Abstract
INTRODUCTION
The impact of blood pressure on brain volumes may be time- or pattern-dependent.
METHODS
In 1678 participants from the Atherosclerosis Risk in Communities Neurocognitive Study, we quantified the association between measures and patterns of blood pressure over three time points (~24 or ~15 years prior and concurrent with neuroimaging) with late life brain volumes.
RESULTS
Higher diastolic blood pressure ~24 years prior, higher systolic and pulse pressure ~15 years prior, and consistently elevated or rising systolic blood pressure from ~15 years prior to concurrent with neuroimaging, but not blood pressures measured concurrent with neuroimaging, were associated with smaller volumes. The pattern of hypertension ~15 years prior and hypotension concurrent with neuroimaging was associated with smaller volumes in regions preferentially affected by Alzheimer’s disease (e.g., hippocampus: −0.27 standard units, 95%CI:−0.51,−0.03).
DISCUSSION
Hypertension 15 to 24 years prior is relevant to current brain volumes. Hypertension followed by hypotension appears particularly detrimental.
Keywords: magnetic resonance imaging, brain volumes, neurodegeneration, Alzheimer’s disease, hypertension, blood pressure, hypotension, epidemiology, cohort study, human
1. INTRODUCTION
Elevated blood pressure in midlife appears to confer excess risk of cognitive impairment [8, 9] while associations with elevated late life blood pressure are typically null or protective.[8, 10] This finding appears attributable, at least in part, to differences in the timing or duration of elevated blood pressure relative to cognitive assessment.[11] Additionally, the pattern of blood pressure over the life-course may be more informative than blood pressure at any single time point.[6, 11] As declining brain volume due to neurodegeneration occurs in dementia and may precede clinically noticeable change in cognition,[12–14] understanding how life-course blood pressure is related to brain volumes may provide mechanistic insights and has implications for treatment decisions. Our objective was to evaluate the relation between life-course blood pressure, including patterns of blood pressure, with brain volumes in late life (i.e. age ≥65) in the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS).
2. METHODS
2.1 STUDY POPULATION
The Atherosclerosis Risk in Communities (ARIC) study recruited persons ages 45 to 65 in 1987–1989 from four United States communities: Minneapolis suburbs, Minnesota; Forsyth County, North Carolina; Washington County, Maryland; and Jackson, Mississippi. We consider information on blood pressure from three study visits spaced at intervals of approximately a decade: Visit 1, 1987–1989; Visit 4, 1996–1998; and Visit 5, 2011–2013. (We do not consider blood pressure data from Visit 2 (1990–1992) or Visit 3 (1993–1995) to limit the number of comparisons.) A sample of Visit 5 participants lacking contraindications for MRI, all of whom were over age 65, were invited to complete brain MRI at Visit 5 as part of ARIC-NCS. In accordance with the pre-specified sampling strategy, all Visit 5 participants with evidence of cognitive impairment or a previous ARIC study brain MRI and a stratified random sample of the remaining participants, with strata based on age and study site, were invited to complete MRI. In addition to excluding persons without relevant MRI data or complete blood pressure and covariate data, we excluded persons with multiple sclerosis, brain tumor, surgery/radiation to the head, or confirmed stroke (n=82), participants who were not black or white (n=6), and black participants from Minnesota or Maryland (n=9). This study was approved by the institutional review boards of all participating institutions. All subjects provided written informed consent to participate at each study visit.
2.2 BLOOD PRESSURE
At each visit, study personnel measured systolic and diastolic blood pressure (SBP and DBP) up to three times according to a standardized protocol; we used the mean of the two final measurements. Antihypertensive medication use was determined through visual inspection of medications and linkage to Medi-Span Therapeutic Classification codes.
We considered those with SBP≥140 mmHg or DBP≥90 mmHg, antihypertensive medication use, or self-report of physician diagnosed hypertension at the current or any past study visit as having a “history of hypertension.” In addition to considering measured SBP, DBP, and pulse pressure (PP, defined as SBP minus DBP) as continuous variables, we also classified persons as hypotensive (SBP<90 mmHg or DBP<60 mmHg), hypertensive (≥140 mmHg SBP or ≥90 mmHg DBP in the absence of hypotension), or normotensive (the absence of hyper- or hypotension) at each study visit.
We further derived summaries of blood pressure patterns, including within-person change in SBP, DBP and PP across pairs of study visits (Visit 1 to 5, Visit 1 to 4, Visit 4 to 5) and a six-category variable describing the pattern of measured blood pressure across each pair of visits (hypotensive or normotensive/hypertensive at the earlier visit by hypotensive/normotensive/hypertensive at the later visit).
2.3 BRAIN VOLUMES
3T brain MRIs were completed following identical protocols at each study center. Each center underwent a qualifying process and phantom scans were completed bi-monthly and after upgrades. All scans included a sagittal T1-weighted 3D volumetric Magnetization Prepared Gradient Echo (MPRAGE) pulse sequence, allowing quantification of brain volumes. The ARIC MRI Reading Center used Freesurfer (version 5.1) to measure grey matter volumes. We report on associations with total brain volume, lobar grey matter volumes (frontal, parietal, temporal, occipital), total volume of the deep grey structures (insula, thalamus, caudate, putamen, and pallidum), total combined volume of the parahippocampal, entorhinal, and inferior parietal lobules, hippocampus, precuneus, and cuneus (denoted as the Alzheimer’s Disease (AD) signature region)[15] and hippocampal volume.
2.4 COVARIATES
We used data obtained at Visits 1, 4, and 5/ARIC-NCS to define covariates. We calculated body mass index (BMI) as measured weight (kg) divided by the square of measured height (m) and mean arterial pressure (MAP) as 2/3 DBP + 1/3 SBP. We defined hypercholesterolemia as measured total cholesterol of >200 mg/dL and diabetes as self-reported diagnosis, ≥126 mg/dL fasting glucose, ≥200 mg/dL non-fasting glucose, or use of diabetes medications. The ARIC MRI Reading Center used in-house algorithms to estimate total brain and intracranial volume.[16] We defined all other covariates based on information recorded about the study visit alone or in combination with information provided via self-report.
2.5 STATISTICAL ANALYSES
We used separate weighted linear regression models to quantify the association between each of our blood pressure and z-scored MRI brain volume measures. Sampling weights were used to account for the stratified random sampling approach used to select Visit 5 participants for MRI; thus we estimate the association in the Visit 5 ARIC participant population. All analyses were adjusted for potential confounders, including both time-invariant confounders --gender, education (<12/12–16/>16 years), race/center (black in Jackson/black in Forsyth County/white in Forsyth County/white in Minneapolis/white in Washington County), estimated total intracranial volume and its interaction with gender -- and confounders that vary over time --body mass index (BMI, <25/25 to <30/≥30 kg/m2), diabetes, smoking status (current/former/never), hypercholesterolemia, and (excluding analyses considering history of hypertension) antihypertensive medication use. Time-varying confounders were assessed at the appropriate study visit for analyses of visit-specific blood pressure (e.g., Visit 4 values if considering Visit 4 SBP). For analyses of patterns of blood pressure, exploratory analyses provided no support for the presence of time-varying confounding[17]; therefore, we adjusted for time-varying confounders by adjusting for status at multiple time points (e.g., both Visit 1 and Visit 5 smoking status if considering change in SBP from Visit 1 to 5). Analyses of within-person change in blood pressure were additionally adjusted for starting blood pressure. To isolate the impact of PP, given that higher blood pressure is associated with greater PP, analyses of PP were additionally adjusted for MAP, and analyses of change in PP were adjusted for change in MAP. All continuous explanatory variables were modeled using linear terms. We did not correct for multiple comparisons in these primary analyses because the association of blood pressure with one imaging feature is likely correlated with other imaging features.
We conducted multiple sensitivity analyses. To evaluate sensitivity to our exclusion criteria, we repeated our analyses (a) allowing participants with less than complete blood pressure data to contribute data, and (b) including persons with stroke. We also repeated our analyses omitting use of sampling weights to understand the influence of the sampling strategy. Finally, we derived and applied inverse probability of attrition weights (IPAW)[11, 18, 19] to address potential selection bias due to attrition from ARIC Visit 1 to Visit 5 (see Supplemental Methods). In combination with the sampling weights, the IPAW weighted estimates are designed to recover the association that would have been observed under either (i) no loss-to-follow-up (i.e. full follow-up for all living participants) or (ii) attrition that is statistically independent of blood pressure (i.e., full follow-up for all living participants and a random mechanism accounting for who dies), under the assumptions that death and drop-out are missing at random conditional on observed data and that the weights models are correctly specified.
We considered effect modification by race, age, and gender for all analyses and by antihypertensive medication use (overall, and by class of medication – angiotension-converting enzyme inhibitors/angiotension II receptor blockers, beta blockers, calcium channel blockers, and diuretics, when the prevalence of use was >5%) for analyses of visit-specific measured blood pressure using multiplicative interaction terms. We evaluated support for effect modification using Benjamini-Hochberg corrected p-values,[20] allowing a false discovery rate of 5%. Throughout we consider p<0.05 to be significant and p<0.10 to be marginally significant and report 95% confidence intervals. All analyses were completed using SAS, Version 9.3 or R, Version 3.0.1.
3. RESULTS
The study sample included up to 1687 participants. The weighted sample population was predominately female (61%) and well-educated (11% with less than a high school diploma or equivalent certification, 48% with a college degree); 31% were white from Minneapolis, 28% were white from Washington County, 21% were white and 1% were black from Forsyth County, and 19% were black from Jackson. Table 1 details additional sample characteristics while blood pressure patterns are described in Table 2 (unweighted versions of Tables 1 and 2 are provided as appendix Tables A.1 and A.2).
Table 1.
Weighted* time-varying characteristics and late life brain volumes of eligible ARIC-NCS participants
| Visit 1(~24 years prior to MRI) |
Visit 4 (~15 years prior to MRI) |
Visit 5 (Concurrent with MRI) |
|
|---|---|---|---|
| Mean (25th, 75th percentile) or % | |||
| Time to MRI (years) | 24 (23, 24) | 15 (14, 15) | 0 (0, 0) |
| Age (years) | 52 (47, 55) | 61 (56, 64) | 75 (70, 79) |
| Body mass index | |||
| Normal | 40% | 28% | 28% |
| Overweight | 38% | 40% | 40% |
| Obese | 22% | 33% | 33% |
| Smoking status | |||
| Current | 16% | 9% | 5% |
| Former | 32% | 47% | 51% |
| Never | 52% | 43% | 44% |
| Diabetes | 4% | 10% | 31% |
| Hypercholesterolemia | 58% | 50% | 35% |
| Systolic blood pressure (mmHg) | 115 (103, 122) | 123 (111, 132) | 130 (117, 140) |
| Diastolic blood pressure (mmHg) | 72 (65, 78) | 71 (64, 77) | 66 (58, 72) |
| Pulse pressure (mmHg) | 43 (35, 48) | 52 (42, 59) | 64 (54, 72) |
| History of hypertension | 31% | 53% | 81% |
| Antihypertensive medication use | 19% | 32% | 72% |
| Beta blockers** | 6% | 8% | 29% |
| Calcium channel blockers** | 2% | 9% | 22% |
| ACE Inhibitors/ARBs** | 2% | 11% | 44% |
| Diuretics** | 12% | 14% | 40% |
| Measured blood pressure | |||
| Hypotensive | 9% | 11% | 28% |
| Normotensive | 83% | 73% | 49% |
| Hypertensive | 8% | 16% | 23% |
| Estimated intracranial volume (cm3) | -- | -- | 1387 (1275, 1488) |
| Total brain volume (cm3) | -- | -- | 1026 (949, 1099) |
| Temporal lobe cortical volume (cm3) | -- | -- | 103 (96, 111) |
| Parietal lobe cortical volume (cm3) | -- | -- | 108 (99, 116) |
| Occipital lobe cortical volume (cm3) | -- | -- | 41 (38, 45) |
| Frontal lobe cortical volume (cm3) | -- | -- | 152 (141, 162) |
| Deep grey matter (cm3) | -- | -- | 43 (40, 45) |
| AD signature region volume (cm3) | -- | -- | 60 (55, 64) |
Abbreviations: ACE, angiotension-converting enzyme; AD, Alzheimer’s disease; ARB, angiotension receptor blockers; ARIC-NCS Atherosclerosis Risk in Communities Neurocognitive Study
Weighting was used to account for the sampling strategy used to select Visit 5 participants for MRI.
Not mutually exclusive
Table 2.
Weighted* time-varying patterns of measured blood pressure among eligible ARIC-NCS participants
| Visit 1 to Visit 4 (~24 to ~15 years prior to MRI) |
Visit 4 to Visit 5 (~15 years prior to concurrent with MRI) |
Visit 1 to Visit 5 (~24 years to concurrent with MRI) |
|
|---|---|---|---|
| Mean (25th, 75th percentile) or % | |||
| Within-person change in mmHg: | |||
| Systolic blood pressure | 8 (−1, 16) | 7 (−7, 19) | 15 (2, 27) |
| Diastolic blood pressure | −1 (−8, 4) | −5 (−13, 2) | −6 (−14, 2) |
| Pulse pressure | 10 (1, 16) | 12 (2, 21) | 21 (12, 29) |
| Pattern of Measured Blood Pressure | |||
| Hypertensive to Hypotensive | <1% | 4% | 2% |
| Normo/hypotensive to Hypotensive | 10% | 24% | 26% |
| Hypertensive to Normotensive | 4% | 6% | 3% |
| Normo/hypotensive to Normotensive | 70% | 43% | 45% |
| Always Hypertensive | 4% | 6% | 3% |
| Normo/hypotensive to Hypertensive | 11% | 17% | 20% |
Abbreviations: ARIC-NCS Atherosclerosis Risk in Communities Neurocognitive Study
Weighting was used to account for the sampling strategy used to select Visit 5 participants for MRI.
3.1 HISTORY OF HYPERTENSION
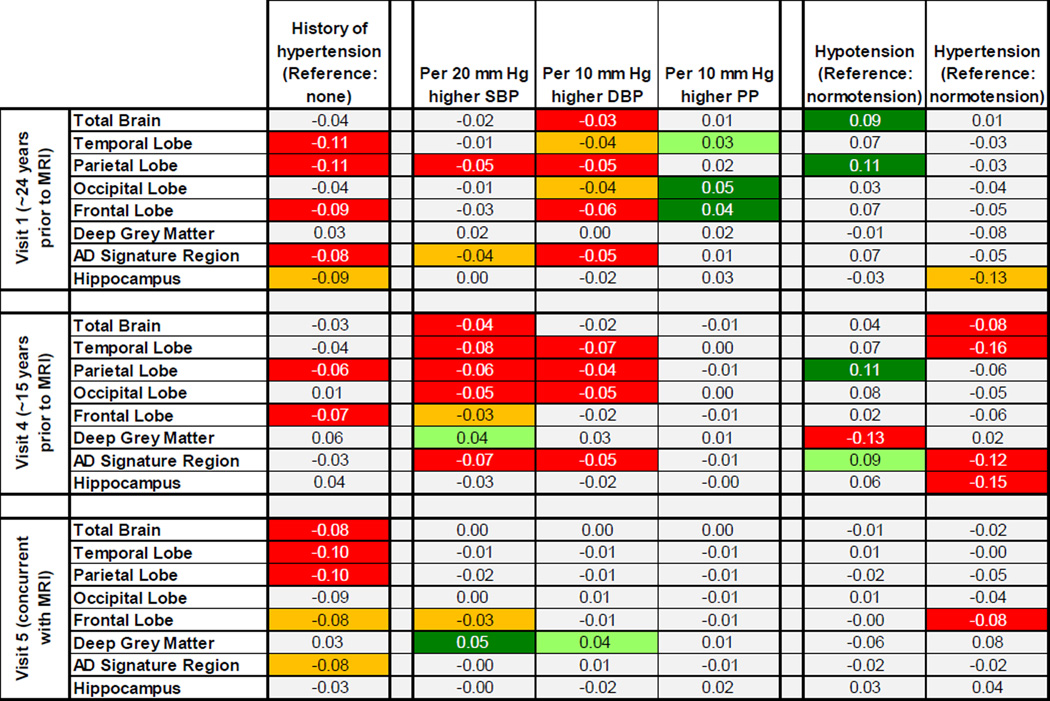
A history of hypertension at any study visit was related to smaller parietal and frontal lobe cortical volumes in late life (Figure 1; appendix Table A.3). A history of hypertension at either Visit 1 or Visit 5, but not Visit 4, was associated with smaller temporal lobe and AD signature region volumes (total combined volume of the parahippocampal, entorhinal, and inferior parietal lobules, hippocampus, precuneus, and cuneus). A history of hypertension at Visit 5, concurrent with neuroimaging, appeared associated with smaller total brain volumes, while a history of hypertension at Visit 1, ~24 years prior, was marginally associated with smaller hippocampal volumes.
Figure 1. P-value heatmap of adjusted average difference in brain volumes in late life for a given contrast in measured blood pressure at each study visit.
Each square contains the beta coefficient, in SD units, corresponding to a given exposure-outcome analysis. Squares containing statistically significant associations are shaded in red (if negative) or green (if positive), while squares containing marginally significant associations are shaded in orange (if negative) or light green (if positive). Unshaded squares denote p-values >0.10. All analyses were weighted to account for sampling and adjusted for gender, race-center, education, age, estimated intracranial volume, BMI, diabetes, high cholesterol, smoking status, and gender*estimated intracranial volume. All analyses exclusive of those considering history of hypertension were also adjusted for antihypertensive medication use and analyses of pulse pressure were additionally adjusted for mean arterial pressure.
3.2 MEASURED BLOOD PRESSURE
3.2.1 Blood Pressure ~24 Years Prior to Neuroimaging (Visit 1)
Higher SBP at Visit 1, ~24 years prior to neuroimaging, was significantly associated with smaller parietal lobe volumes and marginally associated with smaller AD signature region volumes, but was not associated with other late life volumes (Figure 1; appendix Table A.4). Higher DBP at Visit 1 was significantly or marginally associated with smaller volumes in late life for all regions except the deep grey matter and hippocampus. Greater Visit 1 PP was associated with larger frontal, occipital, and temporal lobe cortical volumes.
3.2.2 Blood Pressure ~15 Years Prior to Neuroimaging (Visit 4)
Higher SBP at Visit 4, ~15 years prior to neuroimaging, was significantly or marginally associated with smaller brain volumes in almost all regions; there was no association with hippocampal volume and higher Visit 4 SBP was marginally associated with larger deep grey matter volumes (Figure 1; appendix Table A.4). Higher DBP at Visit 4 was associated with smaller AD signature region and temporal, parietal, and occipital lobe cortical volumes. Visit 4 PP was not associated with volumes.
3.2.3 Blood Pressure Concurrent with Neuroimaging (Visit 5)
There was little support for an association between measures of blood pressure at the time of neuroimaging (Visit 5) and late life brain volumes, with a few exceptions (Figure 1; appendix Table A.4).
3.3 HYPERTENSION AND HYPOTENSION
Associations between categories of measured blood pressure and brain volumes were generally consistent with expectations given the findings considering SBP and DBP separately (Figure 1, appendix Table A.5). At Visit 1, ~24 years prior to neuroimaging, hypotension was associated with larger late life total brain volumes and parietal lobe cortical volumes, while hypertension was marginally associated with smaller hippocampal volumes. At Visit 4, ~15 years prior to neuroimaging, hypotension was associated with larger parietal lobe and AD signature region volumes as well as smaller deep grey volumes, while hypertension was associated with smaller total brain, temporal lobe, AD signature region, and hippocampal volumes. With the exception of an association between concurrent hypertension and smaller frontal lobe volumes, categories of blood pressure assessed concurrent with neuroimaging (Visit 5) were not associated with brain volumes.
3.4 WITHIN-PERSON CHANGE IN BLOOD PRESSURE
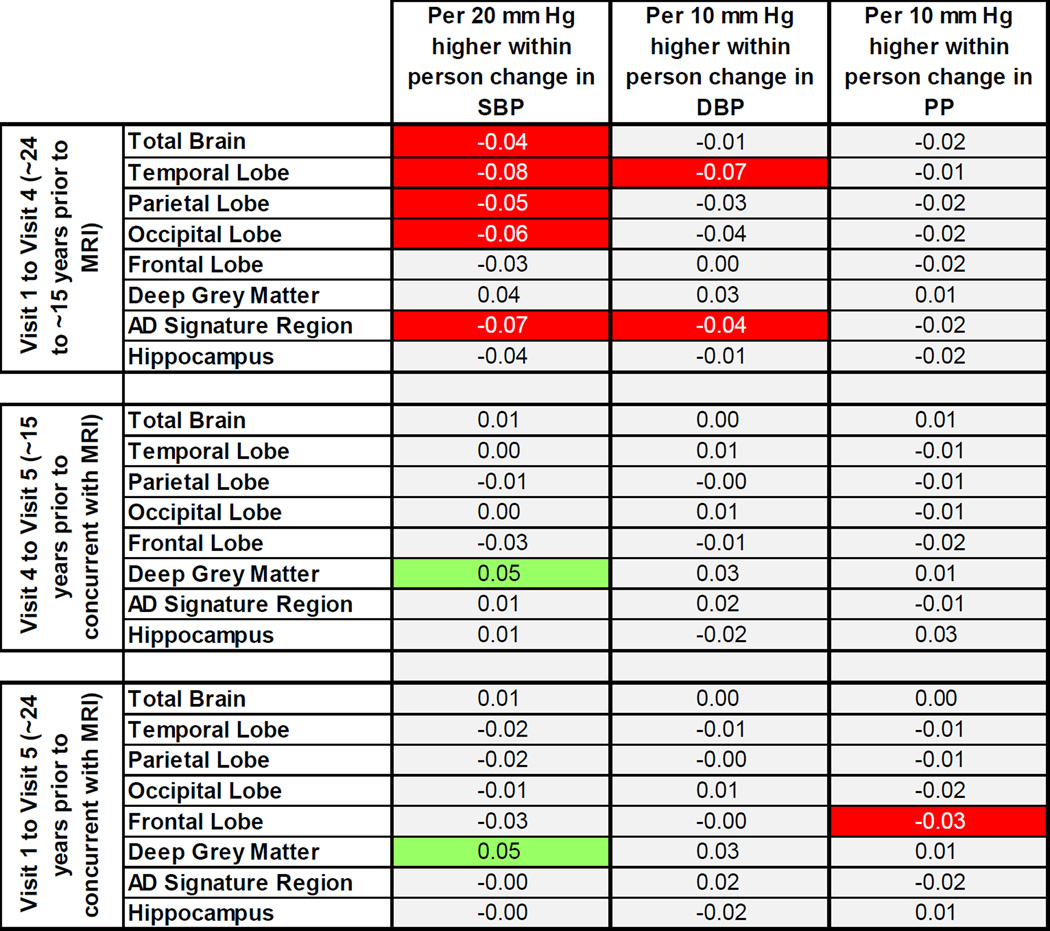
Higher SBP at Visit 4 relative to Visit 1 (i.e., at ~15 years prior relative to ~24 years prior to neuroimaging) was significantly associated with smaller total brain, AD signature region, and temporal, parietal, and occipital lobe cortical volumes (Figure 2; appendix Table A.6). Positive within-person change in DBP from Visit 1 to Visit 4 was associated with smaller temporal lobe and AD signature region volumes. Change in PP from Visit 1 to Visit 4 was not associated with volumes. Change in blood pressure from Visit 1 or Visit 4 to the time of neuroimaging (Visit 5) was not associated with volumes, with a few exceptions (Figure 2; appendix Table A.6).
Figure 2. P-value heatmap of adjusted average difference in brain volumes in late life for a given within-person change in measured blood pressure across study visits.
Each square contains the beta coefficient, in SD units, corresponding to a given exposure-outcome analysis. Squares containing statistically significant associations are shaded in red (if negative) or green (if positive), while squares containing marginally significant associations are shaded in orange (if negative) or light green (if positive). Unshaded squares denote p-values >0.10. Analyses were weighted to account for sampling and adjusted for gender, race-center, education, age, estimated intracranial volume, BMI, diabetes, high cholesterol, smoking status, antihypertensive medication use, starting blood pressure value, gender*estimated intracranial volume, and (pulse pressure only) within-person change in mean arterial pressure.
3.5 PATTERNS OF MEASURED BLOOD PRESSURE OVER TIME
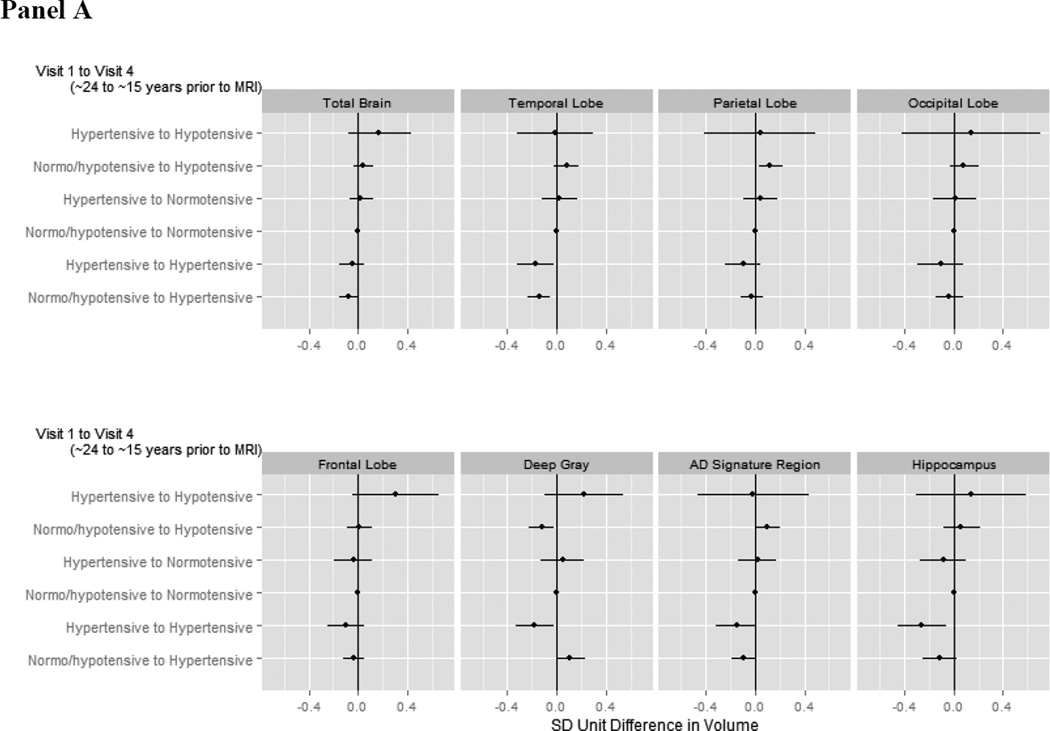
3.5.1 Categorical Patterns from ~24 to ~15 Years Prior to Neuroimaging (Visit 1 to 4)
Persons who were hypertensive at both Visit 1 and Visit 4 (~24 and ~15 years prior to neuroimaging) or newly hypertensive at Visit 4 (~15 years prior to neuroimaging) exhibited significantly or marginally smaller late life temporal lobe, AD signature region, and hippocampal volumes compared to those with “normal” blood pressure throughout (Figure 3a; appendix Table A.7). Being hypertensive at both time points was also significantly associated with smaller deep grey volumes, while being newly hypertensive was marginally associated with larger deep grey volumes. Being newly hypertensive at Visit 4 was also significantly associated with smaller total brain volumes. The pattern of Visit 1 normo- or hypotension with Visit 4 hypotension was significantly associated with greater parietal lobe and AD signature region volumes but smaller deep grey volumes.
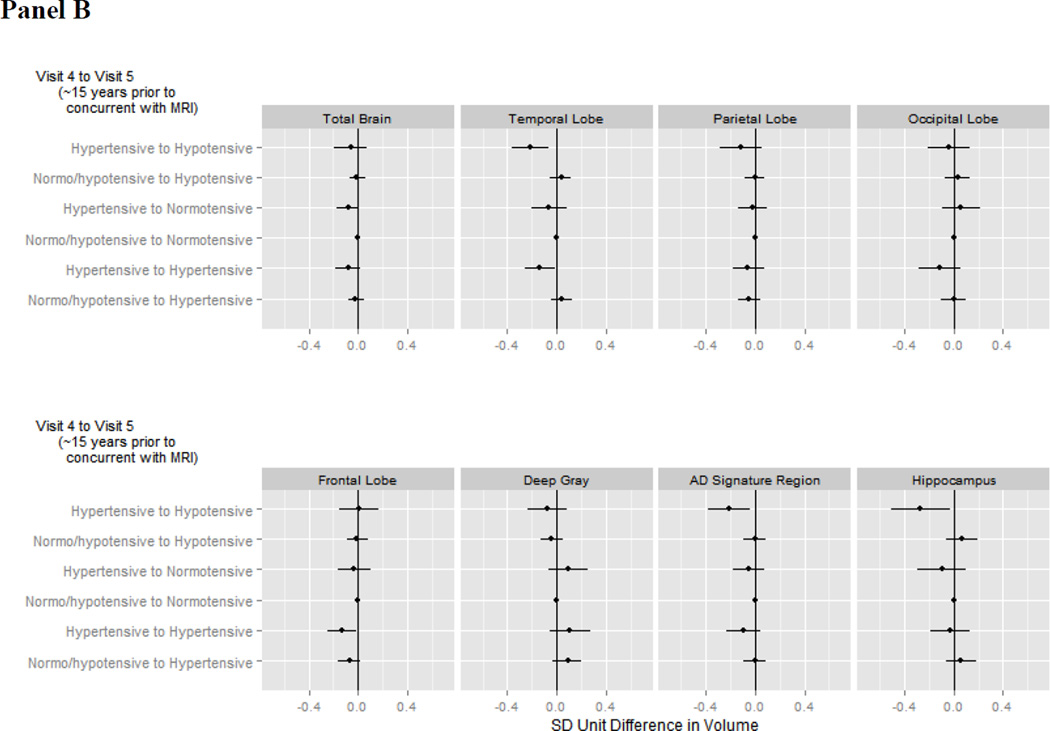
Figure 3. Adjusted average difference in brain volumes in late life for a given pattern of blood pressure.
Panel A illustrates associations with patterns from Visit 1 (~24 years prior to neuroimaging) to Visit 4 (~15 years prior to neuroimaging) while panel B illustrates associations with patterns from Visit 4 (~15 years prior to neuroimaging) to Visit 5 (concurrent with neuroimaging). The reference category is those with earlier normo-or hypotension and later normotension. Analyses were weighted to account for sampling and adjusted for gender, race-center, education, age, estimated intracranial volume, BMI, diabetes, high cholesterol, smoking status, antihypertensive medication use, and gender*estimated intracranial volume.
3.5.2 Categorical Patterns from ~15 Years Prior to Concurrent with Neuroimaging (Visit 4 to 5)
Hypertension ~15 years prior to neuroimaging (Visit 4) followed by hypotension at the time of neuroimaging (Visit 5) was strongly associated with smaller temporal lobe, AD signature region, and hippocampal volumes compared to those with “normal” blood pressure throughout (Figure 3b; appendix Table A.7). Participants who were hypertensive at both Visit 4 and Visit 5 also had smaller temporal and frontal lobe volumes. There was marginal support for smaller total brain volumes in participants who were hypertensive at Visit 4 but normotensive at Visit 5.
3.5.3 Categorical Patterns from ~24 Years Prior to Concurrent with Neuroimaging (Visit 1 to 5)
There was no evidence to support a difference in late life volumes among those with alternate patterns of blood pressure status from Visit 1 to 5 those with “normal” blood pressure throughout, with two exceptions: a significant association between normo- or hypotension at Visit 1, ~24 years prior to neuroimaging, followed by hypertension at Visit 5, concurrent with neuroimaging, and smaller frontal lobe volumes and a marginally significant association between hypertension at Visit 1 with normotension at Visit 5 and smaller deep grey volumes (appendix Table A.7).
3.6 SENSITIVITY ANALYSES AND EFFECT MODIFICATION
All sensitivity analyses were consistent with primary analyses. There was no support for effect modification by any considered characteristic (all corrected p-values >0.05).
4. DISCUSSION
In our study, blood pressure status 15 to 24 years prior appears most relevant to current amount of brain atrophy. Specifically, diastolic hypotension ~24 years prior to neuroimaging appeared associated with larger late life brain volumes, while elevated blood pressure ~15 years prior, particularly higher SBP, appeared associated with smaller late life volumes.
Our analyses of patterns of blood pressure suggested that controlling SBP may preserve brain volumes one to two decades later, as rising SBP and being hypertensive from ~24 years prior to ~15 years prior or being newly hypertensive ~15 years prior were associated with smaller late life brain volumes. Interestingly, compared to persons with normal blood pressure throughout, hypertension ~ 15 years earlier followed by hypotension at the time of neuroimaging was associated with substantially smaller volumes in regions affected early in Alzheimer’s disease.[21]
Strengths of this study include life-course information on blood pressure and a large sample with brain MRI. Our focus on measured blood pressure is also a strength, as our study provides insight into the effects of achieving specific blood pressure targets. However, this can be viewed as a limitation given that we make the assumption that antihypertensive treatments act only or mostly through changing blood pressure, and thus we avoid the complexity of quantifying the effect of treatments and ignore the fact that standard of care changed over the study period. While lack of effect modification by antihypertensive medication class provides reassurance that this approach is reasonable, we acknowledge that we have not formally tested this assumption in our data. While confounding, misclassification, or selection bias cannot be completely discounted, we do not believe they account for our non-null findings; we adjusted for relevant confounders, non-differential measurement error would likely bias towards the null, and sensitivity analyses addressing selection, which assume the data are missing at random conditional on known predictors of death and drop-out, were consistent with the primary analyses. Study limitations include our inability to consider within-individual change in brain volumes and limited power to detect effect modification.
Our findings extend the work of others on the association between life-course blood pressure and late life brain volumes through use of a comprehensive assessment of life-course blood pressure over an extended period and consideration of both broad and targeted brain regions. For example, in prior work in ARIC considering a smaller group of participants with MRI earlier in life, elevated SBP six years prior to MRI was associated with greater qualitative ratings of ventricular size; the association with concurrent SBP was similar but only marginally significant.[1] Conversely, concurrent hypertension was associated with greater qualitative ratings of sulcal size, but blood pressure six years prior to MRI was not.[1] Higher baseline SBP was associated with increases in ventricular and sulcal size over approximately 10 years of follow-up in ARIC participants with serial MRI, while higher DBP appeared to protect against increases in ventricular size.[2] In a subset of Rotterdam Study participants, persons using antihypertensive medications at both 5 years prior to and at the time of MRI, a proxy for longstanding hypertension, exhibited smaller hippocampal and amygdalar volumes compared to those without antihypertensive medication use.[3] Interestingly, elevated DBP five years prior to MRI was associated with smaller hippocampal volumes in those without antihypertensive medication use, while higher DBP at the time of MRI predicted greater amygdalar volumes; SBP was not associated with volumes.[3] In additional analyses using the Rotterdam cohort, baseline DBP, but not SBP, predicted faster declines in left hippocampal volume over approximately 10 years of follow-up.[4] Several studies report an association between midlife hypertension, in the range of assessed approximately 22–30 years prior to neuroimaging, and smaller late life brain volumes.[22–24] However, in the Framingham Offspring Cohort Study, midlife hypertension was not associated with change in total brain volume or temporal horn volume (a surrogate for hippocampal volume) over a period approximately 7–13 years later.[5]
Notably, our results on patterns of blood pressure are consistent with AGES-Reykjavik Study findings,[6] where lower late life DBP was associated with smaller total brain and grey matter volumes only in persons with a history of midlife hypertension. However, our results differ somewhat from those of the Rotterdam Scan Study,[7] where both high and low concurrent DBP, elevated midlife DBP in those without antihypertensive medication use, and 20-year within-person declines in DBP were associated with greater cortical atrophy, while there was little association with any measure of SBP.
Multiple theories may explain the finding that hypotension, when preceded by hypertension ~15 years prior, is associated with substantially smaller brain volumes in Alzheimer’s disease-related regions. Longstanding hypertension can shift the cerebral autoregulatory curve, leading to reduced cerebral blood flow at (relatively) lower pressures[25–27]; therefore, hypotension, possibly induced by overly aggressive blood pressure treatment, may lead to periods of ischemia and associated neurodegeneration.[28] (Note, 97% of persons with this pattern were taking antihypertensives in late life.) Second, areas of the brain that are particularly vulnerable to AD pathology (especially tauopathy) may be selectively vulnerable to a variety of insults. Third, poor health may lead to reductions in both blood pressure and brain volume. (However, of persons with this pattern, only 2% had coronary heart failure and 8% had coronary heart disease.) Finally, atrophy (particularly of the insula) might lead to autonomic dysfunction and reduced blood pressure (i.e. reverse causation).[29]
In light of the findings of the Systolic Blood Pressure Intervention Trial (SPRINT)[30], clinicians are likely to lower blood pressure targets for many patients. While our study suggests this is unlikely to confer excess harm, and may ultimately confer substantial benefit for most individuals, our results also suggest that caution may be warranted when pursuing lower targets in persons with long-standing hypertension when treatment substantially lowers DBP. Given the short study period, as with prior trials,[31, 32] the SPRINT Memory and Cognition In Decreased Hypertension (SPRINT-MIND) study will only be able to comment on whether short-term intensive blood pressure lowering results in a common, immediate harm or benefit. Thus, future epidemiologic work investigating the long-term impact of specific patterns of life-course blood pressure, as well as the impact of life-course treatment, are clearly warranted.
In summary, our study highlights the potential benefit of effective screening and subsequent treatment for hypertension from the time of onset forward on brain health. It also supports a “personalized medicine” approach to blood pressure management incorporating information on prior blood pressure history and the potential for harm due to diastolic hypotension in the chronically hypertensive.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review
We searched PUBMED to identify relevant studies. [1–7] Very few studies have considered the influence of patterns of blood pressure over time on brain volumes. [6, 7]
Interpretation
Our study provides a comprehensive picture of the association between blood pressure and patterns of blood pressure over the life-course on brain volumes. We found hypertension 15 to 24 years prior to neuroimaging to be most relevant to current brain volumes. Our study also suggests that the pattern of hypertension followed by hypotension may be particularly detrimental.
Future directions
Our study highlights the potential benefit on brain health of effective screening and subsequent treatment for hypertension from the time of onset forward. However, it also provides support for a “personalized medicine” approach to blood pressure management that incorporates information on prior blood pressure history and sensitivity to diastolic hypotension.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
Melinda C. Power is supported by the NIA (T32 AG027668). The ARIC is carried out as a collaborative study supported by NHLBI contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). Neurocognitive data is collected by U01 HL096812, HL096814, HL096899, HL096902, HL096917 with previous brain MRI examinations funded by R01-HL70825. The sponsors had no role in the design and conduct of the study; collection management, analysis and interpretation of the data; or preparation review, or approval of the manuscript.
Dr. Clifford R. Jack Jr. serves on a scientific advisory board for Eli Lilly & Company and receives research support from the NIH/NIA (R01-AG011378, U01-HL096917, U01-AG024904, RO1 AG041851, R01 AG37551, R01AG043392, U01-AG06786) and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. Dr. Knopman serves as Deputy Editor for Neurology®; serves on a Data Safety Monitoring Board for Lundbeck Pharmaceuticals and for the DIAN study; is an investigator in clinical trials sponsored by TauRX Pharmaceuticals, Lilly Pharmaceuticals and the Alzheimer’s Disease Cooperative Study; and receives research support from the NIH.
Abbreviations
- AD
Alzheimer’s disease
- ARIC
Atherosclerosis Risk in Communities study
- ARIC-NCS
Atherosclerosis Risk in Communities Neurocognitive Study
- BMI
body mass index
- DBP
diastolic blood pressure
- IPAW
inverse probability of attrition weighting
- MAP
mean arterial pressure
- MPRAGE
Magnetization Prepared Gradient Echo
- MRI
magnetic resonance imaging
- PP
pulse pressure
- SBP
systolic blood pressure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICTS OF INTEREST
Drs. Schneider, Wruck, Griswold, Coker, Alonso, Power, Deal, Sharrett, Mosley, and Gottesman report no conflicts of interest.
REFERENCES
- 1.Knopman DS, Mosley TH, Catellier DJ, Sharrett AR. Cardiovascular risk factors and cerebral atrophy in a middle-aged cohort. Neurology. 2005;65:876–881. doi: 10.1212/01.wnl.0000176074.09733.a8. [DOI] [PubMed] [Google Scholar]
- 2.Knopman DS, Penman AD, Catellier DJ, Coker LH, Shibata DK, Sharrett AR, et al. Vascular risk factors and longitudinal changes on brain MRI: the ARIC study. Neurology. 2011;76:1879–1885. doi: 10.1212/WNL.0b013e31821d753f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.den Heijer T, Launer LJ, Prins ND, van Dijk EJ, Vermeer SE, Hofman A, et al. Association between blood pressure, white matter lesions, and atrophy of the medial temporal lobe. Neurology. 2005;64:263–267. doi: 10.1212/01.WNL.0000149641.55751.2E. [DOI] [PubMed] [Google Scholar]
- 4.den Heijer T, van der Lijn F, Ikram A, Koudstaal PJ, van der Lugt A, Krestin GP, et al. Vascular risk factors, apolipoprotein E, and hippocampal decline on magnetic resonance imaging over a 10-year follow-up. Alzheimers Dement. 2012;8:417–425. doi: 10.1016/j.jalz.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Debette S, Seshadri S, Beiser A, Au R, Himali JJ, Palumbo C, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461–468. doi: 10.1212/WNL.0b013e318227b227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muller M, Sigurdsson S, Kjartansson O, Aspelund T, Lopez OL, Jonnson PV, et al. Joint effect of mid- and late-life blood pressure on the brain: The AGES-Reykjavik Study. Neurology. 2014 doi: 10.1212/WNL.0000000000000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heijer T, Skoog I, Oudkerk M, de Leeuw FE, de Groot JC, Hofman A, et al. Association between blood pressure levels over time and brain atrophy in the elderly. Neurobiol Aging. 2003;24:307–313. doi: 10.1016/s0197-4580(02)00088-x. [DOI] [PubMed] [Google Scholar]
- 8.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurol. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 9.Gottesman RF, Schneider AL, Albert M, Alonso A, Bandeen-Roche K, Coker L, et al. Midlife Hypertension and 20-Year Cognitive Change: The Atherosclerosis Risk in Communities Neurocognitive Study. JAMA neurology. 2014 doi: 10.1001/jamaneurol.2014.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Power MC, Weuve J, Gagne JJ, McQueen M, Viswanathan A, Blacker D. The association between blood pressure and incident Alzheimer disease: a systematic review and meta-analysis. Epidemiology. 2011;22:646–659. doi: 10.1097/EDE.0b013e31822708b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Power MC, Tchetgen EJ, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24:886–893. doi: 10.1097/EDE.0b013e3182a7121c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.NGMRCCFaAS. Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Neuropathology Group of the Medical Research Council Cognitive Function and Ageing Study (MRC CFAS) Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 13.Davis DG, Schmitt FA, Wekstein DR, Markesbery WR. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 14.Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dickerson BC, Stoub TR, Shah RC, Sperling RA, Killiany RJ, Albert MS, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jack CR, Jr, Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, et al. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol. 2014;13:997–1005. doi: 10.1016/S1474-4422(14)70194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Robins JM, Hernan MA, Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. doi: 10.1097/00001648-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, et al. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. doi: 10.1097/EDE.0b013e318230e861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. doi: 10.1097/00001648-200009000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57:289–300. [Google Scholar]
- 21.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathologica. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 22.Swan GE, DeCarli C, Miller BL, Reed T, Wolf PA, Jack LM, et al. Association of midlife blood pressure to late-life cognitive decline and brain morphology. Neurology. 1998;51:986–993. doi: 10.1212/wnl.51.4.986. [DOI] [PubMed] [Google Scholar]
- 23.Korf ES, White LR, Scheltens P, Launer LJ. Midlife blood pressure and the risk of hippocampal atrophy: the Honolulu Asia Aging Study. Hypertension. 2004;44:29–34. doi: 10.1161/01.HYP.0000132475.32317.bb. [DOI] [PubMed] [Google Scholar]
- 24.DeCarli C, Miller BL, Swan GE, Reed T, Wolf PA, Garner J, et al. Predictors of brain morphology for the men of the NHLBI twin study. Stroke. 1999;30:529–536. doi: 10.1161/01.str.30.3.529. [DOI] [PubMed] [Google Scholar]
- 25.Strandgaard S. Cerebral blood flow in the elderly: impact of hypertension and antihypertensive treatment. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 1991;4(Suppl 6):1217–1221. doi: 10.1007/BF00114223. [DOI] [PubMed] [Google Scholar]
- 26.Muller M, van der Graaf Y, Visseren FL, Mali WP, Geerlings MI. Hypertension and longitudinal changes in cerebral blood flow: the SMART-MR study. Ann Neurol. 2012;71:825–833. doi: 10.1002/ana.23554. [DOI] [PubMed] [Google Scholar]
- 27.Barry DI, Strandgaard S, Graham DI, Braendstrup O, Svendsen UG, Vorstrup S, et al. Cerebral blood flow in rats with renal and spontaneous hypertension: resetting of the lower limit of autoregulation. J Cereb Blood Flow Metab. 1982;2:347–353. doi: 10.1038/jcbfm.1982.35. [DOI] [PubMed] [Google Scholar]
- 28.Iadecola C, Davisson RL. Hypertension and cerebrovascular dysfunction. Cell metabolism. 2008;7:476–484. doi: 10.1016/j.cmet.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idiaquez J, Roman GC. Autonomic dysfunction in neurodegenerative dementias. Journal of the Neurological Sciences. 305:22–27. doi: 10.1016/j.jns.2011.02.033. [DOI] [PubMed] [Google Scholar]
- 30.Wright JT, Jr, Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, et al. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Staessen JA, Thijs L, Richart T, Odili AN, Birkenhager WH. Placebo-controlled trials of blood pressure-lowering therapies for primary prevention of dementia. Hypertension. 2011;57:e6–e7. doi: 10.1161/HYPERTENSIONAHA.110.165142. [DOI] [PubMed] [Google Scholar]
- 32.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, et al. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol. 2008;7:683–689. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.