Abstract
Diabetic retinopathy has recently become associated with complications similar to chronic inflammatory diseases. While it is clear that tumor necrosis factor- alpha (TNF-α) is increased in diabetes, the role of innate immunity is only recently being investigated. As such, we hypothesized that diabetes would increase toll-like receptor 4 (TLR4) signaling, which could be inhibited by a β-adrenergic receptor agonist (Compound 49b) previously shown to have anti-inflammatory actions. In order to investigate β-adrenergic receptor signaling and TLR4 in the diabetic retina, streptozotocin-injected diabetic mice, as well as human primary retinal endothelial cells (REC) and rat retinal Müller cells (rMC-1) exposed to high glucose (25mM), were treated with a novel β-adrenergic receptor agonist, Compound 49b (50nM), or PBS (control). TLR4 and its downstream signaling partners (MyD88, IRAK1, TRAF6, total and phosphorylated NF-κB) were examined. In addition, we assessed high mobility box group 1 (HMGB1) protein levels. Our data showed that diabetes or high glucose culture conditions significantly increased TLR4 and downstream signaling partners. Compound 49b was able to significantly reduce TLR4 and related molecules in the diabetic animal and retinal cells. HMGB1 was significantly increased in REC and Müller cells grown in high glucose, which was subsequently reduced with Compound 49b treatment. Our findings suggest that high glucose may increase HMGB1 levels that lead to increased TLR4 signaling. Compound 49b significantly inhibited this pathway providing a potential mechanism for its protective actions.
Keywords: TLR4, diabetic retinopathy, retinal endothelial cells, Müller cells, inflammation
Introduction
Diabetic retinopathy is associated with significant health care costs (245 billion in 2012) which are expected to substantially increase with the prevailing obesity epidemic (American Diabetic Association, diabetes.org). Unfortunately, current therapies to treat this condition remain limited and currently only delay disease progression until the retinopathy has progressed to a proliferative phase. While a plethora of potential causal factors are implicated in diabetic retinopathy, none has yet led to the development of successful therapeutic modalities. Most recently, the role of inflammation and the immune system have become of greater interest in understanding diabetic retinopathy1–5. Work in type 1 diabetic rats has shown that leukocyte activation is induced by diabetes and can be reduced by antibody neutralization of ICAM1 or CD18 6. Others have reported that reductions in TNF-α could decrease diabetic-induced retinal damage 1. Similarly, inhibition of IL-1β prevents vascular complications associated with retinopathy 7. Taken together, these results support the notion that inflammation and the immune response may be involved in the diabetic retina.
Toll-like receptor (TLR) pathways are known to play an essential role in the mediation of immune responses. TLRs were originally identified in Drosophila as key receptors for developing embryos 8. Subsequently it was recognized that these TLRs comprise a group of genetically conserved pattern recognition receptors (PRRs) encoded in germline DNA 9. TLRs bind to pathogen-associated molecular patterns (PAMPs) to initiate a proliferative innate immune response 9 leading to activation of a number of cytokines and inflammatory mediators including TNF-α and IL-1. Each TLR has a pathogen group to which it will mount a response. For example, lipopolysaccride (LPS) as a potent pathogen signal for TLR4 8, 10. In addition to LPS, TLR4 can also respond to endogenous damage associated molecular patterns (DAMPs), such as hyaluronic acid, high mobility group box 1 (HMGB1), receptor for advanced glycation end products (RAGE), and heat shock proteins 9. Recent work has suggested that diabetes may represent a sterile inflammation 11. Extending on this work, it has been shown that whole retinal lysates from type 1 diabetic rats have increased TLR4, MyD88 and other downstream proteins associated with TLR4 activation 12. Recent work has demonstrated that reduced MyD88 levels in diabetic mice can prevent leukostasis and decrease ICAM1 levels, as well as reactive oxygen species (ROS) 13. MyD88 is a common signaling molecule for all TLRs, leading to downstream activation of nuclear factor-kappa beta (NF-κB) 8. Retinal endothelial cells (REC) cultured in high glucose have been shown to express increased levels of TLR2 and TLR4, as well as MyD88 and NF-κB 14. Use of a TLR4 antagonist led to a significant reduction in TNFα, NF-κB, IL-8, ICAM1, and VCAM1, as well as decreased adhesion 14.
It is clear that a number of cell types may be involved in the retinal damage associated with diabetic retinopathy, as high glucose has been reported to induce expression of inflammatory mediators in REC 15 and Müller cells 16, as well. Additionally, evidence suggests that immune cells may also enter the retina, further regulating the inflammatory response 13. We have previously reported that β-adrenergic receptor agonists can reduce inflammatory mediator expression by both REC 17 and Müller cells 18. TLR4 expression has been detected on mouse Müller cells 19; while photoreceptor studies have shown a role for TLR4 regulation of neuronal apoptosis 20, further supporting that TLR4 is likely active in the retina. Work in human peripheral blood mononuclear cells has shown that the β-adrenergic receptor agonist, isoprenaline, reduces TNF-α levels following LPS stimulation 21. Similarly, it has been demonstrated in peritoneal macrophages that ephedrine reduces TNF-α through TLR-mediated pathways 22. Studies using a THP-1 human monocyte cell line demonstrated that fenoterol, a β2-adrenergic receptor agonist, could inhibit LPS-induced TLR4 levels 23. Thus, literature suggests that β-adrenergic receptors can regulate TLR4 signaling in multiple targets.
Previously we reported that the novel β-adrenergic receptor agonist, Compound 49b, reduced TNF-α in both REC 24 and Müller cells 25. Following from this, we sought to ascertain whether this effect occurs through a reduction in TLR4 signaling. To this end, we hypothesized that hyperglycemia would increase TLR4 signaling which would then be inhibited by Compound 49b in diabetic mice, REC and Müller cells.
Results
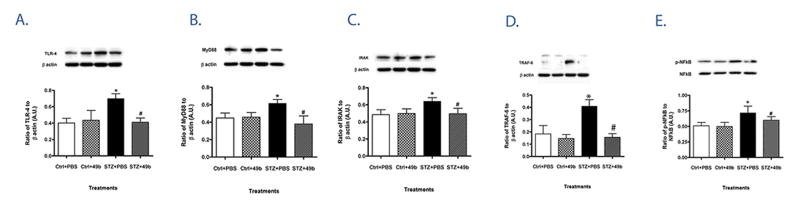
β-adrenergic receptor activation reduces diabetes-induced TLR4 signaling in retinal samples
Diabetic mice had significantly lower bodyweight and increased blood glucose levels when compared to control littermates as noted in Table 1. All mice were on a standard chow and glucose measurements were taken on non-fasted mice. In whole retinal lysates from streptozotocin-induced diabetic mice after 2 months of diabetes, TLR4 expression was significantly increased compared to non-diabetic mice (Fig. 1A). As TLR signaling leads to pro-inflammatory cytokine production, we also examined protein expression of the TLR4 adaptor molecule, MyD88 together with downstream signaling partners IRAK1, TRAF6 and p-NF-κB (Fig 1B–E, respectively). All four molecules were similarly up-regulated in retinas of diabetic versus control animals. In contrast, TLR4, MyD88, IRAK1, TRAF6 and p-NF-κB were significantly decreased in diabetic mice treated with topical Compound 49b, potentially explaining a pathway for Compound 49b’s inhibition of TNF-α levels 24. These data suggest that β-adrenergic receptors can regulate TLR4 signaling in the diabetic retina.
Table 1.
Body weight and blood glucose measurements for the control and diabetic mice.
| n | body weight (g) | Blood Glucose (mg/dL) | |
|---|---|---|---|
| Control (PBS) | 6 | 23±1.6 | 137±6 |
| Control 49b | 6 | 22±1.5 | 134±7 |
| STZ | 6 | 19.5± 2.5* | 508±62* |
| STZ+49b | 6 | 19±1.8* | 421±65* |
Data are mean ± SD
p < 0.05 v.s control
Figure 1.
Diabetes increases TLR4 signaling, which is blocked by Compound 49b. Protein levels of TLR4 (A), MyD88 (B), IRAK-1 (C), TRAF6 (D) and the ratio of phosphorylated NF-κB to total NF-κB (E) were measured by Western blot in retinal lysates from control (Ctrl) and diabetic (STZ) mice +/- Compound 49b treatment (50nM) (Ctrl+49b and STZ+49b). *P<0.05 vs Ctrl, #P<0.05 vs STZ. Data are expressed as mean ± SEM (N=6 for all groups) and show one of two similar experiments.
Compound 49b reduces TLR4 signaling in REC cultured in high glucose
In order to dissect potential cell types involved in β-adrenergic receptor regulation of TLR4 signaling in the diabetic retina, human REC were cultured under normal and high glucose conditions +/- Compound 49b treatment. As demonstrated in Figure 2, high glucose conditions resulted in significant increases not only in levels of TLR4 (A), but also in the adaptor molecule MyD88 (B) and downstream signaling partners, IRAK1 (C), TRAF6 (D) and p-NF-κB (E). Compound 49b treatment significantly decreased expression of TLR4 and aforementioned TLR-related signaling molecules in REC cultured in high glucose conditions. This suggests that TLR4 signaling can be regulated by β-adrenergic receptors on REC.
Figure 2.
TLR4 signaling is enhanced in retinal endothelial cells (REC) exposed to hyperglycemia, which was inhibited by Compound 49b. Protein levels of TLR4 (A), MyD88 (B), IRAK-1 (C), TRAF6 (D) and the ratio of phosphorylated NF-κB to total NF-κB (E) were measured by Western blot in REC grown in normal glucose (NG), high glucose (HG) and after treatment with Compound 49b (NG+49b and HG+49b). *P<0.05 vs NG, #P<0.05 vs HG. Data are show the mean ± SEM (N=4 for all groups) and show one of two similar experiments.
Compound 49b reduces TLR4 levels and MyD88-dependent signaling proteins in Müller cells
Similar to our findings in whole retinal lysates and REC, high glucose culture conditions significantly increased TLR4 levels (Fig. 3A) in Müller cells. In addition, MyD88, IRAK1, TRAF6, and p-NF-κB (Fig. 3B – E, respectively) were increased in response to hyperglycemic conditions. Compound 49b was observed to significantly reduce all of these molecules, indicating that β-adrenergic receptors can regulate the TLR4 signaling pathway in both REC and Müller cells, two key cell types altered in the diabetic retina.
Figure 3.
Müller cells grown in high glucose have increased TLR4 signaling. Protein levels of TLR4 (A), MyD88 (B), IRAK-1 (C), TRAF6 (D) and the ratio of phosphorylated NF-κB to total NF-κB (E) were measured by Western blot in Müller cells grown in normal glucose (NG), high glucose (HG) or normal glucose + Compound 49b (NG+49b) or high glucose + Compound 49b (HG+49b). *P<0.05 vs NG, #P<0.05 vs HG. Data are show the mean ± SEM (N=4 for all groups) and show one of two similar experiments.
Compound 49b significantly reduced HMGB1 levels in both REC and Müller cells
To determine a cellular mechanism by which a β-adrenergic receptor agonist can regulate TLR4 signaling, we measured whether Compound 49b could reduce a known DAMP, HMGB1. High glucose significantly increased HMGB1 levels in both REC and Müller cells, suggesting that HMGB1 may respond to high glucose and respond as a sterile inflammation. Compound 49b was able to significantly reduce HMGB1 in both cell types (Figure 4A–B), indicating that HMGB1 may be the link between Compound 49b and TLR4 signaling.
Figure 4.
REC and Müller cells have increased HMGB1 levels. Western blots of HMGB1 in REC (A) and Müller cells (B) in normal (NG) or high glucose (HG) and treated with Compound 49b (49b). *P<0.05 vs NG, #P<0.05 vs HG. Data are show the mean ± SEM (N=4 for all groups) and show one of two similar experiments.
Discussion
The role of inflammation in diabetes is becoming increasingly evident. It is well established that diabetic retinopathy is associated with elevated leukostasis, and up-regulated levels of classic pro-inflammatory mediators, TNF-α and IL-1β, by retinal cells 6, 7, 26, 27. However, only recently studies have started to investigate whether the innate immune response may be involved in diabetic retinal changes despite that observed retinal abnormalities in diabetes are congruous with events of inflammation. As important mediators of inflammation and innate immunity, TLR signaling pathways have become the focus of diabetic retinopathy studies as of late. Results obtained from MyD88 chimeric mice suggest that TLR signaling may regulate leukostasis and leukocyte-induced endothelial cell death 13. Additionally, it has been demonstrated that TLR4 is increased in the streptozotocin-induced type 1 diabetic rat 12. Similarly, work in human REC has shown that high glucose activates TLR2/4 downstream signaling pathways 14. Literature indicates that REC have increased TLR4 signaling 14, as well as whole retinal lysates from diabetic animals 12. Our data extend those findings to demonstrate that, in addition to REC, Müller cells also demonstrate enhanced TLR4 signaling when cultured under hyperglycemic conditions.
Previous studies have shown that the novel β-adrenergic receptor agonist, Compound 49b, is protective against diabetic retinopathy; ameliorating vascular, neuronal and functional changes in the diabetic retina (24). One mechanism of action for this receptor agonist is by preventing diabetes-induced apoptosis of REC through increased levels of insulin-like growth factor-1 binding protein 3 (IGFBP-3) (24). In addition, Compound 49b has been shown to reduce the potent pro-inflammatory cytokine, TNF-α in REC (25). Since TNF-α is known to be up-regulated upon TLR4 activation, we sought to determine whether this β-adrenergic receptor agonist improves diabetic retinopathy disease pathogenesis, in part, via TLR signaling. As expected, Compound 49b significantly decreased TLR4 levels in both whole retina and in two retinal cells types (REC and Müller cells). Compound 49b also decreased protein levels of several of the positive regulators of inflammation involved in the MyD88-dependent signaling pathway. While Compound 49b may protect the retina through multiple mechanisms, the data presented suggest that these pathways likely include a reduction in TLR4 signaling.
The remaining key question was the pathway by which Compound 49b could regulate TLR4 activation. While little work has investigated β-adrenergic receptor actions on retinal TLR4, studies demonstrated that β1-adrenergic receptors can regulate HMGB1 to attenuate hypoxia/reoxygenation to protect neonatal rat cardiomyocytes 28. These authors demonstrated that dobutamine, a β1-adrenergic receptor agonist used in heart disease, inhibited HMGB1 in a rat model of ischemia/reperfusion 29. Others have reported that HMGB1 may be highly related to autophagy, through its ability to sense stressors and bind TLR2, TLR4, and TLR9 30. Focusing on diabetic retinopathy, HMGB1 can induce cytoxicity in glial cells and mediate cell death of retinal endothelial cells 31. Based on this literature, we showed that HMGB1 is increased by high glucose culturing conditions in both REC and Müller cells. Furthermore, this increase was attenuated following treatment of both cell types with Compound 49b.
While we feel our findings of β-adrenergic receptor regulation of TLR4 and downstream signaling pathways in the diabetic retina, REC and Müller cells are novel, we appreciate the limitations of our work. It is highly likely that Compound 49b may work on other retinal cell types, including microglia or photoreceptor cells, to protect them against increased TLR4 signaling. This will be a focus in future work. Additionally, while beyond the scope of this study, it is clear that β-adrenergic receptors are present in the cornea 32 and that the cornea is significantly affected in diabetes 33. Based upon the existing literature, Compound 49b may have detrimental effects to the diabetic cornea in regards to wound healing 34. Future studies may extend our findings into other ocular tissues and investigate whether they may be protected from diabetes-induced damage by Compound 49b.
In conclusion, these data suggest that high glucose causes an increase in HMGB1, leading to enhanced TLR4 signaling. Compound 49b, a novel β-adrenergic receptor agonist, significantly suppressed HMGB1 levels, as well as downstream MyD88-dependent signaling pathways, resulting in reduced NF-κB phosphorylation.
Methods
Animals
All mouse experiments were approved by the Institutional Animal Care and Use Committee at Wayne State University (Protocol# 11-08-14) and adhere to the Animal Policy of the Association for Research in Vision and Ophthalmology. Eight week old male C57BL/6J (B6) wild type mice were purchased from Charles River Laboratories. Diabetes was induced by injecting 60mg/kg of streptozotocin dissolved in citrate buffer for 5 consecutive days. Control mice received citrate buffer only. Glucose measurements were done twice weekly, with glucose levels > 250mg/dL considered diabetic. Mice were not fasted before blood glucose measurements. Glucose measurements were taken on blood samples obtained from the tail vein using a hand-held measurement device. Table 1 provides body weights and glucose levels of all mice.
After 2 months of diabetes, 6 control and 6 diabetic mice received 1mM Compound 49b eye drops for 14 days (4 uL onto each eye). Control mice received topical PBS. Mice were randomly placed into the treatment or no treatment groups without blinding by the investigators. After 14 days of Compound 49b treatment, all mice were sacrificed for further analysis as described below.
Retinal Endothelial Cell Culture
Primary human retinal microvascular endothelial cells (REC) were acquired from Cell System Corporation (CSC, Kirkland, Washington). Cells were grown in M131 medium containing microvascular growth supplements (MVGS) (Invitrogen), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B but without serum. Three days prior to the experiment, cells were transferred to high (25mM) or normal (5mM) glucose medium (M131 medium with added glucose), supplemented with MVGS and antibiotics. Only primary cells were used within passage 6 only. Cells were quiesced by incubating in high or normal glucose medium without MVGS for 24 hr. Compound 49b treatment was carried out at 50nM, as we have done in the past 24. No signs of mycoplasma contamination were evident.
Müller Cell Culture
Müller cells (rMC-1; kindly provided by Dr. Vijay Sarthy at Northwestern University) were thawed and cultured in DMEM medium under normal glucose (5mM) conditions. Medium was supplemented with 10% FBS and antibiotics. Upon reaching ~ 80% confluency, they were passed into dishes containing high glucose (25mM) or normal glucose medium. Once ready for experimentation, cells were transferred to the appropriate medium without FBS to induce serum starvation for 18–24 hours. Compound 49b (50nM) was then added for 24 hours prior to cell collection. No signs of mycoplasma contamination were evident.
Western Blotting
After appropriate treatments and rinsing with cold phosphate-buffered saline, REC were collected in lysis buffer containing protease/phosphatase inhibitors and scraped into their respective tubes. Retinal extracts were prepared by sonication. Equal amounts of protein from cell or tissue extracts were separated on pre-cast tris-glycine gels (Invitrogen, Carlsbad, CA) and blotted onto a nitrocellulose membrane. After blocking in TBST (10mM Tris-HCl buffer, pH 8.0, 150 mM NaCl, 0.1% Tween 20) and 5% (w/v) BSA, membranes were treated with the following primary antibodies: TLR4, IL-1 receptor-associated kinase 1 (IRAK1), TNF receptor-associated factor 6 (TRAF6), MyD88, HMGB1 (Abcam, San Francisco, CA), NF-κB, phospho-NF-κB (Cell Signaling, Danvers, MA), and β-actin (Santa Cruz, Santa Cruz, CA); then followed by incubation with secondary antibodies (Fisher Scientific, Pittsburgh, PA) labeled with horseradish peroxidase. Antigen-antibody complexes were detected using a chemilluminescence reagent kit (Thermo Scientific, Pittsburgh, PA). Western blot images were collected on an Azure Biosystem C500 machine (Azure Biosystems, Dublin, CA). Mean densitometry of bands for each protein were measured.
Analyses of samples
Streptozotocin treatment
Treatments for all mice were commenced at 16 weeks of age. Mice were randomly assigned to STZ or control groups to induce diabetes. Two months after STZ treatment, Compound 49b was administered to groups of 6 control and diabetic mice which were assigned randomly. Fourteen days after Compound 49b treatment, mice were sacrificed and retinal lysates collected and processed for protein analyses. ELISA was performed on all lysates in parallel and each sample was analyzed in triplicate. Western blot analyses was performed in triplicate and, for each individual blot, a control+PBS, control+49b, STZ+PBS, STZ+49b were run as a group of 4. Blots were then probed for a the protein of interest, then stripped and probed for beta actin. Mean densitometry was done for each band and the ratio of the band of protein of interest to beta actin was calculated. The density for each biological replicate was calculated as the mean of the technical replicates for each sample. A representative blot is shown for all Western blots. Data show the mean ± SEM of 6 independent biological samples for each group.
Cell culture
Samples for REC and Müller cells were derived from 4 separate frozen aliquots of Müller or REC cells. These were then expanded to provide to provide sufficient cells for each of the treatment conditions. Following treatment, all cells were harvested at the same time and protein analyses were done described for the mouse retinal lysates.
Statistics
Data are presented as the mean ± SEM and were analyzed using the Kruskal-Wallis method, followed by Dunn’s test using Prism 7.0 software. P<0.05 was considered significant.
Acknowledgments
NIH grants R01 EY023226 (EAB), R01 EY022045 (JJS), P30EY004068 (Core Grant, PI: Hazlett), Research to Prevent Blindness (RPB, PI: Juzych).
Footnotes
Conflicts of Interest. There are no conflicts of interest by the authors with this study.
References
- 1.Joussen AM, Doehmen S, Le ML, Koizumi K, Radetzky S, Krohne TU, et al. TNF-alpha mediated apoptosis plays an important role in the development of early diabetic retinopathy and long-term histopathological alterations. Molecular vision. 2009;15:1418–1428. [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng L, Du Y, Miller C, Gubitosi-Klug RA, Kern TS, Ball S, et al. Critical role of inducible nitric oxide synthase in degeneration of retinal capillaries in mice with streptozotocin-induced diabetes. Diabetologia. 2007;50:1987–1996. doi: 10.1007/s00125-007-0734-9. [DOI] [PubMed] [Google Scholar]
- 3.Joussen AM, Poulaki V, Le ML, Koizumi K, Esser C, Janicki H, et al. A central role for inflammation in the pathogenesis of diabetic retinopathy. FASEB journal. 2004;18:1450–1452. doi: 10.1096/fj.03-1476fje. [DOI] [PubMed] [Google Scholar]
- 4.Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Exp Diabetes Res. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Du Y, Sarthy VP, Kern TS. Interaction between NO and COX pathways in retinal cells exposed to elevated glucose and retina of diabetic rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R735–741. doi: 10.1152/ajpregu.00080.2003. [DOI] [PubMed] [Google Scholar]
- 6.Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. Am J Path. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vincent JA, Mohr S. Inhibition of caspase-1/interleukin-1beta signaling prevents degeneration of retinal capillaries in diabetes and galactosemia. Diabetes. 2007;56:224–230. doi: 10.2337/db06-0427. [DOI] [PubMed] [Google Scholar]
- 8.Takeda K, Akira S. TLR signaling pathways. Semin Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Mollen KP, Anand RJ, Tsung A, Prince JM, Levy RM, Billiar TR. Emerging paradigm: toll-like receptor 4-sentinel for the detection of tissue damage. Shock. 2006;26:430–437. doi: 10.1097/01.shk.0000228797.41044.08. [DOI] [PubMed] [Google Scholar]
- 10.Kizaki T, Shirato K, Sakurai T, Ogasawara JE, Oh-ishi S, Matsuoka T, et al. Beta2-adrenergic receptor regulate Toll-like receptor 4-induced late-phase NF-kappaB activation. Mol Immunol. 2009;46:1195–1203. doi: 10.1016/j.molimm.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Beutler B. Inferences, questions and possibilities in Toll-like receptor signalling. Nature. 2004;430:257–263. doi: 10.1038/nature02761. [DOI] [PubMed] [Google Scholar]
- 12.Wang YL, Wang K, Yu SJ, Li Q, Li N, Lin PY, et al. Association of the TLR4 signaling pathway in the retina of streptozotocin-induced diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2015;253:389–398. doi: 10.1007/s00417-014-2832-y. [DOI] [PubMed] [Google Scholar]
- 13.Tang J, Allen Lee C, Du Y, Sun Y, Pearlman E, Sheibani N, et al. MyD88-dependent pathways in leukocytes affect the retina in diabetes. PloS one. 2013;8:e68871. doi: 10.1371/journal.pone.0068871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajamani U, Jialal I. Hyperglycemia induces Toll-like receptor-2 and -4 expression and activity in human microvascular retinal endothelial cells: implications for diabetic retinopathy. Journal of diabetes research. 2014;2014:790902. doi: 10.1155/2014/790902. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Busik JV, Mohr S, Grant MB. Hyperglycemia-induced reactive oxygen species toxicity to endothelial cells is dependent on paracrine mediators. Diabetes. 2008;57:1952–1965. doi: 10.2337/db07-1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yego EC, Vincent JA, Sarthy V, Busik JV, Mohr S. Differential regulation of high glucose-induced glyceraldehyde-3-phosphate dehydrogenase nuclear accumulation in Muller cells by IL-1beta and IL-6. Invest ophthal & visl sci. 2009;50:1920–1928. doi: 10.1167/iovs.08-2082. [DOI] [PubMed] [Google Scholar]
- 17.Zhang Q, Jiang Y, Miller MJ, Peng B, Liu L, Soderland C, et al. IGFBP-3 and TNF-alpha Regulate Retinal Endothelial Cell Apoptosis. Invest ophthal & vis sci. 2013;54:5376–5384. doi: 10.1167/iovs.13-12497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker RJ, Anderson NM, Jiang Y, Bahouth S, Steinle JJ. Role of beta-adrenergic receptors regulation of TNF-alpha and insulin signaling in retinal Muller cells. Invest ophthal & vis sci. 2011;52:9527–9533. doi: 10.1167/iovs.11-8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin X, Fang D, Zhou H, Su SB. The expression of Toll-like receptors in murine Muller cells, the glial cells in retina. Neurol Sci. 2013;34:1339–1346. doi: 10.1007/s10072-012-1236-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yi H, Patel AK, Sodhi CP, Hackam DJ, Hackam AS. Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PloS one. 2012;7:e36560. doi: 10.1371/journal.pone.0036560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Link A, Selejan S, Maack C, Lenz M, Bohm M. Phosphodiesterase 4 inhibition but not beta-adrenergic stimulation suppresses tumor necrosis factor-alpha release in peripheral blood mononuclear cells in septic shock. Crit Care. 2008;12:R159. doi: 10.1186/cc7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Y, Guo Z, He W, Yang Y, Li Y, Zheng A, et al. Ephedrine hydrochloride protects mice from LPS challenge by promoting IL-10 secretion and inhibiting proinflammatory cytokines. International immunopharmacology. 2012;13:46–53. doi: 10.1016/j.intimp.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 23.Wang W, Xu M, Zhang YY, He B. Fenoterol, a beta(2)-adrenoceptor agonist, inhibits LPS-induced membrane-bound CD14, TLR4/CD14 complex, and inflammatory cytokines production through beta-arrestin-2 in THP-1 cell line. Acta Pharmacol Sin. 2009;30:1522–1528. doi: 10.1038/aps.2009.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Q, Guy K, Pagadala J, Jiang Y, Walker RJ, Liu L, et al. Compound 49b Prevents Diabetes-Induced Apoptosis through Increased IGFBP-3 Levels. Invest ophthal & vis sci. 2012;53:3004–3013. doi: 10.1167/iovs.11-8779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiang Y, Pagadala J, Miller D, Steinle JJ. Reduced insulin receptor signaling in retinal Muller cells cultured in high glucose. Molecular vision. 2013;19:804–811. [PMC free article] [PubMed] [Google Scholar]
- 26.Abcouwer SF, Lin CM, Shanmugam S, Muthusamy A, Barber AJ, Antonetti DA. Minocycline prevents retinal inflammation and vascular permeability following ischemia-reperfusion injury. Journal of neuroinflammation. 2013;10:149. doi: 10.1186/1742-2094-10-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang Y, Zhang Q, Soderland C, Steinle JJ. TNFalpha and SOCS3 regulate IRS-1 to increase retinal endothelial cell apoptosis. Cell Signal. 2012;24:1086–1092. doi: 10.1016/j.cellsig.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J, Hu X, Xie J, Xu W, Jiang H. Beta-1-adrenergic receptors mediate Nrf2-HO-1-HMGB1 axis regulation to attenuate hypoxia/reoxygenation-induced cardiomyocytes injury in vitro. Cell Physiol Biochem. 2015;35:767–777. doi: 10.1159/000369736. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Yang H, Hu X, Fu W, Xie J, Zhou X, et al. Dobutamine-mediated heme oxygenase-1 induction via PI3K and p38 MAPK inhibits high mobility group box 1 protein release and attenuates rat myocardial ischemia/reperfusion injury in vivo. J Surg Res. 2013;183:509–516. doi: 10.1016/j.jss.2013.02.051. [DOI] [PubMed] [Google Scholar]
- 30.Tsung A, Tohme S, Billiar TR. High-mobility group box-1 in sterile inflammation. Journal of internal medicine. 2014;276:425–443. doi: 10.1111/joim.12276. [DOI] [PubMed] [Google Scholar]
- 31.Santos AR, Dvoriantchikova G, Li Y, Mohammad G, Abu El-Asrar AM, Wen R, et al. Cellular mechanisms of high mobility group 1 (HMGB-1) protein action in the diabetic retinopathy. PloS one. 2014;9:e87574. doi: 10.1371/journal.pone.0087574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elena PP, Denis P, Kosina-Boix M, Saraux H, Lapalus P. Beta adrenergic binding sites in the human eye: an autoradiographic study. J Ocul Pharmacol. 1990;6:143–149. doi: 10.1089/jop.1990.6.143. [DOI] [PubMed] [Google Scholar]
- 33.Yin J, Huang J, Chen C, Gao N, Wang F, Yu FS. Corneal complications in streptozocin-induced type I diabetic rats. Invest ophthal & vis sci. 2011;52:6589–6596. doi: 10.1167/iovs.11-7709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinle JJ. Topical administration of adrenergic receptor pharmaceutics and nerve growth factor. Clin Ophthalmol. 2010;4:605–610. doi: 10.2147/opth.s10992. [DOI] [PMC free article] [PubMed] [Google Scholar]