in conscious animals and potential contribution of NMDAR signaling to the acute cardiovascular effects of ethanol. Nonetheless, future investigations are required to fully elucidate the role of peripheral NMDAR in pathophysiological conditions, and as a potential therapeutic target.
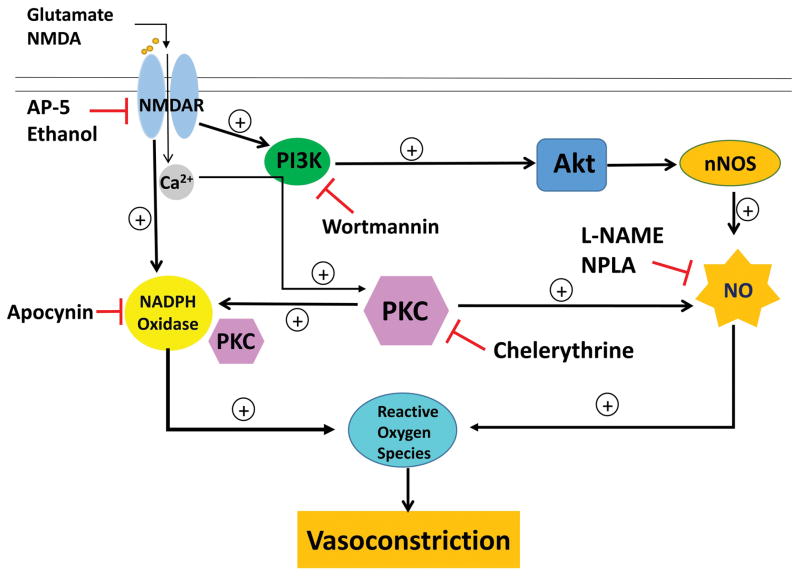
Figure 1.
Schematic presentation of the molecular and blood pressure responses elicited by peripheral NMDAR activation. This model is based on our reported findings in male conscious Sprague-Dawley rats.42,59,118 The peripheral NMDAR can be activated by its ligands, glutamate or NMDA. NMDAR blockade with antagonists, AP-5 or ethanol attenuates peripheral NMDAR-mediated cardiovascular responses. Following activation, Ca2+ influx occurs and multiple signaling cascades are triggered: 1) PI3K-Akt signaling, 2) NOX activation and 3) PKC activation. PI3K-Akt signaling increased nNOS phosphorylation leading to NO generation, which in turn contributes to ROS generation within the vasculature and vasoconstriction. This signaling is inhibited at multiple points within the pathway by either PI3K inhibitor, wortmannin or nitric oxide synthase (NOS) inhibitors, N (ω)-propyl-L-arginine (NPLA) or N(ω)-nitro-L-arginine methyl ester (L-NAME). Peripheral NMDAR activation also activates NOX leading to ROS generation and vasoconstriction; nonspecific NOX inhibitor, apocynin attenuates these responses. Activation of PKC mediates the phosphorylation of NOS leading to NO generation, which contribute to downstream ROS generation and vasoconstriction. Inhibition of PKC with chelerythrine reduces vascular NO and ROS generation and NOX activation as well as the pressor response elicited by peripheral NMDAR activation.
References
- 1.Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- 2.Iacovelli L, Bruno V, Salvatore L, et al. Native group-III metabotropic glutamate receptors are coupled to the mitogen-activated protein kinase/phosphatidylinositol-3-kinase pathways. J Neurochem. 2002;82:216–223. doi: 10.1046/j.1471-4159.2002.00929.x. [DOI] [PubMed] [Google Scholar]
- 3.Niswender CM, Conn PJ. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu Rev Pharmacool Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willard SS, Koochekpour S. Glutamate, glutamate receptors, and downstream signaling pathways. Int J Biol Sci. 2013;9:948–959. doi: 10.7150/ijbs.6426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chater TE, Goda Y. The role of ampa receptors in postsynaptic mechanisms of synaptic plasticity. Front Cell Neurosci. 2014;8:401. doi: 10.3389/fncel.2014.00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lerma J, Marques JM. Kainate receptors in health and disease. Neuron. 2013;80:292–311. doi: 10.1016/j.neuron.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 7.Lomeli H, Sprengel R, Laurie DJ, et al. The rat delta-1 and delta-2 subunits extend the excitatory amino acid receptor family. FEBS Lett. 1993;315:318–322. doi: 10.1016/0014-5793(93)81186-4. [DOI] [PubMed] [Google Scholar]
- 8.Orth A, Tapken D, Hollmann M. The delta subfamily of glutamate receptors: characterization of receptor chimeras and mutants. Eur J Neurosci. 2013;37:1620–1630. doi: 10.1111/ejn.12193. [DOI] [PubMed] [Google Scholar]
- 9.Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels1. Annu Rev Physiol. 2004;66:161–81. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- 10.Paoletti P, Bellone C, Zhou Q. NMDA receptor subunit diversity: impact on receptor properties, synaptic plasticity and disease. Nat Rev Neurosci. 2013;14:383–400. doi: 10.1038/nrn3504. [DOI] [PubMed] [Google Scholar]
- 11.Ulbrich MH, Isacoff EY. Rules of engagement for NMDA receptor subunits. Proc Natl Acad Sci U S A. 2008;105:14163–14168. doi: 10.1073/pnas.0802075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dingledine R, Borges K, Bowie D, et al. The glutamate receptor ion channels. Pharmacol Rev 1999. 1999;51:7–62. [PubMed] [Google Scholar]
- 13.Dryer SE. Glutamate receptors in the kidney. Nephrol Dial Transplant. 2015;0:1–8. doi: 10.1093/ndt/gfv028. [DOI] [PubMed] [Google Scholar]
- 14.Lerma J, Zukin RS, Bennett MV. Glycine decreases desensitization of N-Methyl-D-aspartate (NMDA) receptors expressed in xenopus oocytes and is required for NMDA responses. Proc Natl Acad Sci U S A. 1990;87:2354–2358. doi: 10.1073/pnas.87.6.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martina M, Krasteniakov NV, Bergeron R. D-serine differently modulates NMDA receptor function in rat ca1 hippocampal pyramidal cells and interneurons. J Physiol. 2003;548:411–423. doi: 10.1113/jphysiol.2002.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mayer ML. Glutamate receptor ion channels. Curr Opin Neurobiol. 2005;15:282–288. doi: 10.1016/j.conb.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Priestley T, Laughton P, Myers J, et al. Pharmacological properties of recombinant human N-Methyl-D-aspartate receptors comprising Nr1a/Nr2a and Nr1a/Nr2b subunit assemblies expressed in permanently transfected mouse fibroblast cells. Mol Pharmacol. 1995;48:841–848. [PubMed] [Google Scholar]
- 18.Kemp J, Leeson PD. The glycine site of the NMDA receptor-five years on. Trends Pharmacol Sci. 1993;14:20–25. doi: 10.1016/0165-6147(93)90108-v. [DOI] [PubMed] [Google Scholar]
- 19.Lewis S, Barres C, Jacob H, et al. Cardiovascular effects of the N-Methyl-D-Aspartate receptor antagonist Mk-801 in conscious rats. Hypertension. 1989;13:759–765. doi: 10.1161/01.hyp.13.6.759. [DOI] [PubMed] [Google Scholar]
- 20.Loscher W, Annies R, Honack D. Comparison of competitive and uncompetitive NMDA receptor antagonists with regard to monoaminergic neuronal activity and behavioural effects in rats. Eur J Pharmacol. 1993;242:263–274. doi: 10.1016/0014-2999(93)90250-l. [DOI] [PubMed] [Google Scholar]
- 21.Wolff K, Winstock AR. Ketamine: from medicine to misuse. CNS Drugs. 2006;20:199–218. doi: 10.2165/00023210-200620030-00003. [DOI] [PubMed] [Google Scholar]
- 22.Hu X–J, Ticku MK. Chronic ethanol treatment upregulates the NMDA receptor function and binding in mammalian cortical neurons. Mol Brain Res. 1995;30:347–356. doi: 10.1016/0169-328x(95)00019-o. [DOI] [PubMed] [Google Scholar]
- 23.Williams K. Ifenprodil discriminates subtypes of the N-Methyl-D-aspartate receptor: selectivity and mechanisms at recombinant heteromeric receptors. Mol Pharmacol 1993. 1993;44:851–859. [PubMed] [Google Scholar]
- 24.Poleszak E, Wośko S, Serefko A, et al. The effects of ifenprodil on the activity of antidepressant drugs in the forced swim test in mice. Pharmacol Rep. 2014;66:1031–1036. doi: 10.1016/j.pharep.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 25.Igarashi M, Habata T, Akita H, et al. The Nr2b antagonist, ifenprodil, corrects the L-Dopa-induced deficit of bilateral movement and reduces c-Fos expression in the subthalamic nucleus of hemiparkinsonian rats. J Neurosci Res. 2015;(96):45–53. doi: 10.1016/j.neures.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 26.Nagele P, Metz LB, Crowder CM. Nitrous oxide (N2O) requires the N-Methyl-D-aspartate receptor for its action in caenorhabditis elegans. Proc Natl Acad Sci US A. 2004;101:8791–8796. doi: 10.1073/pnas.0402825101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Traynelis SF, Wollmuth LP, McBain CJ, et al. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62:405–496. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki N, Kuromi H, Gonoi T, et al. Expression and role of ionotropic glutamate receptors in pancreatic islet cells. FASEB J. 1995;9(8):686–891. [PubMed] [Google Scholar]
- 29.Gu Y, Genever PG, Skerry TM, et al. The NMDA type glutamate receptors expressed by primary rat osteoblasts have the same electrophysiological characteristics as neuronal receptors. Calcif Tissue Int. 2002;70:194–203. doi: 10.1007/s00223-001-2004-z. [DOI] [PubMed] [Google Scholar]
- 30.Hinoi E, Fujimori S, Yoneda Y. Modulation of cellular differentiation by N-Methyl-D-aspartate receptors in osteoblasts. FASEB J. 2003;17(11):1532–1534. doi: 10.1096/fj.02-0820fje. [DOI] [PubMed] [Google Scholar]
- 31.Fujita H, Hinoi E, Nakatani E, et al. Possible modulation of process extension by N-Methyl-D-aspartate receptor expressed in osteocytic Mlo-Y4 Cells. J Pharmcol Sci. 2012;119:112–116. doi: 10.1254/jphs.12068sc. [DOI] [PubMed] [Google Scholar]
- 32.Chen J, Zheng Y, Xiong H, et al. NMDA receptors are involved in the regulation of BMP4-mediated survival in rat cochlear epithelial cells. Neurosci Lett. 2014;566(0):275–279. doi: 10.1016/j.neulet.2014.02.067. [DOI] [PubMed] [Google Scholar]
- 33.Morhenn VB, Murakami M, O'Grady T, et al. Characterization of the expression and function of N- Methyl-D-aspartate receptor in keratinocytes. Exp Dermatol. 2004;13:505–511. doi: 10.1111/j.0906-6705.2004.00186.x. [DOI] [PubMed] [Google Scholar]
- 34.Dingledine R, Conn PJ. Peripheral glutamate receptors: molecular biology and role in taste sensation. J Nutr. 2000;130:1039S–1042S. doi: 10.1093/jn/130.4.1039S. [DOI] [PubMed] [Google Scholar]
- 35.Said S. Glutamate receptor activation in the pathogenesis of acute lung injury. In: Bhattacharya J, editor. Cell Signaling in Vascular Inflammation. Humana Press; 2005. pp. 47–50. [Google Scholar]
- 36.Ma MC, Huang HS, Chen YS, et al. Mechanosensitive N-Methyl-D-aspartate receptors contribute to sensory activation in the rat renal pelvis. Hypertension. 2008;52:938–944. doi: 10.1161/HYPERTENSIONAHA.108.114116. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez-Cadavid NF, Ryndin I, Vernet D, et al. Presence of NMDA receptor subunits in the male lower urogenital tract. J Androl. 2000;21:566–578. [PubMed] [Google Scholar]
- 38.Bozic M, Valdivielso JM. The potential of targeting NMDA receptors outside the CNS. Expert Opin Ther Targets. 2015;19:399–413. doi: 10.1517/14728222.2014.983900. [DOI] [PubMed] [Google Scholar]
- 39.Hinoi E, Takarada T, Ueshima T, et al. Glutamate signaling in peripheral tissues. Eur J Biochem. 2004;271:1–13. doi: 10.1046/j.1432-1033.2003.03907.x. [DOI] [PubMed] [Google Scholar]
- 40.Leung JC, Travis BR, Verlander JW, et al. Expression and developmental regulation of the NMDA receptor subunits in the kidney and cardiovascular system. Am J Physiol Regul Integr Comp Physiol. 2002;283:R964–R971. doi: 10.1152/ajpregu.00629.2001. [DOI] [PubMed] [Google Scholar]
- 41.Seeber S, Becker K, Rau T, et al. Transient expression of NMDA receptor subunit NR2B in the developing rat heart. J Neurochem. 2000;75:2472–2477. doi: 10.1046/j.1471-4159.2000.0752472.x. [DOI] [PubMed] [Google Scholar]
- 42.McGee MA, Abdel-Rahman AA. Enhanced vascular neuronal nitric-oxide synthase-derived nitric-oxide production underlies the pressor response caused by peripheral N-Methyl-D-aspartate receptor activation in conscious rats. J Pharmacol Exp Ther. 2012;342:461–471. doi: 10.1124/jpet.112.194464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Fitzgerald R, Brown A, et al. Identification of a homocysteine receptor in the peripheral endothelium and its role in proliferation. J Vasc Surg. 2005;41:853–860. doi: 10.1016/j.jvs.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 44.Sitniewska EM, Wi niewska RJ, Wi niewski K. The role of ionotropic receptors of glutaminic acid in cardiovascular system. Amino Acids. 2003;24:397–403. doi: 10.1007/s00726-002-0342-4. [DOI] [PubMed] [Google Scholar]
- 45.Shi S, Liu TAO, Li Y, et al. Chronic N-Methyl-D-Aspartate receptor activation induces cardiac electrical remodeling and increases susceptibility to ventricular arrhythmias. PACE Pacing Clin Electrophysiol. 2014;37:1367–1377. doi: 10.1111/pace.12430. [DOI] [PubMed] [Google Scholar]
- 46.D'Amico M, Di Filippo C, Rossi F, et al. Arrhythmias induced by myocardial ischaemia-reperfusion are sensitive to ionotropic excitatory amino acid receptor antagonists. Eur J Pharmacol. 1999;366:167–174. doi: 10.1016/s0014-2999(98)00914-5. [DOI] [PubMed] [Google Scholar]
- 47.Jackson MF, Konarski JZ, Weerapura M, et al. Protein kinase c enhances glycine-insensitive desensitization of NMDA receptors independently of previously identified protein kinase c sites. J Neurochem. 2006;96:1509–1518. doi: 10.1111/j.1471-4159.2006.03651.x. [DOI] [PubMed] [Google Scholar]
- 48.Singh P, Doshi S, Spaethling JM, et al. N-Methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit. J Biol Chem. 2012;287:4348–4359. doi: 10.1074/jbc.M111.253740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan JZ, Xu Z, Ren SQ, et al. Protein kinase C promotes N-Methyl-D-aspartate (NMDA) receptor trafficking by indirectly triggering calcium/calmodulin-dependent protein kinase Ii (Camkii) autophosphorylation. J Biol Chem. 2011;286:25187–25200. doi: 10.1074/jbc.M110.192708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lu WY, Xiong ZG, Lei S, et al. G-protein-coupled receptors act via protein kinase C and Src to regulate NMDA receptors. Nat Neurosci. 1999;2:331–338. doi: 10.1038/7243. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L, Rzigalinski BA, Ellis EF, et al. Reduction of voltage-dependent Mg2+ blockade of NMDA current in mechanically injured neurons. Science. 1996;274:1921–1923. doi: 10.1126/science.274.5294.1921. [DOI] [PubMed] [Google Scholar]
- 52.Okada D. Protein kinase C modulates calcium sensitivity of nitric oxide synthase in cerebellar slices. J Neurochem. 1995;64:1298–1304. doi: 10.1046/j.1471-4159.1995.64031298.x. [DOI] [PubMed] [Google Scholar]
- 53.Gunasekar PG, Kanthasamy AG, Borowitz JL, et al. NMDA receptor activation produces concurrent generation of nitric oxide and reactive oxygen species: implications for cell death. J Neurochem. 1995;65:2016–2021. doi: 10.1046/j.1471-4159.1995.65052016.x. [DOI] [PubMed] [Google Scholar]
- 54.Muñoz P, Humeres A, Elgueta C, et al. Iron mediates N-Methyl-D-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. J Biol Chem. 2011;286:13382–13392. doi: 10.1074/jbc.M110.213785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moosmang S, Haider N, Klugbauer N, et al. Role of hippocampal Cav1.2 Ca2+ channels in NMDA receptor-independent synaptic plasticity and spatial memory. J Neurosci. 2005;25:9883–9892. doi: 10.1523/JNEUROSCI.1531-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hardingham GE, Bading H. The yin and yang of NMDA receptor signalling. Trends Neurosci. 2003;26:81–89. doi: 10.1016/S0166-2236(02)00040-1. [DOI] [PubMed] [Google Scholar]
- 57.Gao X, Xu X, Pang J, et al. NMDA receptor activation induces mitochondrial dysfunction, oxidative stress and apoptosis in cultured neonatal rat cardiomyocytes. Physiol Res. 2007;56:559–569. doi: 10.33549/physiolres.931053. [DOI] [PubMed] [Google Scholar]
- 58.Winter CR, Baker RC. L-glutamate-induced changes in intracellular calcium oscillation frequency through non-classical glutamate receptor binding in cultured rat myocardial cells. Life Sci. 1995;57:1925–1934. doi: 10.1016/0024-3205(95)02179-m. [DOI] [PubMed] [Google Scholar]
- 59.McGee MA, Abdel-Rahman AA. Enhanced vascular PI3k/Akt-Nox signaling underlies the peripheral NMDA-mediated pressor response in conscious rats. J Cardiovasc Pharmacol. 2014;63:395–405. doi: 10.1097/FJC.0000000000000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tennyson Andrew G, Lippard Stephen J. Generation, translocation, and action of nitric oxide in living systems. Chem Biol. 2011;18:1211–20. doi: 10.1016/j.chembiol.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 61.Ferraro G, Sardo P. Nitric oxide and brain hyperexcitability. In Vivo. 2004;18:357–366. [PubMed] [Google Scholar]
- 62.Knowles RG, Palacios M, Palmer RM, et al. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci USA. 1989;86:5159–62. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chowdhary S, Townend JN. Role of nitric oxide in the regulation of cardiovascular autonomic control. Clin Sci. 1999;97:5–17. [PubMed] [Google Scholar]
- 64.Tang LJ, Li C, Hu SQ, et al. S-nitrosylation of C-Src via NMDAR-nNOS module promotes C-Src activation and NR2a phosphorylation in cerebral ischemia/reperfusion. Mol Cell Biochem. 2012;365:363–377. doi: 10.1007/s11010-012-1280-4. [DOI] [PubMed] [Google Scholar]
- 65.Lo WC, Lin HC, Ger LP, et al. Cardiovascular effects of nitric oxide and N-Methyl-D-aspartate receptors in the nucleus tractus solitarii of rats. Hypertension. 1997;30:1499–503. doi: 10.1161/01.hyp.30.6.1499. [DOI] [PubMed] [Google Scholar]
- 66.Matsuo I, Hirooka Y, Hironaga K, et al. Glutamate release via NO production evoked by NMDA in the NTS enhances hypotension and bradycardia in vivo. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1285–R1291. doi: 10.1152/ajpregu.2001.280.5.R1285. [DOI] [PubMed] [Google Scholar]
- 67.Lin HC, Wan FJ, Tseng CJ. Modulation of cardiovascular effects produced by nitric oxide and ionotropic glutamate receptor interaction in the nucleus tractus solitarii of rats. Neuropharmacology. 1999;38:935–941. doi: 10.1016/s0028-3908(99)00017-9. [DOI] [PubMed] [Google Scholar]
- 68.Pacher Pl, Beckman JS, Liaudet L. Nitric oxide and peroxynitrite in health and disease. Physiol Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Radi R. Peroxynitrite, a stealthy biological oxidant. J Biol Chem. 2013;288:26464–26472. doi: 10.1074/jbc.R113.472936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Jin R, Loscalso J. Vascular nitric oxide: formation and function. J Blood Med. 2010:147–162. doi: 10.2147/JBM.S7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chen C, Druhan L, Varadharaj S, et al. Phosphorylation of endothelial nitric-oxide synthase regulates superoxide generation from the enzyme. J Biol Chem. 2008;283:27038–27047. doi: 10.1074/jbc.M802269200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Forstermann U, Sessa WC. Nitric oxide synthases: regulation and function. Eur Heart J. 2012;33:829–837. doi: 10.1093/eurheartj/ehr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chianca DA, Jr, Lin LH, et al. NMDA receptors in nucleus tractus solitarii are linked to soluble guanylate cyclase. Am J Physiol Heart Circ Physiol. 2004;286:H1521– H1527. doi: 10.1152/ajpheart.00236.2003. [DOI] [PubMed] [Google Scholar]
- 74.Boldyrev AA, Carpenter DO, Huentelman MJ, et al. Sources of reactive oxygen species production in excitotoxin-stimulated cerebellar granule cells. Biochem Biophys Res Commun. 1999;256:320–324. doi: 10.1006/bbrc.1999.0325. [DOI] [PubMed] [Google Scholar]
- 75.Lafon-Cazal M, Pietri S, Culcasi M, et al. NMDA-dependent superoxide production and neurotoxicity. Nature. 1993;364:535–537. doi: 10.1038/364535a0. [DOI] [PubMed] [Google Scholar]
- 76.Reynolds IJ, Hastings TG. Glutamate induces the production of reactive oxygen species in cultured forebrain neurons following NMDA receptor activation. J Neurosci. 1995;15:3318–3327. doi: 10.1523/JNEUROSCI.15-05-03318.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Choi DW. Ionic dependence of glutamate neurotoxicity. J Neurosci. 1987;7:369–379. doi: 10.1523/JNEUROSCI.07-02-00369.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bedard K, Krause K-H. The Nox family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 79.Wingler K, Hermans JJR, et al. Nox1, 2, 4, 5: counting out oxidative stress. Br J Pharmacol. 2011;164:866–883. doi: 10.1111/j.1476-5381.2011.01249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Brennan AM, Won Suh S, Joon Won S, et al. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Girouard H, Wang G, Gallo EF, et al. NMDA receptor activation increases free radical production through nitric oxide and Nox2. J Neurosci. 2009;29:2545–2552. doi: 10.1523/JNEUROSCI.0133-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kishida KT, Pao M, Holland SM, et al. NADPH oxidase is required for NMDA receptor-dependent activation of ERK in hippocampal area Ca1. J Neurochem. 2005;94:299–306. doi: 10.1111/j.1471-4159.2005.03189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Guemez-Gamboa A, Estrada-Sánchez AM, Montiel T, et al. Activation of Nox2 by the stimulation of ionotropic and metabotropic glutamate receptors contributes to glutamate neurotoxicity in vivo through the production of reactive oxygen species and calpain activation. J Neuropathol Exp Neurol. 2011;70:1020–1035. doi: 10.1097/NEN.0b013e3182358e4e. [DOI] [PubMed] [Google Scholar]
- 84.Cave AC, Brewer AC, Narayanapanicker A, et al. NADPH oxidases in cardiovascular health and disease. Antioxid Redox Signal. 2006;8:691–728. doi: 10.1089/ars.2006.8.691. [DOI] [PubMed] [Google Scholar]
- 85.Sutton G, Chandler LJ. Activity-dependent NMDA receptor-mediated activation of protein kinase B/Akt in cortical neuronal cultures. J Neurochem. 2002;82:1097–1105. doi: 10.1046/j.1471-4159.2002.01031.x. [DOI] [PubMed] [Google Scholar]
- 86.de Araujo Herculano B, Vandresen-Filho S, Martins WC, et al. NMDA preconditioning protects against quinolinic acid-induced seizures via PKA, PI3k and MAPK/ERK signaling pathways. Behav Brain Res. 2011;219:92–97. doi: 10.1016/j.bbr.2010.12.025. [DOI] [PubMed] [Google Scholar]
- 87.Miao B, Yin XH, Pei DS, et al. Neuroprotective effects of preconditioning ischemia on ischemic brain injury through down-regulating activation of JNK1/2 via N-Methyl-D-aspartate receptor-mediated Akt1 activation. J Biol Chem. 2005;280:21693–21699. doi: 10.1074/jbc.M500003200. [DOI] [PubMed] [Google Scholar]
- 88.Brennan-Minnella AM, Shen Y, El-Benna J, et al. Phosphoinositide 3-kinase couples NMDA receptors to superoxide release in excitotoxic neuronal death. Cell Death Dis. 2013;4:e580. doi: 10.1038/cddis.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Viard P, Butcher AJ, Halet G, et al. PI3K promotes voltage-dependent calcium channel trafficking to the plasma membrane. Nat Neurosci. 2004;7:939–946. doi: 10.1038/nn1300. [DOI] [PubMed] [Google Scholar]
- 90.Macrez N, Mironneau C, Carricaburu V, et al. Phosphoinositide 3-kinase isoforms selectively couple receptors to vascular L-type Ca2+ channels. Circ Res. 2001;89:692–699. doi: 10.1161/hh2001.097864. [DOI] [PubMed] [Google Scholar]
- 91.Morello F, Perino A, Hirsch E. Phosphoinositide 3-kinase signalling in the vascular system. Cardiovasc Res. 2009;82:261–271. doi: 10.1093/cvr/cvn325. [DOI] [PubMed] [Google Scholar]
- 92.El-Mas M, Fan M, Abdel-Rahman A. Facilitation of myocardial PI3K/Akt/nNOS signaling contributes to ethanol-evoked hypotension in female rats. Alcohol Clin Exp Res. 2009;33:1158–1168. doi: 10.1111/j.1530-0277.2009.00939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dimmeler S, Fleming I, Fisslthaler B, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 94.Hers I, Vincent EE, Tavaré JM. Akt signalling in health and disease. Cellular Signalling. 2011;23:1515–1527. doi: 10.1016/j.cellsig.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 95.Sala C, Rudolph-Correia S, Sheng M. Developmentally regulated NMDA receptor-dependent dephosphorylation of camp response element-binding protein (CREB) in hippocampal neurons. J Neurosci. 2000;20:3529–3536. doi: 10.1523/JNEUROSCI.20-10-03529.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wang JQ, Fibuch EE, Mao L. Regulation of mitogen-activated protein kinases by glutamate receptors. J Neurochem. 2007;100:1–11. doi: 10.1111/j.1471-4159.2006.04208.x. [DOI] [PubMed] [Google Scholar]
- 97.Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 98.Kurino M, Fukunaga K, Ushio Y, et al. Activation of mitogen-activated protein kinase in cultured rat hippocampal neurons by stimulation of glutamate receptors. J Neurochem. 1995;65:1282–1289. doi: 10.1046/j.1471-4159.1995.65031282.x. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Li B, Wan X, et al. NMDA receptor activation stimulates transcription-independent rapid WNT5a protein synthesis via the MAPK signaling pathway. Mol Brain. 2012;5:1. doi: 10.1186/1756-6606-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tian X, Gotoh T, Tsuji K, et al. Developmentally regulated role for Ras-Grfs in coupling NMDA glutamate receptors to Ras, ERK and CREB. EMBO J. 2004;23:1567–1575. doi: 10.1038/sj.emboj.7600151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ivanov A, Pellegrino C, Rama S, et al. Opposing role of synaptic and extrasynaptic NMDA receptors in regulation of the extracellular signal-regulated kinases (ERK) activity in cultured rat hippocampal neurons. J Physiol. 2006;572:789–798. doi: 10.1113/jphysiol.2006.105510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Perkinton MS, Ip J, Wood GL, et al. Phosphatidylinositol 3-kinase is a central mediator of NMDA receptor signalling to MAP kinase (ERK1/2), Akt/Pkb and Creb in striatal neurones. J Neurochem. 2002;80:239–54. doi: 10.1046/j.0022-3042.2001.00699.x. [DOI] [PubMed] [Google Scholar]
- 103.Xiao L, Hu C, Feng C, et al. Switching of N-Methyl-D-aspartate (NMDA) receptor-favorite intracellular signal pathways from ERK1/2 protein to p38 mitogen-activated protein kinase leads to developmental changes in NMDA neurotoxicity. J Biol Chem. 2011;286:20175–20193. doi: 10.1074/jbc.M110.188854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yoshizumi M, Tsuchiya K, Tamaki T. Signal transduction of reactive oxygen species and mitogen-activated protein kinases in cardiovascular disease. J Med Invest. 2001;48:11–24. [PubMed] [Google Scholar]
- 105.Muslin AJ. MAPK signalling in cardiovascular health and disease: molecular mechanisms and therapeutic targets. Clin Sci (Lond) 2008;115:203–218. doi: 10.1042/CS20070430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Papparella I, Ceolotto G, Lenzini L, et al. Angiotensin II-induced over-activation of P47phox in fibroblasts from hypertensives: which role in the enhanced ERK1/2 responsiveness to angiotensin II. J Hypertens. 2005;23:793–800. doi: 10.1097/01.hjh.0000163148.97459.9d. [DOI] [PubMed] [Google Scholar]
- 107.Roberts R. The extracellular-regulated kinase pathway: a potential therapeutic target in hypertension. J Exp Pharmacol. 2012;4:77–83. doi: 10.2147/JEP.S28907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- 109.Rose BA, Force T, Wang Y. Mitogen-activated protein kinase signaling in the heart: angels versus demons in a heart-breaking tale. Physiol Rev. 2010;90:1507–1546. doi: 10.1152/physrev.00054.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Laukeviciene A, Brecht S, Kevelaitis E, et al. Enhanced contractility of small blood vessels in JNK knockout mice. Eur J Pharm Sci. 2006;29:335–9. doi: 10.1016/j.ejps.2006.06.012. [DOI] [PubMed] [Google Scholar]
- 111.Ju H, Behm DJ, Nerurkar S, et al. p38 MAPK inhibitors ameliorate target organ damage in hypertension: part 1. p38 MAPK-dependent endothelial dysfunction and hypertension. J Pharm Exp Ther. 2003;307:932–938. doi: 10.1124/jpet.103.057422. [DOI] [PubMed] [Google Scholar]
- 112.Martin ED, Bassi R, Marber MS. p38 MAPK in cardioprotection – are we there yet? Br J Pharmacol. 2015;172:2101–2113. doi: 10.1111/bph.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bao W, Behm DJ, Nerurkar SS, et al. Effects of p38 MAPK inhibitor on angiotensin II-dependent hypertension, organ damage, and superoxide anion production. J Cardiovasc Pharmacol. 2007;49:362–368. doi: 10.1097/FJC.0b013e318046f34a. [DOI] [PubMed] [Google Scholar]
- 114.Xu M, Smothers CT, Trudell JR, et al. Ethanol inhibition of spontaneously active N-Methyl-D- aspartate receptors. J Pharm Exp Ther. 2011;17 doi: 10.1124/jpet.111.187179. 111.187179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chandrasekar R. Alcohol and NMDA receptor: current research and future direction. Front Mol Neurosci. 2013;6:14. doi: 10.3389/fnmol.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xiang Y, Kim KY, Gelernter J, et al. Ethanol upregulates NMDA receptor subunit gene expression in human embryonic stem cell-derived cortical neurons. PLoS One. 2015;10:e0134907. doi: 10.1371/journal.pone.0134907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.El-Mas MM, Abdel-Rahman AA. Role of NMDA and non-NMDA receptors in the nucleus tractus solitarius in the depressant effect of ethanol on baroreflexes. J Pharm Exp Ther. 1993;266:602–610. [PubMed] [Google Scholar]
- 118.Mao L, Abdel-Rahman AA. Blockade of L-glutamate receptors in the rostral ventrolateral medulla contributes to ethanol-evoked impairment of baroreflexes in conscious rats. Brain Res Bull. 1995;37:513–521. doi: 10.1016/0361-9230(95)00034-c. [DOI] [PubMed] [Google Scholar]
- 119.Allgaier C. Ethanol sensitivity of NMDA receptors. Neurochem Int. 2002;41:377–382. doi: 10.1016/s0197-0186(02)00046-3. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Y, Ren H, Dwyer DS, et al. Different sites of alcohol action in the NMDA receptor Glun2a and Glun2b subunits. Neuropharmacology. 2015;97:240–250. doi: 10.1016/j.neuropharm.2015.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McGee MA, Abdel-Rahman AA. Ethanol attenuates peripheral NMDA-mediated vascular oxidative stress and pressor response. Alcohol. 2015;49:499–506. doi: 10.1016/j.alcohol.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.El-Mas MM, Abdel-Rahman AA. Sexually dimorphic hemodynamic effects of intragastric ethanol in conscious rats. Clin Exp Hypertens. 1999;21:1429–1445. doi: 10.3109/10641969909070858. [DOI] [PubMed] [Google Scholar]
- 123.Lian C. L'alcoholisme, cause d'hypertension arterielle. Bull Acad Natl Med. 1915;74:525–528. [Google Scholar]
- 124.Marchi KC, Muniz JJ, Tirapelli CR. Hypertension and chronic ethanol consumption: what do we know after a century of study? World J Cardiol. 2014;6:283–294. doi: 10.4330/wjc.v6.i5.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Abdel-Rahman A, Wooles W. Ethanol-induced hypertension involves impairment of baroreceptors. Hypertension. 1987;10:67–73. doi: 10.1161/01.hyp.10.1.67. [DOI] [PubMed] [Google Scholar]
- 126.O'Keefe JH, Bybee KA, Lavie CJ. Alcohol and cardiovascular health: the razor-sharp double-edged sword. J Am Coll Cardiol. 2007;50:1009–1014. doi: 10.1016/j.jacc.2007.04.089. [DOI] [PubMed] [Google Scholar]
- 127.Hoffman PL, Rabe CS, Grant KA, et al. Ethanol and the NMDA receptor. Alcohol. 1990;7:229–231. doi: 10.1016/0741-8329(90)90010-a. [DOI] [PubMed] [Google Scholar]
- 128.Iorio KR, Reinlib L, Tabakoff B, et al. Chronic exposure of cerebellar granule cells to ethanol results in increased N-Methyl-D-aspartate receptor function. Mol Pharmacol. 1992;41:1142–1148. [PubMed] [Google Scholar]
- 129.Rezq S, Abdel-Rahman AA. Central GPR109A activation mediates glutamate-dependent pressor response in conscious rats. J Pharmacol Exp Ther. 2016;356(2):457–466. doi: 10.1124/jpet.115.229146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Husain K. Vascular endothelial oxidative stress in alcohol-induced hypertension. Cell Mol Biol (Noisy-legrand) 2007;53:70–77. [PubMed] [Google Scholar]