Abstract
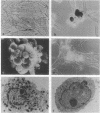
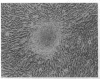
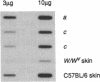
The W/Kit mouse locus, affecting proliferation and survival of pigment cells, blood cells, and germ cells, is known to encode a tyrosine kinase growth factor receptor and is considered a protooncogene; yet it has not heretofore been causally implicated in any malignancies of those cells. The Wf/Wf mutant mouse coat comprises viable and inviable melanoblast clones, seen ultimately as pigmented and white transverse stripes--the latter more prominent. Judging from the pattern, all clones initially expand, and the inviable ones then undergo programmed cell death prenatally. To observe skin melanocytes of the viable clones during extended proliferation, the cells were explanted from individual young mice. An unusually large number of primary explants failed to survive--a result consistent with a growth handicap. In 3 of the 10 surviving cell lines, many cells spontaneously underwent a series of striking changes with the classic features of transformation. The two transformed lines that have been tested by grafting to immunosuppressed hosts formed undifferentiated invasive tumors compatible with malignant amelanotic melanoma. None of our 52 other melanocyte lines of the coisogenic wild-type strain and 13 other natural genotypes have become transformed under the same culture conditions. Molecular analysis of the Wf gene revealed a single change from wild-type: a point mutation affecting the catalytic region in the kinase domain of the Kit protein. The apparent growth disadvantage due to the mutation may allow selection for melanocytes mobilizing more efficient pathways, thus leading to neoplasia. Production of both viable and inviable melanoblast clones is unlikely to be due only to the kinase mutation; possibly the degree, duration, and consistency of expression of this locus may be controlled by cis elements outside the coding region.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Besmer P., Murphy J. E., George P. C., Qiu F. H., Bergold P. J., Lederman L., Snyder H. W., Jr, Brodeur D., Zuckerman E. E., Hardy W. D. A new acute transforming feline retrovirus and relationship of its oncogene v-kit with the protein kinase gene family. Nature. 1986 Apr 3;320(6061):415–421. doi: 10.1038/320415a0. [DOI] [PubMed] [Google Scholar]
- Brooks G., Wilson R. E., Dooley T. P., Goss M. W., Hart I. R. Protein kinase C down-regulation, and not transient activation, correlates with melanocyte growth. Cancer Res. 1991 Jun 15;51(12):3281–3288. [PubMed] [Google Scholar]
- Castagna M., Takai Y., Kaibuchi K., Sano K., Kikkawa U., Nishizuka Y. Direct activation of calcium-activated, phospholipid-dependent protein kinase by tumor-promoting phorbol esters. J Biol Chem. 1982 Jul 10;257(13):7847–7851. [PubMed] [Google Scholar]
- Chabot B., Stephenson D. A., Chapman V. M., Besmer P., Bernstein A. The proto-oncogene c-kit encoding a transmembrane tyrosine kinase receptor maps to the mouse W locus. Nature. 1988 Sep 1;335(6185):88–89. doi: 10.1038/335088a0. [DOI] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., McFarland E. C., Russell E. S. Analysis of pleiotropism at the dominant white-spotting (W) locus of the house mouse: a description of ten new W alleles. Genetics. 1981 Feb;97(2):337–361. doi: 10.1093/genetics/97.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geissler E. N., Ryan M. A., Housman D. E. The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell. 1988 Oct 7;55(1):185–192. doi: 10.1016/0092-8674(88)90020-7. [DOI] [PubMed] [Google Scholar]
- Guenet J. L., Marchal G., Milon G., Tambourin P., Wendling F. Fertile dominant spotting in the house mouse. J Hered. 1979 Jan-Feb;70(1):9–12. doi: 10.1093/oxfordjournals.jhered.a109202. [DOI] [PubMed] [Google Scholar]
- Hanks S. K., Quinn A. M., Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988 Jul 1;241(4861):42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- Hunter T. A tail of two src's: mutatis mutandis. Cell. 1987 Apr 10;49(1):1–4. doi: 10.1016/0092-8674(87)90745-8. [DOI] [PubMed] [Google Scholar]
- Koshimizu U., Watanabe D., Tajima Y., Nishimune Y. Effects of W (c-kit) gene mutation on gametogenesis in male mice: agametic tubular segments in Wf/Wf testes. Development. 1992 Apr;114(4):861–867. doi: 10.1242/dev.114.4.861. [DOI] [PubMed] [Google Scholar]
- Larue L., Mintz B. Pigmented cell lines of mouse albino melanocytes containing a tyrosinase cDNA with an inducible promoter. Somat Cell Mol Genet. 1990 Jul;16(4):361–368. doi: 10.1007/BF01232464. [DOI] [PubMed] [Google Scholar]
- Lassam N., Bickford S. Loss of c-kit expression in cultured melanoma cells. Oncogene. 1992 Jan;7(1):51–56. [PubMed] [Google Scholar]
- Lev S., Yarden Y., Givol D. Receptor functions and ligand-dependent transforming potential of a chimeric kit proto-oncogene. Mol Cell Biol. 1990 Nov;10(11):6064–6068. doi: 10.1128/mcb.10.11.6064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINTZ B., RUSSELL E. S. Gene-induced embryological modifications of primordial germ cells in the mouse. J Exp Zool. 1957 Mar;134(2):207–237. doi: 10.1002/jez.1401340202. [DOI] [PubMed] [Google Scholar]
- Manova K., Bachvarova R. F. Expression of c-kit encoded at the W locus of mice in developing embryonic germ cells and presumptive melanoblasts. Dev Biol. 1991 Aug;146(2):312–324. doi: 10.1016/0012-1606(91)90233-s. [DOI] [PubMed] [Google Scholar]
- Mintz B. Clonal basis of mammalian differentiation. Symp Soc Exp Biol. 1971;25:345–370. [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian differentiation. Annu Rev Genet. 1974;8:411–470. doi: 10.1146/annurev.ge.08.120174.002211. [DOI] [PubMed] [Google Scholar]
- Mintz B. Gene control of mammalian pigmentary differentiation. I. Clonal origin of melanocytes. Proc Natl Acad Sci U S A. 1967 Jul;58(1):344–351. doi: 10.1073/pnas.58.1.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintz B., Silvers W. K. Histocompatibility antigens on melanoblasts and hair follicle cells. Cell-localized homograft rejection in allophenic skin grafts. Transplantation. 1970 May;9(5):497–505. doi: 10.1097/00007890-197005000-00009. [DOI] [PubMed] [Google Scholar]
- Nocka K., Majumder S., Chabot B., Ray P., Cervone M., Bernstein A., Besmer P. Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice--evidence for an impaired c-kit kinase in mutant mice. Genes Dev. 1989 Jun;3(6):816–826. doi: 10.1101/gad.3.6.816. [DOI] [PubMed] [Google Scholar]
- Nocka K., Tan J. C., Chiu E., Chu T. Y., Ray P., Traktman P., Besmer P. Molecular bases of dominant negative and loss of function mutations at the murine c-kit/white spotting locus: W37, Wv, W41 and W. EMBO J. 1990 Jun;9(6):1805–1813. doi: 10.1002/j.1460-2075.1990.tb08305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orr-Urtreger A., Avivi A., Zimmer Y., Givol D., Yarden Y., Lonai P. Developmental expression of c-kit, a proto-oncogene encoded by the W locus. Development. 1990 Aug;109(4):911–923. doi: 10.1242/dev.109.4.911. [DOI] [PubMed] [Google Scholar]
- Porter S., Larue L., Mintz B. Mosaicism of tyrosinase-locus transcription and chromatin structure in dark vs. light melanocyte clones of homozygous chinchilla-mottled mice. Dev Genet. 1991;12(6):393–402. doi: 10.1002/dvg.1020120604. [DOI] [PubMed] [Google Scholar]
- Qiu F. H., Ray P., Brown K., Barker P. E., Jhanwar S., Ruddle F. H., Besmer P. Primary structure of c-kit: relationship with the CSF-1/PDGF receptor kinase family--oncogenic activation of v-kit involves deletion of extracellular domain and C terminus. EMBO J. 1988 Apr;7(4):1003–1011. doi: 10.1002/j.1460-2075.1988.tb02907.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak M. Z., Luger S. M., DeRiel K., Abrahm J., Calabretta B., Gewirtz A. M. Role of the KIT protooncogene in normal and malignant human hematopoiesis. Proc Natl Acad Sci U S A. 1992 Mar 1;89(5):1710–1714. doi: 10.1073/pnas.89.5.1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reith A. D., Rottapel R., Giddens E., Brady C., Forrester L., Bernstein A. W mutant mice with mild or severe developmental defects contain distinct point mutations in the kinase domain of the c-kit receptor. Genes Dev. 1990 Mar;4(3):390–400. doi: 10.1101/gad.4.3.390. [DOI] [PubMed] [Google Scholar]
- Russell E. S. Hereditary anemias of the mouse: a review for geneticists. Adv Genet. 1979;20:357–459. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Tamura A., Halaban R., Moellmann G., Cowan J. M., Lerner M. R., Lerner A. B. Normal murine melanocytes in culture. In Vitro Cell Dev Biol. 1987 Jul;23(7):519–522. doi: 10.1007/BF02628423. [DOI] [PubMed] [Google Scholar]
- Trent J. M., Stanbridge E. J., McBride H. L., Meese E. U., Casey G., Araujo D. E., Witkowski C. M., Nagle R. B. Tumorigenicity in human melanoma cell lines controlled by introduction of human chromosome 6. Science. 1990 Feb 2;247(4942):568–571. doi: 10.1126/science.2300817. [DOI] [PubMed] [Google Scholar]
- Witte O. N. Steel locus defines new multipotent growth factor. Cell. 1990 Oct 5;63(1):5–6. doi: 10.1016/0092-8674(90)90280-r. [DOI] [PubMed] [Google Scholar]
- Yarden Y., Kuang W. J., Yang-Feng T., Coussens L., Munemitsu S., Dull T. J., Chen E., Schlessinger J., Francke U., Ullrich A. Human proto-oncogene c-kit: a new cell surface receptor tyrosine kinase for an unidentified ligand. EMBO J. 1987 Nov;6(11):3341–3351. doi: 10.1002/j.1460-2075.1987.tb02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]