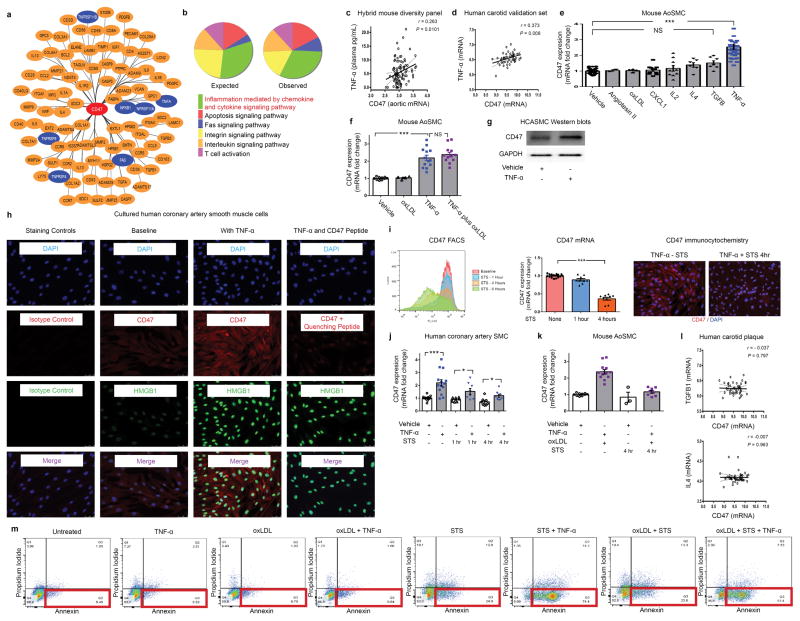
Extended Data Figure 7. Additional bioinformatic and experimental analyses further implicate a central role for the pro-inflammatory cytokine, TNF-α, in vascular CD47 signaling.
(a). Cytoscape network visualization of the genes which are significantly correlated with CD47 expression in both human and murine atherosclerotic plaque reveals a high number of TNF-α-related factors (indicated in blue), including ligands, receptors, and downstream signaling factors. (b). PANTHER pathway analysis of those genes which were (a) significantly associated with CD47 expression in mouse and human vascular tissue and (b) have been previously associated with atherosclerosis through the STAGE study33, identifies “inflammation mediated by chemokine and cytokine signaling pathway” as the most over-abundant pathway associated with CD47 expression in vascular tissue. (c). Using the Hybrid Mouse Diversity Panel (HMDP), which correlates aortic gene expression with Luminex cytokine array data of plasma samples from over 100 inbred strains of mice, we found that vascular CD47 expression is positively correlated with three inflammatory cytokines in vivo, including TNF-α, IL-2 and CXCL1. Correlation data shown for CD47 and TNF-α. (d). Co-expression studies confirm that TNF-α and CD47 expression are positively correlated in human carotid endarterectomy samples from the BiKE validation study. The Pearson correlation coefficient was determined assuming a Gaussian distribution and P values were determined using a two-tailed test. (e). Experiments with primarily cultured mouse aortic SMCs indicate that TNF-α reproducibly induces CD47 mRNA upregulation, while a number of other classical pro-atherosclerotic stimuli have no significant effect. Notably, CXCL1, IL4, TGFβ and IL-2 fail to induce CD47 expression in vitro, as assessed by ANOVA. (f). Additional studies suggest that the effect of TNF-α on CD47 expression persists in the presence of oxidized LDL, as occurs in the atherosclerotic plaque. (g). Western blotting confirms that TNF-α induces CD47 expression in vascular cells at the protein level. For gel source data, see Supplementary Figure 1. (h). Immunocytochemistry studies of HCASMCs confirm that CD47 expression is induced on the cell surface of TNF-α treated cells. TNF-α effect is assessed by co-staining for HMGB1, and antibody specificity is confirmed with isotype control and recombinant CD47 peptide quenching assays. (i). Multiple assays (including FACS, Taqman and immunocytochemistry studies) reveal that CD47 expression is downregulated on vascular SMCs during programmed cell death, as has previously been observed with inflammatory cells. (j). Confirmatory assays in cultured human coronary artery SMC reveal that TNF-α induces changes similar to those observed in murine cells (Fig 3D), including an induction of CD47 under physiological conditions and a blunting of its expected downregulation during apoptosis. (k). TNF-α’s capacity to impair CD47 downregulation during programmed cell death is also observed in mouse SMCs simultaneously exposed to pro-apoptotic stimuli and oxidized LDL. (l). No correlation between CD47 and other candidate cytokines was observed in the BiKE biobank, further supporting a specific relationship between CD47 and TNF-α. (m). Representative FACS-based apoptosis panels from cells exposed to the conditions used in Fig 3G confirm that TNF-α suppresses efferocytosis (Fig 3G) despite increasing programmed cell death. Comparisons made by two-tailed t tests, unless otherwise specified. *** = P < 0.001, * = P < 0.05. Error bars represent the SEM.