Abstract
The use of alternative chemical flame retardants in consumer products is increasing as the result of the phase-out of polybrominated diphenyl ethers. Today, the most commonly detected alternatives in residential furniture include the organophosphate flame retardants (PFRs) and the Firemaster 550® mixture (FM550). Urinary levels of dialkyl and diaryl phosphate esters, and 2-ethylhexyl tetrabromobenzoate (EH-TBB) have been used as biomarkers of human exposure to PFRs and FM550, respectively. In a previous study, we demonstrated that toddlers had significantly higher levels of PFRs relative to their mothers in a cohort from New Jersey; however, it is unclear if there are regional differences in exposure. It is possible that exposure to PFRs may be higher in California relative to other US States due to the California flammability standard, as was seen previously observed for PBDEs. In the current study, we examined urinary levels of PFR metabolites and TBBA in 28 mother-child pairs from California, USA, collected in 2015, and compared them with levels measured in our previous study from New Jersey. Urine samples were extracted using solid-phase extraction and analyzed by liquid chromatography tandem mass spectrometry (LC-MS/MS). Diphenyl phosphate (DPHP), isopropyl-phenyl phenyl phosphate (ip-PPP), bis(1,3-dichloro-2propyl) phosphate (BDCIPP) and BCIPHIPP conjugates were detected in 100% of mother and child urine samples, while bis(1-chloro-2-propyl) phosphate (BCIPP), tert-butyl-phenyl phenyl phosphate (tb-PPP) and TBBA were detected in <50% of samples. Interestingly, BCIPHIPP conjugates were detected in 100% of the urine samples, suggesting ubiquitous exposure to the parent compound, tris(1-chloro-2-propyl) phosphate (TCIPP). The current study found significantly higher BDCIPP levels in California toddlers and higher and ip-PPP levels in mothers as compared to the New Jersey cohort, which may be reflective of California’s furniture flammability standard. For example, BDCIPP levels in California children were 2.4 times higher than those in New Jersey children. Consistent with our previous work, the current study showed higher PFR and EH-TBB exposure in children, likely due to increased hand-mouth behavior. Children’s DPHP and BDCIPP levels, on average, were 5.9 times and 15 times those of their mothers. Positive correlations between paired mothers and their children were shown for DPHP and BCIPHIPP conjugates but not BDCIPP or ip-PPP. In the children, several predictors of hand-mouth behavior were associated with BDCIPP, DPHP and ip-PPP urine levels, but no associations were observed with BCIPHIPP conjugates.
Keywords: organophosphate chemical flame retardants, urine metabolites, mother-child pairs
INTRODUCTION
In recent years the use of chemical flame retardants (FRs) in foams, polymers and textiles has undergone significant evolution. Specifically, the penta-, octa- and deca-polybrominated diphenyl ether (PBDE) mixtures were phased-out in the US as of the mid-2000s (penta- and octaBDE) and 2013 (decaBDE). In the European Union penta- and octa- have been banned and deca- has been restricted1. As a result, there is evidence of declining PBDE levels in consumer products2, abiotic media3–5, wildlife6, 7 and humans8–10. Further, California Technical Bulletin 117 (TB 117) underwent revision in 2014 such that it no longer requires open flame tests for upholstered furniture11. The change to TB 117 is anticipated to reduce, but not eliminate, chemical FR use.
As the chemical FR market continues to shift away from the PBDEs, an increasing diversity of alternative FRs are being employed to meet flammability standards. As such, knowledge about the human health and environmental risks of FR alternatives is essential. A significant class of alternative FRs are the organophosphate flame retardants (PFRs) which are composed of chlorinated alkyl phosphates [e.g. tris(1,3,-dichloro-2-propyl) phosphate (TDCIPP)] and nonhalogenated aryl phosphates [e.g. triphenyl phosphate (TPHP)]. TPHP has also been used as a plasticizer and lubricant12 and thus has multiple exposure sources, complicating source apportionment. Another major FR alternative is the Firemaster® 550 mixture (FM 550) which is comprised of TPHP, isopropylated TPHP isomers, 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (EH-TBB), and bis(2-ethylhexyl)2,3,4,5-tetrabromphtalate (BEH-TEBP). Other fire retardant mixtures containing PFRs are also on the market (e.g. Fyrol HF-513).
In vitro studies show that PFRs are readily metabolized to their diester metabolites as well as various phase II conjugate metabolites14, 15. Predominately detected diester metabolites include diphenyl phosphate (DPHP) and bis(1,3-dichloro-2propyl) phosphate (BDCIPP). As well, recent work has identified conjugates of bis(1-chloro-2-propyl) 1-hydroxy-2-propyl phosphate (BCIPHIPP) as significant metabolites of tris(1-chloro-2-propyl) phosphate (TCIPP)14, 15. Further, studies from our group have shown that EH-TBB is metabolized to 2,3,4,5-tetrabromobenzoic acid (TBBA)16. Therefore, urinary levels of diester PFR metabolites, BCIPHIPP and TBBA have been used as biomarkers for PFR and EH-TBB exposures17–20.
In our previous study, we showed that BDCIPP levels in toddlers living in New Jersey were approximately 5 times greater in children, on average, as compared to their mothers. DPHP and isopropyl-phenyl phenyl phosphate (ip-PPP) were also greater in children but these relationships were not statistically significant. In addition, elevated BDCIPP and DPHP levels in children were associated with several predictors of hand-mouth exposure, suggesting the importance of hand-mouth behavior for PFR exposure. Further work by our group showed that infants (age 6–18 months) also had elevated BDCIPP and DPHP urinary levels as compared to adults. Additional studies, from other regions of the world, have corroborated these findings18, 20.
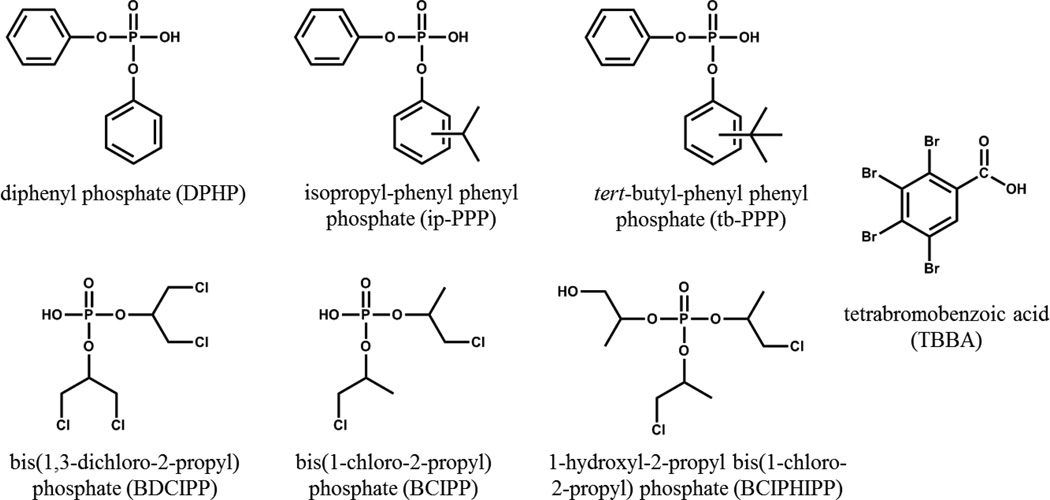
As mentioned earlier, California has had strict flammability standards, and residential use of chemical flame retardants was primarily driven by TB 117 and the revised TB 117–2013. As a result, studies have generally observed higher PBDE levels in California house dust and human serum as compared to other US regions and the world21, 22. For example, one study found elevated PBDE serum levels (2–9 times) in California toddlers as compared to other US regions22. In response to the pentaBDE phase-out in the mid-2000s, research suggests that as PBDE dust levels have decreased from 2006–2011, PFRs and Firemaster® 550 components have increased23. Further, PFR and Firemaster® 550 levels in California dust were shown to be higher than nearly all other global regions. Therefore, the primary aim of this study was to determine if PFR and Firemaster® 550 exposure levels, as measured by urinary metabolites, were higher in individuals from California compared to levels recently reported in a New Jersey cohort24. To accomplish this, we used the same experimental design as our previous study and recruited mother-toddler pairs living in California to measure urinary metabolite levels. In addition, our analyte list was expanded to include the TCIPP metabolite, BCIPHIPP. Figure 1 shows the chemical structures of all target analytes investigated in this study. We tested the hypothesis that a California cohort would have higher PFR metabolite and TBBA levels, as compared to our previously reported New Jersey cohort, due to the flammability regulations in California that were met by the use of fire retardant chemicals. Revised regulations became effective only recently in 2014. As well, consistent with our previous work, we hypothesized that children would have higher PFR exposure levels due to increased hand-mouth activity.
Figure 1.
Chemical structures of target PFR metabolites and TBBA.
EXPERIMENTAL SECTION
Study Group
The cohort consisted of 28 pairs of mothers and their children. In most pairs only one child was sampled, but two children were sampled for 5 adult participants (i.e. total mothers = 28, total children = 33). In addition, mothers completed a questionnaire to evaluate factors that may contribute to PFR/EH-TBB exposure for both themselves and their children (e.g. hand-mouth contact and the presence of furniture that may contain chemical flame retardants). A convenience cohort was recruited by email to targeted lists between February and August 2015. All participants lived in California and had a child 5 years of age or younger. Overall, our cohort was highly educated, of high socioeconomic status and primarily Caucasian (Table 1). Mothers were older than 18 and the mean age for children was 44 months (range: 2–70 months). Most of the mothers had a moderate awareness of chemical FR issues, but 4 of those mothers had extensive knowledge. Informed consent was obtained from all mothers and the mothers provided consent for their children. The study design, consent forms, and recruitment materials were approved by Chesapeake Research Review, Inc (Chesapeake IRB).
Table 1.
Demographic characteristics of the study cohort.
| Mothers (n=28) | |||
| Race | |||
| Asian | 1 | ||
| Hispanic | 1 | ||
| White | 23 | ||
| Multiracial | 3 | ||
| Education | |||
| College Graduate | 7 | ||
| Graduate Degree | 21 | ||
| Income (1000s) | |||
| <50 | 1 | ||
| 50–99 | 8 | ||
| 100–149 | 6 | ||
| >150 | 13 | ||
| Children (n=33) | |||
| Age (months) | Range: 2–70 | Mean=43.9 | |
| <24 | 4 | ||
| 24–35 | 7 | ||
| 36–47 | 8 | ||
| 48–59 | 4 | ||
| 60–70 | 10 | ||
Urine spot samples were collected in-home by study participants in sterile urine specimen collection cups. Urine samples were collected before 9 am for the majority of the participants (84%) for which sample collection time was recorded (56 out of 61 participants). Thus, sample collection time was not likely a confounder for urinary metabolites levels. Samples were immediately frozen at −20°C and shipped overnight to Duke University in insulated containers with ice packs. Samples were kept frozen until extraction and chemical analysis.
Materials
BDCIPP, d10-BDCIPP, and d12-tris(chloroethyl) phosphate (d12-TCEP), TBBA and 13C6-TBBA were purchased from Wellington Laboratories (Guelph, ON). Bis(1-chloro-2-propyl) phosphate (BCIPP) and d10-DPHP were synthesized by the Max Planck Institute for Biophysical Chemistry (Goettingen, Germany). The ip-PPP, tert-butyl-phenyl phenyl phosphate (tb-PPP), 13C2-DPHP were synthesized by the Small Molecule Synthesis Facility at Duke University (Durham, NC). 1-hydroxy-2-propyl bis(1-chloro-2-propyl) phosphate (BCIPHIPP) was a gift from Professor Adrian Covaci, University of Antwerp (Antwerp, Belgium).
Ammonium acetate, trimethylamine, pyrrolidine and 2,3,5-triiodobenzoic acid (TIBA), β-glucuronidase from limpets (>1M units/g) and sulfatase from Helix pomatia (>10,000 units/g) were purchased from Sigma-Aldrich (St. Louis, MO). StrataX-AW (60 mg, 3 ml) solid phase extraction columns (SPE) and the Luna C18(2) (2.5 µm, 50 × 2 mm) analytical column were purchased from Phenomenex (Torrance, CA, USA). Methanol and acetonitrile were HPLC grade (EMD Millipore Corporation, Bellerica, MA).
Extraction and Instrumental Analysis
Extraction methods for the PFR metabolites were adapted from previously published methods20, but adjusted for 5 ml of urine. Briefly, urine was thawed, 5 ml was transferred to a clean glass tube and spiked with the internal standard mixture (10 ng of d10-BDCIPP, 8.8 ng of d10-DPHP; 25 ng of d12-TCEP). After the addition of 1.75 ml of sodium acetate (pH 5, 1 M) and 250 µl of enzyme solution (1000 units/ml of β-glucuronidase, 33 units/ml of sulfatase in 0.2 M sodium acetate buffer), the samples were vortexed and incubated overnight at 37°C in a water bath. Samples were cleaned and concentrated using SPE techniques as previously described20 with the exception that the extracts were reconstituted in 500 µl of methanol:water. Internal standard recovery was quantified by spiking with 13C2-DPHP. Samples were transferred to Mini-UniPrep vials (GE Healthcare Life Sciences) immediately prior to instrumental analysis. Specific gravity measurements were taken with a digital refractometer (Atago USA, Inc., Bellevue, WA) prior to analysis.
Extracts were analyzed by electrospray ionization (ESI) liquid chromatography tandem mass spectrometry (LC-MS/MS) as previously described24, but with the addition of BCIPHIPP and d12-TCEP. Data were acquired under multiple reaction monitoring conditions using optimized parameters24, 25. BCIPHIPP (quantification: m/z 309.0 > 99.1; qualification: m/z 309.0 > 81.1) and d12-TCEP (m/z 297.1 > 67.2) were monitored in positive ESI mode. Analyte responses were normalized to internal standard responses. BCIPP and BDCIPP were normalized using d10-BDCIPP, DPHP, ip-PPP and tb-PPP were normalized using d10-DPHP and BCIPHIPP was normalized using d12-TCEP.
TBBA was isolated and cleaned by liquid-liquid extraction techniques as previously described19. However, the internal standard was 0.5 ng of 13C6-TBBA and internal standard recovery was was quantifying by spiking with TIBA after reconstituting with 1:1 MeOH:water, at end of sample preparation. Similar to our previous study24, extracts were analyzed by ESI(-)-LC-MS/MS.
Quality assurance/quality control
The mean recovery of the mass-labelled standards in the urine samples (n=61) was 125% (standard error = 2.2%) for d10-BDCIPP, 104% (2.8%) for d10-DPHP, 13% (0.8%) for d12-TCEP and 82% (1.3%) for 13C6-TBBA. The low d12-TCEP recovery is presumably related to quantification issues resulting from matrix suppression since the d12-TCEP recovery was 65–80% in the blank samples (clean water). Further, the 13C2-DPHP is likely not a good surrogate for d12-TCEP since these compounds are structurally different (e.g. presence of OH-group on 13C2-DPHP) and experience different ionization in the electrospray source (positive ionization for d12-TCEP and negative ionization for 13C2-DPHP). One replicate and one laboratory blank sample (5 mL Milli-Q water only) was extracted with every batch (n=5 batches). Replicate values were generally within 15%. Low levels of DPHP (mean = 0.34 ng) and ip-PPP (0.10 ng) were consistently detected in the laboratory blanks. Analyte values were blank corrected using the mean laboratory blank levels. Method detection limits (MDLs) were calculated as three times the standard deviation of the laboratory blanks, normalized to the blank volume (5 mL). MDLs were 78 pg/ml for BCIPP, 55 pg/ml for DPHP, 79 pg/ml for BDCIPP, 48 pg/ml for ip-PPP, 122 pg/ml for tb-PPP and 7 pg/ml for BCIPHIPP.
Statistical Analyses
Descriptive statistics were calculated for PFR metabolites measured in urine samples from children and adults. Summary statistics and statistical tests were only performed on analytes with >50% detection frequency and value of MDL/2 was used for all values <MDL. These data indicated that the distributions of PFR metabolite levels were log-normally distributed. Thus, either non-parametric statistical tests or log10-transformed values were used in analyses. Paired t-tests were used to investigate differences in the geometric mean PFR metabolite levels in urine samples collected from children and mothers. Association between maternal and child BDCIPP, DPHP, ip-PPP, and BCIPHIPP levels were assessed with Spearman (non-transformed values) and Pearson correlations (log-transformed values). To evaluate whether there were independence concerns resulting from including multiple children from the same family, we also used the average urinary metabolite levels of the siblings in analyses (t-tests and correlations). The results are not reported because the regression coefficients and statistical significance did not change from the results including all pairs. Generalized estimating equations (GEEs) were used to examine relationships between continuous measures of PFRs in urine and children’s hand washing and hand-to-mouth behaviors. GEEs are an extension of linear regression models that account for potential residual within-family correlations that may arise from including multiple children from the same family in analyses. For mothers, general linear models were used to examine relationships between continuous measures of PFRs in urine and hand washing frequency. Beta coefficients from regression models were exponentiated (10β), producing an estimate of the multiplicative change in urinary PFR levels relative to the reference group (or for a one unit increase in continuous variables). All statistical analyses were performed in SAS (version 9.2; SAS Institute Inc., Cary, NC).
RESULTS AND DISCUSSION
Geographic trends in urinary PFR metabolites
The current study cohort (California) showed overall higher BDCIPP and ip-PPP urine levels in both mothers and children as compared to our previous study from Princeton, New Jersey24 (Table 2). Note that for the comparison between study cohorts, to prevent potential age bias, the California children were restricted to the same age range as the New Jersey cohort (30–42 months). The spatial trends were particularly pronounced for BDCIPP in children, in which levels in the California cohort were 2.4-fold greater than in the New Jersey cohort. However, the elevated BDCIPP and ip-PPP levels in the California cohort were only significant for BDCIPP in children and ip-PPP in mothers, whereas the other relationships were only suggestive. DPHP urine levels in California were actually lower than in New Jersey, but the results were not significant. Overall, the results suggest greater TDCIPP and ip-PDPP exposure in California as compared to New Jersey, which may be driven by the stricter California flammability regulations. This trend has also been observed in elevated serum PBDE levels from California as compared to other states21. While it is possible that urinary levels can vary throughout the day, we do not expect that the differences in the sample collection time will be a confounding factor in our analyses. It has previously been shown the BDCIPP urinary levels are strongly consistent, and DPHP urinary levels are moderately to strongly consistent, over a 5 day period, respectively26. However, potentially confounding the trends are the fact that the current study cohort was recruited from February–August 2015, whereas the New Jersey cohort was recruited from August 2013-January 2014. There is evidence of increasing PFR use2 and thus the more recent sampling time frame may partially explain the higher California cohort levels.
Table 2.
Geographic region (California or New Jersey) as a predictor of urinary BDCIPP, DPHP, ip-PPP and BCIPHIPP concentrations (specific gravity corrected) in mothers and children (CI = confidence interval, SG = specific gravity).
| BDCIPP (SG normalized) | DPHP (SG normalized) | ip-PPP (SG normalized) | |||||
|---|---|---|---|---|---|---|---|
| exponentiated beta (95% CI) |
p-value | exponentiated beta (95% CI) |
p-value | exponentiated beta (95% CI) |
p-value | ||
| Mothers | |||||||
| California | 1.34 (0.83, 2.16) | 0.23 | 0.61 (0.35, 1.07) | 0.08 | 1.85 (1.07, 3.21) | 0.03 | |
| New Jersey | reference | reference | reference | ||||
| Children | |||||||
| California | 2.43 (1.08, 5.50) | 0.03 | 0.82 (0.38, 1.76) | 0.61 | 1.75 (0.75, 4.09) | 0.24 | |
| New Jersey | reference | reference | reference | ||||
A comparison of global DPHP and BDCIPP urine levels was recently presented by Cequier et al.18, but was expanded to include several recent studies14, 17, 26–29 (Table 3). DPHP levels in the current study were similar to those recently published for North American adults and children19, 24, 25, 27–29. In general, DPHP urine levels in North American appear to be higher than in Europe18, 30, but much lower than from Australia20. However, given the limited studies in Europe and Australia, these trends need to be verified. Overall, BDCIPP urine levels in the current study were similar to those from recent studies24, 27 (i.e. post-2013) but higher than older studies (i.e. pre-2013)12, 18–20, 25. The increasing use of TDCIPP as a flame retardant may explain the higher BDCIPP urine levels in the most recent samples. However, due to the relatively small number of research groups performing PFR metabolite analyses, instrumental systematic bias is a possible confounder. The only other study that reported BCIPHIPP urine levels20 did not present age-stratified summary statistics and thus comparisons were only possible when considering both mothers and children. Considering all age groups, the geometric mean BCIPHIPP level in our cohort (1.88 ng/ml, non-specific gravity normalized) was very similar to those from the north-eastern Australia cohort (geometric mean: 1.74–1.86 ng/ml non-specific gravity normalized). Although very limited, this trend suggests similar TCIPP exposures in the United States and Australia.
Table 3.
Urinary PFR metabolite levels from North America, Australia and Europe (ng/ml, specific gravity normalized). Non-SG normalized values shown in parenthesis. GM = geometric mean. a ng/g b cohort includes children and adults. c non-smokers only.
| Region | Year | DPHP | BDCIPP | Reference | |||
|---|---|---|---|---|---|---|---|
| median | GM | median | GM | ||||
| Adults | |||||||
| North America | |||||||
| California, USA (n=28) | 2015 | 1.2 (0.80) | 1.2 (0.75) | 2.8 (1.9) | 3.3 (2.0) | This study | |
| Canada (n=12 pools, 4 individuals) | 2014 | (0.63) a | (0.39) a | Su et al. (2015) | |||
| New Jersey, USA (n=22) | 2013–2014 | 1.9 | 2.4 | Butt et al. (2014) | |||
| North Carolina, USA (n=53) | 2012 | 1.0 | 0.37 | Hoffman et al. (2015) | |||
| North Carolina, USA (n=39) | 2011–2012 | (1.6) | (1.9) | (1.1) | (1.3) | Hoffman et al. (2014) | |
| California, USA (n=16) | 2011 | (0.44) | (0.09) | Dodson et al. (2014) | |||
| United States (n=9) | 2011 | 1.8 (0.80) | 3.0 (1.1) | 0.37 (0.08) | 0.41 (0.15) | Cooper et al. (2011) | |
| Ontario, Canada (n=24) | 2010–2012 | (2.9) | (2.9) | (0.26) | (0.27) | Kosarac et al. (2016) | |
| Massachusetts, USA (n=29) | 2009 | 0.41 | Carignan et al. (2013) | ||||
| Massachusetts, USA (n=45) | 2002–2007 | 0.27 | 0.31 | 0.12 | 0.13 | Meeker et al. (2013) | |
| Australia | |||||||
| Queensland, Australia (n=72) | 2010–2011 | (24.4) b | (1.00) a | Van den Eede et al. (2015) | |||
| Queensland, Australia (n=23) | 2012–2013 | (63.4) b | (0.66) a | Van den Eede et al. (2015) | |||
| Europe | |||||||
| 0.63 | 0.61 | ||||||
| Olso, Norway (n=48) | 2012 | (0.51) | (0.46) | 0.08 (0.12) | 0.15 (0.13) | Cequier et al. (2015) | |
| Belgium (n=59) | 2009–2012 | (0.82) c | Van den Eede et al. (2013) | ||||
| Germany (n=19) | not reported | (1.3) | Reemtsma et al. (2011) | ||||
| Germany (n=30) | not reported | <0.5 | Schindler et al. (2009) | ||||
| Children | |||||||
| North America | |||||||
| California, USA (n=33) | 2015 | 2.5 (1.9) | 2.9 (2.0) | 7.4 (7.7) | 10.9 (7.6) | This study | |
| North Carolina, USA (n=43) | 2014–2015 | 3.2 (1.0) | 7.3 (2.3) | Hoffman et al. (2015) | |||
| New Jersey, USA (n=26) | 2013–2014 | 3.0 | 5.6 | Butt et al. (2014) | |||
| Europe | |||||||
| Olso, Norway (n=54) | 2012 | 1.0 (1.1) | 1.1 (1.1) | 0.23 (0.23) | 0.23 (0.22) | Cequier et al. (2015) | |
| Germany (n=312) | 2011–2012 | (0.8) | Fromme et al. (2014) | ||||
Urinary concentrations in mothers and children
Considering the entire cohort, BDCIPP, DPHP, ip-PPP and BCIPHIPP conjugates were detected in 100% of the mother and child urine samples (Table 4, Table S1 for non-specific gravity normalized values). In general, previous studies have also shown high detection frequencies of BDCIPP, DPHP and ip-PPP and low detection of BCIPP and tb-PPP18, 20, 24, 27. The low detection frequency of BCIPP may be due to the formation of other non-dealkylated TCIPP metabolites, namely BCIPHIPP, as suggested by an in vitro study14. However, further work by the same research group showed that BCIPHIPP formation was 10-fold lower than BCIPP in human liver microsomes15. Thus, the low detection frequency of BCIPP may be due to the higher detection limits. Interestingly, the only other study to monitor BCIPHIPP also showed 100% detection20. However, it should be emphasized that the current and previous study20 are in fact reporting levels of the conjugated BCIPHIPP and not BCIPHIPP itself. Therefore, the reported values likely represent the sum BCIPHIPP glucuronide and sulfate conjugates, and not free BCIPHIPP itself. No attempt was made to specifically identify which BCIPHIPP conjugate was present in the urine since we used an enzyme mixture that contained both glucuronidase and sulfatase.
Table 4.
Paired mother-children urine concentrations of PFR metabolites (ng/ml, specific gravity normalized) and TBBA (pg/mL, specific gravity normalized) a.
| Mothers (n=28) | ||||||||
|
detection frequency (%) |
geometric mean (95% CI) |
min | max | 25% | 50% | 75% | 95% | |
| BCIPP | 11 | NA | <0.08 | 4.0 | NA | NA | NA | NA |
| BDCIPP | 100 | 3.3 (2.5, 4.2) | 0.98 | 14.3 | 2.2 | 2.8 | 5.1 | 12.2 |
| DPHP | 100 | 1.2 (0.97, 1.5) | 0.39 | 3.5 | 0.79 | 1.2 | 1.9 | 3.5 |
| ip-PPP | 100 | 2.0 (1.5, 2.5) | 0.56 | 14.8 | 1.3 | 2.0 | 2.6 | 10.2 |
| tb-PPP | 7 | NA | <0.12 | 0.37 | NA | NA | NA | NA |
| BCIPHIPP | 100 | 3.4 (2.1, 5.6) | 0.42 | 104 | 1.7 | 2.4 | 4.5 | 80 |
| TBBA | 36 | NA | <6.0 | 21 | NA | NA | NA | NA |
| Children (n=33) | ||||||||
|
detection frequency (%) |
geometric mean (95% CI) |
min | max | 25% | 50% | 75% | 95% | |
| BCIPP | 9 | NA | <0.08 | 3.4 | NA | NA | NA | NA |
| BDCIPP | 100 | 10.9 (6.8, 17.6) | 1.7 | 798 | 4.6 | 7.4 | 16.7 | 413 |
| DPHP | 100 | 2.9 (1.9, 4.4) | 0.36 | 82.0 | 1.2 | 2.5 | 5.1 | 52.1 |
| ip-PPP | 100 | 1.8 (1.3, 2.4) | 0.44 | 8.5 | 0.92 | 2.1 | 3.6 | 7.3 |
| tb-PPP | 9 | NA | <0.12 | 0.78 | NA | NA | NA | NA |
| BCIPHIPP | 100 | 2.5 (1.8, 5,5) | 0.37 | 23.2 | 1.3 | 2.0 | 5.1 | 16.5 |
| TBBA | 45 | NA | <6.0 | 225 | NA | NA | NA | NA |
MDL values: BCIPP = 0.08 ng/ml, BDCIPP = 0.08, ng/ml, DPHP = 0.06 ng/ml, ip-PPP = 0.05 ng/ml, tb-PPP = 0.12 ng/ml, BCIPHIPP = 0.01 ng/ml, TBBA = 6 pg/ml. NA = not applicable, CI = confidence interval.
TBBA was detected in 36% of adult urine samples and 45% of children urine samples. The TBBA detection frequency for mothers was similar to our previous study, but much lower for children (70% in the previous study)24. Due to the low detection frequency, geometric mean TBBA levels were not calculated. However, the results suggest that TBBA levels are 1–2 orders of magnitude lower than the PFR metabolites. Additionally, the TBBA concentration range was similar to our previous studies19, 24. Overall, these trends suggest relatively lower exposure to EH-TBB, inefficient EH-TBB uptake or low biotransformation rate of EH-TBB to TBBA.
Correlations between Mothers and Children
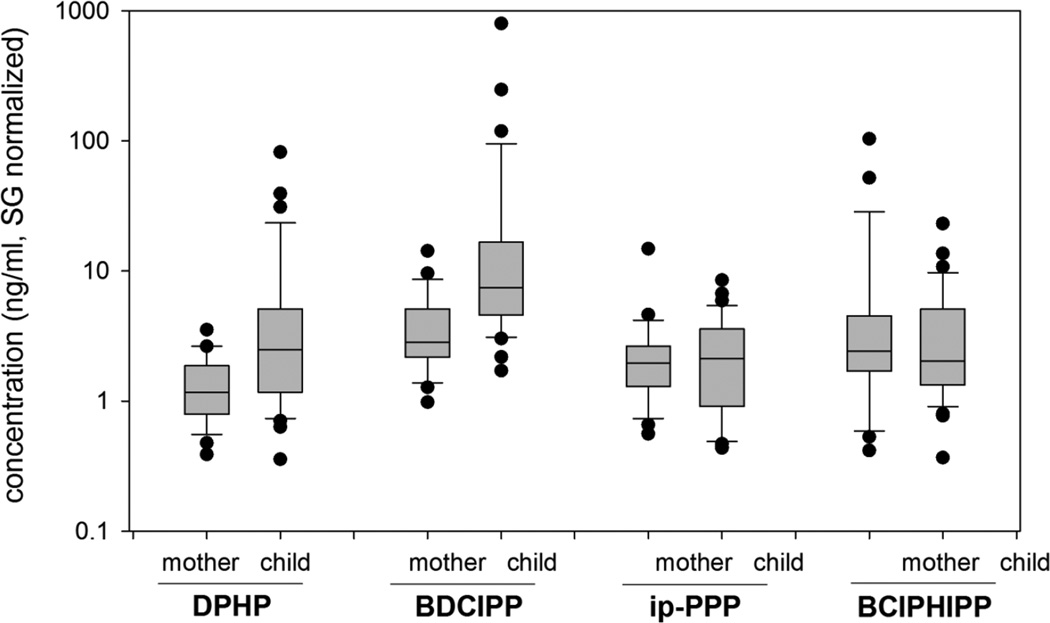
Children’s urine levels of DPHP and BDCIPP in this California cohort were, on average, 5.9 times (p<0.001, t-test using log-transformed data) and 15 times (p<0.0001) than those of their mothers (Figure 2). Similarly, children’s ip-PPP and BCIPHIPP levels were 1.3 times (p=0.63) and 1.4 times (p=0.31), on average, than those of their mothers but these relationships were not statistically significant. Our previous study also showed greater DPHP and BDCIPP levels in children as compared to their mothers24, but the magnitude of difference was much greater in the current study. A cohort of mother-child pairs from Oslo, Norway also showed higher levels in the children, relative to their mothers18. Similarly, our study of PFR metabolite levels in infants (age 2–18 months) from Durham, North Carolina, showed elevated levels as compared to adults27. In addition, a cohort from north-eastern Australia, pooled by age class, showed a negative correlation with age for DPHP and BDCIPP20. BCIPHIPP also showed a significant, but weak negative association with age. The current results, combined with other published studies from different regions of the globe18, 20, 24, 27, show a consistent trend; that is, children have elevated levels of exposure to PFR flame retardants. The exception to this trend may be TCIPP since children’s BCIPHIPP levels were not elevated in our study and the Australian study20, and may suggest an alternative exposure source for TCIPP. For example, a recent study showed that inhalation was likely the dominant source of children’s TCIPP exposure, but dust ingestion was dominant for TDCIPP31. However, since only two studies have reported BCIPHIPP urinary levels, more research is needed for confirmation.
Figure 2.
Box and whisker plot for urinary DPHP, BDCIPP, ip-PPP, BCIPHIPP levels (ng/ml, specific gravity normalized) for mothers (n=28) and their children (n=33).
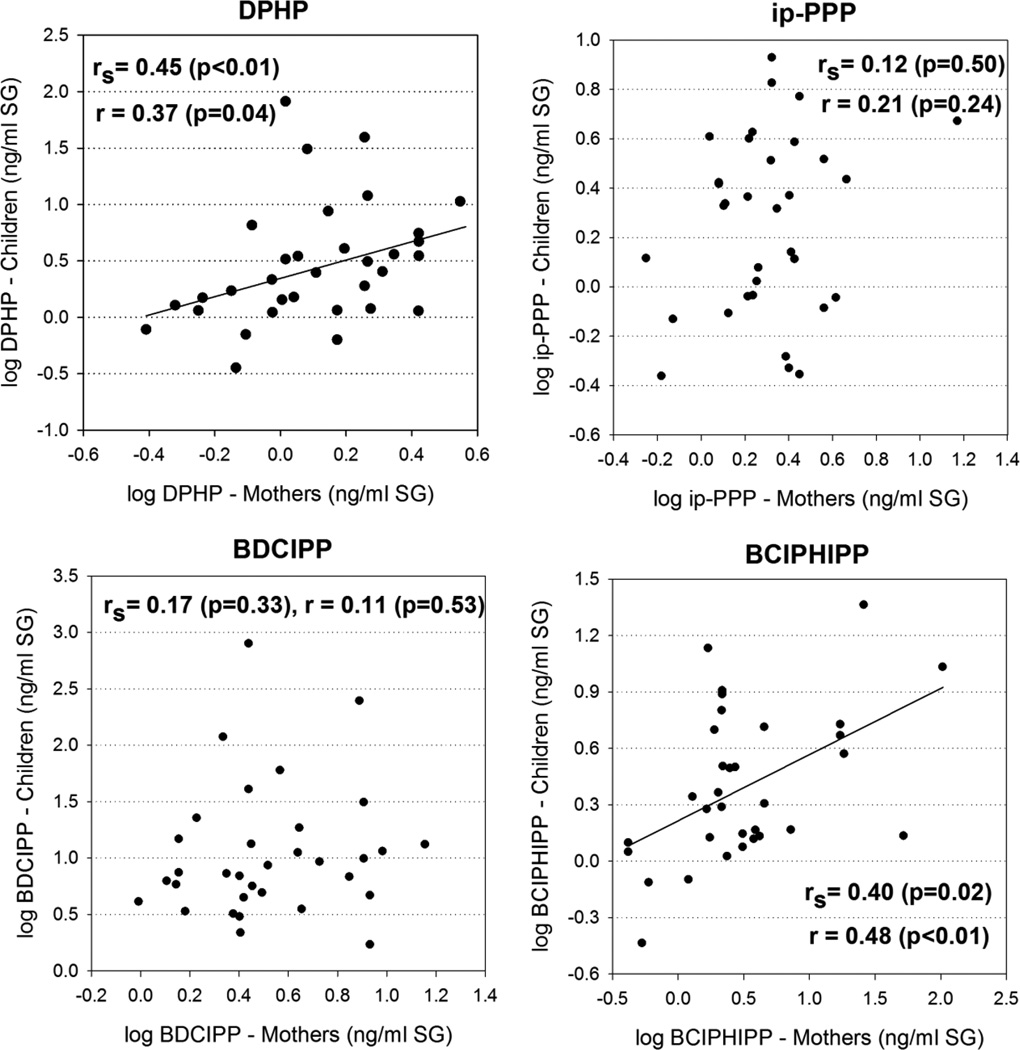
There was a positive correlation in PFR metabolite levels between paired mothers and their children for DPHP (rs=0.45, p<0.01; r=0.37, p=0.04) and BCIPHIPP (rs=0.40, p=0.02; r=0.48, p<0.01) but not BDCIPP (rs=0.17, p=0.33; r=0.11, p<0.53) or ip-PPP (rs=0.12, p=0.50; r=0.21, p=0.24) (Figure 3). These results are in contrast to our previous study which showed a statistically significant correlation between moms and toddlers for BDCIPP and ip-PPP24. The reason for this discrepancy is not known, but may be related to differences in activity patterns or other household characteristics between the cohorts. It was not possible to assess mother-child correlations for TBBA due to the high frequency of <MDL values in the data set – specifically, 16 out of 33 pairs showed <MDL values for both mothers & their children. However, in the remaining 17 pairs, children had higher TBBA concentrations in 13 pairs. This trend suggests that that EH-TBB exposure is greater in children, consistent with our findings from the New Jersey cohort24.
Figure 3.
Relationship between DPHP, ip-PPP, BDCIPP, BCIPHIPP (ng/ml, specific gravity normalized) in mothers and their children.
Predictors of PFR Metabolite Levels in Urine
A questionnaire was used to assess various predictors of PFR exposures (i.e. demographic, behavior and home characteristics). Demographic factors could not be evaluated since the cohort was predominately homogeneous with regards to education, race and socioeconomic status. However, there was sufficient variability in children’s age and it was shown that age was a significant predictor for BDCIPP (p<0.0001) and DPHP (p<0.0001), but not for ip-PPP (p=0.62) or BCIPHIPP (p=0.39) (Table 5). Specifically, BDCIPP levels decreased by ~5%/month (10β= 0.95, 95% confidence interval (CI): 0.92, 0.99) and DPHP levels decreased by ~6%/month (10β= 0.94, 95% CI: 0.94, 0.96). These results are consistent with increased hand-mouth activity in younger children, which results in increased PFR exposures.
Table 5.
Predictors Specific-Gravity Corrected Urinary BDCIPP, DPHP, ip-PPP and BCIPHIPP in Children (n=33).
| SG-Corrected BDCIPP |
SG-Corrected DPHP |
SG-Corrected ip-PPP |
SG-Corrected BCIPHIPP |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Behavior | n | exponentiated beta (95% CI) |
p | exponentiated beta (95% CI) |
p | exponentiated beta (95% CI) |
p | exponentiated beta (95% CI) |
p | |
| Hand washing before eating* | ||||||||||
| Sometimes or hardly ever | 22 | reference | -- | reference | -- | reference | -- | reference | -- | |
| Frequently | 10 | 0.46 (0.22, 0.95) | 0.04 | 0.68 (0.31, 1.46) | 0.32 | 1.55 (0.08, 2.99) | 0.19 | 1.74 (0.89, 3.41) | 0.11 | |
| Hand-to-mouth contacts | ||||||||||
| 0–6 per day | 17 | reference | -- | reference | -- | reference | -- | reference | -- | |
| >6 per day | 16 | 7.86 (2.22, 27.83) | 0.001 | 3.31 (1.78, 6.18) | 0.0002 | 0.50 (0.19, 1.29) | 0.15 | 0.57 (0.24, 1.32) | 0.27 | |
| Object-to-mouth contacts* | ||||||||||
| 0–3 per day | 20 | reference | -- | reference | -- | reference | -- | reference | -- | |
| ≥4 per day | 12 | 1.44 (0.72, 2.90) | 0.30 | 1.43 (0.65, 3.14) | 0.37 | 0.68 (0.44, 1.05) | 0.08 | 0.76 (0.46, 1.24) | 0.27 | |
| Thumb sucking* | ||||||||||
| Hardly ever | 26 | reference | -- | reference | -- | reference | -- | reference | -- | |
| Sometimes or frequently | 6 | 1.47 (0.72, 2.99) | 0.29 | 1.11 (0.43, 2.86) | 0.83 | 1.79 (1.11, 2.88) | 0.02 | 0.97 (0.59, 1.61) | 0.92 | |
| Age (continuous) | 0.95 (0.92, 0.99) | 0.005 | 0.94 (0.94, 0.96) | <0.001 | 1.01 (1.00, 1.03) | 0.06 | 1.01 (0.99, 1.02) | 0.45 | ||
not recorded for the participant that was 2 months of age.
In the children, several predictors of hand-mouth behavior were associated with BDCIPP, DPHP and ip-PPP urine levels, but no associations were observed with BCIPHIPP (Table 5). For example, children with “frequent” handing washing before eating had BDCIPP levels that were 0.46 times (95% CI: 0.22, 0.95, p=0.04) those of children reporting “sometimes/hardly ever”. Interesting, the effect of hand washing on BDCIPP levels was similar to our previous study (~50% reduction with more hand washing)24. In addition, increased frequency of hand-to-mouth contacts was significantly associated with higher BDCIPP levels (p=0.001) and DPHP levels (p=0.0002). Specifically, children with >6 hand-to-mouth contacts per day had BDCIPP and DPHP levels that were 7.9 times (95% CI: 2.2–27.8) and 3.3 times (95% CI: 1.8, 6.2), respectively, those of children with 0–6 hand-to-mouth contacts. Finally, children with reported “sometimes/frequent” thumb sucking had ip-PPP levels that were 1.8 times (95% CI: 1.1, 2.9) those reported to “hardly ever” suck their thumbs. Conversely, no associations were observed with object-to-mouth contacts. As noted above, age was strongly correlated with BDCIPP and DPHP levels and thus age is an apparent confounder for these metabolites. However, it was not possible to control for age due to the limited sample size as well as the fact that the behavior characteristics likely are correlated with age.
In general, adults did not exhibit sufficient variability to adequately evaluate behavioral predictors. Further, two behavioral characteristics that were evaluated (hand washing: 0–4 times day versus >4 times per day, and working outside of the home) did not show associations with BDCIPP, DPHP, ip-PPP or BCIPHIPP urine levels. These trends are consistent with our previous study which did not find any associations between hand-mouth behavior and adult PFR metabolite levels24.
Environmental Implications
In summary, the current study suggests that Californians have greater TDCIPP and ip-PDPP exposure, as compared to New Jersey. The elevated PFR exposure is consistent with the higher PBDE levels in California, as relative to other states, and may be a result of stricter California flammability standards. Future research is needed to determine if these trends will persist after the 2014 TB 117 revision. Further, the current paper corroborates our previous studies, and those from other research groups, which show children are ubiquitously exposed to PFRs, that children’s urinary metabolite levels are greater than adults and that this appears to be an international trend. The elevated children’s levels are consistent with other chemical flame retardants (i.e. PBDEs), and are most likely associated with increased hand-mouth activity in children. For example, in the current study we showed that some PFR metabolites were associated with hand-mouth behaviors. This is supported by previous studies showing that urinary PFR metabolite levels are correlated with their parent PFR levels in indoor dust and hand wipes18, 26, 32, 33. However, this study did not investigate relationships between the observed metabolite levels and health effects. A growing body of literature has indicated that PFR and EH-TBB compounds are potential carcinogens12, 34, endocrine disruptors35–37 and neurodevelopmental toxicants38, 39, thus justifying future human health studies and exposure reduction strategies.
Supplementary Material
HIGHLIGHTS.
Urinary BDCIPP levels higher in California children than New Jersey children
Regional differences in exposure may be driven by California flammability standard
Urinary DPHP and BCIPHIPP were correlated between mother-child pairs
Hand-to-mouth frequencies were significantly associated with exposure
Acknowledgments
The Environmental Working Group provided funding for this project. Effort for C.M.B., K.H. and HMS were also provided by a grant from the National Institute of Environmental Health Sciences (R01 ES016099). Professor Adrian Covaci (University of Antwerp) is thanked for donation of the BCIPHIPP standard.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
SUPPORTING INFORMATION
Table showing non-specific gravity normalized urine concentrations in mothers and their children.
REFERENCES
- 1.European Chemicals Agency. [Accessed: March 28, 2016];SEAC concludes on Bisphenol A, DecaBDE and PFOA restrictions and finalises two opinions for authorisation. 2015 Sep 15; http://echa.europa.eu/view-article/-/journal_content/title/seac-concludes-on-bisphenola-decabde-and-pfoa-restrictions-and-finalises-two-opinions-for-authorisation. [Google Scholar]
- 2.Stapleton HM, Sharma S, Getzinger G, Ferguson PL, Gabriel M, Webster TF, Blum A. Novel and high volume use flame retardants in US couches reflective of the 2005 PentaBDE phase out. Environ. Sci. Technol. 2012;46(24):13432–13439. doi: 10.1021/es303471d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sutton R, Sedlak MD, Yee D, Davis JA, Crane D, Grace R, Arsem N. Declines in polybrominated diphenyl ether contamination of San Francisco Bay following production phase-outs and bans. Environ. Sci. Technol. 2015;49(2):777–784. doi: 10.1021/es503727b. [DOI] [PubMed] [Google Scholar]
- 4.Marvin C, Waltho J, Jia J, Burniston D. Spatial distributions and temporal trends in polybrominated diphenyl ethers in Detroit River suspended sediments. Chemosphere. 2013;91(6):778–783. doi: 10.1016/j.chemosphere.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 5.Schuster JK, Gioia R, Moeckel C, Agarwal T, Bucheli TD, Breivik K, Steinnes E, Jones KC. Has the burden and distribution of PCBs and PBDEs changed in european background soils between 1998 and 2008? Implications for sources and processes. Environ. Sci. Technol. 2011;45(17):7291–7297. doi: 10.1021/es200961p. [DOI] [PubMed] [Google Scholar]
- 6.Braune BM, Letcher RJ, Gaston AJ, Mallory ML. Trends of polybrominated diphenyl ethers and hexabromocyclododecane in eggs of Canadian Arctic seabirds reflect changing use patterns. Environ. Res. 2015;142:651–661. doi: 10.1016/j.envres.2015.08.010. [DOI] [PubMed] [Google Scholar]
- 7.Miller A, Elliott JE, Elliott KH, Guigueno MF, Wilson LK, Lee S, Idrissi A. Brominated flame retardant trends in aquatic birds from the Salish Sea region of the west coast of North America, including a mini-review of recent trends in marine and estuarine birds. Sci. Total Environ. 2015;502:60–69. doi: 10.1016/j.scitotenv.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Guo W, Holden A, Smith SC, Gephart R, Petreas M, Park JS. PBDE levels in breast milk are decreasing in California. Chemosphere. 2015 doi: 10.1016/j.chemosphere.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 9.Toms LML, Guerra P, Eljarrat E, Barceló D, Harden FA, Hobson P, Sjodin A, Ryan E, Mueller JF. Brominated flame retardants in the Australian population: 1993–2009. Chemosphere. 2012;89(4):398–403. doi: 10.1016/j.chemosphere.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 10.Zota AR, Linderholm L, Park JS, Petreas M, Guo T, Privalsky ML, Zoeller RT, Woodruff TJ. Temporal comparison of PBDEs, OH-PBDEs, PCBs, and OH-PCBs in the serum of second trimester pregnant women recruited from San Francisco General Hospital, California. Environ. Sci. Technol. 2013;47(20):11776–11784. doi: 10.1021/es402204y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Technical Bulletin 117–2013. Requirements, test procedure and apparatus for testing the smolder resistance of materials used in upholstered furniture. Sacramento, CA: State of California, Department of Consumer Affairs; 2013. [Google Scholar]
- 12.van der Veen I, de Boer J. Phosphorus flame retardants: Properties, production, environmental occurrence, toxicity and analysis. Chemosphere. 2012;88(10):1119–1153. doi: 10.1016/j.chemosphere.2012.03.067. [DOI] [PubMed] [Google Scholar]
- 13.ICL Industrial Products; 2012. Jul 18, Material Safety Data Sheet. Fyrol HF-5. http://www.triiso.com/documents/ICL_Fyrol_HF-5_MSDS.pdf. [Google Scholar]
- 14.Van den Eede N, Maho W, Erratico C, Neels H, Covaci A. First insights in the metabolism of phosphate flame retardants and plasticizers using human liver fractions. Toxicol. Lett. 2013;223(1):9–15. doi: 10.1016/j.toxlet.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 15.Van den Eede N, Tomy G, Tao F, Halldorson T, Harrad S, Neels H, Covaci A. Kinetics of tris (1-chloro-2-propyl) phosphate (TCIPP) metabolism in human liver microsomes and serum. Chemosphere. 2016;144:1299–1305. doi: 10.1016/j.chemosphere.2015.09.049. [DOI] [PubMed] [Google Scholar]
- 16.Roberts SC, Macaulay LJ, Stapleton HM. In vitro metabolism of the brominated flame retardants 2-ethylhexyl-2,3,4,5-tetrabromobenzoate (TBB) and bis(2-ethylhexyl) 2,3,4,5-tetrabromophthalate (TBPH) in human and rat tissues. Chem. Res. Toxicol. 2012;25(7):1435–1441. doi: 10.1021/tx300086x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dodson RE, Van Den Eede N, Covaci A, Perovich LJ, Brody JG, Rudel RA. Urinary biomonitoring of phosphate flame retardants: Levels in california adults and recommendations for future studies. Environ. Sci. Technol. 2014;48(23):13625–13633. doi: 10.1021/es503445c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cequier E, Sakhi AK, Marcé RM, Becher G, Thomsen C. Human exposure pathways to organophosphate triesters - A biomonitoring study of mother-child pairs. Environ. Int. 2015;75:159–165. doi: 10.1016/j.envint.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman K, Fang M, Horman B, Patisaul HB, Garantziotis S, Birnbaum LS, Stapleton HM. Urinary tetrabromobenzoic acid (TBBA) as a biomarker of exposure to the flame retardant mixture Firemaster (R) 550. Environ. Health Perspect. 2014 doi: 10.1289/ehp.1308028. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Eede N, Heffernan AL, Aylward LL, Hobson P, Neels H, Mueller JF, Covaci A. Age as a determinant of phosphate flame retardant exposure of the Australian population and identification of novel urinary PFR metabolites. Environ. Int. 2014;74:1–8. doi: 10.1016/j.envint.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Zota AR, Rudel RA, Morello-Frosch RA, Brody JG. Elevated house dust and serum concentrations of PBDEs in California: Unintended consequences of furniture flammability standards? Environ. Sci. Technol. 2008;42(21):8158–8164. doi: 10.1021/es801792z. [DOI] [PubMed] [Google Scholar]
- 22.Rose M, Bennett DH, Bergman AKE, Fängström B, Pessah IN, Hertz-Picciotto I. PBDEs in 2–5 year-old children from California and associations with diet and indoor environment. Environ. Sci. Technol. 2010;44(7):2648–2653. doi: 10.1021/es903240g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dodson RE, Perovich LJ, Covaci A, Van Den Eede N, Ionas AC, Dirtu AC, Brody JG, Rudel RA. After the PBDE phase-out: A broad suite of flame retardants in repeat house dust samples from California. Environ. Sci. Technol. 2012;46(24):13056–13066. doi: 10.1021/es303879n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Butt CM, Congleton J, Hoffman K, Fang M, Stapleton HM. Metabolites of organophosphate flame retardants and 2-ethylhexyl tetrabromobenzoate in urine from paired mothers and toddlers. Environ. Sci. Technol. 2014;48(17):10432–10438. doi: 10.1021/es5025299. [DOI] [PubMed] [Google Scholar]
- 25.Cooper EM, Covaci A, Van Nuijs ALN, Webster TF, Stapleton HM. Analysis of the flame retardant metabolites bis(1,3-dichloro-2-propyl) phosphate (BDCPP) and diphenyl phosphate (DPP) in urine using liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2011;401(7):2123–2132. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffman K, Garantziotis S, Birnbaum LS, Stapleton HM. Monitoring indoor exposure to organophosphate flame retardants: Hand wipes and house dust. Environ. Health Perspect. 2015;123(2):160–165. doi: 10.1289/ehp.1408669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hoffman K, Butt CM, Chen A, Limkakeng AT, Stapleton HM. High Exposure to Organophosphate Flame Retardants in Infants: Associations with Baby Products. Environ. Sci. Technol. 2015;49(24):14554–14559. doi: 10.1021/acs.est.5b03577. [DOI] [PubMed] [Google Scholar]
- 28.Kosarac I, Kubwabo C, Foster WG. Quantitative determination of nine urinary metabolites of organophosphate flame retardants using solid phase extraction and ultra performance liquid chromatography coupled to tandem mass spectrometry (UPLC-MS/MS) Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2016;1014:24–30. doi: 10.1016/j.jchromb.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 29.Su G, Letcher RJ, Yu H. Determination of organophosphate diesters in urine samples by a high-sensitivity method based on ultra high pressure liquid chromatography-triple quadrupole-mass spectrometry. J. Chromatogr. A. 2015;1426:154–160. doi: 10.1016/j.chroma.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 30.Van den Eede N, Neels H, Jorens PG, Covaci A. Analysis of organophosphate flame retardant diester metabolites in human urine by liquid chromatography electrospray ionisation tandem mass spectrometry. J. Chromatogr. A. 2013;1303:48–53. doi: 10.1016/j.chroma.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 31.Schreder ED, Uding N, La Guardia MJ. Inhalation a significant exposure route for chlorinated organophosphate flame retardants. Chemosphere. 2016;150:499–504. doi: 10.1016/j.chemosphere.2015.11.084. [DOI] [PubMed] [Google Scholar]
- 32.Fromme H, Lahrz T, Kraft M, Fembacher L, Mach C, Dietrich S, Burkardt R, Völkel W, Göen T. Organophosphate flame retardants and plasticizers in the air and dust in German daycare centers and human biomonitoring in visiting children (LUPE 3) Environ. Int. 2014;71:158–163. doi: 10.1016/j.envint.2014.06.016. [DOI] [PubMed] [Google Scholar]
- 33.Hammel S, Hoffman K, Webster TF, Anderson KA, Stapleton HM. Measuring personal exposure to organophosphate flame retardants using silicone wristbands and hand wipes. Environ. Sci. Technol; ASAP; 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.State of California Environmental Protection Agency. Office of Environmental Health Hazard Assessment; 2014. Mar 28, Safe Drinking Water and Toxic Enforcement Act of 1986. Chemicals Known to the State to Cause Cancer or Reproductive Toxicity. [Google Scholar]
- 35.Kojima H, Takeuchi S, Van den Eede N, Covaci A. Effects of primary metabolites of organophosphate flame retardants on transcriptional activity via human nuclear receptors. Toxicol. Lett. 2016;245:31–39. doi: 10.1016/j.toxlet.2016.01.004. [DOI] [PubMed] [Google Scholar]
- 36.Patisaul HB, Roberts SC, Mabrey N, McCaffrey KA, Gear RB, Braun J, Belcher SM, Stapleton HM. Accumulation and Endocrine Disrupting Effects of the Flame Retardant Mixture Firemaster® 550 in Rats: An Exploratory Assessment. J. Biochem. Mol. Toxicol. 2013;27(2):124–136. doi: 10.1002/jbt.21439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Q, Lai NLS, Wang X, Guo Y, Lam PKS, Lam JCW, Zhou B. Bioconcentration and transfer of the organophorous flame retardant 1,3-dichloro-2-propyl phosphate causes thyroid endocrine disruption and developmental neurotoxicity in zebrafish larvae. Environ. Sci. Technol. 2015;49(8):5123–5132. doi: 10.1021/acs.est.5b00558. [DOI] [PubMed] [Google Scholar]
- 38.Noyes PD, Haggard DE, Gonnerman GD, Tanguay RL. Advanced morphological - behavioral test platform reveals neurodevelopmental defects in embryonic zebrafish exposed to comprehensive suite of halogenated and organophosphate flame retardants. Toxicol. Sci. 2015;145(1):177–195. doi: 10.1093/toxsci/kfv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dishaw LV, Hunter DL, Padnos B, Padilla S, Stapleton HM. Developmental exposure to organophosphate flame retardants elicits overt toxicity and alters behavior in early life stage zebrafish (danio rerio) Toxicol. Sci. 2014;142(2):445–454. doi: 10.1093/toxsci/kfu194. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.