Abstract
Two thermally activated ruthenium(II) polypyridyl complexes, cis-Ru(bpy)2Cl2 and trans-Ru(qpy)Cl2 were investigated to determine the impact of the geometric arrangement of the exchangable ligands on the potential of the compounds to act as chemotherapeutics. In contrast to the geometry requirements for cisplatin, trans-Ru(qpy)Cl2 was 7.1–9.5× more cytotoxic than cis-Ru(bpy)2Cl2. This discovery could open up a new area of metal-based chemotherapeutic research.
Graphical Abstract
Cisplatin, cis-[PtCl2(NH3)2] has received worldwide acceptance as a clinical drug for the treatment of various neoplastic diseases,1 while its isomer transplatin, trans-[PtCl2(NH3)2] was found to be therapeutically inactive.2 This observation was considered a paradigm for the structure-activity relationships (SAR) of Pt-based antitumor compounds, according to which the antitumor activity requires a neutral square-planar platinum center with two ammine ligands and two leaving groups in cis-geometry.2 The lack of antitumor activity in transplatin has been associated with the formation of intrastrand cross-links between purine-pyrimidine3 residues instead of purine-purine (major DNA adducts formed by cisplatin)4 due to stereochemical constraints.
In addition to Pt based compounds, other metal complexes have been shown to have biological activity in vitro, including potency in cisplatin-resistant tumor cells.5 Ruthenium has received particular attention in the present search for therapeutic agents, and ruthenium compounds exhibit antitumor effects as well as antibiotic, antiviral, and antimalarial activity.5b Two anionic Ru(III) coordination compounds, NAMI-A and KP1019, possess a strong ability to inhibit metastases of solid invasive cancers and successfully completed phase I clinical trials but ultimately failed in phase II clinical trials.6 Generally, antitumor Ru(II) complexes can be divided in two primary families, i.e. the half-sandwich “piano-stool” and polypyridyl-types.5b The later family has been gaining attention due to their appealing physicochemical properties, which offer the possibility to use them in photodynamic therapy (PDT) and photoactivated chemotherapy (PACT).7 However, all the Ru(II) compounds able to form covalent bonds to biomolecules exhibit a cis geometry, and no examples of trans polypyridyl Ru(II) isomers with biological activity are yet known, in spite of their interesting photophysical and catalytic properties.8 But, does geometry matter in the design of anticancer Ru(II) complexes? Here we report that geometry appears to play a very important role, and moreover, a Ru(II) polypyridyl complex with exchangeable ligands with trans geometry exhibits in vitro anticancer activity significantly superior to the cis compound.
In order to determine the behavior of trans Ru(II) polypyridyl complexes, trans-Ru(qpy)Cl2 (2a, qpy = 2,2′:6′,2″:6″,2‴-quaterpyridine) was synthesized and the Cl− ligand exchange rate, DNA binding, cytotoxicity, and cellular uptake were investigated in comparison to cis-Ru(bpy)2Cl2 (1a, bpy = 2,2′-bipyridine) (Chart 1). The qpy ligand was chosen to generate a complex with exchangeable sites only in the trans arrangement; this was required as trans-Ru(bpy)2Cl2 is known to be highly insoluble.9 Alternatively, attempting a comparison of the cis- and trans-Ru(bpy)2(H2O)2 complexes is not viable as the two systems photoisomerize.10 The qpy ligand was synthesized via an oxidative coupling reaction with 6-chloro-2,2′-bipyridine; coordination to RuCl3 then yielded 2a.8d
Chart 1.
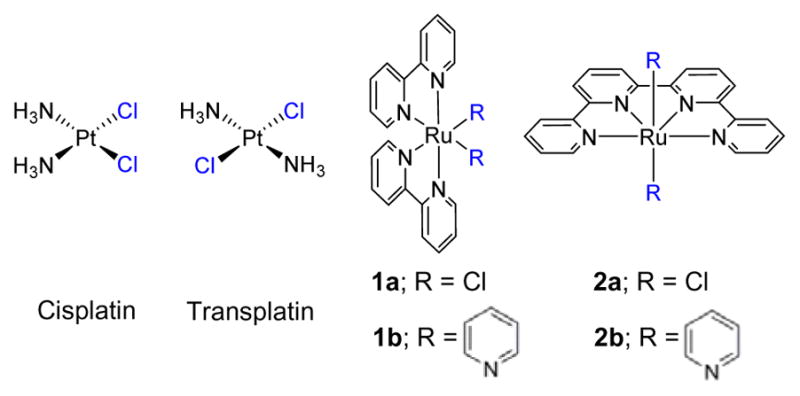
Structures of compounds included in this study. Exchangeable ligands are highlighted in blue
The qpy ligand and derivatives have been reported as a tetradentate ligand in mononuclear Ru(II) complexes,8d, 8e, 11 but some derivatives may act as a bridging ligand in dinuclear Ru(II) complexes.8a, 12 Thus, the mono-metallic structure of the complex 2a was confirmed by the further synthesis and characterization of trans-[Ru(qpy)(py)2]2+ (2b, py = pyridine) from 2a.8d As complex 2b is not capable of ligand exchange, it also served as a control compound to assess if the addition of the qpy ligand itself to the Ru(II) center was responsible for the observed biological activity. Similarly, cis-[Ru(bpy)2(py)2]2+ (1b) was used as a nonexchanging control for the cis geometry.
To determine the efficacy of 1a, 1b, 2a, and 2b in cancer cells, cytotoxicity studies were performed in HL-60 leukemia cells and A549 lung cancer cells and compared to cisplatin and transplatin (Figure 1 and Table 1). For square planar platinum compounds with exchangeable Cl− ligands, the cis geometry is known to be more potent. Here, cisplatin was cytotoxic with IC50 values of 1.5 and 1.7 μM in HL-60 and A549 cells. As anticipated, transplatin had no effect on either cell line.
Figure 1.
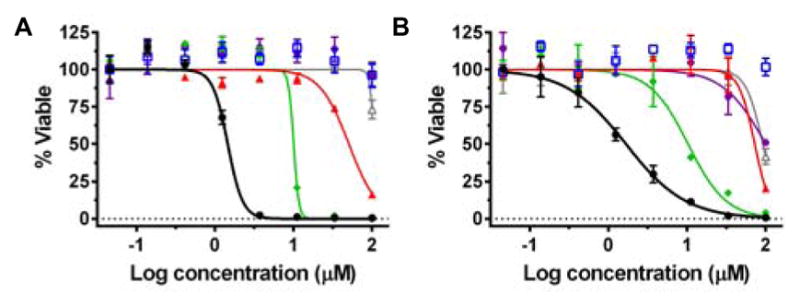
Cytotoxicity of cisplatin (black, ●), transplatin (blue, □), 1a (red, ▲), 1b (purple, ⬣), 2a (green, ◆), and 2b (grey, △) in (A) HL-60 and (B) A549.
Table 1.
Cytotoxicity IC50 values for HL-60 and A549 cell lines.
| Compound | HL-60 IC50 (μM) |
A549 IC50 (μM) |
|---|---|---|
| Cisplatin | 1.5 ± 0.1 | 1.7 ± 0.5 |
| Transplatin | >100 | >100 |
| 1a | 96 ± 1 | 73 ± 1 |
| 1b | >100 | 98 ± 1 |
| 2a | 10.1 ± 1.1 | 10.3 ± 1.2 |
| 2b | >100 | 93 ± 1 |
In marked contrast, for octahedral ruthenium complexes with exchangeable Cl− ligands, the opposite relationship between geometry and cytotoxicity was observed. The complex with exchangeable ligands in the trans geometry was 7.1–9.5× more cytotoxic than the cis geometry. For 1b and 2b, the control compounds that are incapable of ligand exchange, the cytotoxicity was eliminated in HL-60 cells, and only minimal toxicity was observed at 100 μM in A549 cells. Two hypotheses were proposed to rationalize the differences in cytotoxicity for the complexes: 1) the trans geometry interacts with different in vivo targets from the cis geometry; and 2) thermally exchangeable ligands are required for the cytotoxic effect to occur.
The DNA binding behavior for each compound was assessed using agarose gel electrophoresis to compare cis and trans Pt(II) versus Ru(II). Dose responses were performed with cisplatin, transplatin, 1a, 1b, 2a, and 2b, with pUC19 plasmid DNA after reaction at 37 °C for 12 hours (Figure 2). Cisplatin interacts with plasmid DNA at very low concentrations (15 μM) and effectively crosslinks the DNA. The adducts were visualized by the reduced mobility of the DNA at low concentrations of cisplatin, followed by increased mobility of the DNA at higher concentrations. In contrast, transplatin has minimal interaction with the DNA, even at high concentrations, where the migration of the DNA is only effected at ≥ 125 μM transplatin. Surprisingly, when incubated with plasmid DNA, 1a and 2a only showed minimal perturbation of DNA mobility, suggesting that either they do not interact strongly with plasmid DNA or the interaction does not cause significant changes to the supercoiled plasmid structure. Replacement of the Cl− ligands with py ligands resulted in an even smaller effect, with only a slight decrease in mobility at the highest concentration of 1b and 2b.
Figure 2.
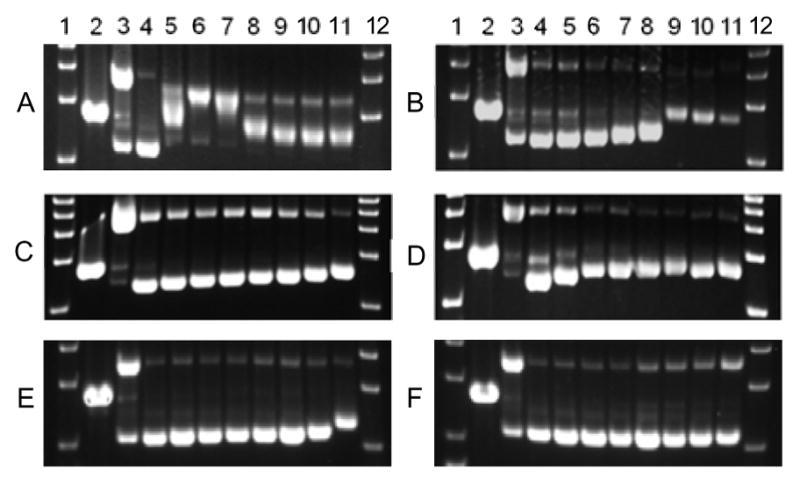
Agarose gel electrophoresis showing the dose response of (A) cisplatin, (B) transplatin, (C) 1a, (D) 2a, (E) 1b, and (F) 2b with 40 μg mL−1 pUC19 DNA incubated at 37 °C. Lanes 1 and 12: DNA ladder; Lane 2: EcoRI; Lane 3: Cu(OP)2; Lane 4–11: 0, 7.8, 15.6, 31.3, 62.5, 125, 250, and 500 μM compound. EcoRI and Cu(OP)2 were used as controls to represent linear DNA and relaxed circle DNA, respectively.
Given that both 1a and 2a contain thermally labile chloride ligands, their ligand exchange rates were monitored using UV/Vis absorption spectroscopy. Compounds 1b and 2b were also studied, and were anticipated to be less susceptible to thermal exchange. Initially, the thermal exchange of 1a, 1b, 2a, and 2b was determined under different buffer and media conditions (Figures 3, S1–S4). Full spectrum absorbance was measured in 96 well plates incubated at 37 °C over the course of 15 hours. To determine the half-life (t1/2) of ligand exchange, the change in absorbance was plotted as a function of time. While a t1/2 could be determined for 1a, compound 2a underwent a very slow ligand exchange and the reaction never reached completion; therefore, the half-life could not be accurately determined. Not surprisingly, 1b and 2b show minimal changes in UV/Vis spectra following 15 hour incubation in aqueous solutions, confirming that they are essentially kinetically inert.
Figure 3.
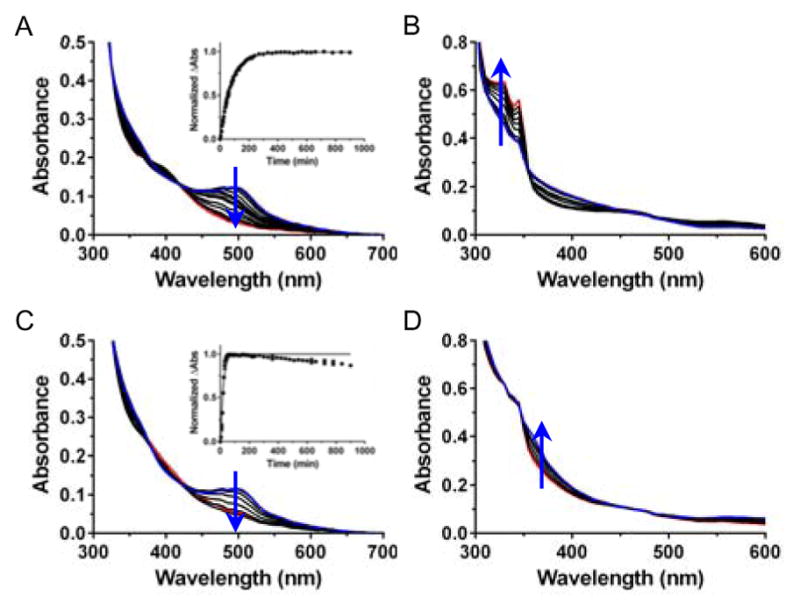
Thermal exchange studies of 40 μM 1a and 2a at 37 °C showing rapid exchange for 1a and slow exchange for 2a. (A) 1a in water, (B) 2a in water, (C) 1a in Opti-MEM, 1% FBS, and (D) 2a in Opti-MEM, 1% FBS. Insets show the change in absorbance fit to a one phase decay equation. Note: 2a undergoes incomplete conversion over the course of 15 hours.
To compare the rates of ligand exchange under different conditions for 1a, 1b, 2a, and 2b, the spectral change at 500, 450, 345 and 325 nm, respectively, was determined. These wavelengths represent the maximal signal change for the majority of conditions tested. Striking differences were seen for 1a and 2a, where 1a exhibited the fastest exchange in Opti-MEM supplemented with 1% FBS (fetal bovine serum, t1/2 = 12.8 min; used as a control for the cell cytotoxicity experiments), and slowest in water (t1/2 = 53 min, Figures 3 and S1, Table S2). On the other hand, 2a had the largest spectral change in water (Δabs = 0.19) yet the smallest change in Opti-MEM with 1% FBS (Δabs = 0.011, Figure 3 and S3, Table S4). Compounds 1b and 2b were studied under the same conditions, but once more only showed minimal change (Δabs < 0.08) over the course of 15 hrs due to the thermally stable pyridine ligands.
The fast exchange for 1a and minimal exchange for 2a in Opti-MEM with 1% FBS may help to explain the drastic differences in cytotoxicity. The slow/minimal reaction of 2a with cell culture media would potentially allow the complex to enter the cell without reaction with media components, whereas the fast reaction of 1a could essentially deactivate the complex prior to entering the cell. In addition to aqueous media, the thermal exchange in the presence of duplex DNA and small molecules, used to mimic the side chains of amino acids, was tested.13 The results from these studies revealed differences in the reactivity profiles for 1a compared to 2a, supporting that fast exchange reactions for 1a prevents it from either entering the cell, or enables unintended side reactions with other biomolecules upon entering the cell.13
One of the causes for transplatin’s inactivity is its high chemical reactivity, where it becomes deactivated through reactions with plasma and tissue proteins before entering a cell.14 In a recent publication, transplatin was successfully internalized as the inactive molecule by encapsulation into nanocapsules, essentially preventing deactivation; following intracellular release it was able to induce a cytotoxic effect.15 Likewise, we have previously reported Ru(II) complex prodrugs that are inactive, but when irradiated with light produce a cis-Ru(bpy)2L2 (L = H2O or Cl−) and are quite cytotoxic.16 It appears that caging the “inactive” compound to allow uptake into cancer cells renders these compounds “active”.
In order to test our deactivation hypothesis, cellular uptake of 1a and 2a was determined in HL-60 cells. The HL-60 cells were incubated in the presence of 20 μM 1a or 2a for 12 hours; following this time 90–95% of cells remained viable. The cells were then harvested and the ruthenium content was determined using graphite furnace atomic absorption spectrometry (GFAAS) for the media and cells separately. The total uptake for 2a (475.8 ng) was 49× greater than the uptake for 1a (9.8 ng); this represents 15% cellular uptake for 2a (Figure S5). These results provide support for the deactivation hypothesis, where the fast reaction of 1a in Opti-MEM renders the complex unable to accumulate in cells. On the other hand, the slow reaction of 2a correlated to significant cellular accumulation, ultimately leading to the cytotoxic effect. Furthermore, flow cytometry confirmed the mechanism of cell death for 2a occurs via apoptosis with no visible sign of necrosis.13 Thus, the damage induced by compound 2a triggers the programmed cell death pathway.
It is possible that the number of exchangeable ligands differed for the cis and trans complexes, and this also could contribute to the disparate biological activities. As both 1a and 2a react with imidazole, the complexes were incubated with this heterocycle until there was no further change in the absorption spectra, and then samples were analyzed by HPLC and mass spectrometry. Both complexes produced new species with longer retention times than the products that form in buffer alone. The reaction of complex 1a with imidazole resulted in full conversion to cis-[Ru(bpy)2(imidazole)2]2+ with the same retention time and absorption spectrum of the molecule produced by chemical synthesis (Figure S8). Mass spectrometry also confirmed that two imidazole ligands replaced the chloride ligands in both the cis complex 1a (Figure S6) and the trans complex 2a (Figure S7). Thus, both the cis and trans complexes can form biadducts, and should be capable of crosslinks, either between DNA bases, DNA and proteins, or within a protein.
SAR studies for cytotoxic metal compounds rarely address the impact of geometry. The history of research in platinum compounds and the inactivity of transplatin were interpreted as a demonstration that a particular geometric arrangement was required for efficacy. However, replacement of the NH3 ligand in ineffective transplatin by planar N-heterocyclic amines produced trans-platinum complexes with significantly improved cytotoxicity due to enhanced rate of bifunctional interstrand adduct formation and altered sequence specificity.17 Not only the geometry requirements for platinum species have been lifted; many compounds with “non-conventional” structures, including polynuclear,18 monofunctional,19 Pt(IV)20 and organometallic21 complexes have displayed anticancer potential. These findings highlighted that more chemical space is available for exploration among platinum compounds than previously thought. The same appears to be true for ruthenium compounds, or, alternatively, different geometries should be investigated than are currently reflected in the literature.
In conclusion, we have synthesized two trans Ru(II) complexes and studied their binding interactions and cytotoxicities in comparison to their cis analogues. The trans complex containing exchangeable ligands is 7.1–9.5× more potent than the cis compound. In addition, the trans complex is accumulated in cells 49× more than the cis compound. We hypothesize that the slower rate of reactivity of 2a is crucial for the cytotoxic activity. The fast and highly reactive cis complex 1a can easily react with media and non-essential biomolecules, becoming inactivated before it is able to enter the cancer cell. On the other hand, the slow exchange of the trans complex 2a allows it to avoid side reactions and reach a vulnerable target within the cell. Lastly, the ability of the Cl− ligands of 2a to exchange is crucial to the biological activity, as evidenced by the absence of activity for the complex incapable of ligand exchange. To the best of our knowledge, this is the first report of the cytotoxic behavior of a thermally activated trans polypyridyl Ru(II) complex. These results have the potential to open up a new area of research to develop a metal-based chemotherapeutic.
Supplementary Material
Acknowledgments
This work was supported by the National Institutes of Health (5R01GM107586). We would like to thank the University of Kentucky Environmental Research Training Laboratory (ERTL) for their assistance in some chemical analysis. Ana Zamora was supported by Fundación Séneca-CARM (Exp. 19020/FPI/13). The Spanish Ministerio de Economía y Competitividad and FEDER (Project CTQ2015-64319-R) is also acknowledged.
Footnotes
Electronic Supplementary Information (ESI) available: experimental details, UV/Vis absorption profiles for thermal exchange and kinetic analysis. See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Kelland L. Nat Rev Cancer. 2007;7:573–584. doi: 10.1038/nrc2167. [DOI] [PubMed] [Google Scholar]
- 2.Farrell N. Transition Metal Complexes as Drugs and Chemotherapeutic Agents. Kluwer; Dordrecht, The Netherlands: 1989. [Google Scholar]
- 3.(a) Brabec V, Leng M. Proc Natl Acad Sci U S A. 1993;90:5345–5349. doi: 10.1073/pnas.90.11.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Brabec V, Síp M, Leng M. Biochemistry. 1993;32:11676–11681. doi: 10.1021/bi00094a025. [DOI] [PubMed] [Google Scholar]; (c) Žákovská A, Nováková O, Balcarová Z, Bierbach U, Farrell N, Brabec V. Eur J Biochem. 1998;254:547–557. doi: 10.1046/j.1432-1327.1998.2540547.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Huang H, Zhu L, Reid BR, Drobny GP, Hopkins PB. Science. 1995;270:1842–1845. doi: 10.1126/science.270.5243.1842. [DOI] [PubMed] [Google Scholar]; (b) Fichtinger-Schepman AM, van der Veer JL, den Hartog JHJ, Lohman PHM, Reedijk J. Biochemistry. 1985;24:707–713. doi: 10.1021/bi00324a025. [DOI] [PubMed] [Google Scholar]
- 5.(a) Cutillas N, Yellol GS, de Haro C, Vicente C, Rodríguez V, Ruiz J. Coord Chem Rev. 2013;257:2784–2797. [Google Scholar]; (b) Medici S, Peana M, Nurchi VM, Lachowicz JI, Crisponi G, Zoroddu MA. Coord Chem Rev. 2015;284:329–350. [Google Scholar]; (c) Murray BS, Babak MV, Hartinger CG, Dyson PJ. Coord Chem Rev. 2016;306:86–114. [Google Scholar]
- 6.(a) Bergamo A, Sava G. Chem Soc Rev. 2015;44:8818–8835. doi: 10.1039/c5cs00134j. [DOI] [PubMed] [Google Scholar]; (b) Leijen S, Burgers SA, Baas P, Pluim D, Tibben M, van Werkhoven E, Alessio E, Sava G, Beijnen JH, Schellens JH. Invest New Drugs. 2015;33:201–214. doi: 10.1007/s10637-014-0179-1. [DOI] [PubMed] [Google Scholar]
- 7.Mari C, Pierroz V, Ferrari S, Gasser G. Chem Sci. 2015;6:2660–2686. doi: 10.1039/c4sc03759f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.(a) Zong R, Thummel RP. J Am Chem Soc. 2004;126:10800–10801. doi: 10.1021/ja047410y. [DOI] [PubMed] [Google Scholar]; (b) Zhang G, Zong R, Tseng H-W, Thummel RP. Inorg Chem. 2008;47:990–998. doi: 10.1021/ic701798v. [DOI] [PubMed] [Google Scholar]; (c) Zong R, Wang B, Thummel RP. Inorg Chem. 2012;51:3179–3185. doi: 10.1021/ic202648h. [DOI] [PubMed] [Google Scholar]; (d) Liu Y, Ng S-M, Yiu S-M, Lam WWY, Wei X-G, Lau K-C, Lau T-C. Angew Chem Int Ed Engl. 2014;53:14468–14471. doi: 10.1002/anie.201408795. [DOI] [PubMed] [Google Scholar]; (e) Rudd JA, Brennaman MK, Michaux KE, Ashford DL, Murray RW, Meyer TJ. J Phys Chem A. 2016;120:1845–1852. doi: 10.1021/acs.jpca.6b00317. [DOI] [PubMed] [Google Scholar]
- 9.Walsh JL, Durham B. Inorg Chem. 1982;21:329–332. [Google Scholar]
- 10.Durham B, Wilson SR, Hodgson DJ, Meyer TJ. J Am Chem Soc. 1980;102:600–607. [Google Scholar]
- 11.(a) Renouard T, Fallahpour R-A, Nazeeruddin MK, Humphry-Baker R, Gorelsky SI, Lever ABP, Grätzel M. Inorg Chem. 2002;41:367–378. doi: 10.1021/ic010512u. [DOI] [PubMed] [Google Scholar]; (b) Chan C-W, Lai T-F, Che C-M. J Chem Soc Dalton Trans. 1994:895–899. [Google Scholar]
- 12.Constable EC, Cathey CJ, Hannon MJ, Tocher DA, Walker JV, Ward MD. Polyhedron. 1999;18:159–173. [Google Scholar]
- 13.Wachter E, Zamora A, Nease LA, Heidary DK, Glazer EC. manuscript in preparation. [Google Scholar]
- 14.(a) Cleare MJ, Hoeschele JD. Bioinorg Chem. 1973;2:187–210. [Google Scholar]; (b) Trynda-Lemiesz L, Kozlowski H, Keppler BK. J Inorg Biochem. 1999;77:141–146. doi: 10.1016/s0162-0134(99)00183-x. [DOI] [PubMed] [Google Scholar]
- 15.Vrana O, Novohradsky V, Medrikova Z, Burdikova J, Stuchlikova O, Kasparkova J, Brabec V. Chem Eur J. 2016;22:2728–2735. doi: 10.1002/chem.201504671. [DOI] [PubMed] [Google Scholar]
- 16.Howerton BS, Heidary DK, Glazer EC. J Am Chem Soc. 2012;134:8324–8327. doi: 10.1021/ja3009677. [DOI] [PubMed] [Google Scholar]
- 17.(a) Bierbach U, Qu Y, Hambley TW, Peroutka J, Nguyen HL, Doedee M, Farrell N. Inorg Chem. 1999;38:3535–3542. doi: 10.1021/ic981181x. [DOI] [PubMed] [Google Scholar]; (b) Pérez JM, Fuertes MA, Alonso C, Navarro-Ranninger C. Crit Rev Oncol Hematol. 2000;35:109–120. doi: 10.1016/s1040-8428(00)00053-6. [DOI] [PubMed] [Google Scholar]; (c) Coluccia M, Natile G. Anticancer Agents Med Chem. 2007;7:111–123. doi: 10.2174/187152007779314080. [DOI] [PubMed] [Google Scholar]; (d) Aris SM, Farrell NP. Eur J Inorg Chem. 2009;2009:1293–1302. doi: 10.1002/ejic.200801118. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Kasparkova J, Brabec V. J Inorg Biochem. 2015;153:206–210. doi: 10.1016/j.jinorgbio.2015.07.014. [DOI] [PubMed] [Google Scholar]; (f) McGowan G, Parsons S, Sadler PJ. Inorg Chem. 2005;44:7459–7467. doi: 10.1021/ic050763t. [DOI] [PubMed] [Google Scholar]
- 18.(a) Billecke C, Finniss S, Tahash L, Miller C, Mikkelsen T, Farrell NP, Bögler O. Neuro Oncol. 2006;8:215–226. doi: 10.1215/15228517-2006-004. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mangrum JB, Farrell NP. Chem Commun. 2010;46:6640–6650. doi: 10.1039/c0cc01254h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.(a) Lovejoy KS, Todd RC, Zhang S, McCormick MS, D’Aquino JA, Reardon JT, Sancar A, Giacomini KM, Lippard SJ. Proc Natl Acad Sci U S A. 2008;105:8902–8907. doi: 10.1073/pnas.0803441105. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Park GY, Wilson JJ, Song Y, Lippard SJ. Proc Natl Acad Sci U S A. 2012;109:11987–11992. doi: 10.1073/pnas.1207670109. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Johnstone TC, Wilson JJ, Lippard SJ. Inorg Chem. 2013;52:12234–12249. doi: 10.1021/ic400538c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han X, Sun J, Wang Y, He Z. Med Res Rev. 2015;35:1268–1299. doi: 10.1002/med.21360. [DOI] [PubMed] [Google Scholar]
- 21.Zamora A, Pérez SA, Rodríguez V, Janiak C, Yellol GS, Ruiz J. J Med Chem. 2015;58:1320–1336. doi: 10.1021/jm501662b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


