Abstract
BACKGROUND
A minimum male hemoglobin (Hb) of 13.0 g/dL will become an FDA requirement in May 2016. In addition, extending whole blood (WB) interdonation intervals (IDIs) beyond 8 weeks has been considered in order to reduce iron depletion in repeat blood donors. This study estimates the impact these changes might have on blood availability and donor iron status.
STUDY DESIGN AND METHODS
Six blood centers participating in REDS-II collected information on all donation visits from 2006–09. Simulations were developed from these data using a multi-stage approach that first sought to adequately reproduce the patterns of donor return, Hb and ferritin levels, and outcomes of a donor’s visit (successful single or double RBC donation, deferral for low Hb) observed in REDS-II datasets. Modified simulations were used to predict the potential impact on the blood supply and donor iron status under different Hb cutoff and IDI qualification criteria.
RESULTS
More than 10% of WB donations might require replacement under many simulated scenarios. Longer IDIs would reduce the proportion of donors with iron depletion, but 80% of these donors may remain iron-depleted if minimal IDIs increased to 12 or 16 weeks.
CONCLUSION
Higher Hb cutoffs and longer IDIs are predicted to have a potentially large impact on collections but only a modest impact on donor iron depletion. Efforts to address iron depletion should be targeted to at-risk donors, such as iron supplementation programs for frequent donors, and policy makers should try to avoid broadly restrictive donation requirements that could substantially reduce blood availability.
Keywords: blood donor, iron depletion, anemia, low hemoglobin, donation interval, deferral
INTRODUCTION
Approximately 13 million (M) red cell components are transfused annually in the United States.1 Ensuring an adequate blood supply requires blood centers to recruit new donors while also relying heavily on those with an established commitment. Measurement of donor hemoglobin (Hb) is intended to prevent collection of blood from anemic donors, but is often erroneously considered a good proxy for iron status. Until recently, a common Hb requirement of 12.5 g/dL has applied to male and female donors in the US; effective May 2016 a minimum hemoglobin (Hb) of 13.0 g/dL for male donors is the new FDA requirement.2
This report uses donor information collected between 2006–2009 at REDS-II blood centers and data from the REDS-II RISE study to model the impact of changes in Hb cutoff and potential changes in the minimal interdonation interval (IDI) on the US blood supply, and on the prevalence of low iron in the blood donor population.
MATERIALS AND METHODS
Two primary sources of REDS-II data were used for this analysis: The Donation/Deferral database and the RISE study dataset.
Donation/Deferral Database: Six blood centers, accounting for >8% of annual US blood collections, participating in the National Heart, Lung, and Blood Institute (NHLBI) REDS-II program provided data. During 2006–2009, data on donor demographics (age, gender, race/ethnicity), visit date and eligibility outcome (donation vs. deferral), donation procedure (whole blood, apheresis platelets, etc.) and phlebotomy outcome (successful, quantity not sufficient, etc.), and cause for deferral were collected. Supplemental information was compiled for > 90% of donor visits, including height and weight, and a quantitative value for the fingerstick Hb or hematocrit (Hct) test of record during 2008 and 2009. Overall, 1.9M donors made 6.1M presentations during the 4-year period; 1.8M donor visits in 2008–2009 had a quantitative Hb or Hct available.
RISE Study Dataset: The RISE study of iron deficiency in blood donors, has been described elsewhere.3,4 Briefly, 2425 donors were recruited into 4 cohorts stratified by gender and recent donation history and followed for 15–24 months. Donors with no prior donation history (FT), or not within the last two years (RA) entered the “First-Time/Reactivated” donor cohort (FT/RA), while females with 2 or males with 3 WB donations in the prior 12 months entered the Frequent Repeat (RPT) cohorts. Two measures of iron depletion were assessed: 1) Iron Deficient Erythropoiesis (IDE), an intermediate stage of iron depletion was defined as the upper 2.5% of the distribution of log(soluble transferrin receptor/ferritin) in the FT/RA male cohort at enrollment, corresponding roughly to ferritin < 26 ng/mL; and 2) Absent Iron Stores (AIS), defined as ferritin < 12 ng/mL.
A detailed summary was prepared of donor presentations for allogeneic WB or RBC apheresis donation and productivity over the full 4-year REDS-II data collection. Donors were classified into 8 sub-groups based on gender and 4 similar-sized age groups at their first appearance during 2006–2009. Number of donors, red cell components donated, and low Hb deferrals were summed by group, and measures of donor return and productivity were derived. As a first approach to estimating impact on blood availability, a parallel dataset was created that artificially lengthened return IDIs to a hypothetical new minimum duration (12, 16, or 20 weeks). Observed donation visits were mapped to new dates, with all IDIs shorter than the “new” minimum extended to exactly the new minimum and those at or above the new minimum maintained. Any donations that shifted in time beyond the end of the 4-year observation period (12/31/09) were counted as “lost” donations. Lost donations were summed and normalized as a proportion of red cell components donated during the 2006–2009 period for all six REDS-II blood centers in aggregate, for both genders, and for each of the 8 age-gender cohorts.
The impact of adjusted Hb cutoff values was also estimated. Quantitative Hb or Hct values were tabulated for the 5 REDS-II blood centers that captured the test results electronically. Hct values were converted to Hb values by dividing by 3.04. The proportion of successful donation visits from male donors with qualifying Hb below hypothetical alternate cutoffs of 13.0 and 13.5 g/dL was derived. Since the Final Rule contemplates lowering female donor cutoffs to 12.0 g/dL,2 a level within physiologic norms, the proportion of female deferral visits with Hb values between 12.0 and 12.5 was similarly calculated.
To assess predictors of Hb deferral observed in the Donation/Deferral Database, we developed a repeated measures logistic regression model with potential predictor variables limited to data that routinely are (demographics) or could reasonably be (recent donation intensity) operationally available at the time of donor presentation or scheduling.
Simulation models were developed using a multi-stage approach that first sought to establish a baseline simulation such that it adequately reproduced the patterns of donor return and visit outcome observed in the REDS-II database under existing criteria for Hb cutoffs (12.5 g/dL) and IDI (8 weeks). Five input models contributed to this effort. Details of each model are found in the online Appendix:
A “Donor Return Model” used survival methods to predict the timing of a donor’s return visit following presentation to donate. Simulated outcomes at each donor visit were a function of the combined results of Models 2–4 to follow. Simulated iron status was based on Model 5.
A “Hemoglobin Model” that used longitudinal linear regression to predict absolute Hb values in donors at a given visit based on demographic characteristics and recent donation activity.
An “Other Deferral Model” that used longitudinal logistic regression to estimate the probability of being deferred for something other than low Hb as a function of factors relating to blood center procedures, donor demographics, and prior donation history.
An “R2 Model” that used longitudinal logistic regression to predict donation of two units of red cells at a single donation visit; and
A “Ferritin Model” that used longitudinal linear regression to predict donor log10 ferritin levels on a continuous scale at each presentation.
The first 4 models were developed from the REDS-II Donation/Deferral Database; Model 5 was based on parameters developed from the RISE study. Upon development of the baseline simulation, 10 additional scenarios were modeled that specified longer minimum IDIs (altered assumptions to Model 1) and/or the minimum Hb required to qualify for donation (altered cutoff for classification of outcome in Model 2). The results from 38 simulation runs were averaged to predict the potential impact on the blood supply and on donor iron status under different donor acceptance criteria. Aggregate outcomes of red cell collections and low iron in donors are shown here for all scenarios. Results by gender and race are available in an online Appendix.
RESULTS
Table 1 shows that females accounted for 55% of unique donors and 46% of red cell collections over the 4-year period. For both genders, the two older age groups (35–50 and 50+) showed higher return rates and greater donation productivity than the two younger age groups (≤ 20 and 20–35 years old). The average annual donation rate varied widely, with the oldest donors donating twice as frequently as those in the youngest age group. The proportion donating twice or more on an annualized basis varied roughly four-fold (4.5% in youngest vs. 20.6% in oldest age group for females, 8% vs. 31.5% in males).
Table 1.
REDS-II Donor Return Behavior, Donation Productivity, and Estimated Future Loss of Red Cell Units with Longer Minimum IDIs
| Characterization at 1st visit during REDS-II |
Donors (N)+ |
RC components (N) |
Median Return Interval > RC* |
% w/ Return Visit** |
Ave. RC/Yr** |
% Donate ≥ 2x/yr** |
% w/ Hb deferral |
N Hb deferrals |
% Estimated Red Cell loss extending interval to |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12 wks | 16 wks | 20 wks | ||||||||||
| Females | 1,022,099 | 2,303,232 | 17.4 | 57.7 | 0.89 | 13.6 | 26.7 | 460,324 | 2.2 | 5.5 | 8.9 | |
| ≤ 20 yrs | 296,555 | 435,602 | 22.3 | 50.6 | 0.61 | 4.5 | 23.8 | 97,441 | 0.5 | 1.5 | 2.6 | |
| 20+ to 35 | 250,661 | 455,085 | 18.6 | 50.6 | 0.72 | 7.1 | 24.5 | 96,021 | 1.2 | 3.3 | 5.6 | |
| 35+ to 50 | 276,749 | 733,394 | 17.4 | 62.7 | 1.00 | 13.8 | 31.1 | 158,590 | 2.5 | 6.3 | 10.2 | |
| 50+ | 198,134 | 679,151 | 15.4 | 68.9 | 1.26 | 20.6 | 27.8 | 108,272 | 3.6 | 8.7 | 13.7 | |
| Males | 843,698 | 2,713,875 | 16.7 | 59.7 | 1.25 | 23.1 | 2.9 | 41,551 | 3.9 | 8.9 | 13.4 | |
| ≤ 20 yrs | 234,465 | 431,368 | 22.9 | 46.9 | 0.76 | 8.0 | 0.7 | 1,830 | 0.7 | 1.9 | 3.3 | |
| 20+ to 35 | 187,412 | 467,446 | 17.7 | 50.3 | 1.00 | 13.6 | 1.1 | 3,031 | 2.4 | 5.8 | 9.2 | |
| 35+ to 50 | 226,555 | 881,225 | 16.9 | 66.5 | 1.44 | 24.4 | 2.8 | 10,342 | 4.3 | 10.0 | 15.1 | |
| 50+ | 195,266 | 933,836 | 14.7 | 73.3 | 1.70 | 31.5 | 7.5 | 26,348 | 5.6 | 12.8 | 18.6 | |
|
Totals (all donors) |
1,865,797 | 5,017,107 | 17.0 | 58.6 | 1.05 | 14.9 | 16.0 | 501,875 | 3.1 | 7.3 | 11.3 | |
Excludes 62,212 donors who donated only platelets or plasma or who made autologous or directed donation at first visit.
Median interval until next donor visit following RC donation, in weeks (excludes those who fail to return).
Restricted to those with ≥ 1 year observation time (i.e. donated 2006–2008).
The estimated aggregate impact of longer IDIs was modest, with loss of 3% of red cell collections at 12-week intervals, 7% at 16-week intervals, and 11% at 20-week intervals. However, it varied considerably with observed donation productivity across donor sub-groups. In those groups with lower return rates and less frequent donations, longer IDIs had a relatively modest impact; e.g, with a 20-week IDI, component loss was only 3% from donors ≤20 years of age. In contrast, the estimated impact ranged from 10 to 18% in male and female donors 35 years of age and older.
Table 2a presents the percentage of male donor donations made during 2008–2009 for which the fingerstick Hb value was below 13.0 or 13.5 g/dL. These unadjusted data suggest that raising the Hb level could reduce blood collections from male donors from 5 to 14% for alternate cutoffs of 13.0 and 13.5 g/dL, respectively, with an even larger effect in Black male donors, reflecting the lower Hb values in a healthy Black population compared to Caucasians. These data serve as a useful benchmark for the more sophisticated modeling approaches shown in subsequent tables.
Table 2.
| a: Distribution of pre-donation fingerstick Hb values for donations from REDS-II male donors, 2008–2009 | ||||||||
|---|---|---|---|---|---|---|---|---|
| White | Black | Total | ||||||
| N | < 13 g/dL | < 13.5 g/dL | N | < 13 g/dL | < 13.5 g/dL | N | < 13 g/dL | < 13.5 g/dL |
| 752,851 | 5.0% | 14.4% | 21,535 | 7.3% | 18.6% | 810,103 | 5.0% | 14.3% |
| Applied to 2,713,875 red cell donations from male donors during REDS-II, 2006–2009 | 135,694 | 388,084 | ||||||
| b: Distribution of pre-donation fingerstick Hb values for low Hb deferrals of REDS-II female donors, 2008–2009 | |||||
|---|---|---|---|---|---|
| White | Black | Total | |||
| N | 12.0 – 12.49 g/dL | N | 12.0 – 12.49 g/dL | N | 12.0 – 12.49 g/dL |
| 114,987 | 42% | 1,224 | 39% | 129,833 | 42% |
| Applied to 460,324 low Hb deferrals in female donors during REDS-II, 2006–2009 | 193,336 | ||||
Much of the expected donation loss in male donors could potentially be offset by lowering the female donor Hb cutoff to 12.0 g/dL. Table 2b shows that 42% of females with a low Hb deferral had recorded Hb values between 12.0 and 12.4 g/dL. Recovery of these ineligible donors yields an additional 193,000 units over the 4-year period. This surpasses the estimated loss of red cell collections from males at a 13.0 g/dL Hb cutoff (135,694 donations), but is only half that at a 13.5 g/dL cutoff (388,084 donations).
Table 3 provides results from three longitudinal models evaluating the association of demographic factors, donation history, and blood center with Hb deferral and blood donor Hb and iron levels. The estimated risk of low Hb deferral was 16-fold higher in female than in male donors, declined in females after childbearing years, and in males climbed steadily with age. It was more than twice as high in Black than White donors, was lower with increased weight in male donors, varied considerably across centers, and decreased with higher donation intensity. Additionally, the elevated risk for Hb deferral at next presentation persisted for 6 months or more, considerably longer than the current minimum 8-week IDI (Fig 1A–B).
Table 3.
Models for Hemoglobin Deferral, Hemoglobin Values, and Ferritin Values
| Data source | REDS-II Donation Data | RISE Donation Data | |||||
|---|---|---|---|---|---|---|---|
| Hb deferral model (Odds Ratios) |
Linear Hb model* (Δg/dL) |
Ferritin log-linear model* (% difference) |
|||||
| Intercept (reference value) | 0.9% | 15.28 g/dL | 132 ng/mL | ||||
| Race/Ethnicity | |||||||
| Race/Ethnicity | |||||||
| Asian vs. White | 1.17 | −0.07 | 26% | ||||
| Black vs. White | 2.62 | −0.52 | 0% | ||||
| Hispanic vs. White | 1.39 | −0.14 | −11% | ||||
| Other vs. White | 1.38 | −0.12 | 0% | ||||
| Gender | |||||||
| Female vs. Male | 16.17 | −1.83 | −63% | ||||
| Age (in years) | Male | Female | Male | Female | Male | Female | |
| < 30 vs. 40–49 | 0.57 | 1.09 | 0.35 | 0.00 | −7% | −7% | |
| 30–39 vs. 40–49 | 0.74 | 1.00 | 0.14 | 0.00 | −28% | −28% | |
| 50–59 vs. 40–49 | 1.56 | 0.73 | −0.13 | 0.16 | −13% | 46% | |
| 60 + vs. 40–49 | 2.96 | 0.72 | −0.32 | 0.18 | −15% | 43% | |
| Weight | Male | Female | Male | Female | |||
| <150 vs. 150–174 | 1.38 | 1.13 | −0.07 | −0.05 | −9% | ||
| 175–199 vs. 150–174 | 0.79 | 0.98 | 0.08 | 0.02 | 2% | ||
| 200+ vs. 150–174 | 0.72 | 1.04 | 0.13 | −0.01 | 28% | ||
| Red Cell Donations in previous 2 years | Male | Female < 50 | Female ≥ 50 | ||||
| 1–3 donations vs. 0 donations | 1.02 | −0.03 | −12% | −61% | −52% | ||
| 4–6 donations vs. 0 donations | 1.00 | −0.09 | −50% | −69% | −36% | ||
| 7–9 donations vs. 0 donations | 0.83 | −0.14 | −60% | −71% | −44% | ||
| 10+ donations vs. 0 donations | 0.59 | −0.19 | −64% | −72% | −48% | ||
| Center | |||||||
| A vs. F | 1.60 | −0.56 | 2% | ||||
| B vs. F | 1.87 | NA | 8% | ||||
| C vs. F | 0.94 | 0.18 | −1% | ||||
| D vs. F | 1.26 | 0.02 | −6% | ||||
| E vs. F | 1.16 | 0.13 | −16% | ||||
| Double red cell | |||||||
| last DR vs last single | 0.79 | 0.07 | −6% | ||||
| Interval since donation | See Fig 1a–1b | See Fig 1c–1d | See Fig 1e–1f | ||||
The linear Hb and log-linear ferritin models contribute to the simulation modeling.
Fig 1.
Modeled estimates for change in hemoglobin deferral risk, hemoglobin levels, and iron levels in low- and high-intensity donors following donation
Red line = Males; Green line = Females < 50 years-old; Blue line = Females ≥ 50 years-old. Panels 1A-B show estimated change in risk for Hb deferral (Odds ratio) for those with 1–3 donations (1A) and 4+ donations in prior 2 years (1B); comparisons for Hb deferral risk are intra-group. For low- and high-intensity donors (similarly defined), Panels 1C–D show estimated recovery of Hb levels (g/dL) and Panels 1E-F recovery of iron levels (% decrease in ferritin). For Panels 1C-F, the vertical line reflects the current deferral period.
The association between demographic and other factors and absolute Hb levels in the linear hemoglobin model mostly reflected the corresponding increase or decrease in risk for Hb deferral. For example, Hb levels were essentially flat in women during childbearing years, but increased in women aged 50 and older, the point at which Hb deferral risk declines. In men, Hb levels declined steadily with each successive age group, spanning a range of 0.67 g/dL from youngest to oldest; this corresponded to a six-fold higher risk for Hb deferral in the oldest group.
The ferritin model presented in Table 3 indicates much lower iron stores in female donors than in males. Controlling for race/ethnicity, recent donation history, weight and blood center, females in the reference group of 40–49 years of age had levels of ferritin estimated at 49 ng/mL, 63% lower than comparably aged male donors (132 ng/mL). As with Hb, iron stores were increased in females after age 50. Recent donation history was an important contributor to iron status. In females of childbearing age, as few as 1–3 donations in two years was associated with a 61% decline in ferritin values. A similar decline was found in males, albeit reaching that magnitude only with 7–9 donations in two years. In females >50 years of age, the impact of donation on ferritin levels appeared less pronounced than for other donors.
Table 4 compares the observed to the simulated distributions of donation visits and outcomes for 2008–2009. The donor count is fixed by design, while the remaining elements reflect probabilistic transitions between visits, i.e. a stochastic process. Overall, the simulation model underestimated the observed visit count in the actual REDS-II database by about 7% (see discussion); however the proportional distribution of visits across the four possible outcomes (successful donation of one or two red cell components, and Hb or other deferral) matched the observed REDS-II data fairly well. Thus, the simulated distributions represented the observed distributions on a slightly smaller scale and the baseline simulation model was judged satisfactory to serve as the foundation for comparisons under alternate Hb deferral thresholds and IDI scenarios.
Table 4.
The baseline simulation closely approximates observed distributions
| Count | Percent (standard error) | |||
|---|---|---|---|---|
| Observed | Baseline Simulation (standard error1) |
Observed | Baseline Simulation (standard error1) |
|
| Donors | 1,074,313 | 1,074,313 (N/A) | NA | NA |
| Total visits | 2,463,431 | 2,299,735 (132) | NA | NA |
| R1 visits | 2,033,994 | 1,877,990 (136) | 82.57% | 81.63% (.31%) |
| R2 visits | 111,595 | 117,254 (45) | 4.53% | 5.12% (.19%) |
| Other deferrals | 151,979 | 143,567 (27) | 6.17% | 6.25% (.13%) |
| Hemoglobin deferrals | 165,863 | 160,924 (46) | 6.73% | 7.00% (.19%) |
Based on 38 simulations
Baseline simulation models 2008–2009 data for donation visits and outcomes under existing donor qualification criteria.
Ten modeled scenarios are presented in Table 5 in addition to the baseline scenario. In simulations 1–3, the IDI was lengthened. In simulations 4–6, Hb cutoffs were altered for males (simulations 5 and 6), or for both male and female donors (simulation 4). Simulations 7–10 show results from combined changes to both IDI and Hb cutoff. All scenarios but one were associated with decreased component availability. Only simulation 4 - maintaining an 8-week IDI while shifting male and female Hb cutoffs to 13.0 and 12.0 g/dL, respectively - had a net positive effect on availability (+3.5%), Raising the male donor Hb level to 13.0 g/dL (simulation 5) or 13.5 g/dL (simulation 6) without altering the minimum IDI was predicted to lead to a 1.1% or 4.0% drop in red cell components collected, respectively; this was half the magnitude of the estimated loss suggested by Table 2a.
Table 5.
Simulated Change in Red Cell Collection, Hb Deferrals, and Donor Iron Depletion Under Modified Policies1
| SimID | Hgb cutoff | Donation interval | Red Cell Components Donated2 |
Hb Deferrals2 |
% of Red Cell Donation Visits with3 |
|||
|---|---|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Ferr<12 | Ferr<26 | |||
|
Baseline (cv) |
12.5 | 12.5 | 8 | 8 |
2,112,497 0.04% |
160,924 0.02% |
13.1% 0.02% |
34.0% 0.01% |
| Δ to Baseline simulation | % RC Visits Ferr< 12 or 26 | |||||||
|
Sim 1 Sim 2 Sim 3 |
12.5 | 12.5 | 12 | 12 | −7.7% | −4.9% | 11.6% | 31.9% |
| 12.5 | 12.5 | 16 | 12 | −9.5% | −9.1% | 11.0% | 31.0% | |
| 12.5 | 12.5 | 16 | 16 | −13.3% | −9.3% | 10.8% | 30.7% | |
|
Sim 4 Sim 5 Sim 6 |
12.0 | 13.0 | 8 | 8 | 3.5% | −27% | 13.8% | 35.2% |
| 12.5 | 13.0 | 8 | 8 | −1.1% | 5.4% | 13.1% | 34.0% | |
| 12.5 | 13.5 | 8 | 8 | −4.0% | 21% | 13.1% | 34.1% | |
|
Sim 7 Sim 8 Sim 9 Sim 10 |
12.0 | 13.0 | 12 | 12 | −4.8% | −31% | 12.1% | 32.8% |
| 12.5 | 13.5 | 12 | 12 | −10.0% | 13% | 11.6% | 32.1% | |
| 12.0 | 13.0 | 16 | 12 | −7.3% | −32% | 11.3% | 31.7% | |
| 12.5 | 13.5 | 16 | 12 | −11.8% | 8.7% | 11.0% | 31.1% | |
Shaded cells represent conditions altered from baseline (status quo) scenario
Standard error estimates and approximations were calculated for each cell based on 38 simulations for each level of the parameters. All standard error estimates were less than .32%.
Standard errors were approximated using the Taylor series expansion which included variance terms for the baseline and alternative simulations.
Standard errors were calculated by pooling variance terms from the baseline and alternative simulations and assuming that they were equal.
In comparison, lengthening the IDI was associated with a larger drop in collections. With no change to Hb requirements, a 4-week extension of the 8-week IDI for both genders (simulation 1) was associated with a drop of 7.7% in red cell components. Extending the IDI in women to 16 weeks (simulation 2) increased the red cell loss to 9.5%, while extending the IDI in both men and women to 16 weeks (simulation 3) resulted in an estimated 13.3% loss.
Table 5 suggests that iron depletion is a relatively common condition in those accepted for blood donation, with roughly one in eight donors having absent iron stores (AIS, or ferritin < 12 ng/mL) and one in three having ferritin below 26 ng/mL, an intermediate stage of iron depletion. Raising the minimum Hb requirement for men had virtually no effect on these proportions, while lowering female Hb cutoffs to 12.0 g/dL raised the estimate by less than a percentage point to 13.8% (simulation 4). Lengthening the IDI had a more appreciable effect, with adoption of a 12-week IDI (simulation 1) predicted to result in a drop from 13.1% to 11.6% for AIS (11% difference). Extending the IDI to 16 weeks for both genders (simulation 3) resulted in a further small reduction in AIS to 10.8%. Though meaningful in relative terms (18% decline in AIS compared to baseline), these results indicate that even with such a change, about 80% of the AIS present in blood donors would not be reversed, while such an intervention might result in a 13% loss of red cell components.
This can be explained by the extended time required to recover the iron lost in a typical WB donation, shown in Panels E and F of Figure 1. Graphed separately for pre- and post-menopausal females and for male donors, as well as by recent donation intensity, the return to pre-donation ferritin levels takes far longer than 8-weeks, extending well beyond more than12 months in some groups. More generally, these iron recovery curves and other results in Figure 1 suggest considerable heterogeneity in impact of and physiological response to blood donation after controlling for demographic factors and for blood center, similar to findings of other modeling efforts5,6 and to rigorously controlled trials, as discussed below.7 Panels A-B reflect change in risk for Hb deferral, within each group (Males, Females < 50, Females ≥ 50) compared to the risk for a donor with similar demographics and no prior donation history. Panels 1C and 1D present the modeled recovery curves of Hb in absolute terms following blood donation. The salient conclusion from all three models are that differences in Hb and iron recovery vary by age, gender, and donation intensity, and for many donors a return to baseline iron status occurs long after 8 weeks.
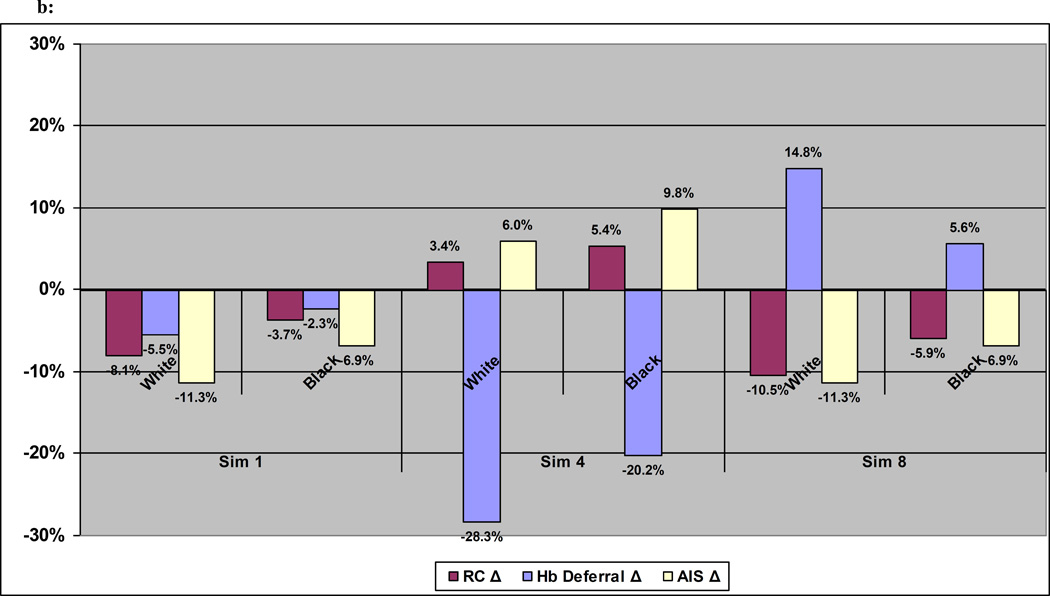
The extent to which changes in Hb cutoffs or lengthened IDIs might have variable impacts on sub-groups of donors is shown in Figure 2. Relative changes to baseline for red cell collections, Hb deferrals, and AIS in blood donors are shown by gender (2a) and race/ethnicity (2b). Aggregate change in red cell collections or Hb deferrals can mask considerable underlying differences across sub-groups of donors. This heterogeneity in the modeled results reflects that seen in observed patterns of donor presentation behavior and donation outcomes seen in Table 1, where two- to four-fold differences were seen across the different measures of donor productivity.
Fig 2.
a: Change in Red Cell Collections, Hb Deferrals, and AIS at Donation by Gender under 3 alternate scenarios
b: Change in Red Cell Collections, Hb Deferrals, and AIS at Donation by Race under 3 alternate scenarios
Sim 1: Male Hb = 12.5, Female Hb = 12.5, Male Interval = 12 weeks, Female Interval = 12 weeks
Sim 4: Male Hb = 13.0, Female Hb = 12.0, Male Interval = 8 weeks, Female Interval = 8 weeks
Sim 8: Male Hb = 13.5, Female Hb = 12.5, Male Interval = 12 weeks, Female Interval = 12 weeks
DISCUSSION
Ensuring the well-being of blood donors is a priority for blood centers and regulators. Two areas of concern are the need to protect donors from anemia with appropriate Hb screening eligibility values and to limit donation-induced iron depletion. Analysis of large population databases indicates that Hb reference ranges for healthy males are higher than for females, suggesting that Hb cutoffs for blood donation could reasonably be set at different levels for each gender.8 Our modeled data (see Hemoglobin Model -Table 3), indicated that males in the reference age group 40–49 have predicted Hb levels that are 1.8g/dL higher than females. Our results add to available evidence that the large majority of male donors have Hb levels well above the minimum 12.5 g/dL. In addition, they provide quantitative estimates of the proportion of presenting female donors with Hb between 12.0 and 12.5 g/dL. Data reported here and elsewhere9 suggest that an offsetting 0.5 g/dL change (an increase for males and a decrease for females) would be neutral or even result in a net positive impact on red cell inventories. In the FDA final rule, the male Hb cutoff has been raised to 13 g/dL but the female cutoff has been maintained at 12.5 g/dL. However, a provision has been made for obtaining an FDA variance to lower the female Hb cutoff to 12 g/dL provided that an institution can demonstrate that it has taken appropriate steps to protect female donors with Hb between 12 and 12.5g/dl from iron deficiency as a result of blood donation. Whether female donors in this Hb range are more likely to be iron deficient than females with higher Hb has not been rigorously studied. One might expect that to be the case since some female donors in this range could have experienced drops in Hb levels due to insufficient iron levels; however, ferritin testing for females in this range would differentiate between non-anemic donors and those with iron deficiency anemia.
An AABB Association Bulletin recommended measures for reducing or preventing iron deficiency in blood donors.10 One potential strategy discussed was to adopt longer IDIs or otherwise restrict donation frequency. This latter point included a significant caution that due consideration should be given to the impact on blood availability for which, until now, little data were available for consideration. One recent analysis11 and another commentary12 indicated a significant impact on availability with lengthened IDIs, especially for high-demand components such as Group O products, but both reports were limited by evaluating only one blood center over a single year’s time. In contrast, the simulation results presented herein represent projections from 6 geographically dispersed and demographically diverse blood centers. Importantly, they control for dynamic (time-varying) variables and explicitly take account of the lower risk for Hb deferral conferred by longer IDIs. The same variability that leads to divergent productivity over time in different sub-groups of donors has implications for setting an appropriate IDI. Historically, the original 8-week IDI was established following studies in predominantly young men,13 even though 25% of this presumably iron-replete population failed to fully recover pre-donation Hb levels within an 8-week period. However, healthy young males are a minority of modern blood donors, with females contributing nearly half of red cell collections and those older than 35 being the most productive donors for both genders. In these donor subgroups, our results show that the estimated recovery time for both Hb and iron appears to extend well beyond 8 weeks and potentially as long as several months, and as shown in Figure 1 differs by gender, age, and recent donation activity. Substantial heterogeneity across donor subgroups is reinforced by other modeling studies5,6 and by the randomized controlled trial, HEIRS.7 In HEIRS, the time to recovery of the Hb and iron lost in a single donation varied widely, and donors not assigned iron supplements on average took longer than 24 weeks to return to pre-donation levels. In contrast, donors assigned iron pills (38 mg) had uniformly accelerated recovery of both Hb and iron levels, with modest variability across donors. Similarly, the STRIDE study, another recently completed RCT, demonstrated that low-dose iron (19 mg) equivalent to over-the-counter formulations available in the US strongly improved donor Hb and iron levels and sharply reduced their Hb deferrals compared to controls.14 Importantly, it suggested that merely providing donors information about their iron status following a donation might be sufficient to stimulate donor initiation of iron supplementation. In sum, while lengthening IDI seems a reasonable approach for reducing iron deficiency in blood donors, its impact is marginal on the prevalence of AIS in blood donors. For donors not taking supplemental iron, longer IDIs are usually insufficient to recover the Hb and iron lost in a donation; for those who do take iron, longer IDIs confer little to no benefit. Hence, given the potentially substantial impact on red cell availability of longer IDI, alternate solutions such as recommending or providing iron supplements to repeat donors, with or without targeted ferritin testing, appear to be preferable approaches. The growing prevalence of patient blood management programs and concomitant decline of red cell products transfused1 together may allow blood centers to diminish reliance on high frequency donors, even without regulatory changes requiring longer IDI. For those donors who return frequently, however, iron supplementation will likely be needed to avoid iron depletion.
One should interpret our simulation studies with appropriate caution. There may be important respects in which RED-II blood centers are not representative of the national donor population. Second, if to any extent our modeled relationships of ferritin and Hb as a function of donor characteristics and donation frequency are imprecise, such inaccuracies would be embedded in our simulations. Third, the estimates of impact on availability are highly sensitive to assumptions about blood donor return behavior, a complex phenomenon influenced by motivation, convenience, quality of the donation experience, health status, and other factors, such as changes in blood center scheduling. Fourth, model validation was driven primarily by approximating aggregate empirical results; results from sub-populations might be subject to greater levels of uncertainly. Fifth, the baseline simulation only approximates the actual REDS-II database, resulting in a7% underestimate of donor visits (whereas baseline simulation standard deviation of donor visits is about 5%). This could in part be due to chance, as well as the challenges in estimating donor return behavior, but in any case should have minimal impact on findings since alternative scenarios are compared to baseline simulation and not actual REDS-II database. Finally, we have not attempted to account for any response by blood centers to the simulated changes. One should anticipate that tighter restrictions on donor frequency will lead to enhanced recruitment efforts by blood centers to replace the units of red cells they might otherwise lose. In this respect, the references to “lost” components should be understood to reflect units “potentially lost” in the absence of additional blood center efforts to maintain collections at a level required by their hospital customers. Hence, the results should be considered informative as to the direction and relative magnitude of changes in red cell collections, Hb deferrals, or donor iron status and not as reflecting absolute precision of likely outcomes.
Ideally, methods to limit or reduce iron deficiency secondary to blood donation will strike a balance that targets interventions to higher risk populations and appropriately weighs the importance of component availability to patients, especially high demand products such as Group O units and scarce products such as those for sickle cell patients. Two recent RCTs (HEIRS and STRIDE) have addressed these issues in detail, with both studies indicating a more active role for blood centers in helping donors replace the iron lost in a successful donation.
Supplementary Material
Acknowledgments
The authors thank the staff at all six participating blood centers. Without their help, this study would not have been possible. The Retrovirus Epidemiology Donor Study (REDS)- II (Donation/Deferral Database and RISE) was the responsibility of the following persons: Blood Centers: 1) American Red Cross Blood Services, New England Region: R. Cable, J. Rios, RJ Benjamin; 2) American Red Cross Blood Services, Southern Region / Emory University: J.D. Roback; 3) Blood Center of Wisconsin: J. Gottschall, A.E. Mast; 4) Hoxworth Blood Center, University of Cincinnati Academic Health Center: R.A. Sacher, P.M. Carey, S. Wilkinson; 5) Regents of the University of California/Blood Centers of the Pacific/BSRI: E.L. Murphy, M.P. Busch, B. Custer; 6) The Institute for Transfusion Medicine (ITxM)/LifeSource Blood Services: D. Triulzi, R. Kakaiya, J. Kiss; Central Laboratory: Blood Systems Research Institute: M.P. Busch, P. Norris; Coordinating Center: Westat, Inc.: J Shulman, M. King; National Heart, Lung, and Blood Institute, NIH: G. Nemo; Steering Committee Chairman: R.Y. Dodd.
The RISE Analysis Group, which includes the named authors and other individuals, provided invaluable contributions through frequent discussion of study design, data analysis, and manuscript review. Special appreciation is given to RJ Benjamin, R Forshee, MP Busch, RY Dodd, JE Kiss, and KS Schlumpf.
Financial support: This study was supported by contracts N01HB47168; N01HB47169; N01HB47170; N01HB47171; N01HB47172; N01HB47174; N01HB47175; N01HB57181 from the National Heart, Lung, and Blood Institute.
Footnotes
Conflict of interest: The authors have no conflicts of interest relevant to this manuscript.
REFERENCES
- 1.AABB. The 2013 Blood Collection, Utilization, and Patient Blood Management Survey Report. Bethesda, MD: 2015. [Google Scholar]
- 2.Federal Register. [cited January 17, 2016];80(99) Available from website: https://federalregister.gov/a/2015-12228. [Google Scholar]
- 3.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2012;52:702–711. doi: 10.1111/j.1537-2995.2011.03401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cable RG, Glynn SA, Kiss JE, et al. Iron deficiency in blood donors: the REDS-II Donor Iron Status Evaluation (RISE) study. Transfusion. 2011;51(3):511–522. doi: 10.1111/j.1537-2995.2010.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Custer B, Bravo M, Bruhn R, Land K, Tomasulo P, Kamel H. Predictors of hemoglobin recovery or deferral in blood donors with an initial successful donation. Transfusion. 2014;51(9):2267–2275. doi: 10.1111/trf.12628. [DOI] [PubMed] [Google Scholar]
- 6.Nasserinejad K, van Rosmalen J, de Kort W, Rizopoulos D, Lesaffre E. Prediction of hemoglobin in blood donors using a latent class mixed-effects transition model. Statist Med. 2015 doi: 10.1002/sim.6759. [DOI] [PubMed] [Google Scholar]
- 7.Kiss Je, Brambilla D, Glynn SA, Mast AE, Spencer BR, Stone M, Kleinman SH, Cable RG. Oral iron supplementation after blood donation: a randomized clinical trial. JAMA. 2015;6:575–583. doi: 10.1001/jama.2015.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beutler E, Waalen J. The definition of anemia: what is the lower limit of normal of the blood hemoglobin concentration? Blood. 2006;107:1747–1750. doi: 10.1182/blood-2005-07-3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eder Anne. Proposed changes to hemoglobin and donation interval criteria for whole blood donors : projected impact on current donor base. Paper presented at: 98th meeting of Blood Products Advisory Committee, U.S. Food and Drug Administration; 2008 July 28; Gaithersburg, MD. [Cited December 23, 2013]. Minutes available from website http://www.fda.gov/ohrms/dockets/ac/cber08.html#BloodProducts. [Google Scholar]
- 10.AABB. Association Bulletin 12-03: Strategies to monitor, limit or prevent iron deficiency in blood donors. [September 21, 2012]; Available at URL: http://www.aabb.org/programs/publications/bulletins/Pages/ab12-03.aspx. [Google Scholar]
- 11.Gandhi MJ, Duffy K, Benike M, et al. Effect of increasing hemoglobin cutoff in male donors and increasing interdonation interval in whole blood donors at a hospital-based blood donor center. Transfusion. 2012;52:1880–1888. doi: 10.1111/j.1537-2995.2011.03533.x. [DOI] [PubMed] [Google Scholar]
- 12.Sayers M, Centilli J. Contemplating the effect on blood availability if the interdonation interval of 56 days is prolonged. Transfusion. 2013;53:1132–1136. doi: 10.1111/j.1537-2995.2012.03900.x. [DOI] [PubMed] [Google Scholar]
- 13.Fowler WM, Barer AP. Rate of hemoglobin regeneration in blood donors. JAMA. 1942;118:421–427. [Google Scholar]
- 14.Mast AE, Bialkowski W, Bryant BJ, Wright DJ, Birch R, Kiss JE, D’Andrea P, Cable RG, Spencer BR. A randomized, blinded, placebo-controlled trial of education and iron supplementation for mitigation of iron deficiency in regular blood donors. Transfusion. 2015 doi: 10.1111/trf.13469. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.