Abstract
Macrophage receptor with collagenous structure (MARCO) is a Class A Scavenger Receptor (cA-SR) that recognizes and phagocytoses of a wide variety of pathogens. Most cA-SRs that contain a C-terminal Scavenger Receptor Cysteine Rich (SRCR) domain use the proximal collagenous domain to bind ligands. In contrast, for the role of the SRCR domain of MARCO in phagocytosis, adhesion and pro-inflammatory signalling is less clear. The discovery of a naturally-occurring transcript variant lacking the SRCR domain, MARCOII, provided the opportunity to study the role of the SRCR domain of MARCO. We tested whether the SRCR domain is required for ligand binding, promoting downstream signalling, and enhancing cellular adhesion. Unlike cells expressing full-length MARCO, ligand binding was abolished in MARCOII-expressing cells. Furthermore, co-expression of MARCO and MARCOII impaired phagocytic function, indicating that MARCOII acts as a dominant negative variant. Unlike MARCO, expression of MARCOII did not enhance Toll-Like Receptor 2 (TLR2)-mediated pro-inflammatory signalling in response to bacterial stimulation. MARCO-expressing cells were more adherent and exhibited a dendritic-like phenotype, while MARCOII-expressing cells were less adherent and did not exhibit changes in morphology. These data suggest the SRCR domain of MARCO is the key domain in modulating ligand binding, enhancing downstream pro-inflammatory signalling, and MARCO-mediated cellular adhesion.
Keywords: Scavenger Receptor, MARCO, SRCR, Phagocytosis, Cell Adhesion, Macrophage, Host-Pathogen Interaction
INTRODUCTION
The Class A Scavenger Receptors (cA-SRs) are a diverse group of multi-functional pattern recognition receptors (PRRs) that are required for anti-bacterial immunity, adhesion, motility, and homeostasis1. cA-SRs bind a wide array of polyanionic ligands, including, but not limited to, modified low density lipoproteins (LDLs), inorganic particulates, and bacterial ligands, such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA)2. The “ligand-promiscuous” cA-SRs play a major role in recognizing conserved pathogen-associated molecular patterns (PAMPs), and, thus, are critical in anti-bacterial immunity3. Despite belonging to a single class, the ligand binding site(s) of the cA-SRs are not identical and remain controversial. The cA-SRs Scavenger Receptor Class A (SRA), MAcrophage Receptor with COllagenous structure (MARCO) and SCAvenger Receptor class A, member 5 (SCARA5) all contain a C-terminal Scavenger Receptor Cysteine Rich (SRCR) domain. SRCR domains are found in many protein families and are often involved in ligand binding, but the exact role(s) of the SRCR domain within the cA-SRs remains enigmatic4. The SRCR domain of MARCO has been proposed as the primary ligand binding site and was shown to bind ligands directly via two highly conserved arginine residues, termed the RxR motif5,6. While the SRCR domains of SRA, SCARA5, and MARCO all exhibit a high degree of evolutionary conservation, SRA does not contain a RxR motif and has been shown to primarily bind ligands within the proximal collagenous domain6,7. In addition to SRA, the collagenous domain of SCARA3 has also very recently been identified as a putative ligand binding site for bacteria and modified LDL8.
SRA has been shown to exist as multiple splice variants; full-length (SRA-I), lacking the SRCR domain (SRA-II), and a dominant negative isoform trapped in the endoplasmic reticulum (SRA-III)9. While the expression of SRA splice variants is differentially regulated, SRA-I/II have been shown to bind ligands with similar specificity and affinity, providing evidence that the SRCR domain is not required for ligand binding10,11. Multiple splice variants of MARCO have been deposited to public databases, but have never been functionally characterized. Using publically available transcript databases, we have identified a naturally-occurring splice variant of MARCO lacking the exons coding for the SRCR domain12. To further understand the role of the SRCR domain of MARCO, we have functionally characterized this splice variant, herein referred to as MARCOII. To our knowledge, this is the first study to functionally characterize any splice variant of MARCO. We have shown that loss of the SRCR domain abrogates binding of ligands, diminishes the ability for MARCO to enhance pro-inflammatory signalling, and abolishes MARCO-mediated cellular adhesion.
RESULTS
Identification, Cloning & Expression of Alternatively-Spliced MARCO Transcripts
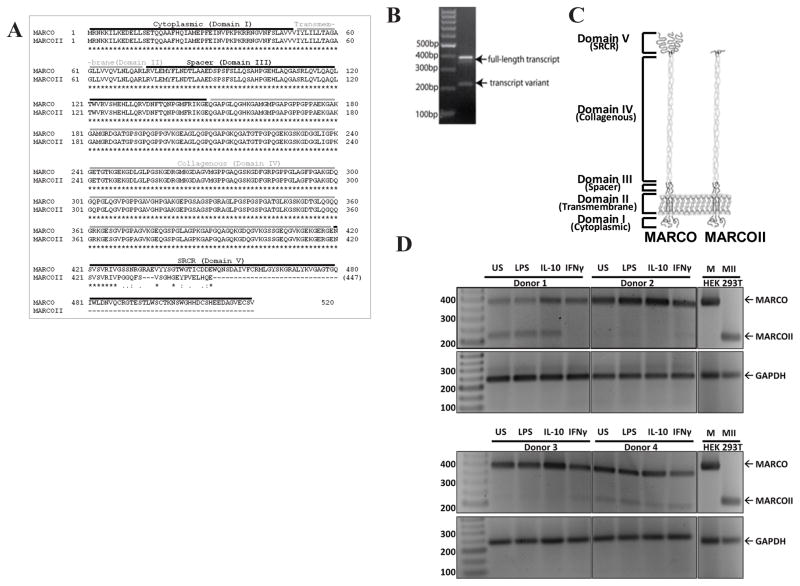
In order to functionally characterize the functional importance of the SRCR domain of MARCO, we cloned a transcript variant lacking the SRCR domain using the Aceview human 2010 transcript database (GenBank Accession Number CR603381), which we call MARCOII. The domain structure of MARCO consists of a N-terminal cytoplasmic domain (aa 1–50) followed by a transmembrane domain (aa 51–74), spacer domain (aa 75–149), collagenous domain (aa 150–419), and SRCR domain (aa 420–520). In comparison, the predicted structure of MARCOII was identical to full-length MARCO for the first four domains, but was considerably different in the SRCR domain. The SRCR domain of MARCOII contained only the first 8 residues followed by an out-of-frame region of 19 residues (Figure 1A).
Figure 1. Characterization of a MARCO splice variant.
The MARCOII transcript variant is truncated, resulting in a loss of almost the entire SRCR domain. (A)Amino acid sequences of MARCO and MARCOII illustrating a near total loss of the SRCR domain within the MARCOII transcript. (B)PCR amplification of PBMC cDNA. Primers surround exons 16/17. (C)Hypothesized structure of MARCO and MARCOII. (D)PCR amplification of cDNA from unstimulated (US), lipopolysaccharide (LPS), interleukin-10 (IL-10) or interferon-gamma (IFN-γ) stimulated PBMCs. Primers surround exons 14/17. All experiments were performed a minimum of three times.
A full-length cDNA clone (CS0DM004YJ08) was generously provided by Wu-Bo Li (Life Technologies, Maryland). To validate the presence of MARCO transcript variants in primary human samples, we used PCR to amplify exons 16 and 17 (surrounding the putative SRCR domain) in human peripheral blood mononuclear cells (PBMCs) followed by gel electrophoresis analysis. As indicated by the presence of a small band (approximately 200 bp) in addition to a large band (approximately 400 bp) (Figure 1B), we confirmed the presence of MARCO transcript variant (MARCOII) with a truncation of the putative SRCR domain. To determine if expression of the MARCOII transcript could be induced, PBMCs were stimulated for 48 h with either PBS (unstimulated, US), lipopolysaccharide (LPS), IL-10 or IFN-γ. Following RNA isolation, cDNA preparation and semi-quantitative PCR, transcripts were analyzed by semi-quantitative PCR using primers surrounding exons 14/17. While MARCO transcript was detected in all samples, the MARCOII transcript was not expressed in 2/4 donors. Sequences of both transcripts were excised, purified and sequenced to confirm the identity of both transcripts.
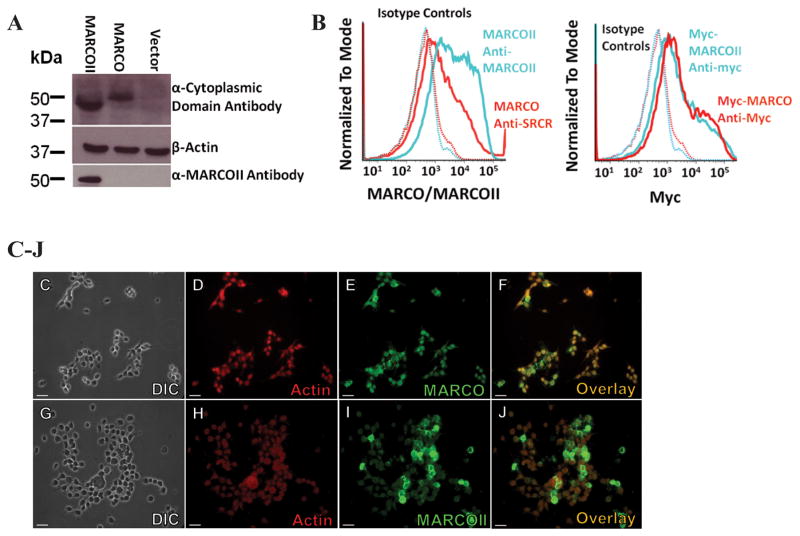
To better understand the functional properties of MARCOII as well as the importance of the SRCR domain, the MARCOII cDNA was subcloned into pcDNA3.1/Hygro(+). Expression of MARCOII was assessed in transiently transfected HEK 293T cells first by Western blot analysis using antibodies targeting the cytoplasmic domain and out-of-frame “SRCR region” of MARCOII. MARCOII was shown to have comparable expression to full-length MARCO (Figure 2A). Next, we added a C-terminal myc tag to better compare surface expression of MARCO and MARCOII by immunofluorescence microscopy (IF) flow cytometry. In our transient transfection system, myc-MARCOII was shown to be expressed at the cell surface (Figure 2C–J) and at similar levels as full-length MARCO (Figure 2B). Myc-tagged constructs were used for subsequent IF experiments.
Figure 2. Expression of MARCO and MARCOII.
HEK 293T cells were transfected with empty vector, MARCO, or MARCOII and analyzed for expression of MARCO/MARCOII. (A) Western blot analysis of MARCO/MARCOII-expressing HEK 293T cell lysates suggests MARCOII can be expressed as protein. (B) c-Myc tagged MARCO and MARCOII show similar levels of surface expression when analyzed by flow cytometry. (C–J) Immunofluorescence analysis of myc-MARCO (C–F)and myc-MARCOII (G–J)highlighting surface expression. Red = Phalloidin, Green = Myc. Scale bars represent 25 μM. All experiments were performed a minimum of 3 times with a minimum of 3 technical replicates.
MARCOII Shows Abrogated Ligand Binding Ability
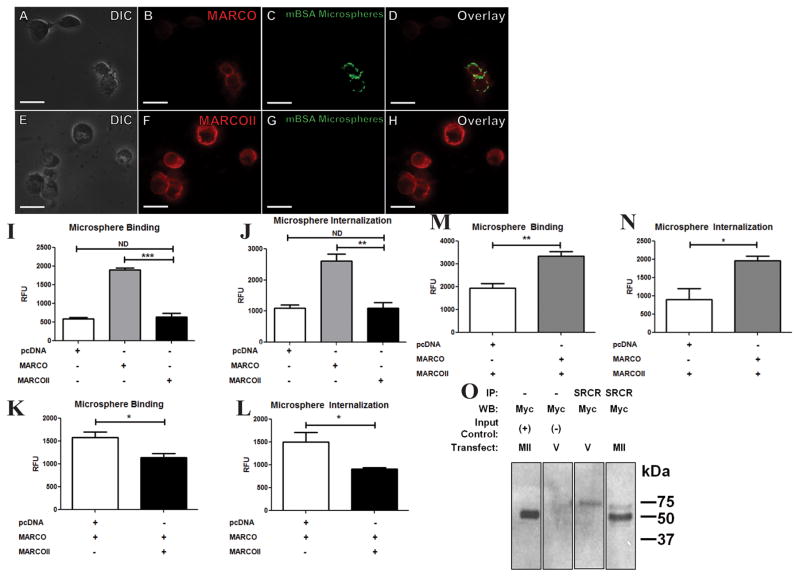
We compared the ability of MARCO and MARCOII to bind polystyrene microspheres (500 nM) which were passively coated with maleylated Bovine Serum Albumin (Mal-BSA; a previously confirmed MARCO ligand)2. Transiently-transfected HEK 293T cells expressing MARCO showed a 300% increase in binding Mal-BSA-coated microspheres when compared to MARCOII-expressing cells (Figure 3A–I). Similarly, MARCO-transfected cells showed a 230% increase in internalization of Mal-BSA-coated microspheres when compared to MARCOII-expressing cells or empty vector control cells (Figure 3J). The same trend was observed for myc-tagged constructs, suggesting that the addition of a C-terminal myc tag does not interfere with ligand binding (data not shown). In order to confirm these quantitative data were due to microsphere binding to MARCO expressing cells, we performed immunofluorescence (IF) microscopy, (Figure 3A–H).. This phenomenon was not observed in myc-MARCOII-expressing cells, further indicating that microsphere binding was dependent on the SRCR domain of MARCO.
Figure 3. MARCOII-expressing cells show decreased binding and internalization of Mal-BSA-coated microspheres.
HEK 293T cells were transfected with empty vector, MARCO, or MARCOII, incubated with 500 nm Mal-BSA-coated fluorescent microspheres at 37°C, and analyzed for bead binding and internalization. (A–H) myc-MARCOII-transfected cells (E–H) show abrogated ligand binding and uptake when compared to myc-MARCO (A–D). Red = Myc, Green = Mal-BSA Microspheres. Scale bars represent 15 μM. (I, J) Quantification of microsphere binding (I) and internalization (J). (K–M) Knockdown/rescue of endogenous ligand binding (K,N) and internalization (L,M) of stably-expressing MARCO or MARCOII HEK 293T cells, respectively.(O) Co-immunoprecipitation of myc-MARCOII from stably-expressing MARCO HEK 293T cells. Cells were transfected with either myc-MARCOII or an empty vector negative control. MARCO was immunoprecipitated with an anti-SRCR antibody, followed by an immunoblot for myc-MARCOII using anti-myc antibodies. Positive and negative input controls were loaded at 10% of IP samples. Statistical significance was calculated by Student’s t-Test. Statistical significance was calculated by Student’s t-Test. Error bars indicate Mean ± Standard Error of the Mean (SEM). * = p <0.05, ** = p <. 0.01, *** = p <0.001. All experiments were performed a minimum of 3 times with a minimum of 3 technical replicates.
To determine if expression of MARCOII can affect endogenous MARCO function, we transiently transfected a HEK 293T cell line stably expressing MARCO with MARCOII and performed ligand-coated microsphere binding and uptake assays as above. This resulted in a 72% reduction in ligand binding and a 61% reduction in ligand internalization (Figure 3K,L). We used a similar approach to determine if expression of MARCO could rescue the function of MARCOII-expressing cells. Conversely, we transfected a stably-expressing MARCOII HEK 293T cell line with MARCO and performed the above assay. We observed a 172% increase in ligand binding and a 218% increase in ligand internalization (Figure 3M,N). It has been shown that the collagenous domain of the cA-SRs is critical for homotrimerization11. Given that MARCO and MARCOII share identical collagenous domains, we performed a co-Immunoprecipitation (Co-IP) and showed that MARCO and MARCOII can form heterotrimeric complexes in HEK 293T cells (Figure 3O).
Taken together, these data demonstrate that the SRCR domain of MARCO is required to bind and internalize Mal-BSA coated microspheres.
The SRCR Domain of MARCO Binds Streptococcus pneumoniae and Enhances TLR2/CD14-mediated NF-κB Activity
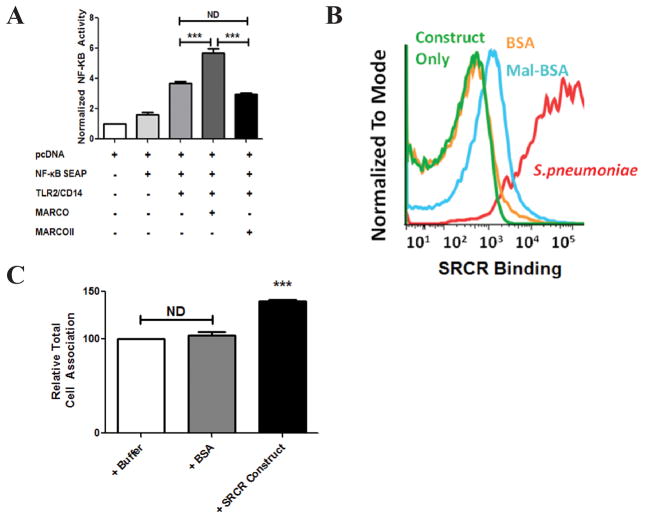
In addition to our microsphere binding assay, we sought to determine whether the SRCR domain could directly bind Streptococcus pneumoniae, a pathogenic bacterium that we have previously shown is cleared from the murine nasopharynx in a MARCO-dependent fashion13. To do so, we created a recombinant, soluble SRCR trimer. Following incubation of the SRCR-construct with BSA- or Mal-BSA-coated microspheres, analysis by flow cytometry confirmed the SRCR domain binds Mal-BSA-coated microspheres. In addition, we confirmed that the SRCR domain binds Streptococcus pneumoniae (Figure 4B).
Figure 4. The SRCR domain binds S. pneumoniae and enhances NF-κB activity via TLR2/CD14.
(A)HEK 293T cells were transfected with combinations of NF-κB SEAP reporter plasmid, TLR2/CD14, and MARCO or MARCOII. Cells were then stimulated with S. pneumoniae for 48 h followed by quantification of NF-κB activity. MARCOII-transfected cells show no significant enhancement of NF-κB activity when compared to MARCO. (B) A soluble SRCR construct binds S. pneumoniae. (C) S. pneumoniae was pre-incubated with folding buffer, BSA or SRCR construct for 2 h. Bacteria were then incubated with peritoneal macrophages at an MOI of 25 for 30 min. Percent bacterial association was calculated as the bacteria recovered at 30 min. The relative total cell association was normalized to buffer pre-treated S. pneumoniae. Statistical significance was calculated by 1-way ANOVA with Tukey’s post-hoc test. Error bars indicate Mean ± Standard Error of the Mean (SEM). *** = p < 0.001. All experiments were performed a minimum of 3 times with a minimum of 3 technical replicates.
While MARCO has never been shown to directly signal in response to ligand binding, it has been shown to enhance TLR2/CD14 signalling in response to Streptococcus pneumoniae13. We hypothesized that the SRCR domain of MARCO might be required to enhance the activation of other PRRs such as TLR2. To test this, we used a NF-κB Secreted Embryonic Alkaline Phosphatase (NF-κB SEAP) reporter assay to assess pro-inflammatory signals in response to S. pneumoniae stimulation. HEK 293T cells transfected with MARCO, TLR2, and CD14 showed a significant increase in NF-κB response when stimulated with heat-killed, lysozyme-digested S. pneumoniae for 48 h when compared to TLR2 and CD14 alone (Figure 4A). Cells transfected with MARCOII, TLR2 and CD14 showed no significant change in NF-κB activation when compared with cells transfected with TLR2 and CD14 alone (Figure 4A). This suggests that the SRCR domain of MARCO is critical for enhancing NF-κB activity via TLR2.
To assess whether the soluble SRCR trimer alone could alter endogenous binding and phagocytosis of Streptococcus pneumoniae by primary murine macrophages, we pre-incubated the bacterium with either folding buffer, BSA or the SRCR construct. It was determined that incubation with the SRCR construct enhanced total cell association by approximately 40% compared to controls, rather than blocking function (Figure 4C).
The SRCR Domain of MARCO Alters Cellular Morphology and Enhances Cellular Adhesion
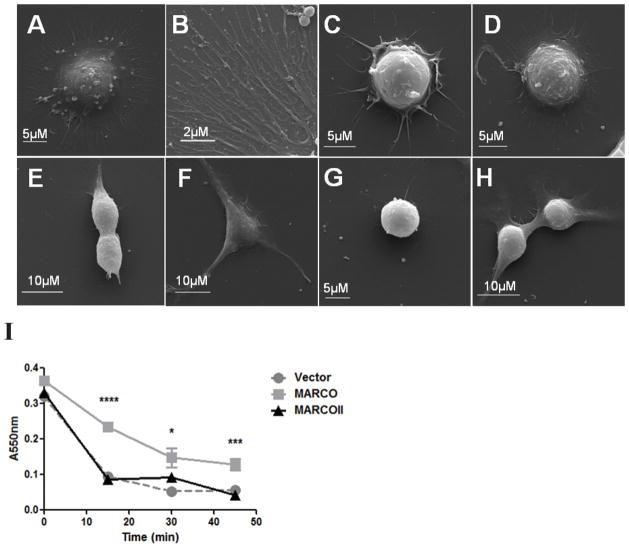
To determine whether the SRCR domain of MARCO contributed to the altered cell morphology that is observed in MARCO-expressing cells, we visualized HEK 293T cells transfected with myc-MARCO, myc-MARCOII, or empty vector control by SEM. MARCO-transfected cells produced a large number of thin (<1 μm), branched, dendritic-like processes (Figure 5A, B). This phenotype was not observed in MARCOII-transfected cells (Figure 5C, D), indicating that the SRCR domain is required for the production of dendritic-like processes.
Figure 5. The SRCR domain is required for MARCO-mediated cellular adhesion.
HEK 293T cells were transfected with vector control, MARCO, or MARCOII, treated with Accutase (Millipore) at 37°C for the indicated time points and stained with crystal violet to quantify adhesion. Data is representative of three independent experiments. (A–D) SEM analysis of transiently transfected HEK 293T identified a large morphological difference in MARCO-transfected cells (A, B) when compared to MARCOII (C, D). (E) MARCO-expressing cells were significantly more adherent after 15, 30, and 45 min of Accutase incubation when compared to MARCOII-expressing cells. Statistical significance was calculated by 2-way ANOVA with Tukey’s post-hoc test. Error bars indicate Mean ± Standard Error of the Mean (SEM). Stars indicate comparisons between MARCO and MARCOII where * = p <0.05, ** = p < 0.01, *** = p <0.001, **** = p < 0.0001. All experiments were performed a minimum of 3 times with a minimum of 3 technical replicates.
To further understand the role of the SRCR domain in cellular adhesion, we quantified cellular adhesion using transiently transfected HEK 293T cells. HEK 293T are weakly adherent to tissue culture-treated plastic but were observed to increase in adherence when transfected with MARCO. When adherence was directly quantified by an adhesion assay, MARCO-transfected cells showed a 300% increase in adherence when compared to MARCOII-transfected cells after 45 min of Accutase treatment (Figure 5E). This indicates that MARCO can enhance cellular adhesion via the SRCR domain.
Taken together, these results place an emphasis on the importance of the SRCR domain of MARCO in ligand binding, enhancing pro-inflammatory signalling, and modulating cellular adhesion.
DISCUSSION
The class A family of Scavenger Receptors contains five members including SRA, MARCO, SCARA3, SCARA4, and SCARA5. Despite belonging to the same class, the cA-SRs share varying degrees of protein domain homology and, importantly, function1,6. The functional heterogeneity of the cA-SRs has made it difficult to assign unifying functions to shared protein domains. This is especially true in the case of the Scavenger Receptor Cysteine Rich (SRCR) domain, a domain shared by SRAI, MARCO, and SCARA5.
Several MARCO transcript variants have been identified via Aceview human 2010 transcript database (NCBI), yet have never been functionally characterized12. In this study, we sought to characterize the functional importance of the SRCR domain of MARCO using a naturally-occurring transcript variant. We began by confirming that the MARCOII transcript variant existed in human peripheral blood mononuclear cells (Figure 1A). It has been well demonstrated that MARCO expression is enhanced in macrophages polarized to an M2 phenotype via IL-10 treatment14–16. Therefore, we sought to determine if M1 or M2 polarization of macrophages would alter the expression of the MARCOII transcript. Both M1 polarization via LPS or IFN-γ treatment and M2 polarization via IL-10 treatment did not change the expression of the MARCOII transcript (Figure 1D). Interestingly, we do not observe universal or consistent expression between donors implying that there may be additional genetic regulation of MARCOII (Fig 1D). Indeed a number of polymorphisms within introns of MARCO have been associated with increased susceptibility to M. tuberculosis, but whether this is due to differences in MARCO or MARCOII expression is not known17,18.
Previous work by Brännström et al. highlighted the importance of the RxR motif within the SRCR domain of MARCO for ligand binding using artificially truncated or mutated constructs5; however, some constructs were not expressed on the cell surface5. Others have reported similar findings when expressing artificial constructs of SRA19. We have demonstrated that MARCO and MARCOII are expressed at comparable levels in our transient transfection system by Western blotting and that they both expressed on the cell surface by flow cytometry (Figure 2A,B) and immunofluorescence microscopy (Figure 2C–J). Therefore, differences in function are not due to differences in protein expression.
Identification of the ligand binding site of MARCO has remained especially controversial for multiple reasons. As stated above, Brännström et al have suggested that the unique RxR motif within the SRCR domain is essential for ligand binding due to positive charge clustering5,20. These findings are consistent with its high affinity for polyanionic ligands. However, SRAI/II and SCARA3 also bind many of the same polyanionic ligands (including modified LDLs and bacteria) within the proximal collagenous domain7,8,11. Thus, both receptors may use regions of positive charge for ligand binding but these regions are localized to different areas of the receptor. To determine the role of the SRCR domain of MARCO in ligand binding, we performed studies using 500 nM Mal-BSA-coated polystyrene microspheres as a ligand for both MARCO and MARCOII. We showed that cells expressing MARCO bound and internalized 230% more microspheres when compared to MARCOII (Figure 3I,J). Furthermore, only cells expressing myc-MARCO bound microspheres when analyzed by IF microscopy. Neither myc-MARCOII-expressing nor vector-transfected cells bound a significant number of microspheres (Figure 3A–H).
We hypothesized that MARCOII might act as a dominant negative given that the collagenous domain of cA-SRs is critical for homotrimerization and that both MARCO and MARCOII share identical collagenous domains11. To test this, we first knocked down ligand binding and internalization of stably-expressing MARCO HEK 293T cells by transfection with MARCOII. We observed a 72% reduction in ligand binding and a 61% reduction in ligand internalization (Figure 3K,L).. Conversely, we rescued endogenous function by transfecting stably-expressing MARCOII HEK 293T cells with MARCO and observed a 172% increase in ligand binding and a 218% increase in ligand internalization (Figure 3M,N). Second, we showed that myc-MARCOII can be immunoprecipitated from stably-expressing MARCO HEK 293T cells (Figure 3O). Together, this suggests that MARCO and MARCOII can form heterotrimers and that MARCOII can act as a dominant negative isoform of the receptor. It has been previously shown that other members of the cA-SRs, namely SRA, can also exist in multiple splice forms, including as a dominant negative isoform, SRA-III9. Unlike SRA-III, which is trapped intracellularly, MARCOII can be expressed at the cell surface and likely exerts any dominant negative function by reducing the number of SRCR domain-containing moieties within a MARCO/MARCOII heterotrimer. Taken together, these data demonstrate that the SRCR domain of MARCO is the ligand binding site17,18.
MARCO has been shown to play a vital role in the recognition and clearance of bacterial infections in non-opsonic environments, as well as tethering bacterial ligands to other complexes to initiate an inflammatory response13,21. This tethering interaction between MARCO and other PRRs (such as TLR2) is a critical step in initiating an innate immune response, as MARCO has never been shown to signal directly. We have previously shown that MARCO is important in the pathway leading to Nod2 and TLR2-dependent NF-κB activation in response to S. pneumoniae13. Given the important role MARCO plays in pro-inflammatory responses to pathogens, we first sought to determine if the SRCR domain of MARCO is required to directly bind S. pneumoniae irrespective of the collagenous domain. We showed by flow cytometry that in addition to Mal-BSA-coated microspheres, the SRCR construct bound S. pneumoniae (Figure 4A). This both confirms that MARCO binds S. pneumoniae and additionally identifies the SRCR domain as the ligand binding site. In fact, the SRCR enhances cell association with S. pneumoniae. This finding is in agreement with previous findings that the SRCR binds Escherichia coli and enhances phagocytosis of Haemophilus ducreyi by human macrophages15,22. Ligand binding is a critical step not only in phagocytosis, but also for the receptor interactions that are required to signal in response to pathogens. MARCO has been shown to play a vital role in enhancing the NF-κB pro-inflammatory response to Mycobacterium tuberculosis by tethering the cell wall glycolipid trehalose 6,6′-dimycolate (TDM) to TLR2/CD1421. We have also shown that MARCO is required for Nucleotide Oligomerization Domain (NOD2)- and TLR2-mediated responses to unidentified pneumococcal ligands. To determine if the SRCR domain was required to enhance TLR2-dependent NF-κB activity, we performed an NF-κB reporter assay using cells expressing MARCO or MARCOII. Unsurprisingly, cells transfected with MARCO showed a significant increase in NF-κB activity in response to S. pneumoniae stimulation when compared to cells transfected with TLR2 and CD14 alone. Cells transfected with MARCOII showed no significant change in NF-κB response to S. pneumoniae stimulation when compared to cells transfected with TLR2 and CD14 alone. (Figure 4A). These data indicate that the SRCR domain of MARCO is essential to indirectly enhance NF-κB activity via TLR2/CD14.
Apart from enhanced ligand binding, uptake, and downstream inflammatory responses, expression of MARCO has also been shown to drastically alter cellular morphology and adhesion23. Pikkarainen et al. highlighted that MARCO-mediated cellular adhesion is likely modulated by a proximal region (in later publications, identified as the RxR motif) of the SRCR domain23. Similar to later studies by the same group, an experimental caveat occurred due to various truncated MARCO constructs showing different levels of expression. In contrast, SRA-mediated adhesion has been shown to be dependent on the collagenous domain, so we sought to characterize the role of the SRCR domain of MARCO in both SR-induced morphological changes and cellular adhesion24–26. Analysis of MARCO-transfected cells by Scanning Electron Microscopy showed that MARCO-transfected cells formed thin (<1 μm), highly-branched, dendritic-like processes, as previously observed by Pikkarainen et al23. This morphology was not observed in MARCOII-transfected or vector control cells, which adhered by large, unbranched processes (Figure 5A–D). We next sought to confirm whether the SRCR domain of MARCO directly enhances cellular adhesion of weakly adherent cells. Ojala et al. previously showed that transfection of HEK 293 cells with SCARA5 greatly enhances cellular adhesion27. SCARA5-mediated adhesion is likely not mediated by the SRCR domain, as it does not contain an RxR motif, but this remains to be tested. Preliminary evidence for the role of MARCO in cellular adhesion was observed during passaging of stable MARCO-expressing HEK 293T cells and lifting of transient transfectants. MARCO-expressing cells required significantly more force and repetitions when using forceful pipetting of media as a lifting method. This phenomenon was not observed in vector control or MARCOII-transfected cells. Given that HEK 293T cells are weakly adherent, we decided to directly quantify if transfection with MARCO enhances cellular adhesion. Over a 45-min time course of Accutase treatment, MARCO-transfected cells remained three times more adherent when compared to MARCOII-transfected cells (Figure 5E). These data suggest that unlike SRA, MARCO relies on the SRCR domain to adhere to surfaces. Additionally, our data confirm that the SRCR domain is required to induce MARCO-mediated morphological changes.
These findings show that the SRCR domain of MARCO is critical for receptor function. Given that SRA binds ligands and enhances cellular adhesion independently of the SRCR domain, we can conclude that cA-SR functions are not universally tied to the SRCR domain.
METHODS
Cell Lines
HEK 293T (ATCC #CRL-3216) cells were maintained in complete Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% Heat Inactivated Fetal Bovine Serum (HI-FBS), 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 37°C and 5% CO2. Immunoprecipitation (IP) experiments were performed using HEK 293T cells stably expressing human MARCO. All cell lines were regularly tested for mycoplasma contamination.
Plasmids and Antibodies
Human MARCO plasmids were provided by Timo Pikkarainen. Human MARCOII cDNA (clone CS0DM004YJ08) was subcloned from pCMVSPORT 6 plasmid provided by Wu-Bo Li (GenBank Accession Number CR603381) into pcDNA 3.1/Hygro(+) (Invitrogen, Carlsbad, CA, USA) by standard techniques. Human TLR2 and CD14 plasmids were provided by Dr. Cynthia Leifer (Cornell University, Ithaca, NY). NF-κB SEAP reporter plasmid was purchased from InvivoGen. All plasmids were amplified by chemically competent Escherichia coli DH5-α cells (Invitrogen) and purified using a HiPure Plasmid Filter Midiprep Kit (Invitrogen).
Addition of C-terminal myc tags to MARCO and MARCOII was performed by PCR amplification with primers that contained the myc sequence and restriction enzyme sites to facilitate cloning.
Primary antibodies that were used included; monoclonal mouse anti-beta actin (Sigma Aldrich, Oakville, ON, Canada), monoclonal mouse anti-Myc (9E10), and a rabbit polyclonal anti-MARCO (cytoplasmic domain), as in28. MARCO (SRCR) and MARCOII-specific rabbit polyclonal IgG was produced by immunizing a New Zealand White (NZW)rabbit with a hapten consisting of the 21 C-terminal amino acids of MARCO or MARCOII with adventitious K and Y residues for KLH coupling. Serum was collected and purified using hapten-coupled Affigel-10 beads (Bio-Rad) followed by dialysis in PBS. All procedures were performed in accordance with the McMaster Animal Research Ethics Board guidelines and Institutional Animal Care. Secondary antibodies included Alexa Fluor 633 goat anti-mouse IgG (Invitrogen) and horseradish peroxidase-conjugated goat anti-mouse IgG (Genway, San Diego, CA, USA). IP was performed using lysates from HEK 293T cells stably expressing MARCO that were transiently transfected to express myc-MARCOII or with an empty vector control. MARCO (SRCR)-specific rabbit polyclonal IgG was used for immunoprecipitation, and western blotting for myc-MARCOII was performed with monoclonal mouse anti-Myc (9E10).
Human Macrophage Culture and Transcript Detection
Peripheral Blood Mononuclear Cells (PBMCs) were collected from donors who provided informed written consent. All studies were approved by the Hamilton Integrated Research Ethics Board. PBMCs were isolated from buffy-coat preparations by ficoll density gradient centrifugation and differentiated for 7 d in X-VIVO 10 culture media (Lonza, Basel, Switzerland) supplemented with 5% human AB serum (Lonza) and 20ng/mL Granulocyte-Macrophage Colony Stimulating Factor (GM-CSF).
PBMCs were stimulated for 48 h with either PBS (unstimulated, US), lipopolysaccharide (LPS; 100ng/mL), IL-10 (20ng/mL) or IFN-γ (20ng/mL) Following stimulation, cells were lysed and RNA was isolated using a GENEzol TriRNA Pure Kit (Froggabio, North York, ON, Canada) following manufacturers protocol. cDNA was synthesized from 2 μg PBMC RNA using Moloney Murine Leukemia Virus (M-MuLV) reverse transcriptase (New England Biolabs, Ipswitch, MA, USA) following manufacturers protocol. Semi-quantitative PCR was then performed for 30 cycles using primers surrounding exons 14/17 of the human MARCO mRNA transcript or Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) as a housekeeping control transcript (Table 1). PCR products were run on a 2% agarose gel, stained with Ethidium Bromide and imaged on an AlphaImager Imaging System (Alpha Innotech, San Leandro, CA, USA). To confirm each transcript identity, bands were excised and purified using a GenElute Gel extraction kit (Sigma Aldrich). Sequencing was performed at the McMaster University MOBIX facility.
Table 1.
Primers sequences used for semi-quantitative PCR and transcript sequencing.
| Gene | Forward (5′) | Reverse (3′) |
|---|---|---|
| hMARCO | AGGTGTGAAGGGAGAACAGG | GTGCAGCTCCACAGGGTACT |
| hGAPDH | GAGTCAACGGATTTGGTCGT | TTGATTTTGGAGGGATCTCG |
Production of Recombinant SRCR Domain
The SRCR region of MARCO (residues 400–520) was subcloned into a modified pET15b bacterial expression plasmid including a 6x His tag followed by a Tobacco Etch Virus (TEV) protease cleavage site. The integrity of the resulting HIS6-SRCR construct was confirmed by DNA sequencing (MOBIX, McMaster University). Escherichia coli Rosetta-Gami2 cells (Novagen, San Diego, CA, USA) were transformed and grown in Luria-Bertani media to an OD600~0.7. Protein production was induced with isopropyl b-D-1-thiogalactopyranoside (IPTG) to a final concentration of 0.5 mM and the culture was incubated at 37°C for 3 h with orbital agitation. Cells were harvested by centrifugation at 3,500 g for 15 min. Cell pellets were resuspended in lysis buffer (20 mM Tris pH 8, 500 mM NaCl, 2.8 mM β-mercaptoethanol, 5% v/v glycerol) supplemented with protease inhibitors (phenylmethanesulfonyl fluoride (PMSF), leupeptin, benzamidine and pepstatin A) and lysed by sonication. Since the SRCR domain was found in the insoluble fraction of the lysate, pellets were washed twice with lysis buffer and resuspended in denaturing buffer (20 mM Tris pH 8, 500 mM NaCl, 2.8 mM β-mercaptoethanol, 6 M urea and 5% v/v glycerol). The solution was then centrifuged at 40 000 g for 40 min to remove insoluble cell debris. Unfolded SRCR was then loaded onto a HiTrap Ni-chelating column (GE Healthcare, Mississauga, ON, Canada) equilibrated with denaturing buffer and eluted with a step gradient (0.3 M imidazole). Denatured SRCR was concentrated to 5.0 mg/mL and refolded by massive dilution in refolding buffer (50 mM Tris pH 8, 800mM L-arginine and 10 mM β-mercaptoethanol). Refolded SRCR was concentrated to 0.5 mg/mL, dialyzed in storage buffer (50 mM Tris pH 8, 200 mM NaCl, 400 mM L-arginine, 10 mM β-mercaptoethanol and 5% v/v glycerol) and fractionated by size exclusion chromatography using a Superdex200 10/300 GL column (GE Healthcare). The fractions containing the SRCR trimer were pooled and concentrated to 1 mg/mL in storage buffer. Monodispersity of the refolded SRCR trimer was confirmed by dynamic light scattering on a Zetasizer NanoS (Malvern, Malvern, UK).
Microsphere Binding Assays
HEK 293T cells were seeded in complete Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% HI-FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 mg/ml streptomycin at 1×105 cells/well into 6-well plates. Plates were transfected each with pcDNA3.1/Hygro(+), MARCO, or MARCOII plasmids at 2 μg/well using polyethyleneimine (PEI) (Sigma Aldrich). For knockdown and rescue-of-function experiments, stably-expressing MARCO or MARCOII HEK 293T cells were transiently transfected as above with either 2 μg pcDNA3.1/Hygro(+), 0.5 μg MARCO + 1.5 μg pcDNA3.1/Hygro(+) or 0.5 μg MARCOII + 1.5 μg pcDNA3.1/Hygro(+). At 48 h post transfection, cells were lifted, pooled and centrifuged at 400 g for 10 min. Media was removed and cells were resuspended in Opti-Mem (Invitrogen). Cell numbers were normalized in 1 mL aliquots of each transfectant and aliquotted into replicates with conditions of +/− microspheres and 4°C or 37°C. 0.5 μM polystyrene yellow fluorescent microspheres (Polysciences, Warrington, PA, USA) passively coated with maleylated Bovine Serum Albumin (Mal-BSA) were added to the cells at approximately 320 microspheres/cell. The tubes were then incubated with gentle agitation at 4°C and 37°C for 1.5 h. Following incubation, the cells were centrifuged for 10 min at 500 g. The media was removed from each tube and cells were washed 2x with 1 mL phosphate buffered saline (PBS) to remove all unbound microspheres. Following washes, the cells were centrifuged at 500 g for 10 min at room temperature and resuspended in 200 μL PBS. Samples were added to a black 96-well plate and fluorescence was measured on a Spectramax M3 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA) at Ex441nm/Em486nm. Given that ligand binding, but not phagocytosis occurs at 4°C, we calculated microsphere internalization by subtracting the relative fluorescence of cells incubated at 4°C (bound microspheres) from cells incubated at 37°C (total microsphere association; bound and internalized microspheres). We performed microsphere binding and internalization experiments using chlorpromazine (CPZ), which inhibits clathrin mediated internalization to demonstrate that microsphere internalization occurred only at 37°C (Figure S2).
NF-κB Reporter Assays
HEK 293T cells were seeded in complete DMEM at 104 cells/well in 96 well plates. Cells were transfected with combinations of plasmids as in21. Streptococcus pneumoniae serotype 23F, clinical isolate P1121 was grown and prepared as in13,29. Cells were stimulated 48 h post-transfection using HEK Blue detection media (InvivoGen, San Diego, CA, USA) supplemented with 1 μg/mL of TLR2 agonist Pam3Csk4 (InvivoGen) as a positive control, or heat-killed, lysozyme-digested Streptococcus pneumoniae(multiplicity of infection, MOI = 50). NF-κB activation was measured for Pam3Csk4-stimulated cultures after 24 h and Streptococcus pneumoniae-stimulated cultures after 48 h. Cultures were first analyzed on a Typhoon Trio variable mode imager and quantified using ImageQuant software (ImageMaster, Ann Arbor, MI, USA) to determine GFP expression. The cultures were then read on a SpectraMax 384 Plus spectrophotometer (Molecular Devices) at 655nm absorbance to determine SEAP expression (NF-κB activity). NF-κB activity (Abs630nm) was normalized by dividing SEAP activity by GFP expression.
Cellular Adhesion Assays
HEK 293T cells were seeded in complete DMEM at 1×105 cells/well into 6-well plates. Cells were transfected with plasmids expressing MARCO, MARCOII or with an empty vector control. At 48 h post-transfection, a confluent monolayer was observed. In order to measure the strength of adhesion, cells were treated with 1 mL Accutase (BD Biosciences, Mississauga, ON, Canada), an enzymatic cocktail containing EDTA which eliminates integrin-mediated, but not MARCO-mediated adhesion20. After 0, 15, 30 and 45 min of accutase treatment, cells were washed and adherent cells were quantitated. Each well was stained with 1 mL crystal violet for 2 min and washed 3 times with water to remove excess dye. Plates were dried overnight at room temperature. Following drying, 1 mL 0.2% SDS solution was added to each well. Relative amount of cell adhesion was quantitated by measuring theAbs550nmon a Nanovue spectrophotometer (GEHealthcare).
Immunofluorescence Microscopy
Immunofluorescence microscopy (IF) was performed using cells adherent on poly L-lysine-coated 24-well glass cover slips. Samples were fixed in 2% paraformaldehyde (pH 7.4) at 37°C for 10 min followed by 3 washes with PBS for 10 min. Slides were blocked with 5% BSA for 1 h at room temperature and stained overnight with mouse anti-Myc at 4°C. Samples were then washed 3 times with PBS, and stained with Alexa Fluor 488 or 633 goat anti-mouse IgG (Invitrogen) or Texas Red Phalloidin (Invitrogen) for 30 min at room temperature. Samples were washed a final 3 times with PBS for 10 min. Slides were mounted with ProLong Gold (Invitrogen) and were imaged on a Leica DM IRE2 inverted fluorescence microscope (Leica, Wetzlar, Germany) and adjusted for brightness and contrast using OpenLab 5.5.0 and ImageJ (NIH). All adjustments were applied equally to all images.
Electron Microscopy
Samples were immersed overnight in 2% glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4). The samples were rinsed twice in buffer solution and post-fixed for 1 h in 1% osmium tetroxide in 0.1 M sodium cacodylate buffer. After the second fixation step, the samples were dehydrated through a graded ethanol series (50, 70, 70, 95, 95, 100%, 100%, and 100%) and then dried in a critical point dryer. After drying, the samples were mounted onto scanning electron microscopy (SEM) stubs. The stubs were sputter coated with gold and viewed with a Tescan Vega II LSU scanning electron microscope (Tescan, Brno, Czech Republic).
Flow Cytometry& SRCR Construct Experiments
To evaluate surface expression of MARCO and MARCOII, cells were stained with 9E10 mouse anti-myc antibodies in 5% BSA for 1 h at room temperature followed by two washes with PBS. Secondary staining was performed using Alexa Fluor 633 goat anti-mouse IgG (Invitrogen) antibodies in 5% BSA for 30 min at room temperature in the dark. Following staining, cells were washed twice with PBS, filtered, and assayed with a BD FacsCantoII flow cytometer (BD Biosciences).
Streptococcus pneumoniae was prepared as above. 5×107 bacteria or BSA/Mal-BSA-coated microspheres were incubated with 40 μg SRCR construct in folding buffer at room temperature for 2 h. Cells were washed with PBS and stained as above using a rabbit polyclonal anti-SRCR antibody as in28. Secondary staining was performed as above using Alexa Fluor 633 goat anti-rabbit IgG (Invitrogen) antibodies. Antibody specificity was validated using isotype controls in Figure S3.
Data were gathered using FACSDiva software (BD Biosciences) and analyzed using FlowJo version 7.6.2 software (TreeStar, Ashland, OR, USA).
Quantification of bacterial association was performed by incubating 5×107 Streptococcus pneumoniae with either folding buffer alone, 40 μg BSA or 40 μg SRCR construct in folding buffer at 4°C for 2 h. Cells were washed twice in Hanks Balanced Salt Solution (HBSS). Biogel elicited peritoneal macrophages were collected from wildtype C57Bl/6 mice as in13. Approximately 2×105 macrophages were infected at an MOI of 25 for 30 min. Macrophages were washed once with PBS to remove non-associated bacteria followed by lysis in sterile water. Serial dilutions were performed in water and plated on sheep’s blood agar supplemented with 10 μg/mL neomycin as in13. Colonies were counted after overnight incubation at 37°C.
Statistics
Statistical analyses were performed using GraphPad Prism 5.01 (Graphpad Software, San Diego, CA, USA). Results were considered statistically significant if p <0.05.
Supplementary Material
Comparison of MARCO and MARCOII cDNA demonstrating regions of homology and putative alternative splicing.
Figure S2: Validation of differential fluorescence as a method to detect internalized microspheres. HEK 293T cells transiently transfected with MARCO or an empty vector control were incubated with Mal-BSA coated microspheres in the presence of 28.8 μM chlorpromazine (CPZ) at 4°C and 37°C over a 1.5 h time course. (A) Microsphere binding (4°C) with CPZ-treated cells. (B) Microsphere internalization was abolished in MARCO-transfected cells treated with CPZ. Statistical significance was calculated by 2-way ANOVA with Bonferroni post-hoc test. Error bars indicate Mean ± Standard Error of the Mean (SEM). * = p <0.05, ** = p <. 0.01, *** = p < 0.001. All experiments were performed a minimum of 3 times with a minimum of 3 technical replicates.
Figure S3: Specificity of soluble SRCR construct staining. Isotype control experiments highlighting specific detection of SRCR-bound S. pneumoniae.
Acknowledgments
We thank the McMaster Central Animal Facility staff for maintaining and immunizing the rabbit used in our experiments. A special thank you to Dr. Susan Collins, Dr. Karen Mossman, and Marcia Wallace for assistance with microscopy. The authors would also like to thank Dr. Mark McDermott for critical reading of the manuscript. Work in the Bowdish laboratory is supported in part by the McMaster Immunology Research Centre and the M.G. DeGroote Institute for Infectious Disease Research. Kyle Novakowski is supported by an Ontario Graduate Scholarship. Kyle Novakowski and Dr. Kaori Sakamoto are supported by a National Institutes of Health grant (AI094436-01A1). Work in the Guarné laboratory is supported by operating grants from the Canadian Institutes of Health Research and the Natural Sciences and Engineering Council of Canada. Dr. Dawn M.E. Bowdish is supported by a Natural Sciences and Engineering Council of Canada grant.
Footnotes
The authors declare that they have no conflicts of interest with the contents of this article.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
AUTHORS CONTRIBUTIONS
K.E.N. designed, performed and analyzed the experiments and wrote the manuscript. A.H. created and purified the construct used in Figure 4B and Figure S3. SJ.H. performed and analyzed the experiments shown in Figure 4A. M.G.D. performed and analyzed the experiments shown in Figure 4B and Figure S3. C.Y. designed the experiments shown in Figure 4B and Figure S3. Z.T. designed and performed the experiments in Figure S2. P.P. and P.W. designed the procedure used to generate polyclonal rabbit antibodies. A.G. and K.S. edited the manuscript and provided experimental critique. D.M.E.B. designed the experiments and planned and edited the manuscript. All authors reviewed the results and approved the final version of the manuscript.
References
- 1.Canton J, Neculai D, Grinstein S. Scavenger receptors in homeostasis and immunity. Nat Rev Immunol. 2013;13:621–34. doi: 10.1038/nri3515. [DOI] [PubMed] [Google Scholar]
- 2.Greaves DR, Gordon S. The macrophage scavenger receptor at 30 years of age: current knowledge and future challenges. J Lipid Res. 2009;50(Suppl):S282–6. doi: 10.1194/jlr.R800066-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kraal G, van der Laan LJ, Elomaa O, Tryggvason K. The macrophage receptor MARCO. Microbes Infect. 2000;2:313–6. doi: 10.1016/s1286-4579(00)00296-3. [DOI] [PubMed] [Google Scholar]
- 4.Martínez VG, Moestrup SK, Holmskov U, Mollenhauer J, Lonzo F. The conserved scavenger receptor cysteine-rich superfamily in therapy and diagnosis. Pharmacol Rev. 2011;63:967–1000. doi: 10.1124/pr.111.004523. [DOI] [PubMed] [Google Scholar]
- 5.Brännström A, Sankala M, Tryggvason K, Pikkarainen T. Arginine residues in domain V have a central role for bacteria-binding activity of macrophage scavenger receptor MARCO. Biochem Biophys Res Commun. 2002;290:1462–9. doi: 10.1006/bbrc.2002.6378. [DOI] [PubMed] [Google Scholar]
- 6.Whelan FJ, Meehan CJ, Golding GB, McConkey BJ, Bowdish DME. The evolution of the class a scavenger receptors. BMC Evol Biol. 2012;12:227. doi: 10.1186/1471-2148-12-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rohrer L, Freeman M, Kodama T, Penman MMK. Coiled-coil fibrous domains mediate ligand binding by macrophage scavenger receptor type II. Nature. 1990;343:570–572. doi: 10.1038/343570a0. [DOI] [PubMed] [Google Scholar]
- 8.Mori K, Ohtani K, Jang S, Kim Y, Hwang I, Roy N, et al. Scavenger receptor CL-P1 mainly utilizes a collagen-like domain to uptake microbes and modified LDL. Biochim Biophys Acta. 2014:1–12. doi: 10.1016/j.bbagen.2014.08.015. [DOI] [PubMed] [Google Scholar]
- 9.Gough PJ, Greaves DR, Gordon S. A naturally occurring isoform of the human macrophage scavenger receptor (SR-A) gene generated by alternative splicing blocks modified LDL uptake 1. J Lipid Res. 1998;39:531–543. [PubMed] [Google Scholar]
- 10.Józefowski S, Arredouani M, Sulahian T, Kobzik L. Disparate regulation and function of the class A scavenger receptors SR-AI/II and MARCO. J Immunol. 2005;175:8032–41. doi: 10.4049/jimmunol.175.12.8032. [DOI] [PubMed] [Google Scholar]
- 11.Ashkenas J, Penman M, Vasile E, Acton S, Freeman M, Krieger M. Structures and high and low affinity ligand binding properties of murine type I and type II macrophage scavenger receptors. J Lipid Res. 1993;34:983–1000. [PubMed] [Google Scholar]
- 12.Thierry-Mieg D, Thierry-Mieg J. AceView: a comprehensive cDNA-supported gene and transcripts annotation. Genome Biol. 2006;7(Suppl 1):S12.1–14. doi: 10.1186/gb-2006-7-s1-s12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorrington MG, Roche AM, Chauvin SE, Tu Z, Mossman KL, Weiser JN, et al. MARCO Is Required for TLR2-and Nod2-Mediated Responses to Streptococcus pneumoniae and Clearance of Pneumococcal Colonization in the Murine Nasopharynx. J Immunol. 2013;190:250–8. doi: 10.4049/jimmunol.1202113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montoya D, Cruz D, Teles RMB, Lee DJ, Ochoa MT, Krutzik SR, et al. Divergence of macrophage phagocytic and antimicrobial programs in leprosy. Cell Host Microbe. 2009;6:343–353. doi: 10.1016/j.chom.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Li W, Katz BP, Spinola SM. Haemophilus ducreyi-induced interleukin-10 promotes a mixed M1 and M2 activation program in human macrophages. Infect Immun. 2012;80:4426–34. doi: 10.1128/IAI.00912-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 17.Bowdish DM, Sakamoto K, Lack Na, Hill PC, Sirugo G, Newport MJ, et al. Genetic variants of MARCO are associated with susceptibility to pulmonary tuberculosis in a Gambian population. BMC Med Genet. 2013;14:47. doi: 10.1186/1471-2350-14-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ma M-J, Wang H-B, Li H, Yang J-H, Yan Y, Xie L-P, et al. Genetic variants in MARCO are associated with the susceptibility to pulmonary tuberculosis in Chinese Han population. PLoS One. 2011;6:e24069. doi: 10.1371/journal.pone.0024069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang F-L, Shiao Y-J, Hou S-J, Yang C-N, Chen Y-J, Lin C-H, et al. Cysteine-rich domain of scavenger receptor AI modulates the efficacy of surface targeting and mediates oligomeric Abeta internalization. J Biomed Sci. 2013;20:54. doi: 10.1186/1423-0127-20-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ojala JRM, Pikkarainen T, Tuuttila A, Sandalova T, Tryggvason K. Crystal structure of the cysteine-rich domain of scavenger receptor MARCO reveals the presence of a basic and an acidic cluster that both contribute to ligand recognition. J Biol Chem. 2007;282:16654–66. doi: 10.1074/jbc.M701750200. [DOI] [PubMed] [Google Scholar]
- 21.Bowdish DME, Sakamoto K, Kim M-J, Kroos M, Mukhopadhyay S, Leifer Ca, et al. MARCO, TLR2, and CD14 are required for macrophage cytokine responses to mycobacterial trehalose dimycolate and Mycobacterium tuberculosis. PLoS Pathog. 2009;5:e1000474. doi: 10.1371/journal.ppat.1000474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sankala M, Brännström A, Schulthess T, Bergmann U, Morgunova E, Engel J, et al. Characterization of recombinant soluble macrophage scavenger receptor MARCO. J Biol Chem. 2002;277:33378–85. doi: 10.1074/jbc.M204494200. [DOI] [PubMed] [Google Scholar]
- 23.Pikkarainen T, Brannstrom A, Tryggvason K. Expression of Macrophage MARCO Receptor Induces Formation of Dendritic Plasma Membrane Processes. J Biol Chem. 1999;274:10975–10982. doi: 10.1074/jbc.274.16.10975. [DOI] [PubMed] [Google Scholar]
- 24.Huang J, Zheng D, Qin F, Cheng N, Chen H, Wan B. Genetic and epigenetic silencing of SCARA5 may contribute to human hepatocellular carcinoma by activating FAK signaling. J Clin Invest. 2010;120:223–241. doi: 10.1172/JCI38012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan N, Zhang S, Yang Y, Cheng L, Li C, Dai L, et al. Therapeutic upregulation of Class A scavenger receptor member 5 inhibits tumor growth and metastasis. Cancer Sci. 2012;103:1631–9. doi: 10.1111/j.1349-7006.2012.02350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, van Vreden C, et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med. 2014;6:219ra7. doi: 10.1126/scitranslmed.3007563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ojala JRM, Pikkarainen T, Domogatskaya A, Tryggvason K, Rodin S. A novel scavenger receptor 5-based antibiotic-independent selection method for generation of stable recombinant protein-producing mammalian cell lines especially suitable for proteins affecting cell adhesion. Biotechniques. 2012;53:221–30. doi: 10.2144/0000113936. [DOI] [PubMed] [Google Scholar]
- 28.Elomaa O, Sankala M, Pikkarainen T, Bergmann U, Tuuttila a, Raatikainen-Ahokas a, et al. Structure of the human macrophage MARCO receptor and characterization of its bacteria-binding region. J Biol Chem. 1998;273:4530–8. doi: 10.1074/jbc.273.8.4530. [DOI] [PubMed] [Google Scholar]
- 29.McCool TL, Cate TR, Moy G, Weiser JN. The immune response to pneumococcal proteins during experimental human carriage. J Exp Med. 2002;195:359–65. doi: 10.1084/jem.20011576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of MARCO and MARCOII cDNA demonstrating regions of homology and putative alternative splicing.
Figure S2: Validation of differential fluorescence as a method to detect internalized microspheres. HEK 293T cells transiently transfected with MARCO or an empty vector control were incubated with Mal-BSA coated microspheres in the presence of 28.8 μM chlorpromazine (CPZ) at 4°C and 37°C over a 1.5 h time course. (A) Microsphere binding (4°C) with CPZ-treated cells. (B) Microsphere internalization was abolished in MARCO-transfected cells treated with CPZ. Statistical significance was calculated by 2-way ANOVA with Bonferroni post-hoc test. Error bars indicate Mean ± Standard Error of the Mean (SEM). * = p <0.05, ** = p <. 0.01, *** = p < 0.001. All experiments were performed a minimum of 3 times with a minimum of 3 technical replicates.
Figure S3: Specificity of soluble SRCR construct staining. Isotype control experiments highlighting specific detection of SRCR-bound S. pneumoniae.