Abstract
Wolbachia infections have been described in several Drosophila species but relatively few have been assessed for phenotypic effects. Cytoplasmic incompatibility (CI) is the most common phenotypic effect that has been detected, while some infections cause male killing or feminization, and many Wolbachia infections have few host effects. Here we describe two new infections in a recently described species, D. pandora, one of which causes near-complete CI and near-perfect maternal transmission (the “CI” strain). The other infection is a male killer (the “MK” strain), which we confirm by observing re-initiation of male production following tetracycline treatment. No incompatibility was detected in crosses between CI strain males and MK strain females, and rare MK males do not cause CI. Molecular analyses indicate that the CI and MK infections are distantly related and the CI infection is closely related to the wRi infection of D. simulans. Two population surveys indicate that all individuals are infected with Wolbachia, but the MK infection is uncommon. Given patterns of incompatibility among the strains, the infection dynamics is expected to be governed by the relative fitness of the females, suggesting that the CI infection should have a higher fitness. This was evidenced by changes in infection frequencies and sex ratios in population cages initiated at different starting frequencies of the infections.
Keywords: Wolbachia, male killing, cytoplasmic incompatibility, phenotypic effects, Drosophila, MLST
Introduction
Infections of the endosymbiotic bacterium Wolbachia in Drosophila species were first described in D. simulans Sturtevant (Hoffmann et al. 1986) and they have subsequently been found to be widespread across the genus (Rousset and Solignac 1995; Werren and Jaenike 1995; Mateos et al. 2006; Miller and Riegler 2006; Bennett et al. 2012; Müller et al. 2013) although the majority of these infections have only been detected at the molecular level and not characterized for their phenotypic effects on the host (Salzberg et al. 2005; Mateos et al. 2006). The first infection described in Drosophila was shown to cause cytoplasmic incompatibility (CI) (Hoffmann et al. 1986), where embryo death occurred in matings between uninfected females and infected males. Cytoplasmic incompatibility has been detected for several other Drosophila infections since (Giordano et al. 1995; Bourtzis et al. 1996; Charlat et al. 2002; Miller et al. 2010). Infections have also been detected that cause male killing (MK) rather than CI (Dyer and Jaenike 2004) but this phenotype appears rare in Drosophila (Montenegro et al. 2006; Sheeley and McAllister 2009). Other infections have been detected that cause neither CI nor MK in Drosophila hosts (Hoffmann et al. 1996; Zabalou et al. 2004; Hamm et al. 2014); these endosymbionts may spread by generating positive fitness effects in the host (Kriesner et al. 2013; Hamm et al. 2014) although they may also exist transiently in populations if they have very high levels of maternal transmission with minimal effects on fitness.
Male killer infections are relatively common in insects generally (Hurst and Jiggins 2000) and have been identified from a small number of Drosophila (Montenegro et al. 2006) where Wolbachia has been determined as one of the responsible agents (e.g. Hurst and Jiggins 2000; Dyer and Jaenike 2004; Sheeley and McAllister 2009). Models suggest that MK strains have the potential to displace CI infections under some scenarios when they maintain resistance to CI in females and when they are still able to cause CI through males and produce more surviving female offspring (Hurst et al. 2002). However, there is also a region of parameter space where both CI and MK infections are expected to persist, depending on the relative magnitude of CI, rate of male killing and fitness benefits. On the other hand, MK infections are expected to spread most easily in populations where there is strong competition or other interactions among host siblings, or where there is marked inbreeding depression, providing an advantage for hosts that exhibit male killing (Hurst and Majerus 1993). Hosts may also benefit directly from Wolbachia in ways that are unrelated to the male killing phenotype (Unckless and Jaenike 2012). Male killer strains might therefore be relatively more common in Drosophila species where there are strong sibling interactions, although a combination of mechanisms may be required (Unckless and Jaenike 2012).
We are presently exploring the effects and population dynamics of a wide range of Wolbachia infections in Drosophila and related genera to understand factors dictating the dynamics of different types of infections and their ecological context. Here we characterize two newly discovered infections in D. pandora McEvey and Schiffer, a member of the ananassae complex in the melanogaster species group. This species was only recently described and occurs sympatrically within the distribution of D. ananassae (McEvey and Schiffer 2015). Currently D. pandora is known from locations in Papua New Guinea, the Torres Strait Islands, and tropical locations in mainland Australia ranging from the north of Cape York Peninsula to Rockhampton, and as far West as Darwin (McEvey and Schiffer 2015).This species (at least in Australia) appears to prefer urban and disturbed habitats.
The Drosophila pandora – Wolbachia situation seems atypical of Drosophila infections in that our preliminary data pointed to a very high proportion of individuals in a population being infected by Wolbachia, unlike in other Drosophila species, where infected and uninfected individuals typically occur within populations (Hoffmann et al. 1996; Merçot and Charlat 2004; Dyer et al. 2005; Montenegro et al. 2006).
We therefore investigated these Wolbachia infections in detail by: 1) determining the infection frequency of Wolbachia in two field populations of D. pandora from northern Australia; 2) characterizing the phenotype of the Wolbachia infections through experiments investigating cytoplasmic incompatibility, maternal transmission and fecundity; and 3) typing the strains with different phenotypes through PCR and sequencing, and examining their genetic relationships in a phylogenetic context. We show that the D. pandora situation is unusual in having coexisting MK and CI infections present in populations, and we therefore investigate the dynamics of the two infections in a population experiment. Results are interpreted in the context of models that help to explain the frequencies of the two coexisting infections.
Methods
D. pandora field collections and species identification
Population samples of D. pandora were obtained in April 2014 by initiating 112 isofemale lines from Woodlands Big4 Tourist Park, Townsville (19.241°S, 146.664°E) and a further 33 lines from Lake Placid Tourist Park in Cairns (16.269° E, 145.674° S). Species identification was based on configuration of the sex combs on tarsomeres I and II of the male foreleg, and examination of the male Terminalia (see McEvey and Schiffer 2015). Lines were screened for Wolbachia infection by PCR as outlined below. The F1 progeny were collected and stored in 100% ethanol. These collections yielded two female-biased lines of an ananassae-complex species which were initially unidentifiable due to the complete absence of male progeny. By introducing identified males from various ananassae complex species and recording which crosses produced progeny, we were able to determine that these Wolbachia-infected lines were both D. pandora.
To further confirm that the female-biased lines were indeed D. pandora and not a cryptic species, we used the Drosophila nuclear markers Ddc and Pgi (Matsuda et al. 2009) to screen a single F1 individual from ten of the isofemale lines from Cairns and ten from Townsville in addition to the single female-biased line from each location (one individual for the Cairns line, two individuals for the Townsville line). A further two female-biased lines collected at the same location in Cairns in November 2014 were also included, bringing the total female-biased lines to four. DNA extractions were performed using a 5% Chelex (BioRad) based plate method on single individuals. This involved extracting a whole fly per well in a deep well plate using 2.5 µL of Proteinase K (Roche), 250 µL of 5% Chelex solution and two 2 mm glass beads. Samples were homogenized in a mixer mill, 150 µl of the resulting solution was transferred to a standard 96 well PCR plate which was then incubated for 30 minutes at 65°C followed by 10 minutes at 90°C. The PCR conditions for Ddc were: 3 mins at 94°C; 37 cycles of 30 seconds at 94°C, 45 seconds at 65°C, 90 secs at 72°C; and a final extension for 10 mins at 72°C followed by holding at 4°C. PCR conditions for Pgi were the same except for 30 cycles and an annealing time of 75 seconds. PCR products were sequenced using Sanger sequencing by Macrogen (Korea). The chromatograms were checked and edited manually in Geneious v6.1.4 http://geneious.com (Kearse et al. 2012) and double peaks indicating heterozygosity were recorded as IUPAC ambiguity codes. The resulting sequences were aligned and used to build neighbour-joining trees for each marker separately using the Tamura-Nei distance with 100 bootstrap replicates.
Establishment of laboratory lines
Several laboratory lines of D. pandora were used in crosses and experiments. The first line was established by pooling flies from three isofemale lines collected in May 2011 near Lake Placid, Cairns (16.870°S, 145.676°E and 16.869°S, 145.671°E), each of which did not show any bias in sex ratio. These lines were mixed in November 2013 to create a single line for experiments, denoted by pl+ in this paper. Individuals from this combined line were all found to be infected by Wolbachia based on PCR characterization (see below). A line cured of this infection (denoted by pl−) was created from the pl+ line following treatment with 0.03% tetracycline (Sigma) in cornmeal media for a generation as outlined in Hoffmann et al (1986). Tetracycline treatment was undertaken on offspring of the adults that were pooled to form the pl+ line. Lines were maintained in the laboratory on cornmeal media at 19°C with a 12:12 L:D cycle.
The third laboratory line used denoted by C168+ represented an isofemale line that was also positive for Wolbachia but that produced only female offspring when it was first set up. This line and other female biased lines could only be maintained by introducing males from the non-female-biased pl+ D. pandora line each generation.
To assess whether the sex ratio bias we observed in the C168+ line of D. pandora was related to Wolbachia infection, we treated this line with tetracycline as outlined above to create a C168− line which was scored for sex ratio of the emerging progeny. The absence of Wolbachia in this line was verified by PCR (see below).
Wolbachia infection frequency and strain typing
A preliminary screen for Wolbachia was conducted for all field isofemale lines. DNA extractions were performed using the 5% Chelex method outlined above and Wolbachia infection was assessed via standard PCR using the gatB primers for the Multilocus Sequence Typing System (MLST) for Wolbachia outlined in Baldo et al (2006). The PCR conditions were: 3 mins at 94°C; 37 cycles of 30 seconds at 94°C, 45 seconds at 54°C, 90 secs at 72°C; and a final extension for 10 mins at 72 °C followed by holding at 4°C.
Presence of the gatB amplicon was confirmed by running 5 µl of PCR product on a 2% molecular biology grade agarose gel (Scientifix) and observing a clear 471 bp band. To confirm infection status, we also screened a subset of samples using the wsp validation primers wsp_val (Lee et al. 2012; Kriesner et al. 2013) with the cycling regime outlined above for gatB, and an annealing temperature of 59°C.
To further investigate the Wolbachia infections involved, additional PCR amplifications were conducted for two individuals each from two infected non-female-biased isofemale lines and two infected female-biased isofemale lines (including C168+), with the forward and reverse coxA, hcpA, ftsZ, fpbA and gatB MLST primers (Baldo et al. 2006) and wsp_val primers (Lee et al. 2012; Kriesner et al. 2013). The cycling regime was the same as outlined above for gatB, with an annealing temperature of 54°C for coxA, ftsZ and hcpA and 59°C for fpbA and wsp_val. As above, agarose gels were used to visualise clear bands that confirmed the presence of amplicons of the appropriate size for each primer pair. PCR products were sent to Macrogen (Korea) for purification and Sanger sequencing. Sequencing chromatograms were examined and processed using Finch TV v1.4.0 (Geospiza, Seattle, WA) and MEGA version 6 (Tamura et al. 2013). Allelic profiles were compared to those in the MLST database at http://pubmlst.org/wolbachia/ (Jolley and Maiden 2010).
For routine rapid scoring of Wolbachia infections in experiments, we used the genotyping assay developed by Lee et al (Lee et al. 2012; Kriesner et al. 2013) which distinguishes Wolbachia infections on the basis of melting temperature (Tm) difference of the wsp validation PCR amplicons. High-resolution melt analysis on the Roche LightCycler® 480 system produced distinct Tm clusters for female-biased and non-female-biased wsp amplicons that differed by Tm ~0.5 °C. While the clusters are always distinct, the Tm range can differ slightly between runs so the use of controls is essential (e.g. Run 1 female-biased: 81.88 – 82.21°C and non-female-biased: 82.65–82.79 °C; Run 2 female-biased: 82.24–82.51°C and non-female-biased: 82.96–83.28 °C). Sanger sequencing of representative PCR products (14 non-female-biased lines and all 4 female-biased lines) confirmed that individuals from non-female-biased lines clustered at a high Tm whereas those from the female-biased lines clustered at the lower Tm.
To investigate Wolbachia colonization history we also screened female biased and non-female biased lines for mtDNA variation using the mitochondrial marker CO1 (Matsuda et al. 2009). Samples were those outlined above for the nuclear markers Ddc and Pgi comprising 20 individuals from non-female biased lines and four from female biased lines. PCR conditions were: 3 mins at 94°C; 32 cycles of 30 seconds at 94°C, 45 seconds at 55°C, 90 secs at 72°C; and a final extension for 10 mins at 72°C followed by holding at 4°C. However the results revealed very low variation and did not warrant phylogenetic analysis. Sequences have been deposited in Genbank (KX234146- KX234169).
Wolbachia tree-building
We built an unrooted species tree to investigate the genetic relationships of the two D. pandora infections in the context of broader Wolbachia diversity. Representative sequences were obtained for taxa from each Wolbachia supergroup with all five MLST markers and wsp sequences available. Taxa included the most highly-scoring BLAST matches to our D. pandora Wolbachia sequences and several previously described MK infections. The majority of sequences were obtained from GenBank (http://www.ncbi.nlm.nih.gov/genbank/ Benson et al. 2011), and several unpublished sequences for Folsomia candida were kindly provided by Laura Baldo from the University of Barcelona and John H Werren from the University of Rochester. Details of sequences used in the tree-building are presented in Table S1. For each marker region, sequences were aligned using Geneious v6.1 and alignments were trimmed to ensure coverage of all taxa and to remove primer regions. PartitionFinder v1.0.1 (Lanfear et al. 2012) was used to determine appropriate codon position partitioning and nucleotide evolution models for each partition; these were chosen according to the best BIC value.
An initial run of the Bayesian phylogenetics software BEAST v2.3.1 (Bouckaert et al. 2014) for each gene alignment then provided estimates of substitution rate and other nucleotide evolution model parameters used as non-estimated priors in a *BEAST run (Heled and Drummond 2010; Bouckaert et al. 2014) incorporating all genes (see Table S2 for details). The *BEAST approach co-estimates an overall species tree along with individual gene trees for each marker. In this case, molecular clocks and gene trees were linked across partitions within each marker, but maintained as unlinked between markers. Although the markers should in theory share the same tree because they are inherited together, the individual gene trees showed some differences in topology that caused convergence problems in trial *BEAST runs. Leaving gene trees unlinked also allows for processes like horizontal gene transfer that may have occurred during evolution of these taxa. Ploidy was set to ‘Y or mitochondrial’ for each marker; strict molecular clocks were used, and all clock rates were assigned gamma-distribution priors (shape = 2, scale = 2); the species tree was modelled with a Yule model with birthrate assigned a 1/X distribution (default settings) and species tree population size was set to ‘linear’ with population mean estimated during the run. All other priors and operators were maintained at default settings, except the SubtreeSlide operator size was reduced to 0.02 for each gene tree, and the treeScaler scale operator scale factor was set to 0.9 for the hcpA and ftsZ gene trees.
Five independent runs were performed with these settings, each run for 10M generations, sampling trees every 10,000 generations. Traces were checked in Tracer v1.6 (Rambaut et al. 2014) to confirm all ESS values were >200 for the combined runs, and species trees were visualized in DensiTree v2.0 to check that the independent runs agreed on species tree topology. Finally, all five runs were combined in LogCombiner (Bouckaert et al. 2014), removing 20% of each run as burnin (leaving 4204 trees in total). A Maximum Clade Credibility tree was calculated in TreeAnnotator (Bouckaert et al. 2014) retaining median branch lengths. This tree was visualized with the ape package v3.4 (Paradis et al. 2004) in R v3.2.2 (R_Core_Development_Team 2015).
Further phenotype characterization
The infection phenotypes were characterized in the pl+ (non-female-biased) and C168 (female-biased) infected D. pandora lines by conducting a series of experiments investigating cytoplasmic incompatibility, fitness and maternal transmission of Wolbachia. Experiments were conducted at 19°C and/or 25°C with a 12:12 L:D cycle and involved lines 2–20 generations after tetracycline treatment.
Cytoplasmic Incompatibility and Crosses
Cytoplasmic incompatibility associated with the pl+ line was assessed in two identical experiments, performed four generations apart in January and April 2014 in which the pl+ and pl− lines were reciprocally crossed in addition to control crosses within lines (Cross 1. ♀− × ♂+,2. ♀+ × ♂−, 3. ♀+ × ♂+, 4. ♀− × ♂−). Virgins were collected within six hours of emergence and holding vials were checked for absence of progeny after 10 days to confirm virgin status. Fifteen replicates of each cross were set up when virgins were five days old; mating was observed and males were stored in ethanol. Females were provided with spoons containing treacle media brushed with yeast paste to encourage egg-laying. Spoons were checked for eggs the following day; females that had laid >10 eggs were stored in ethanol and the spoons removed, with new spoons provided to flies that had not laid or that had laid fewer than 10 eggs. A minimum of 10 eggs were used, following Hoffmann et al (1986). Spoons were scored for hatched and unhatched eggs 24 hours after collection and put in vials containing 6 ml cornmeal media to provide an appropriate food source for growing larvae. Progeny took approximately 10 days to develop and upon eclosion were stored in 100% ethanol for scoring of sex ratio.
Incompatibility relationships between the pl+ line and C168 line were assessed in an experiment conducted in March 2015 at 25°C. Infected and uninfected individuals of the C168+ and C168− lines were crossed with infected and uninfected individuals of the pl+ and pl− lines (Cross1. C168− ♀ × pl+ ♂, 2. C168+ ♀ × pl− ♂, 3. C168+ ♀ × pl+ ♂, 4. C168− ♀ × pl− ♂, 5. C168+ ♀ × C168− ♂, 6. C168− ♀ × C168− ♂). No cross could be performed between pl− females and C168+ males owing to the lack of male progeny for C168+ at 25°C. Virgin collection, mating, egg collection, egg scoring and progeny scoring were conducted as for the pl+ CI experiment outlined above.
Maternal transmission
Maternal transmission for the pl+ line was assessed following a three step protocol: 1) eggs were collected from an individual female from the pl+ line; 2) 25 sub-lines were created by crossing the resulting female progeny with uninfected males from the pl− line; and 3) four male progeny from each sub-line were crossed with an equivalent number of uninfected females from the pl− line. Individuals for steps 2 and 3 of this experiment were collected as virgins and crossed when they were 5 days old. Eggs were collected on spoons over 24 hours and scored for hatched and unhatched eggs after a further 24 hours. Progeny were collected into 100% ethanol and scored for sex ratio. Crosses with high hatch rates were screened for Wolbachia as outlined above, meaning that maternal transmission was determined from a combination of CI and PCR detection (i.e. males from the final step should show CI when crossed to pl− and also be positive for Wolbachia where maternal transmission has succeeded).
Maternal transmission of the C168+ line was assessed by collecting 10 C168+ females and conducting pairwise matings with C168− males at 25°C. Ten progeny per cross were collected and screened for Wolbachia status via PCR to determine the rate of maternal transmission. We were unable to replicate the three part experiment that we did for the pl+ line owing to the absence of males for this line at 25°C.
Temperature and male emergence
Hurst et al (2000) noted that exposure to elevated temperatures reduced male killing in D. bifasciata Pomini resulting in some male emergence. To test this in the C168+ D. pandora line, we crossed females from the C168+ line with infected and uninfected males from the pl+ line (12 and 13 replicates respectively) at five days old. We maintained the crosses at 28°C for two weeks and scored the sex ratio of progeny. This temperature was chosen as an intermediate between a standard Drosophila rearing temperature of 25°C and the thermal extreme for the majority of Drosophila species of 32°C (Chakir et al. 2002). We chose two weeks as the termination point for the experiment based on the results of a pilot study investigating the effect of C168+ female age on male emergence at high temperature which indicated no males were produced after females were more than one to two weeks old.
To investigate the effect of any emerging males from the C168+ line on egg hatch rates and progeny sex ratios, we collected males from the pl+, pl−, C168+ and C168− lines of D. pandora that were reared at 28°C and reciprocally crossed them with females from the same lines reared at 25°C. After emergence, virgin flies were placed at 25°C and remained at this temperature for all subsequent work. Crosses were conducted at five days of age, mating occurrence in the first six hours was recorded, eggs were collected and egg hatch rates and progeny sex ratios were scored. Males (N = 41) from C168+ were screened for Wolbachia infection via PCR as outlined above using the gatB primers.
Fecundity
Fecundity was assessed for pl+, pl−, C168+ and C168− lines at 25°C in September 2015. Fifteen crosses per line were set up using five day old virgins. C168+ females were crossed with males from pl+ owing to the lack of males for ‘C168+ at 25°C. Crosses and egg collection were conducted as for CI experiments by replacing spoons every day over four days and scoring egg number. Fecundity was compared among crosses with a one way ANOVA given that egg number data were normally distributed.
Population monitoring
The population dynamics of the two infections were investigated in a population experiment running over several months in late 2015. A replicate consisted of a bottle containing 200 flies (1:1 sex ratio).The females were collected as virgins and at two days old were sorted into treatments made up of 10%, 25%, 50%, 75% and 90% ‘C168+ females with the remainder from the pl+ line. All males were collected as virgins from the pl+ line and were added to each bottle after females to reduce initial mating bias. Eleven replicates were set up per treatment and were maintained for five generations using 100 ml standard cornmeal media. Bottles were density controlled to approximately 200 individuals at the beginning of each generation.
Random samples of one hundred generation five progeny from each replicate were scored for sex ratio. In addition, 50 females from all replicates of the 10%, 50% and 90% treatments were extracted and screened for infection type using the 5% Chelex extraction method and Tm-based genotyping assay of the wsp validation PCR amplicon, both outlined above. Sex ratio and infection frequency were tested against the expectation that the infections would be maintained at the same frequency as in the starting population. Deviations from expectations were tested through one sample t-tests (with proportions being angular transformed). Expectations for sex ratio were based on the notion that MK flies produced only female offspring whereas CI females produced an equal number of males and females.
Results
Wolbachia infection in natural populations
To confirm that the two Wolbachia infection types were indeed occurring in the same species and that this was not a case of cryptic species within D. pandora, we obtained nucleotide sequences for 25 individuals from two sites with the Drosophila nuclear markers Ddc and Pgi. (Genbank accession numbers KX234098 - KX234145).Both markers showed variation (Ddc: 20/600 bp variable; Pgi: 11/760 bp) although much of this appeared to be present as heterozygosity within individuals and so was of little utility for determining individual relationships. The resulting neighbour-joining trees (Figs. S1, S2) show no clustering of infection type (MK vs CI) and no clustering of source location (Cairns vs Townsville). This provided additional evidence that the two distinct Wolbachia strains are segregating within D. pandora.
To assess Wolbachia infection frequencies in D. pandora, we screened a variety of laboratory lines in addition to 112 isofemale lines from Townsville and 36 from Cairns. All lines tested were infected with Wolbachia (binomial confidence intervals of 97–100% for Townsville and 90–100% for Cairns). Of these, one female-biased line was collected in Cairns (2.78% of all Cairns lines) and one in Townsville (0.89%).
Our initial line characterization focussed on the pl+ line. The PCR assay confirmed that after tetracycline treatment the pl− line lacked Wolbachia. Crosses between pl− females and Wolbachia infected pl+ males had low or non-existent hatch rates compared to controls (generalized linear model, binomial error, P < 0.001 for cross term), indicating almost complete CI in the pl+ line of D. pandora (Table 1), but no strong male bias. We therefore refer to this as a CI Wolbachia infection.
Table 1.
Results of crosses between pl+ and pl− lines of D. pandora for crosses in a) Jan 2014 and b) April 2014.
| Cross | N | Average % Hatch ± SD |
Range of Egg Numbers Scored |
Average % Males ± SD |
|---|---|---|---|---|
| a) | ||||
| ♀− × ♂+ | 15 | 0 | 22–67 | no progeny |
| ♀+ × ♂− | 13 | 77.39 ± 29.15 | 13–37 | 41.57 ± 17.71 |
| ♀+ × ♂+ | 14 | 81.14 ± 23.65 | 12–33 | 50.18 ± 14.73 |
| ♀− × ♂− | 15 | 77.16 ± 24.24 | 12–40 | 52.02 ± 12.44 |
| b) | ||||
| ♀− × ♂+ | 9 | 2.42 ± 4.01 | 10–53 | 1♀, 1♂ |
| ♀+ × ♂− | 10 | 78.24 ± 31.37 | 12–30 | 46.15 ± 18.92 |
| ♀+ × ♂+ | 9 | 86.99 ± 19.98 | 11–26 | 53.72 ± 17.15 |
| ♀− × ♂− | 5 | 83.54 ± 16.08 | 12–22 | 34.76 ± 21.77 |
N, the number of replicates after excluding those which did not mate, or which had fewer than 10 eggs.
We then carried out initial characterization of the C168+ line given its female biased sex ratio which was not observed in the pl+ line. Treating a copy of the female-biased C168+ with tetracycline resulted in emergence of male progeny and more equal sex ratios (20 ♀, 16 ♂ progeny) compared to a copy of the line that was not treated with tetracycline (27 ♀, 0 ♂). The C168− line became self-sustaining and no longer required the introduction of males, while PCR confirmed its uninfected status, suggesting that the female-bias is indeed related to Wolbachia infection.
Crosses between pl+, pl−, C168+ and C168− showed that the female bias is strongly maintained when C168+ female is present, regardless of the male’s infection status (Table 2). Average egg hatch rates for crosses involving these females were roughly half of those in control crosses indicating that the Wolbachia phenotype for C168+ is male killing, as opposed to feminization. We therefore refer to this as an MK infection.
Table 2.
Results of crosses between infected (+) and uninfected (−) individuals of the C168+ and pl+ D. pandora lines for crosses conducted in March 2015.
| Cross | N | Average % Hatch ± SD |
Range of Egg Numbers Scored |
Average % Male Progeny ± SD |
|---|---|---|---|---|
| C168−♀ × pl+♂ | 12 | 0 | 11–26 | no progeny |
| C168+♀ × pl−♂ | 16 | 44.60 ± 18.50 | 10–27 | 0 |
| C168+♀ × pl+♂ | 13 | 45.36 ± 22.56 | 11–42 | 0 |
| C168−♀ × pl−♂ | 13 | 96.41 ± 4.25 | 11–26 | 55.66 ± 11.27 |
| C168+♀ × C168−♂ | 15 | 48.40 ± 13.56 | 10–33 | 2.56 ± 9.25 |
| C168−♀ × C168−♂ | 12 | 89.00 ± 14.47 | 10–34 | 49.82 ± 14.63 |
N, the number of replicates after excluding those which did not mate, or which had fewer than 10 eggs.
To characterize the infections further, nucleotide sequences for the two Wolbachia strains were obtained for the five MLST loci and wsp (Genbank accession numbers KU686364-KU686375). The Wolbachia strain associated with the CI lines was identical to the wRi strain, while the MK strain had an allelic configuration that did not match any known Wolbachia stains in the MLST database or Genbank. Chromatograms were examined for evidence of co-infection by looking for double peaks or a sudden drop in sequence quality, however none were detected. Moreover, sequences obtained from individuals were always homogeneous. Alignment of the wsp validation PCR amplicon revealed 22 SNPs and one 3-bp insertion-deletion polymorphism fixed between the non-female-biased and female-biased variants. These fixed differences formed the molecular basis of two distinct melt profiles and the Tm-based genotyping assay used in the population dynamics experiments.
Wolbachia tree-building
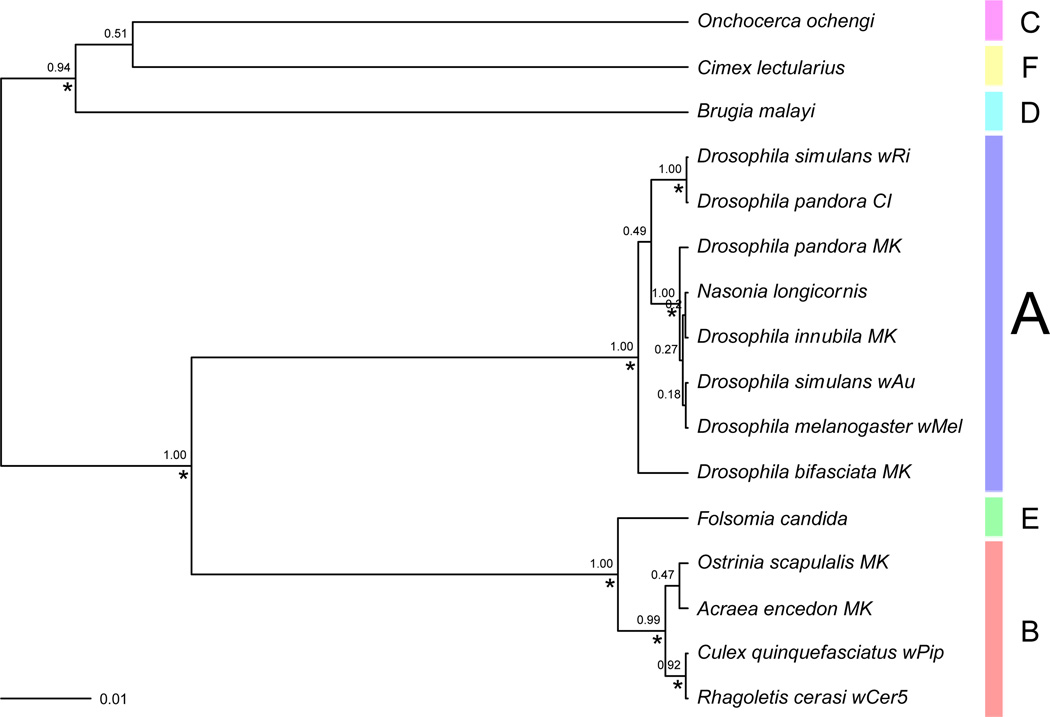
To investigate the similarity of the two D. pandora infections in the context of broader Wolbachia diversity, we calculated an unrooted Bayesian species tree. Species tree estimation revealed both Wolbachia strains in D. pandora are members of Supergroup A (Fig 1). The CI infection clustered with the D. simulans wRi strain (as expected given their 100% sequence identity for the markers tested), whereas the MK infection clustered with several other Drosophila strains including D. melanogaster Meigen wMel, D. simulans wAu and the D. innubila Spencer MK infection, as well as the Nasonia longicornis Darling infection. These relationships were strongly supported with > 0.99 posterior probability support in each case. The D. bifasciata MK strain included was also assigned to Supergroup A but did not cluster strongly with either of the two D. pandora infections. Outside Supergroup A, the representatives of the five other supergroups all formed distinct groupings as expected. Interestingly, supergroups B and E appeared relatively closely related, as evidenced by the short branch length separating them. The non-Drosophila MK sequences both occurred in Supergroup B, suggesting that the MK phenotype appears to have evolved several times across Wolbachia evolutionary history.
Figure 1.
Unrooted Wolbachia species tree highlighting the known supergroups and the position of the newly described D. pandora non-female-biased (CI) and female-biased (MK) infections. This is the maximum clade credibility (MCC) tree produced by combining five independent Bayesian *BEAST runs that co-estimated a species tree with gene trees for each MLST marker coxA, fpbA, ftsZ, gatB, hcpA and wsp. The MCC tree was estimated from 4204 species trees sampled over a total of 40M generations, after removing 20% burnin from each run, using median branch heights (see Methods for details). Bayesian posterior probability support values are shown for each clade, with values over 0.9 (strong support) indicated with an asterisk. Supergroup assignment is highlighted with coloured bars. Scale bar indicates branch length in substitutions per site.
Further phenotypic characterization
To characterize the CI and MK lines from D. pandora further, we considered maternal transmission of the infections, as well as temperature effects on MK and fecundity of both strains.
Maternal transmission
We assessed maternal transmission of Wolbachia for the CI infection in D. pandora by generating 25 maternal sublines from an individual female, crossing their male progeny with uninfected females and assessing the hatch rates of their eggs. If transmission was high, the majority of crosses should have a low hatch rate consistent with the high CI that occurs when an infected male mates with an uninfected female. Maternal transmission was very high at 98%; of 82 crosses involving the pl+ line (25 sublines × 2–4 replicates), only two replicates from different mothers showed a high hatch rate consistent with imperfect maternal transmission of Wolbachia. Later PCR screening showed that the males involved in these crosses were indeed uninfected, despite their mothers being infected. The remainder of replicates had hatch rates ranging between 0 and 13% which were consistent with findings from previous CI assays, and these remaining hatch rates did not differ significantly among the lines (Kruskal-Wallis test, df = 24, P = 0.689).
Maternal transmission of the MK Wolbachia infection was assessed by collecting and PCR screening 10 progeny from each of 10 mothers from the C168+ line. Maternal transmission was 100% of 100 female progeny screened from 10 mothers, all were infected. Screening of the rare males produced at 28°C (N = 41) showed 98% were infected, suggesting a low level of imperfect transmission may occur when populations are at sufficiently high temperatures for males to emerge.
Temperature and male emergence
We tested the potential for exposure to elevated temperatures to reduce male killing, as occurs in D. bifasciata Pomini (Hurst and Jiggins 2000), by crossing females from the C168+ line with infected and uninfected males from the pl+ line at 28°C. Elevated temperature resulted in some male emergence for C168+, however sex ratios were far from equalized. When held at 28°C, 8.5% of progeny (73 out of 853 in total) were male when a C168+ female was crossed with an infected pl+ male, while 6.2% (45 out of 729 in total) were male when a ‘C168+ female was crossed with a pl− male. The majority of these males (60%) emerged on the first day when they comprised 14% of the offspring, whereas only 4.2% of the males emerged on the last two days, when they comprised 3% of offspring. Differences in males and females emerging across the five days differed significantly (G = 77.91, df = 4, P<0.001) by a contingency test.
When male flies reared at 28°C were used in crosses, those from the C168+ line did not perform well, showing much lower mating rates (18–43% mated) than those from the pl+ line or either uninfected line (75–93% mated). The few that mated did not appear to induce CI (for example when mated with pl− females average hatch rate of 87.6%, SD 16.7, N = 6 crosses) however their low mating success resulted in inadequate data to assess this in a meaningful way (when mated with C168− females, mating occurred in only three crosses). In crosses with C168+ females, only 1 male was produced out of 65 progeny. In terms of failing to generate CI or prevent MK, these males therefore behaved the same as uninfected males, despite the majority of them being infected as noted above.
Fecundity
Fecundity was similar for all D. pandora lines irrespective of infection status and infection type (Table 3). An ANOVA showed no significant difference among the four cross types (F(3,52) = 0.552, p = 0.649).
Table 3.
Fecundity of D. pandora lines scored over four days.
| Cross | N | Mean Eggs Laid ± SD |
|---|---|---|
| C168+ ♀ × pl+ ♂ | 15 | 106.07 ± 29.27 |
| pl+ ♀+ × pl+ ♂ | 14 | 112.14 ± 19.28 |
| C168− ♀− × C168−♂ | 14 | 117.57 ± 26.18 |
| pl− ♀ × pl− ♂ | 13 | 112.00 ± 19.26 |
Population monitoring
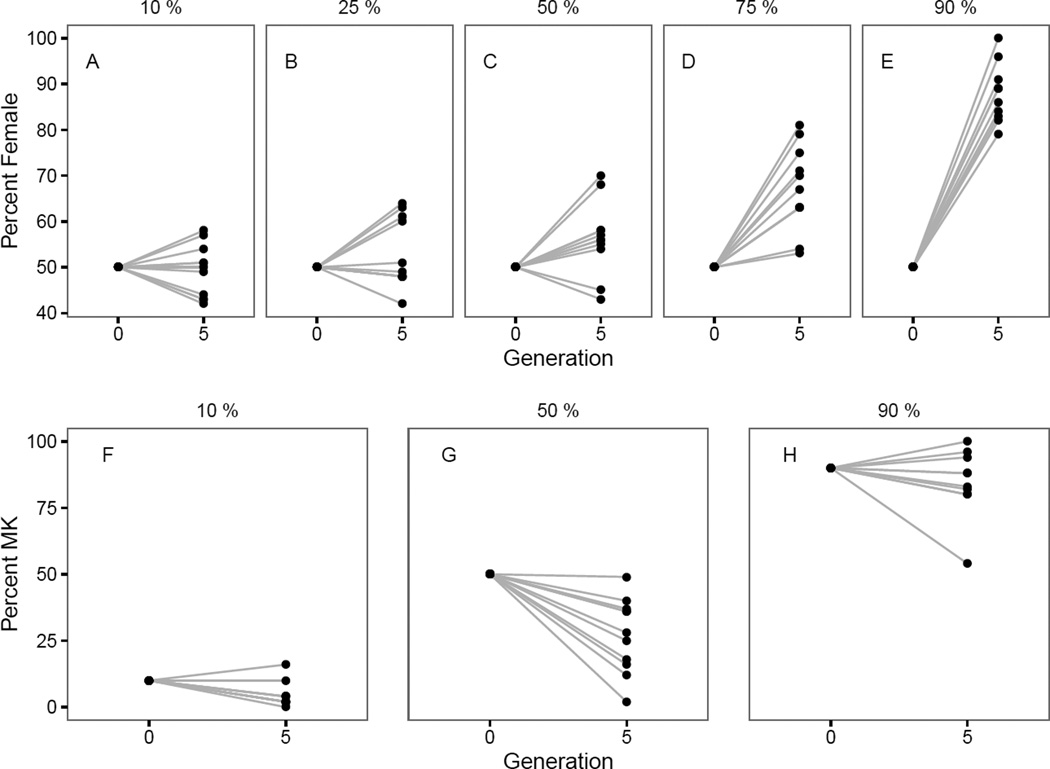
To investigate the population dynamics of the two infections, we set up treatments made up of 10%, 25%, 50%, 75% and 90% C168+ females with the remainder from the pl+ line, and cultured the populations over several months. After five generations, the sex ratio ranged from approximately equal for the 10% C168+ treatment to substantially female-biased for the 90% treatment (Fig 2a). Indeed one 90% replicate contained only females while another failed to produce any progeny, suggesting that there may have been only females in the previous generation. Screening results indicated that there were 0–16%, 2–49% and 54–100% C168+ females remaining in the 10%, 50% and 90% treatments respectively (Fig 2b).
Figure 2.
Results of population monitoring experiment showing percentage of females in samples of one hundred generation five progeny from each replicate for; A) 10%, B) 25%, C) 50%, D) 75%, and E) 90% treatments; and percentage C168+ MK females for samples of fifty females screened from each replicate for F) 10%, G) 50%, and H) 90% treatments.
Based on the assumption that: the infections have a similar fitness; the MK infection produced complete male killing; and an infinite population size, we expected the sex ratios in the population cages after five generations to be dictated by the frequency of the C168+ infection at the start of the experiment. We used one sample t-tests (2 sided) to compare the expected proportion of females of 0.526, 0.571, 0.667, 0.800 and 0.909 for the 10%, 25%, 50%, 75% and 90% ‘C168+ treatments respectively against the proportions we observed. For instance, for the 10% treatment we expected 90% of the population to show a 50:50 sex ratio, and 10% of the population to produce only females, leading to an expected female proportion of (0.9*0.5)+(0.1*0.5) divided by the total proportion emerging (1−(0.1*0.5)), resulting in a final proportion of 0.526. These sex ratios should not change if the frequencies of the infection do not change in the populations. We observed lower proportions of females than expected in all except the 90% treatment with: a female proportion of 0.493 (t = 1.94, df = 10, p = 0.081) for the 10% treatment, with a significant difference in two cases: 0.529 (t = 1.833, df = 10, p = 0.097) for the 25% treatment; 0.564 (t = 4.28, df = 10, p = 0.002) for the 50% treatment; 0.672 (t = 4.63, df = 10, p = 0.001) for the 75% treatment; and 0.879 (t = 1.46, df = 9, p = 0.179) for the 90% treatment.
We also expected the frequency of C168+ females to remain at 10%, 50% and 90% on average for these three treatments if the relative fitness of the two infections was equal and assuming only drift effects. However one way t-tests revealed the observed frequencies were lower than expected, averaging 4.4% for the 10% treatment (t = 4.04, df = 10, p = 0.002), 27.2% for the 50% treatment (t = 5.40, df = 10, p < 0.001) and 84.4% for the 90% treatment (t = 1.39, df = 9, p = 0.198), suggesting an advantage for the CI infection which appears to have increased in frequency relative to the MK infection. Based on the observed MK frequencies, we expected the proportion of females after five generations to be 0.511, 0.578 and 0.865 for the 10%, 50% and 90% populations respectively, and the observed proportions of 0.492, 0.564 and 0.879 respectively, showed no significant departure from expectations.
Discussion
We found a male-killing Wolbachia that appears to be coexisting with a CI Wolbachia at a low frequency in D. pandora populations (assuming that these infections are at equilibrium frequencies). Male killer infections have the capacity to spread rapidly in populations (Hurst et al. 2002), however they tend to occur at variable frequencies in natural populations (Hurst and Majerus 1993). Some male killers can occur at a relatively high frequency including Wolbachia MK strains in Drosophila species. For instance, the male killing Wolbachia from D. innubila is present at intermediate frequencies in natural populations and appears to have persisted in populations as a consequence of a balance between maternal leakage of the infection and a selective advantage (Dyer and Jaenike 2004). This infection can also increase to fixation under some conditions (Jaenike et al. 2003).
For D. pandora, the population dynamics of the MK and CI infections will depend on the impact each infection has on host fitness (see Hurst et al. 2002). The maternal transmission experiments indicated very high levels of transmission of both infections, at or near 100%, at least under laboratory conditions when transmission can be higher than in the field (Turelli et al. 1995). If some uninfected individuals did occur in the field they would likely express very high CI, maintaining a very low frequency of uninfected individuals in field populations, as reflected by a very high frequency of Wolbachia in the field population. Moreover, the crosses between MK infected females and CI males (Table 2) show that there is no CI between these infections, indicating that incompatibility is unlikely to influence the outcome of their interactions. We were able to generate some MK males by rearing flies at a higher temperature than usual, however these males mated poorly compared to controls and did not induce CI when crossed with uninfected females, suggesting they will not influence the dynamics of the infections in natural populations. This contrasts with the situation in Hypolimnas butterflies where CI was induced by infected males that were produced following the evolution of suppression of the MK phenotype (Hornett et al. 2008).
With respect to fitness effects generated by MK and CI infections, we did not find any difference in fecundity between females from these strains or their tetracycline cured counterparts (Table 3). The MK females did show the lowest fecundity, but we had limited power to detect small differences among the lines. For instance we only had around 40% power to detect a difference of around 10% between two treatments at the 5% significance level (based on average means and SDs of non-MK females), and the detection of small effects would require several hundred replicates. We also did not detect any obvious differences in hatch rate among MK and CI infected females; once hatch rates are doubled for the females positive for the MK infection (Table 2) they are similar to the hatch rates observed in the crosses with the CI infection (Table 1).
In contrast to the fecundity results, the population monitoring experiment suggested a fitness advantage of the CI infection, which appeared to increase in frequency relative to the MK infection. This advantage might contribute to the predominance of the CI infection in natural populations, although our cages did not consider one possible advantage of MK, namely reduced larval competition at a high density. Because we followed changes in infection frequencies across five generations, there was the potential for small fitness effects of the infections associated with fecundity and other traits to accumulate. For example, if the MK had a fitness cost of 10% in a generation, in the 50% treatment we would have expected the MK infection frequency to decline to around 34% after five generations. This is only a little higher (and not significantly different from) the observed mean frequency of 27% for the 50% treatment. Following the same logic, the MK infection frequency for the 90% treatment would be expected to have declined to 82.7% MK (similar to the observed mean frequency of 84%), while the MK infection frequency for the 10% treatment would be expected to decline to 5.6%, which was again similar (and not significantly different) to the observed value of 4.4%.
It is expected that MK infections might invade CI infections in cases where there is sib competition or another factor that might increase the fitness of female offspring (Hurst et al. 2002). In D. pandora, as in other Drosophila species that use fruit as breeding sites, sib competition may occur in some situations, assuming both that a limited number of females oviposit on fruit (as in the case of D. melanogaster (Hoffmann and Nielsen 1985), and that there is strong intraspecific competition (as demonstrated in several species (Atkinson 1985)). The MK infection may be persisting in natural populations due to the competitive abilities of its larvae under some conditions, however this remains to be demonstrated in natural populations. Likewise, in mushroom feeding D. innubila that lay eggs in clutches, MK infections may be particularly likely to invade as a consequence of sib competition (Jaenike et al. 2003).
The Wolbachia tree clearly indicates that the infections are different and not immediate relatives. Across the genetic regions assayed, the CI infection is identical to wRi, which causes CI in D. simulans (Turelli et al. 1995), and the MK infection of D. pandora groups with the MK infection of D. innubila (Fig 2). However, the MK infection is also closely related to wAu, which does not cause CI (Hoffmann et al. 1996), and to wMel, which only causes CI in young males (Reynolds and Hoffmann 2002). These patterns suggest that the MK and CI phenotypes are not particularly constrained phylogenetically, a finding consistent with observations of other researchers. For instance, Jaenike et al (2007) previously noted a CI infection from D. recens Wheeler that became an MK infection following transfer to the related D. subquinaria Spencer, although it also acted as a CI infection in some nuclear backgrounds of D. subquinaria.
The infections described in this paper contrast with those in other Drosophila species where within-population Wolbachia polymorphism typically involves infected and uninfected individuals, as documented in detail in the wRi infection of D. simulans (Hoffmann et al. 1986; Turelli et al. 1995). In D. pandora, all studied individuals are naturally infected, and the polymorphic status refers to the mixture of MK and CI-causing Wolbachia strains within host populations. It is not yet clear to what extent MK versus CI (or other) infections predominate in Drosophila species (Hamm et al. 2014). While many instances of Wolbachia infections have been detected in Drosophila species (Clark et al. 2005; Salzberg et al. 2005; Müller et al. 2013), relatively few infections have been characterized at the phenotypic level, particularly in natural situations (Hoffmann et al. 1996; Dyer and Jaenike 2004; Hamm et al. 2014). The majority of Drosophila infections characterized do appear to show CI rather than MK (Bourtzis et al. 1996) but many remain to be tested. Many of the Drosophila lines that have been tested come from fly stock centres that likely select against MK strains, none of which are easily maintained in the lab, and detailed screenings of natural populations remain rare. Studying the variety of Wolbachia that infect Drosophila in the wild has the potential to reveal a wealth of information on the range of phenotypic effects exerted by Wolbachia on its hosts and the evolutionary dynamics associated with Wolbachia infections.
Supplementary Material
Acknowledgments
This research was supported by grants from the National Institute of Health (1R01GM104325-01) and the National Health and Medical Research Council (1007003). The authors would like to thank Jennifer Shirriffs for laboratory assistance and Emily Thomson for administrative assistance. The authors would also like to Laura Baldo from the University of Barcelona and John H Werren from the University of Rochester for providing some of the sequences used in this publication, as well as Michael Turelli and reviewers for comments.
Footnotes
Data archival location: Sequences to deposited in Genbank; accession numbers KU686364-KU686375, KX234098-KX234169 and KX453286-KX453288. Data submitted to Dryad; doi:10.5061/dryad.vq6m6, journal manuscript number 16-0064.R1.
Literature cited
- Arthofer W, Riegler M, Schuler H, Schneider D, Moder K, Miller WJ, Stauffer C. Allele intersection analysis: a novel tool for multi locus sequence assignment in multiply infected hosts. PloS ONE. 2011:E22198. doi: 10.1371/journal.pone.0022198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson WD. Coexistence of Australian rainforest Diptera breeding on fallen fruit. J Anim Ecol. 1985;54:507–518. [Google Scholar]
- Baldo L, Hotopp JCD, Jolley KA, Bordenstein SR, Biber SA, Choudhury RR, Hayashi C, Maiden MCJ, Tettelin H, Werren JH. Multilocus sequence typing system for the endosymbiont Wolbachia pipientis. Appl Environ Microbiol. 2006;72:7098–7110. doi: 10.1128/AEM.00731-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett G, Pantoja N, O’Grady P. Diversity and phylogenetic relationships of Wolbachia in Drosophila and other native Hawaiian insects. Fly. 2012;6:273–283. doi: 10.4161/fly.21161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson D, Karsch-Mizrachi I, Lipman D, Ostell J, Sayers E. GenBank. Nucleic Acids Res. 2011;39:D32–D37. doi: 10.1093/nar/gkq1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordenstein SR, Paraskevopoulos C, Hotopp JC, Sapountzis P, Lo N, Bandi C, Tettelin H, Werren JH, Bourtzis K. Parasitism and mutualism in Wolbachia: what the phylogenomic trees can and cannot say. Mol Biol Evol. 2009;26:231–241. doi: 10.1093/molbev/msn243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouckaert R, Heled J, Kühnert D, Vaughan T, Wu C-H, Xie D, Suchard M, Rambaut A, Drummond AJ. BEAST 2: A Software platform for Bayesian evolutionary analysis. PLoS Comput Biol. 2014;10:e1003537. doi: 10.1371/journal.pcbi.1003537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtzis K, Nirgianaki A, Markakis G, Savakis C. Wolbachia infection and cytoplasmic incompatibility in Drosophila species. Genetics. 1996;144:1063–1073. doi: 10.1093/genetics/144.3.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakir M, Chafik A, Moreteau B, Gibert P, David JR. Male sterility thermal thresholds in Drosophila: D. simulans appears more cold-adapted than its sibling D. melanogaster. Genetica. 2002;114:195–205. doi: 10.1023/a:1015154329762. [DOI] [PubMed] [Google Scholar]
- Charlat S, Nirgianaki A, Bourtzis K, Merçot H. Evolution of Wolbachia-induced cytoplasmic incompatibility in Drosophila simulans and D. sechellia. Evolution. 2002;56:1735–1742. doi: 10.1111/j.0014-3820.2002.tb00187.x. [DOI] [PubMed] [Google Scholar]
- Clark ME, Anderson CL, Cande J, Karr TL. Widespread prevalence of Wolbachia in laboratory stocks and the implications for Drosophila research. Genetics. 2005;170:1667–1675. doi: 10.1534/genetics.104.038901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby AC, Armstrong SD, Bah GS, Kaur G, Hughes MA, Kay SM, Koldkjaer P, Rainbow L, Radford AD, Blaxter ML, Tanya VN, Trees AJ, Cordaux R, Wastling JM, Makepeace BL. Analysis of gene expression from the Wolbachia genome of a filarial. Genome Research. 2012;22:2467–2477. doi: 10.1101/gr.138420.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer KA, Jaenike J. Evolutionarily stable infection by a male-killing endosymbiont in Drosophila innubila: Molecular evidence from the host and parasite genomes. Genetics. 2004;168:1443–1455. doi: 10.1534/genetics.104.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer KA, Minhas MD, Jaenike J. Expression and modulation of embryonic male-killing in Drosophila innubila: Opportunities for multilevel selection. Evolution. 2005;59:838–848. [PubMed] [Google Scholar]
- Giordano R, O’Neill SL, Robertson HM. Wolbachia infections and the expression of cytoplasmic incompatibility in Drosophila sechellia and D. muritiana. Genetics. 1995;140:1307–1317. doi: 10.1093/genetics/140.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm CA, Begun DJ, Vo A, Smith CCR, Saelao P, Shaver AO, Jaenike J, Turelli M. Wolbachia do not live by reproductive manipulation alone: infection polymorphism in Drosophila suzukii and D. subpulchrella. Mol Ecol. 2014;23:4871–4885. doi: 10.1111/mec.12901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heled J, Drummond AJ. Bayesian inference of species trees from multilocus data. Mol Biol Evol. 2010;27:570–580. doi: 10.1093/molbev/msp274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann AA, Clancy D, Duncan J. Naturally-occurring Wolbachia infection in Drosophila simulans that does not cause cytoplasmic incompatibility. Heredity. 1996;76:1–8. doi: 10.1038/hdy.1996.1. [DOI] [PubMed] [Google Scholar]
- Hoffmann AA, Nielsen KM. The effect of resource subdivision on genetic-variation in Drosophila. Am Nat. 1985;125:421–430. [Google Scholar]
- Hoffmann AA, Turelli M, Simmons GM. Unidirectional incompatibility between populations of Drosophila simulans. Evolution. 1986;40:692–701. doi: 10.1111/j.1558-5646.1986.tb00531.x. [DOI] [PubMed] [Google Scholar]
- Hornett EA, Duplouy AMR, Davies N, Roderick GK, Wedell N, Hurst GDD, Charlat S. You can't keep a good parasite down: evolution of a male-killer suppressor uncovers cytoplasmic incompatibility. Evolution. 2008;62:1258–1263. doi: 10.1111/j.1558-5646.2008.00353.x. [DOI] [PubMed] [Google Scholar]
- Hurst GDD, Jiggins FM. Male-killing bacteria in insects: Mechanisms, incidence, and implications. Emerg Infect Dis. 2000;6:329–336. doi: 10.3201/eid0604.000402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst GDD, Jiggins FM, Pomiankowski A. Which way to manipulate host reproduction? Wolbachia that cause cytoplasmic incompatibility are easily invaded by sex ratio-distorting mutants. Am Nat. 2002;160:360–373. doi: 10.1086/341524. [DOI] [PubMed] [Google Scholar]
- Hurst GDD, Majerus MEN. Why do maternally inherited organisms kill males. Heredity. 1993;71:81–95. [Google Scholar]
- Jaenike J. Spontaneous emergence of a new Wolbachia phenotype. Evolution. 2007;61:2244–2252. doi: 10.1111/j.1558-5646.2007.00180.x. [DOI] [PubMed] [Google Scholar]
- Jaenike J, Dyer KA, Reed LK. Within-population structure of competition and the dynamics of male-killing Wolbachia. Evol Ecol Res. 2003;5:1023–1036. [Google Scholar]
- Jolley KA, Maiden MCJ. BIGSdb: Scalable analysis of bacterial genome variation at the population level. BMC Bioinformatics. 2010;11:595. doi: 10.1186/1471-2105-11-595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Mentjies P, Drummond A. Geneious Basic: an integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klasson L, Walker T, Sebaihia M, Sanders MJ, Quail MA, Lord A, Sanders S, Earl J, O'Neill SL, Thomson N, Sinkins SP, Parkhill J. Genome evolution of Wolbachia strain wPip from the Culex pipiens group. Mol Biol Evol. 2008;25:1877–1887. doi: 10.1093/molbev/msn133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriesner P, Hoffmann AA, Lee SF, Turelli M, Weeks AR. Rapid sequential spread of two Wolbachia variants in Drosophila simulans. PLoS Pathog. 2013;9:e1003607. doi: 10.1371/journal.ppat.1003607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfear R, Calcott B, Ho S, Guindon S. PartitionFinder: combined selection of partitioning schemes and substitution models for phylogenetic analyses. Mol Biol Evol. 2012;29:1695–1701. doi: 10.1093/molbev/mss020. [DOI] [PubMed] [Google Scholar]
- Lee SF, White VL, Weeks AR, Hoffmann AA, Endersby NM. High-throughput PCR assays to monitor Wolbachia infection in the dengue mosquito (Aedes aegypti) and Drosophila simulans. Appl Environ Microbiol. 2012;78:4740–4743. doi: 10.1128/AEM.00069-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos M, Castrezana Sergio J, Nankivell Becky J, Estes Anne M, Markow TA, Moran NA. Toward a Wolbachia multilocus sequence typing system: Discrimination of Wolbachia strains present in Drosophila species. Genetics. 2006;174:363–376. [Google Scholar]
- Matsuda M, Ng CS, Doi M, Kopp A, Tobari YN. Evolution in the Drosophila ananassae species subgroup. Fly. 2009;3:157–169. doi: 10.4161/fly.8395. [DOI] [PubMed] [Google Scholar]
- McEvey SF, Schiffer M. New species in the Drosophila ananassae subgroup from northern Australia, New Guinea and the South Pacific (Diptera: Drosophilidae), with historical overview. Records of the Australian Museum. 2015;67:129–161. [Google Scholar]
- Merçot H, Charlat S. Wolbachia infections in Drosophila melanogaster and D. simulans: polymorphism and levels of cytoplasmic incompatibility. Genetica. 2004;120:51–59. doi: 10.1023/b:gene.0000017629.31383.8f. [DOI] [PubMed] [Google Scholar]
- Miller W, Riegler M. Evolutionary dynamics of wAu-Like Wolbachia variants in neotropical Drosophila spp. Appl Environ Microbiol. 2006;72:826–835. doi: 10.1128/AEM.72.1.826-835.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WJ, Ehrman L, Schneider D. Infectious speciation revisited: Impact of symbiont-depletion on female fitness and mating behavior of Drosophila paulistorum. PLoS Pathog. 2010;6:e1001214. doi: 10.1371/journal.ppat.1001214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montenegro H, Hatadani LM, Medeiros HF, Klaczko LB. Male killing in three species of the tripunctata radiation of Drosophila (Diptera: Drosophilidae) J Zool Syst Evol Res. 2006;44:130–135. [Google Scholar]
- Müller MJ, Dörr NCD, Deprá M, Schmitz HJ, Valiati VH, Valente VLdS. Reevaluating the infection status by the Wolbachia endosymbiont in Drosophila Neotropical species from the willistoni subgroup. Infect Genet Evol. 2013;19:232–239. doi: 10.1016/j.meegid.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Paradis E, Claude J, Strimmer K. APE: analyses of phylogenetics and evolution in R language. Bioinformatics. 2004;20:289–290. doi: 10.1093/bioinformatics/btg412. [DOI] [PubMed] [Google Scholar]
- R_Core_Development_Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. https://www.R-project.org/ [Google Scholar]
- Rambaut A, Suchard M, Xie D, Drummond A. Tracer v1.6. 2014 Available from http://beast.bio.ed.ac.uk/Tracer. [Google Scholar]
- Reynolds KT, Hoffmann AA. Male age, host effects and the weak expression or nonexpression of cytoplasmic incompatibility in Drosophila strains infected by maternally transmitted Wolbachia. Genet Res. 2002;80:79–87. doi: 10.1017/s0016672302005827. [DOI] [PubMed] [Google Scholar]
- Rousset F, Solignac M. Evolution of single and double Wolbachia symbioses during speciation in the Drosophila simulans complex. Proc Natl Acad Sci USA. 1995;92:6389–6393. doi: 10.1073/pnas.92.14.6389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzberg SL, Hotopp JCD, Delcher AL, Pop M, Smith DR, Eisen MB, Nelson WC. Serendipitous discovery of Wolbachia genomes in multiple Drosophila species. Genome Biology. 2005;6:R23. doi: 10.1186/gb-2005-6-3-r23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeley SL, McAllister BF. Mobile male-killer: similar Wolbachia strains kill males of divergent Drosophila hosts. Heredity. 2009;102:286–292. doi: 10.1038/hdy.2008.126. [DOI] [PubMed] [Google Scholar]
- Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Hoffmann AA. Cytoplasmic incompatibility in Drosophila simulans: dynamics and parameter estimates from natural populations. Genetics. 1995;140:1319–1338. doi: 10.1093/genetics/140.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unckless RL, Jaenike J. Maintenance of a male-killing Wolbachia in Drosophila innubila by male-killing dependent and male-killing independent mechanisms. Evolution. 2012;66:678–689. doi: 10.1111/j.1558-5646.2011.01485.x. [DOI] [PubMed] [Google Scholar]
- Werren J, Jaenike J. Wolbachia and cytoplasmic incompatibility in mycophagous Drosophila and their relatives. Heredity. 1995;75:320–326. doi: 10.1038/hdy.1995.140. [DOI] [PubMed] [Google Scholar]
- Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, McGraw EA, Martin W, Esser C, Ahmadinejad N, Wiegand C, Madupu R, Beanan MJ, Brinkac LM, Daugherty SC, Durkin AS, Kolonay JF, Nelson WC, Mohamoud Y, Lee P, Berry K, Young MB, Utterback T, Weidman J, Nierman WC, Paulsen IT, Nelson KE, Tettelin H, O'Neill SL, Eisen JA. Phylogenomics of the reproductive parasite Wolbachia pipientis wMel: a streamlined genome overrun by mobile genetic elements. PLoS Biology. 2004;2:E69. doi: 10.1371/journal.pbio.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabalou S, Charlat S, Nirgianaki A, Lachaise D, Merçot H, Bourtzis K. Natural Wolbachia infections in the Drosophila yakuba species complex do not induce cytoplasmic incompatibility but fully rescue the wRi modification. Genetics. 2004;167:827–834. doi: 10.1534/genetics.103.015990. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.