SUMMARY
Hunger is a powerful drive that stimulates food intake. Yet the mechanism that determines how the energy deficits that result in hunger are represented in the brain and promote feeding is not well understood. We previously described SLC5A11 – a sodium/solute co-transporter-like – (or cupcake) in Drosophila melanogaster, which is required for the fly to select a nutritive sugar over a sweeter nonnutritive sweetener after periods of food deprivation. SLC5A11 acts on approximately 12 pairs of ellipsoid body (EB) R4 neurons to trigger the selection of nutritive sugars, but the underlying mechanism is not understood. Here, we report that the excitability of SLC5A11-expressing EB R4 neurons increases dramatically during starvation and that this increase is abolished in the SLC5A11 mutation. Artificial activation of SLC5A11-expresssing neurons is sufficient to promote feeding and hunger-driven behaviors; silencing these neurons has the opposite effect. Notably, SLC5A11 transcript levels in the brain rose significantly when flies were starved, and dropped shortly after starved flies were refed. Furthermore, expression of SLC5A11 is sufficient for promoting hunger-driven behaviors and enhancing the excitability of SLC5A11-expressing neurons. SLC5A11 inhibits the function of Drosophila KCNQ potassium channel in a heterologous expression system. Accordingly, a knock-down of dKCNQ expression in SLC5A11-expressing neurons produces hunger-driven behaviors even in fed flies, mimicking the overexpression of SLC5A11. We propose that starvation increases SLC5A11 expression, which enhances the excitability of SLC5A11-expressing neurons by suppressing dKCNQ channels, thereby conferring the hunger state.
Graphical Abstract
INTRODUCTION
The properly regulated intake of nutrients is the key to survival in the animal kingdom. The regulation of food intake in animals is mediated by neural processes through which homeostatic control influences both physiology and behavior [1, 2]. These processes include complex mechanisms that monitor energy reservoirs in the body and generate a sensation of hunger when these reservoirs diminish. Hunger, in turn, promotes the stimulation of food intake [3, 4] and selection of nutritious foods [5]. It also produces enhanced sensory sensitivities and elevated locomotion in insects [6, 7] and mammals [8, 9]. Despite the fact that this process is basic to existence among all types of animals, the mechanism(s) by which hunger triggers feeding and hunger-driven behaviors is not well understood in any animal system.
Flies, like mammals, constantly adapt their energy needs to nutritional status through metabolic regulation [10]. Flies also select food based on its nutritional value, in addition to taste input [5, 11, 12]. The fruit fly Drosophlia melanogaster is a particularly useful model for studying the neural correlates of hunger. Its genetically amenable system allows the unbiased screening of genes and cells in the adult brain and the dissection of neural mechanisms underlying feeding behaviors [13]. This model system has already proven useful in helping us advance our understanding of how peripheral sensory neurons mediate the detection of food sources and generate behavioral responses to food [14, 15]. It may also be useful for identifying the elusive components of the central nervous system that monitor the depleting energy levels in the body and trigger feeding behaviors when they diminish.
In this study, we found that the activity of central SLC5A11-expressing ellipsoid body R4 neurons (SLC5A11 neurons) and the expression of SLC5A11 transcripts in Drosophila increase significantly following periods of food deprivation. We also found that SLC5A11 is necessary and sufficient for elevating the activity of SLC5A11 neurons, and for promoting food intake and hunger-driven behaviors. We also found that SLC5A11 suppresses potassium currents generated by dKCNQ channel [16] activity when co-expressed in Xenopus oocytes. Consistent with this finding, we observed that the knock-down of dKCNQ expression in SLC5A11 neurons produces hunger-driven behaviors, even in fed flies. These results support our hypothesis that increased levels of SLC5A11 during starvation increase the activity of SLC5A11 neurons by suppressing dKCNQ activity, thereby promoting feeding behavior.
RESULTS
The activity of SLC5A11 neurons is stimulated by food deprivation
We previously determined that SLC5A11 neurons in the Drosophila brain are required for these flies to select a nutritive sugar over a sweeter nonnutritive sweetener after periods of starvation in a two-choice assay (D-glucose versus L-glucose) from a screen [17]. SLC5A11 (or cupcake) acts on approximately 12 pairs of R4 neurons to trigger the selection of nutritive sugars, but the mechanism underlying this process is not understood. We proposed two mechanisms by which SLC5A11 neurons may mediate the selection of nutritive sugars: (1) by detecting the nutritional value of sugar through direct activation; or (2) monitoring the internal energy reserves of the fly with a direct nutrient sensor located elsewhere; starved flies lacking functional SLC5A11 neurons cannot sense the deprived metabolic state and, thus, would not select the nutritive D-glucose. We initially proposed that the first mechanism – direct neuronal activation – occurs during the postprandial rise in hemolymph sugar levels. Through calcium imaging using two-photon microscopy and a more sensitive electrophysiology approach, we tested whether SLC5A11 neurons respond to solutions containing nutritive sugars. However, we failed to observe any responses, either excitatory or inhibitory to glucose or any other sugars (data not shown).
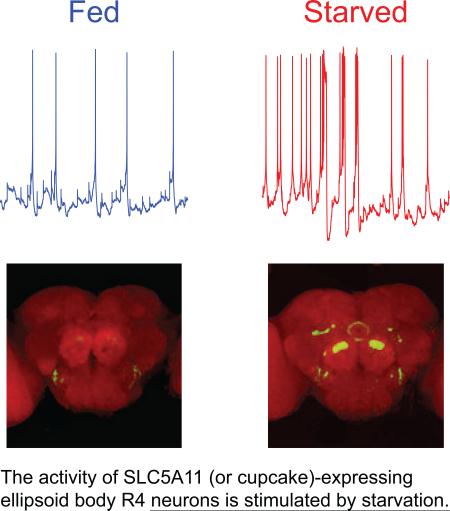
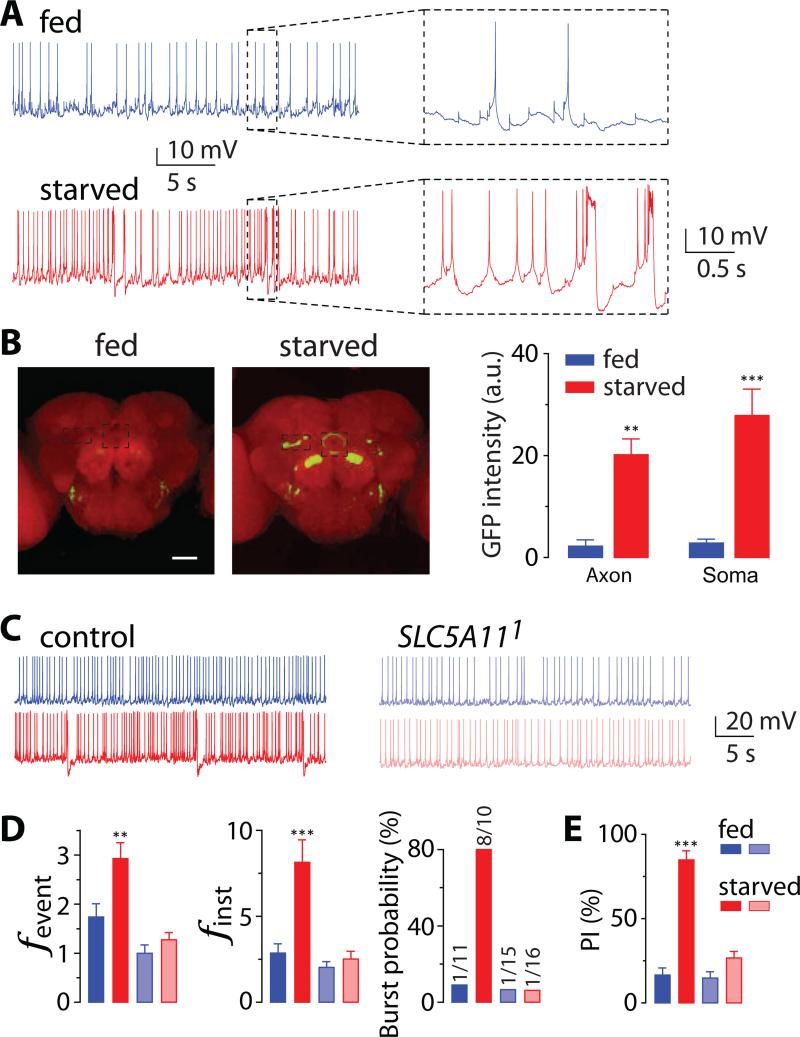
The second mechanism was proposed based on the hypothesis that SLC5A11 neuronal activity changes when the energy reservoir in the fly is depleted. To test this hypothesis, we conducted whole-cell patch-clamp recordings of SLC5A11 neurons in the brains of wild-type (WT) flies carrying PSLC5A11-GAL4 and UAS-GFP that were fed or had been starved for 22 hours. Spontaneous activity in the SLC5A11 neurons was significantly altered following periods of starvation (Figure 1A), with the firing frequency and burst probability increased considerably (Figures S1A and S2A). We also monitored the rate at which action potentials fire in fed versus starved flies in response to step-current injections into their SLC5A11 neurons. We observed that the firing frequency and burst events increased with the magnitude of the current injection and that these rates were high in starved animals compared with fed animals (Figures S1BS1D). Consistent with these findings, the brains of starved flies carrying PSLC5A11-GAL4 and UAS-CaLexA/GFP system [18] showed GFP expression in SLC5A11-expressing ellipsoid body R4 neurons whereas the brains of fed flies displayed no detectable GFP expression in SLC5A11 neurons (Figures 1B and S3).
Figure 1. Starvation enhances the excitability of SLC5A11 neurons, which requires SLC5A11 gene.
(A) Representative traces of spontaneous activity recorded from the soma of SLC5A11 neurons in isolated brain preparations obtained from either 5-hr (fed, blue upper trace) or 22-hr (starved, red lower trace) food deprived adult fly. (Right) Enlarged firing patterns: regular spiking (top) or bursting (bottem). See also Figures S1 and S2. (B) Representative brain images of 5-hr (fed) or 22-hr (starved) deprived flies carrying PSLC5A11-GAL4, UAS-mLexA-VP16-NFAT and LexAop-CD8-GFP-2A-CD8-GFP stained with the antibody to GFP in green and the neuropil marker nc82 in magenta, and the quantification of the intensity of GFP fluorescence in SLC5A11 neurons – the dotted boxes. Scale bar, 50 μm. See also Figure S3. (C) Representative traces of spontaneous activity and (D) quantification of firing frequency and bursting probability of SLC5A11 neurons in control and SLC5A111 mutant flies. See also Figure S2. (E) Behavior responses of 5-hr (fed) or 22-hr (starved) deprived control and SLC5A111 mutant flies in the two-choice assay. Flies were given a choice between 50mM D-glucose and 300mM L-glucose (n = 8-11) in this and subsequent behavior experiments unless indicated otherwise. fevent, event frequency; finst, instantaneous frequency. The numbers of bursting neurons out of the total number of recorded neurons are indicated above the bars. **P < 0.01, ***P < 0.001 vs. fed in the same genotype; error bars indicate SEM.
To determine the contribution of SLC5A11 to neuronal excitability, we measured the activity of SLC5A11 neurons in SLC5A11 mutant versus WT control flies. We targeted these cells for recording using PSLC5A11-GAL4 and UAS-tdTomato in a different genetic background from the previous experiment. Analogous to the traces shown in Figure 1A, the excitability of SLC5A11 neurons in WT flies carrying UAS-tdTomato was significantly enhanced during starvation (Figure 1C). This starvation-induced enhancement of neuronal activity, however, was essentially abolished in SLC5A11 mutants (Figure 1C), with no significant difference found between fed and starved flies (Figures 1D and S2B). The similarity in neuronal activity in fed and starved mutant flies paralleled their similarity in food preference in the two-choice assay (Figure 1E).
SLCA11 neurons are essential for hunger-driven behaviors
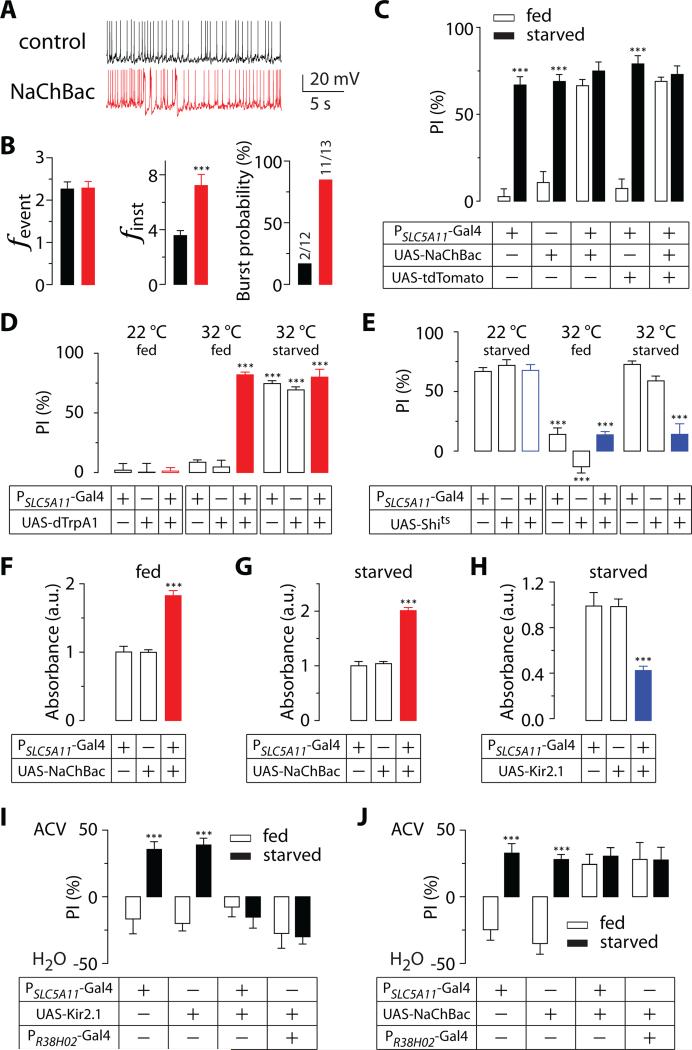
Having shown that SLC5A11 neuronal excitability is enhanced during starvation, we sought to determine whether manipulating SLC5A11 neuronal activity alters feeding behaviors. We artificially activated SLC5A11 neurons by expressing a voltage-gated bacterial sodium channel, NaChBac [19], in these neurons. As a result, neuronal activity increased dramatically, as indicated by the change in instantaneous frequency and burst probability (Figures 2A, 2B, and S2C). Flies with activated SLC5A11 neurons were evaluated using a number of feeding-related assays. In the two-choice assay, these flies demonstrated a strong preference for nutritive D-glucose even when they were fed, whereas the control flies showed little or no preference for D-glucose (Figure 2C). We confirmed this finding by acutely activating SLC5A11 neurons using UAS-dTrpA1[20] at 32°C and observing the flies’ behavior in the two-choice assay (Figure 2D). By contrast, inactivating SLC5A11 neurons by expressing UAS-Shibirets [21] in these neurons at a restrictive temperature (32°C) nearly abolished the starvation-induced preference for D-glucose (Figure 2E). These results suggest that the activity of SLC5A11 neurons is necessary and sufficient for mediating the selection of nutritive sugar. They would also argue against the possibility that SLC5A11 neurons function as a nutrient sensor that directly monitors glucose levels in the hemolymph. The activation of such a sensor would render flies to perceive both D- and L-glucose to be equally nutritious and display no preference for either sugar. Flies with activated SLC5A11 neurons still chose nutritive D-glucose, however, thereby providing additional evidence that SLC5A11 neurons are involved in encoding hunger rather than sensing nutrients directly.
Figure 2. The activity of SLC5A11 neurons is necessary and sufficient for hunger-driven behaviors.
(A) Representative traces of spontaneous activity and (B) quantification of firing frequency and bursting probability recorded from SLC5A11 neurons in the brains of fed flies carrying PSLC5A11-GAL4 alone (control) and fed flies carrying PSLC5A11-GAL4 and UAS-NaChBac (n = 12-13; ***P < 0.001). See also Figure S2. (C-E) Behavior responses of 5-hr (fed) or 22-hr (starved) food deprived flies carrying PSLC5A11-GAL4 and either UAS-NaChBac (n = 8-12; ***P < 0.001 vs. fed in the same genotype) (C), UAS-dTrpA1 (n = 6-8; ***P < 0.001 vs. 22 °C in the same genotype) (D), or UAS-Shits (n = 6-9; ***P < 0.001 vs. 22 °C in the same genotype) (E) in the two-choice assay. (F-H) Relative amounts of food consumed by 5-hr (fed) or 22-hr (starved) deprived flies bearing PSLC5A11-GAL4, and either UAS-NaChBac (F,G) or UAS-Kir2.1 (H) (n = 9-17; ***P < 0.001 vs. control). (I,J) Olfactory responses of 5-hr (fed) or 22-hr (starved) deprived flies bearing PSLC5A11-GAL4 or PR38H02-GAL4 with either UAS-Kir2.1 (I) or UAS-NaChBac (J) to 1% ACV versus water in a T-maze preference assay (n = 7-15; ***P < 0.001 vs. fed in the same genotype). Flies harboring either PSLC5A11-GAL4 or UAS-responder alone were used as controls. Error bars indicate SEM. See also Figure S4.
We next examined whether manipulating the activity of SLC5A11 neurons causes flies to alter their food intake. Indeed, artificial stimulation of SLC5A11 neurons resulted in both fed and starved flies consuming twice as much food as control flies (Figures 2F and 2G). Conversely, inactivating SLC5A11 neurons by expressing UAS-Kir2.1 [22] in these cells significantly decreased food intake in starved flies (Figure 2H). These observations suggest that the impaired food selection during the two-choice assay was due to a change in hunger state associated with the manipulations of SLC5A11 neuronal activity.
To examine whether the change in hunger state extends to olfactory behavior, we explored the possibility that SLC5A11 neuronal activity helps mediate hunger-driven olfactory attraction to food odor. It has been shown that flies are attracted to food odor when they are starved in a four-field arena [23]. In a binary choice assay using a T-maze, flies also preferred the side containing apple cider vinegar (ACV) when they were starved but avoided the same odor when they were fed (Figures 2I and 2J). Accordingly, this assay also measures their state of hunger or satiety. Intriguingly, flies with inactivated SLC5A11 neurons displayed an avoidance response to a tube containing ACV, even after being starved for prolonged periods of time (Figure 2I), i.e., these flies behaved as if they were fed. Furthermore, inactivating these neurons using R4-specific PR38H02-GAL4 driver resulted in a similar avoidance response. Flies carrying the SLC5A11 mutation were also repelled by ACV even when they were starved (Figure S4A). These results are consistent with the hypothesis that inactivation of SLC5A11 neurons reduces the sensitivity to the deprived state and thus, causes flies to reduce their food intake and their preference for a nutritive sugar.
By contrast, flies with activated SLC5A11 neurons were attracted to a tube containing ACV in a T-maze even when they were fed (Figure 2J), i.e., they behaved as if they had been starved. These flies also demonstrated a dramatic increase in the rate of their proboscis extension response (PER), even in the absence of food (Figure S4B). The natural feeding pattern in the fly is characterized by the repeated extension and retraction of the proboscis, which is evoked by contact with food. Starved flies often exhibit an increase in PER responses without appetitive stimuli. These results, together with the consumption of larger amounts of food and selection of nutritive sugars over nonnutritive sugars by flies with activated SLC5A11 neurons, support the claim that activation of SLC5A11 neurons enhances the motivational state of hunger.
Up-regulation of SLC5A11 transcripts during starvation
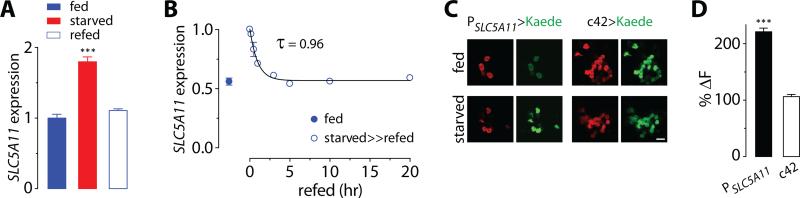
Having demonstrated that SLC5A11 neuronal activity is essential for producing hunger-driven behaviors, we next sought to understand how SLC5A11 regulates the excitability of these neurons. The excitability of SLC5A11 neurons is diminished by the SLC5A11 mutations and is enhanced by the overexpression of SLC5A11 (Figures 1C, 1D, 5B, 5C, and S2C). To investigate a possibility that the expression of SLC5A11 is modulated by the hunger state, we used quantitative reverse transcription polymerase chain reaction (qRT-PCR) to examine its transcript levels in vivo. We found that the expression of SLC5A11 transcripts was upregulated in the brain of flies that had been starved, but this increase dropped significantly after the starved flies were fed again for 30-60 minutes (Figures 3A and 3B). It is worth noting that SLC5A11 transcripts began to decrease only after the starved animals were fed for 10 minutes, illustrating the rapid regulation by changes in the internal energy state (Figure 3B).
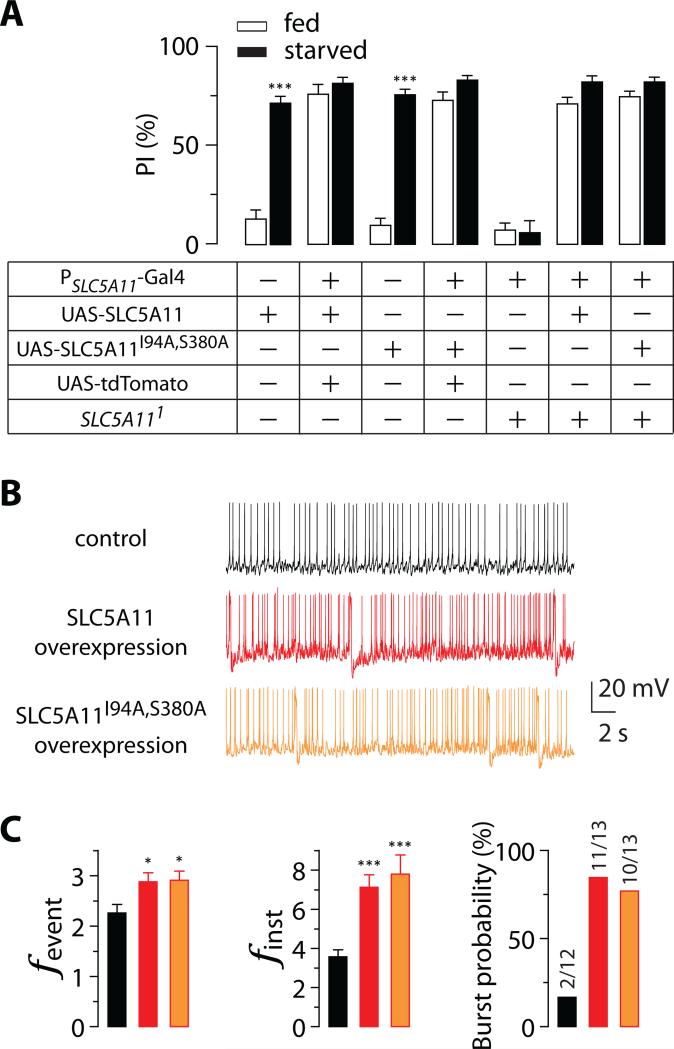
Fig. 5. The leak current is not required for hunger-driven food choice behavior and the excitability of SLC5A11 neurons.
(A) Behavior responses of 5-hr (fed) or 22-hr (starved) deprived flies carrying PSLC5A11-GAL4 and either UAS-SLC5A11 or UAS-SLC5A11I94A,S380A in the two-choice assay (n = 6-10; ***P < 0.001 vs. fed in the same genotype). (B) Representative traces of spontaneous activity and (C) quantification of firing frequency and bursting probability in SLC5A11 neurons of fed flies carrying PSLC5A11-GAL4 and either UAS-SLC5A11 or UAS-SLC5A11I94A,S380A, and flies carrying PSLC5A11-GAL4 only (control). (n = 12-13; *P < 0.05, ***P < 0.001 vs. control). Note that the bars from control group were reproduced for the purposed of comparison (see Figure 2B). fevent, event frequency; finst, instantaneous frequency. The numbers of bursting neurons out of the total number of recorded neurons are indicated above the bars. Error bars indicate SEM. See also Figure S2.
Fig. 3. Starvation increases the expression of SLC5A11 transcript.
(A) Quantitative real-time PCR assay for SLC5A11 transcripts using the SYBR green method from the brains of flies that were food deprived for 5 hours (fed) and for 22 hours (starved), and had been deprived for 22 hours, but were refed for 24 hours (refed). The transcript levels in starved and refed flies are normalized to those in fed flies. GAPDH is used as a reference (n = 9, three independent biological replicates; ***P < 0.001 vs. fed). (B) SLC5A11 transcript levels of fed flies (fed), and 22-hr starved flies that were refed for 0, 10, 30, 60, 180, 300, 600 and 1,200 minutes (starved>>refed). The transcript levels of the starved>>refed flies are normalized to those in starved flies. The rate constant is predicted by a single exponential decay function. (C) Starvation-induced transcriptional activity of the SLC5A11 promoter in SLC5A11 neurons is visualized by monitoring de novo synthesis of green fluorescence in flies carrying PSLC5A11-GAL4 and UAS-Kaede. After preexisting green fluorescence was photoconverted to red fluorescence, the flies were reared in either fed or starved conditions prior to fluorescence imaging. Scale bar, 20μm. (D) Relative amount of newly synthesized green KAEDE in SLC5A11 neurons in starved condition. The values were calculated by normalizing de novo KAEDE to preexisting red KAEDE (ΔF) followed by normalizing the measurements from starved condition to those from fed condition (%ΔF; n = 26-42; ***P < 0.001). Note that the control c42-Gal4 labeled neurons comprise a subset of R2 and R4 neurons. Error bars indicate SEM.
To determine whether the induction of SLC5A11 transcripts occurs specifically in SLC5A11-expressing R4 cells, we expressed the photoconvertible fluorescent protein, Kaede [24], in SLC5A11 neurons, where its normal green fluorescence is photoconverted to red, then monitored the appearance of Kaede's native green fluorescence under the control of the SLC5A11 promoter in fed versus starved flies. Consistent with the qRT-PCR results, the expression of green Kaede by the SLC5A11 promoter, but not by the control c42 promoter, was enhanced in SLC5A11 neurons when the flies were kept in a vial without food compared with flies that were allowed to feed ad libitum (Figures 3C and 3D). This observation indicates that the SLC5A11 promoter stimulates the expression of SLC5A11 transcripts when the internal energy reservoir of the fly is depleted.
SLC5A11 unlikely functions as a sodium/glucose co-transporter
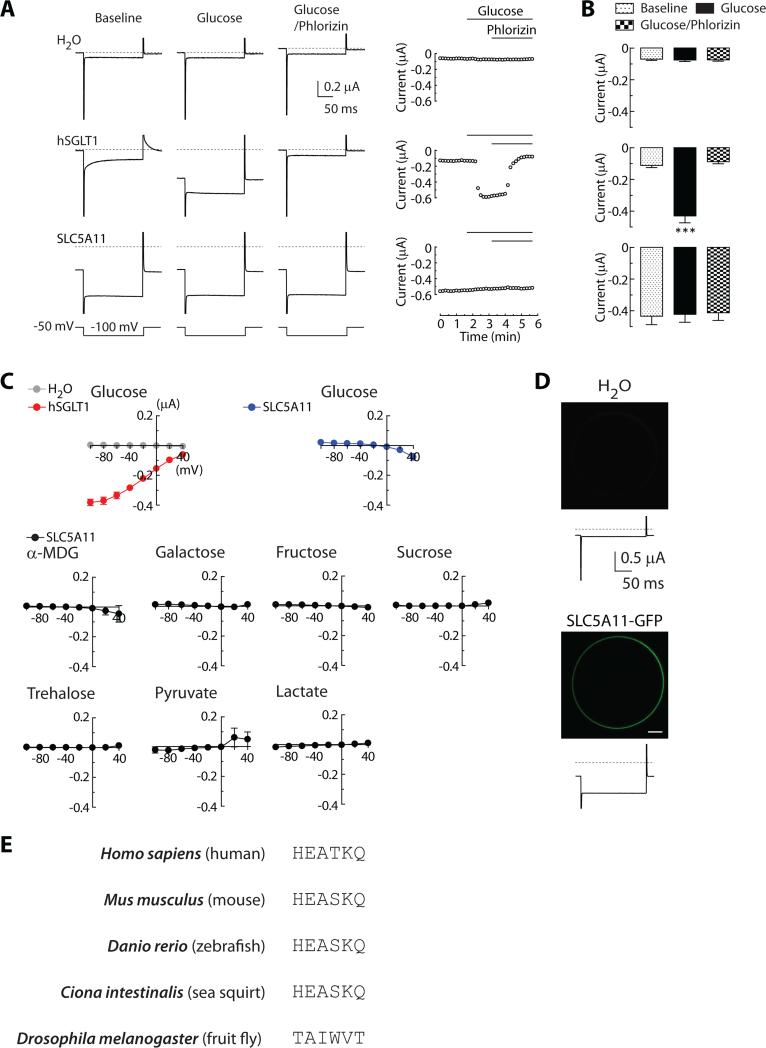
SLC5A11 (or sodium/solute co-transporter-like) encodes a transmembrane protein that is homologous to the sodium/glucose co-transporter [17]. To reconstitute the proposed function of SLC5A11 in a heterologous system, we expressed SLC5A11 in Xenopus oocytes, and then measured glucose-dependent co-transport currents using the two-electrode voltage-clamp technique. As a control, we measured the co-transport currents in oocytes expressing human sodium/glucose co-transporter 1 (hSGLT1), which were rapidly abolished by the presence of phlorizin, a SGLT inhibitor (Figures 4A and 4B). However, oocytes injected with SLC5A11 cRNA did not develop any recognizable glucose-dependent co-transport currents. Nor did we observe co-transport current when other sugars were applied (Figure 4C). The abundant amount of SLC5A11 protein has been translated and localized properly in the plasma membrane of the oocytes (Figure 4D), ruling out the possibility that the SLC5A11 protein had been misfolded or mislocalized. While carrying out sequence alignment, we unexpectedly found that the putative sugar-binding site of SGLTs found in other species is completely absent from the fly SLC5A11 (Figure 4E). This revelation is consistent with our inability to reconstitute sugar-dependent co-transport currents.
Fig. 4. SLC5A11 does not produce sugar-dependent co-transport currents.
(A) Representative current traces in response to -100 mV test pulse from a holding potential of −50 mV in oocytes injected with H2O, hSGLT1, or SLC5A11 cRNA. Only hSGLT1 expressing oocytes show glucose-dependent co-transport currents, which are blocked by the application of phlorizin, an antagonist of SGLT1. Corresponding time courses of peak current amplitude (right). (B) Quantification of current amplitudes recorded from oocytes injected with H2O, hSGLT1, or SLC5A11 cRNA (n = 9-11; ***P < 0.001 vs. baseline). (C) Sugar- or monocarboxylate-dependent co-transport current and voltage relationship measured in oocytes expressing SLC5A11 or hSGLT1, exposed to different stimuli (n = 3-4). (D) Confocal images of oocytes injected with H2O or EGFP-tagged SLC5A11 (upper). Current traces recorded from the corresponding oocytes (lower). Scale bar, 200μm. (E) Sequence alignment of the putative sugar-binding site of sodium/glucose co-transporter homologs in different species. See also Figure S5.
A notable feature of SLC5A11-expressing oocytes is a large leak current (Figures 4A, 4B and S5A), which requires its conserved sodium-binding site (Figure S5B) and cations including sodium ions (Figures S5C and S5D). This prominent characteristic is not important for SLC5A11 neurons to promote hunger-driven behaviors, however. Expression of a SLC5A11 cDNA transgene containing sodium-binding site mutations, UAS-SLC5A11I94A,S380A, in SLC5A11 neurons completely rescued the behavioral defects of SLC5A111 mutant (Figure 5A). Its dispensability was supported further when we observed that the expression of the mutant UAS-SLC5A11I94A,S380A in SLC5A11 neurons has dominant effects similar to those of the WT UAS-SLC5A11, which causes the flies to exhibit a strong preference for nutritive D-glucose even when they were fed (Figure 5A). Consistent with these results, whole-cell recordings showed that overexpression of either UAS-SLC5A11 or UAS-SLC5A11I94A,S380A significantly potentiated membrane excitability (Figure 5B, 5C, and S2C), validating that the sodium-binding site is not essential for mediating the neuronal excitability.
SLC5A11 inhibits K+ channel activity
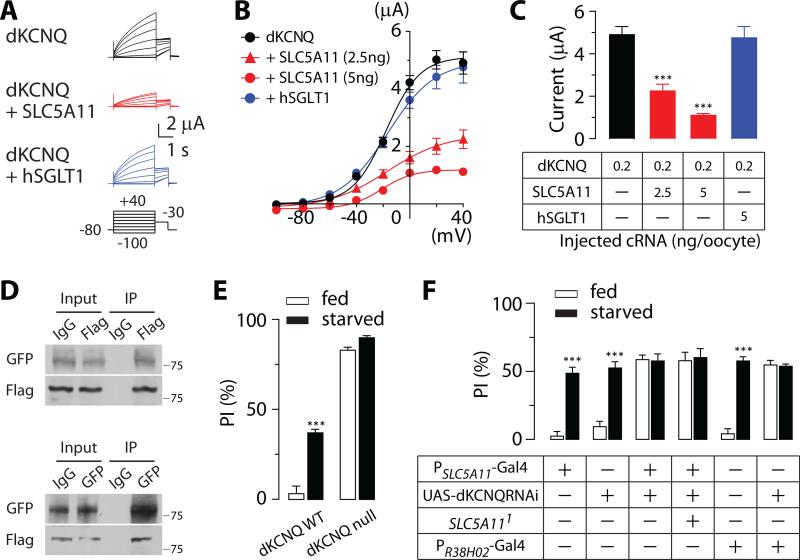
To understand how an increase in SLC5A11 expression enhances the excitability of SLC5A11 neurons, we investigated the possibility that SLC5A11 modulates K+ currents generated by the Drosophila KCNQ (dKCNQ) channel [16]. It was recently shown that a sodium/solute co-transporter, the human sodium/myoinositol co-transporter 1 (hSMIT1) interact with the human KCNQ1 (hKCNQ1) channel to modify its activity [25]. We found that expression of dKCNQ in Xenopus oocytes results in the generation of large outward K+ currents (Figure 6A-6C). This effect is significantly inhibited by the coexpression of SLC5A11, whereas hSGLT1 has little or no effect on the dKCNQ currents (Figure 6A-6C). The effect of SLC5A11 coexpression on the dKCNQ currents was dependent on the amount of cRNAs that was injected, which further corroborates the specificity of its effect (Figure 6C). This effect cannot be accounted for by any change in the voltage dependence of activation and activation time constant (Figure S6). Based on this finding, we explored a possibility that SLC5A11 and dKCNQ physically interact with each other. After expressing SLC5A11-GFP and dKCNQ-Flag in HEK293 cells, reciprocal co-immunoprecipitation using anti-GFP or anti-Flag antibody reveals that SLC5A11 does co-assemble with dKCNQ (Figure 6D).
Fig. 6. Decreased dKCNQ channel activity mediated by SLC5A11 triggers hunger-driven food choice behavior.
(A) Representative current traces and (B) current-voltage relationships in oocytes expressing dKCNQ only, dKCNQ + SLC5A11, and dKCNQ + hSGLT1. (C) Average values for the dKCNQ current amplitude measured at 40mV with oocytes injected with different amounts of the corresponding cRNA (n = 17-20; ***P < 0.001 vs. dKCNQ only). See also Figure S6. (D) Coimmnoprecipitation from HEK293 cells expressing both dKCNQ-Flag and SLC5A11-GFP by using anti-Flag (upper) or anti-GFP (lower) antibodies, followed by the Western analysis. The result is a representative of three independent experiments (n = 3). (E) Behavior responses of dKCNQ mutant and control WT flies (n = 6-7; ***P < 0.001 vs. fed in the same genotype) and (F) behavior responses of flies in which dKCNQ was knocked down in SLC5A11 neurons by the expression of dKCNQ RNAi using PSLC5A11-GAL4 or PR38H02-GAL4 (n = 7-9; ***P < 0.001 vs. fed in the same genotype) in the two-choice assay. These flies were starved for 5 hours (fed) or 22 hours (starved) and were given a choice between 50mM D-glucose and 220mM (E) or 270mM (F) L-glucose. Flies bearing either PSLC5A11-GAL4, PR38H02-GAL4, or UAS-responder alone were used as controls. Error bars indicate SEM.
dKCNQ is required in SLC5A11 neurons to mediate hunger
These unanticipated findings raised a question of whether dKCNQ regulates feeding behavior by controlling the excitability of SLC5A11 neurons. Indeed, dKCNQ mutants [26] strongly preferred nutritive D-glucose to nonnutritive L-glucose even when they were fed (Figure 6E). To determine whether this defect is a result of impaired activity of SLC5A11 neurons, we knocked down the endogenous dKCNQ transcript by expressing dKCNQ RNAi in SLC5A11 neurons using PSLC5A11-GAL4 or R4-specific PR38H02-GAL4 driver. Similar to the dKCNQ mutant phenotype, flies expressing the dKCNQ RNAi in SLC5A11 neurons exhibited a robust preference for the nutritive sugar even when fed. This suggests that dKCNQ is required in these neurons to regulate appropriate food selection (Figure 6F).
DISCUSSION
In this work, we have presented three key findings: (1) the activity of SLC5A11 R4 neurons and the expression of SLC5A11 transcripts are stimulated by starvation; 2) the activity of SLC5A11 neurons and the function of SLC5A11 are necessary and sufficient for promoting food intake and hunger-driven behaviors; 3) the starvation-induced expression of SLC5A11 enhances the excitability of SLC5A11 neurons by inhibiting dKCNQ potassium channel activity in these neurons. Together, we propose that SLC5A11 neurons encode a motivational state of hunger and respond to it by promoting feeding behaviors.
We identified an SLC5A11 mutant through a genetic screen for flies that failed to respond to a nutritive sugar during starvation [17]. Because of its homology to sodium/glucose co-transporters, we initially hypothesized that SLC5A11 is a sensor that detects the nutritional value of sugar. Our current experiments suggest, however, that SLC5A11 is a prescriptive sensor of hunger rather than sugar, monitoring the internal energy levels in the fly and transmitting information about the hunger state to induce feeding and other hunger-driven behaviors. First, the spontaneous activity of SLC5A11 neurons was not changed by the application of sugar solutions, but was significantly altered with an increase in firing frequency and bursting probability following periods of starvation. Second, the artificial activation of SLC5A11 neurons resulted in a preference for the nutritive D-glucose over the nonnutritive, yet sweeter L-glucose even in fed flies. If SLC5A11 neurons function as a nutrient sensor, flies with activated SLC5A11 neurons would not exhibit a preference for either D- or L-glucose, because both enantiomers would be perceived as nutritious. Our laboratory recently identified a bona fide nutrient sensor in the Drosophila brain that consists of DH44-expressing neurons, which are activated specifically by nutritive sugars [27]. Flies with activated DH44 neurons did not display a preference for either D- or L-glucose because activation of the nutrient sensor was sufficient to communicate the “reward” of the nutrient [27]. Furthermore, both activation and inactivation of SLC5A11 neurons had substantial effects on the amount of food intake, hunger-driven olfactory attraction to food odor and PER response. These effects were probably a result of SLC5A11 neurons mediating the state of hunger rather than detecting the nutritional value of the sugar. In fact, the manipulation of DH44 neurons had little or no effect on the amount of food intake.
Drosophila SLC5A11 is structurally similar to mammalian sodium/glucose co-transporters, with approximately 25% of its amino acid sequence identical to that of the human SGLTs [17]. While human SGLT1 transports glucose into cells along a sodium gradient, SGLT3 acts as a glucose-activated channel that induces changes in the membrane potential [28]. In our experiments, Drosophila SLC5A11 did not function as a sodium/glucose co-transporter, i.e., it did not generate glucose-dependent co-transport currents and was not found to contain a canonical sugar-binding site. Interestingly, it did not appear to function as a channel in SLC5A11 R4 neurons, either. Mutations in its sodium-binding site that completely abolished cationic currents were able to potentiate membrane excitability and rescue the behavioral defects seen in SLC5A11 mutant. These observations further support our claim that the starvation-induced expression of SLC5A11 indirectly modulates the excitability of SLC5A11 neurons by targeting an intermediary key factor, a potassium channel. Our findings that SLC5A11 interacts physically with dKNCQ and that a knock-down of dKCNQ in SLC5A11 neurons results in hunger-driven behaviors even in the SLC5A11 mutant background suggest that SLC5A11 presumably functions upstream of dKCNQ.
Ion channels on the plasma membrane often show unexpected properties in vivo as their interactions with various signaling or scaffolding proteins influence the function and localization of ion channels, thereby modulating the signaling potential [29-31]. This was demonstrated in a recent study in which KCNQ1 is coimmunoprecipitated with SMIT1 in the epithelium of the choroid plexus and that KCNQ1 activity is modulated by SMIT1 in a heterologous expression system [25]. Similarly, we found that SLC5A11 interacts physically with dKCNQ and inhibits its potassium channel activity when they are co-expressed in Xenopus oocytes. A knock-down of dKCNQ in SLC5A11 neurons phenocopied the overexpression of SLC5A11 in the two-choice assay. This suggests that SLC5A11 acts selectively as a spatially restricted regulator of the potassium channel in neurons that are encoded to detect hunger. Despite the substantial expression of SGLTs in the mammalian brains, including the hypothalamus [32], their physiological significance in feeding and other behaviors is not known. Our finding may shed light on understanding their role in the mammalian brain.
The function of SLC5A11 neurons in the fly resembles that of NPY/AgRP neurons in the mammalian hypothalamus. Similar to SLC5A11 neurons, the hypothalamic NPY/AgRP neurons are essential for promoting feeding behavior that can restore the animal's energy balance and nutrient storage levels, by transducing metabolic signals into electrical activity in mice [33, 34]. Similar to SLC5A11 neurons, the activity of NPY/AgRP neurons increases following periods of food deprivation [35-37]. It has been demonstrated that the intrinsic membrane properties are the key to starvation-induced changes in firing frequency occurred in these neurons [35]. While these studies showed synaptic inputs are not necessary, others reported that the internal energy state causes changes in excitatory glutamatergic transmission onto NPY/AgRP neurons through a presynaptic or postsynaptic mechanism [36, 37]. Our studies in Drosophila show that starvation-induced changes in intrinsic excitability are highly responsible for modulating the firing frequency and bursting probability in the hunger-encoding SLC5A11 neurons. The non-inactivating KCNQ-dependent M-current functions as a “brake” on repetitive neuronal activity and, thus, is important for regulating the excitability of various neurons [38]. Recently, the M-current in NPY/AgRP neurons was shown to decrease during starvation; this is consistent with the decreased expression of KCNQ [39]. Whether KCNQ activity in NPY/AgRP neurons is required for feeding behavior to be triggered remains to be seen.
In summary, our findings suggest that SLC5A11 neurons comprise an essential component of the neural representation of the response to hunger and act through the coordinated activities of SLC5A11 and dKCNQ. Their activity leads to a series of behavioral changes that allow animals to obtain sufficient nutrients and calories to meet their needs for growth, tissue maintenance, and homeostasis.
Experimental Procedures
Electrophysiological recordings of the Drosophila brains
Ex vivo whole-cell patch clamp recordings were performed on dissected adult fly brains as reported previously[42]. Each brain was allowed to rest in continuously flowing saline (in mM: 103 NaCl, 3 KCl, 5 TES, 8 Trehalose, 10 Glucose, 26 NaHCO3, 1 NaH2PO4, 4 MgCl2, 1.5 CaCl2, bubbled with 95% O2/ 5% CO2 to a pH of 7.3) for at least 10 minutes before recording and the superfusion was continued throughout the recording period. One neuron per brain was recorded. The internal solution for patch-clamp pipettes were contained the following (in mM): 140 K-gluconate, 10 HEPES, 1 EGTA, 4 MgATP, 0.5 Na3GTP, pH7.3. Recordings were made using a MultiClamp 700B amplifier, a Digidata 1440A, and pClamp 10 software (Molecular Devices). Recorded voltages were low-pass filtered at 5 kHz and digitized at 10 kHz. After whole cell break-in, followed by stabilizing membrane properties for 5 minutes, spontaneous neuronal activity was measured for 5 minutes in current clamp configuration and action potential frequencies (event frequency, fevent, and instantaneous frequency, finst) and burst probability were analyzed. Event frequency was calculated by counting the number of action potentials divided by a given period of recording time (in second). Instantaneous frequency is the average inverse of the time interval between two successive action potentials. To obtain the burst probability, the bursting neurons were arbitrarily defined as the neurons demonstrating at least 5 burst events during a recording period (5 minutes). From control flies carrying PSLC5A11-GAL4 and UAS-tdTomato (Fig. 1d), the mean numbers of burst events in fed condition and starved condition were 1.7 and 18.4 respectively.
Two-choice assay
The two-choice preference assay was performed as previously reported [5]. For details, see the Supplemental Experimental Procedures.
T-maze preference assay
T-maze assay was performed as described previously [40, 41] and outlined in the Supplemental Experimental Procedures.
Measurement of food intake
Food intake was estimated using food labeling with a colorimetric dye. For details, see the Supplemental Experimental Procedures.
Other Methods
See the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgements
We are grateful to Drs. ___ and the Suh laboratory for critical comments on the manuscript. We thank Rolf Bodmer for providing dKCNQ186 and dKCNQ97 flies, and Irwin Levitan for dKCNQ cDNA. We thank Jason Lai, Tomohiro Yumita, and Nicholas Cuvelier for their generous assistance. This work is supported by NIH RO1 grant (NINDS NS30989) to B.R., and NIH RO1 grants (NIGMS RO1GM08946, NIDCD RO1DC01279, NIDDK RO1DK106636) and the Irma T. Hirschl/Weill Caulier Trust Award to G.S.B.S.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions. J.P. performed electrophysiology, qRT-PCR, and two-choice behavior experiments. M.D. performed food intake, T-maze, PER, and Kaede experiments. S.K. conducted co-IP experiments. F.A. performed CaLexA experiments. M.I.K. helped with imaging. J.P. and G.S.B.S. designed and analyzed experiments, and wrote the manuscript with input from all authors.
References
- 1.Cannon WB. The wisdom of the body. W.W. Norton & Company, Inc; New York, NY: 1939. [Google Scholar]
- 2.Morton GJ, Cummings DE, Baskin DG, Barsh GS, Schwartz MW. Central nervous system control of food intake and body weight. Nature. 2006;443:289–295. doi: 10.1038/nature05026. [DOI] [PubMed] [Google Scholar]
- 3.Adolph EF. Urges to eat and drink in rats. The American journal of physiology. 1947;151:110–125. doi: 10.1152/ajplegacy.1947.151.1.110. [DOI] [PubMed] [Google Scholar]
- 4.Dethier VG. The Hungry Fly. Harvard University Press; Cambridge, MA: 1976. [Google Scholar]
- 5.Dus M, Min S, Keene AC, Lee GY, Suh GS. Taste-independent detection of the caloric content of sugar in Drosophila. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:11644–11649. doi: 10.1073/pnas.1017096108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Su CY, Wang JW. Modulation of neural circuits: how stimulus context shapes innate behavior in Drosophila. Current opinion in neurobiology. 2014;29:9–16. doi: 10.1016/j.conb.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee G, Park JH. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics. 2004;167:311–323. doi: 10.1534/genetics.167.1.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pager J, Giachetti I, Holley A, Le Magnen J. A selective control of olfactory bulb electrical activity in relation to food deprivation and satiety in rats. Physiology & behavior. 1972;9:573–579. doi: 10.1016/0031-9384(72)90014-5. [DOI] [PubMed] [Google Scholar]
- 9.Pirke KM, Broocks A, Wilckens T, Marquard R, Schweiger U. Starvation-induced hyperactivity in the rat: the role of endocrine and neurotransmitter changes. Neuroscience and biobehavioral reviews. 1993;17:287–294. doi: 10.1016/s0149-7634(05)80012-0. [DOI] [PubMed] [Google Scholar]
- 10.Leopold P, Perrimon N. Drosophila and the genetics of the internal milieu. Nature. 2007;450:186–188. doi: 10.1038/nature06286. [DOI] [PubMed] [Google Scholar]
- 11.Sclafani A, Ackroff K. Role of gut nutrient sensing in stimulating appetite and conditioning food preferences. American journal of physiology. Regulatory, integrative and comparative physiology. 2012;302:R1119–1133. doi: 10.1152/ajpregu.00038.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Araujo IE, Oliveira-Maia AJ, Sotnikova TD, Gainetdinov RR, Caron MG, Nicolelis MA, Simon SA. Food reward in the absence of taste receptor signaling. Neuron. 2008;57:930–941. doi: 10.1016/j.neuron.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 13.Venken KJ, Simpson JH, Bellen HJ. Genetic manipulation of genes and cells in the nervous system of the fruit fly. Neuron. 2011;72:202–230. doi: 10.1016/j.neuron.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thorne N, Chromey C, Bray S, Amrein H. Taste perception and coding in Drosophila. Current biology : CB. 2004;14:1065–1079. doi: 10.1016/j.cub.2004.05.019. [DOI] [PubMed] [Google Scholar]
- 15.Wang Z, Singhvi A, Kong P, Scott K. Taste representations in the Drosophila brain. Cell. 2004;117:981–991. doi: 10.1016/j.cell.2004.06.011. [DOI] [PubMed] [Google Scholar]
- 16.Wen H, Weiger TM, Ferguson TS, Shahidullah M, Scott SS, Levitan IB. A Drosophila KCNQ channel essential for early embryonic development. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2005;25:10147–10156. doi: 10.1523/JNEUROSCI.3086-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dus M, Ai M, Suh GS. Taste-independent nutrient selection is mediated by a brain-specific Na+ /solute co-transporter in Drosophila. Nature neuroscience. 2013;16:526–528. doi: 10.1038/nn.3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Masuyama K, Zhang Y, Rao Y, Wang JW. Mapping neural circuits with activity-dependent nuclear import of a transcription factor. Journal of neurogenetics. 2012;26:89–102. doi: 10.3109/01677063.2011.642910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nitabach MN, Sheeba V, Vera DA, Blau J, Holmes TC. Membrane electrical excitability is necessary for the free-running larval Drosophila circadian clock. Journal of neurobiology. 2005;62:1–13. doi: 10.1002/neu.20053. [DOI] [PubMed] [Google Scholar]
- 20.Parisky KM, Agosto J, Pulver SR, Shang Y, Kuklin E, Hodge JJ, Kang K, Liu X, Garrity PA, Rosbash M, et al. PDF cells are a GABA-responsive wake-promoting component of the Drosophila sleep circuit. Neuron. 2008;60:672–682. doi: 10.1016/j.neuron.2008.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitamoto T. Conditional modification of behavior in Drosophila by targeted expression of a temperature-sensitive shibire allele in defined neurons. Journal of neurobiology. 2001;47:81–92. doi: 10.1002/neu.1018. [DOI] [PubMed] [Google Scholar]
- 22.Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- 23.Semmelhack JL, Wang JW. Select Drosophila glomeruli mediate innate olfactory attraction and aversion. Nature. 2009;459:218–223. doi: 10.1038/nature07983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ando R, Hama H, Yamamoto-Hino M, Mizuno H, Miyawaki A. An optical marker based on the UV-induced green-to-red photoconversion of a fluorescent protein. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12651–12656. doi: 10.1073/pnas.202320599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbott GW, Tai KK, Neverisky DL, Hansler A, Hu Z, Roepke TK, Lerner DJ, Chen Q, Liu L, Zupan B, et al. KCNQ1, KCNE2, and Na+-coupled solute transporters form reciprocally regulating complexes that affect neuronal excitability. Science signaling. 2014;7:ra22. doi: 10.1126/scisignal.2005025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ocorr K, Reeves NL, Wessells RJ, Fink M, Chen HS, Akasaka T, Yasuda S, Metzger JM, Giles W, Posakony JW, et al. KCNQ potassium channel mutations cause cardiac arrhythmias in Drosophila that mimic the effects of aging. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:3943–3948. doi: 10.1073/pnas.0609278104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dus M, Lai JS, Gunapala KM, Min S, Tayler TD, Hergarden AC, Geraud E, Joseph CM, Suh GS. Nutrient Sensor in the Brain Directs the Action of the Brain-Gut Axis in Drosophila. Neuron. 2015;87:139–151. doi: 10.1016/j.neuron.2015.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diez-Sampedro A, Hirayama BA, Osswald C, Gorboulev V, Baumgarten K, Volk C, Wright EM, Koepsell H. A glucose sensor hiding in a family of transporters. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11753–11758. doi: 10.1073/pnas.1733027100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang J, Bal M, Bierbower S, Zaika O, Shapiro MS. AKAP79/150 signal complexes in G-protein modulation of neuronal ion channels. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:7199–7211. doi: 10.1523/JNEUROSCI.4446-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuller MD, Fu Y, Scheuer T, Catterall WA. Differential regulation of CaV1.2 channels by cAMP-dependent protein kinase bound to A-kinase anchoring proteins 15 and 79/150. The Journal of general physiology. 2014;143:315–324. doi: 10.1085/jgp.201311075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu XY, Chu XP, Mao LM, Wang M, Lan HX, Li MH, Zhang GC, Parelkar NK, Fibuch EE, Haines M, et al. Modulation of D2R-NR2B interactions in response to cocaine. Neuron. 2006;52:897–909. doi: 10.1016/j.neuron.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 32.Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev. 2011;91:733–794. doi: 10.1152/physrev.00055.2009. [DOI] [PubMed] [Google Scholar]
- 33.Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310:683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- 34.Aponte Y, Atasoy D, Sternson SM. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nature neuroscience. 2011;14:351–355. doi: 10.1038/nn.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi KA, Cone RD. Fasting induces a large, leptin-dependent increase in the intrinsic action potential frequency of orexigenic arcuate nucleus neuropeptide Y/Agouti-related protein neurons. Endocrinology. 2005;146:1043–1047. doi: 10.1210/en.2004-1397. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Atasoy D, Su HH, Sternson SM. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell. 2011;146:992–1003. doi: 10.1016/j.cell.2011.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu T, Kong D, Shah BP, Ye C, Koda S, Saunders A, Ding JB, Yang Z, Sabatini BL, Lowell BB. Fasting activation of AgRP neurons requires NMDA receptors and involves spinogenesis and increased excitatory tone. Neuron. 2012;73:511–522. doi: 10.1016/j.neuron.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown DA, Passmore GM. Neural KCNQ (Kv7) channels. British journal of pharmacology. 2009;156:1185–1195. doi: 10.1111/j.1476-5381.2009.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roepke TA, Qiu J, Smith AW, Ronnekleiv OK, Kelly MJ. Fasting and 17beta-estradiol differentially modulate the M-current in neuropeptide Y neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2011;31:11825–11835. doi: 10.1523/JNEUROSCI.1395-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh GS, Wong AM, Hergarden AC, Wang JW, Simon AF, Benzer S, Axel R, Anderson DJ. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 41.Ai M, Min S, Grosjean Y, Leblanc C, Bell R, Benton R, Suh GS. Acid sensing by the Drosophila olfactory system. Nature. 2010;468:691–695. doi: 10.1038/nature09537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gu H, O'Dowd DK. Cholinergic synaptic transmission in adult Drosophila Kenyon cells in situ. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:265–272. doi: 10.1523/JNEUROSCI.4109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.