Abstract
Few studies have explored the relationship between coarse particles (PM10-2.5) and adverse birth outcomes. We examined associations between gestational exposure of PM10-2.5 and birth weight. U.S. birth certificates data (1999-2007) were acquired for 8,017,865 births. Gestational and trimester exposures of PM10-2.5 were estimated using co-located PM10 and PM2.5 monitors ≤ 35 km from the population-weighted centroid of mothers’ residential counties. A linear regression model was applied, adjusted by potential confounders. As sensitivity analyses, we explored alternative PM10-2.5 estimations, adjustment for PM2.5, and stratification by regions. Gestational exposure to PM10-2.5 was associated with 6.6 g (95% Confidence Interval: 5.9, 7.2) lower birth weight per interquartile range increase (7.8 μg/m3) in PM10-2.5 exposures. All three trimesters showed associations. Under different exposure methods for PM10-2.5, associations remained consistent but with different magnitudes. Results were robust after adjusting for PM2.5, and regional analyses showed associations in all four regions with larger estimates in the South. Our results suggest that PM10-2.5 is associated with birth weight in addition to PM2.5. Regional heterogeneity may reflect differences in population, measurement error, region-specific emission pattern, or different chemical composition within PM10-2.5. Most countries do not set health-based standards for PM10-2.5, but our findings indicate potentially important health effects of PM10-2.5.
Keywords: air pollution, birth weight, coarse PM, particulate matter
1. Introduction
In scientific research and policy, particulate matter (PM) is often defined by size range, but not chemical constituents. There are many size types of PM (PM with aerodynamic diameter ≤ 10 μm (PM10), ≤ 2.5 μm (PM2.5), etc.), and many studies reported associations between these PM metrics and adverse health outcomes, including pregnancy outcomes, across the world. For instance, gestational exposure to PM10 and PM2.5 were associated with low birth weight in U.S. and Europe (1, 2). Furthermore, short-term exposure to PM10 and PM2.5 were associated with mortality and morbidity (3, 4).
Consistent findings have accumulated for linking PM10 and PM2.5 with health, as summarized in systematic reviews (5-7), and the U.S. Environmental Protection Agency (EPA) set National Ambient Air Quality Standards (NAAQS) for these PM metrics. On the other hand, coarse particulate matter (PM with aerodynamic diameter between 2.5 μm and 10 μm: PM10-2.5) is not directly regulated (although is indirectly regulated through the separate regulations for PM10 and PM2.5). While EPA concluded that scientific evidence of PM10-2.5 and health outcomes are suggestive of a causal relationship, the agency notes that the number of studies is limited, and knowledge of biological plausibility is insufficient (8).
Several multi-city studies have been performed for short-term exposure to PM10-2.5 and various health outcomes. While many studies identify associations, findings are inconsistent. Peng et al. did not find associations of PM10-2.5 with cardiovascular and respiratory disease hospital admissions in 108 U.S. counties after controlling for PM2.5 (9). However, Powell et al., with additional data to the study by Peng et al., observed increased cardiovascular hospitalization in association with PM10-2.5 (10). A study using 47 U.S. counties found that PM10-2.5 was associated with mortality (11), but a study conducted in 12 European cities did not observe a statistically significant association with mortality (12).
Only a few studies have explored birth outcomes in association with PM10-2.5. Three of them examined a single city or state (i.e. Florida, Atlanta, and Barcelona) (13-15), and only one national study has been conducted (16). Results of these studies are inconsistent and they evaluated different exposure windows. EPA concluded that “evidence is inadequate to determine if a causal relationship exists between long-term exposure to PM10-2.5 and developmental and reproductive outcomes,” and that further studies are warranted (8).
One of the challenging issues of PM10-2.5 is how to estimate PM10-2.5 levels. A direct measurement using dichotomous samplers is preferred. There are several studies used that sampling method, but the monitors were set by investigators (17, 18). Therefore, such an approach may be practical for single-city study, but not for a multi-city study. EPA does not measure PM10-2.5 directly, resulting in PM10-2.5 estimation based on the difference of the same location monitor of PM10 and PM2.5 (co-located monitor) values for many multi-city studies (9, 10). Another approach is averaging county-wide PM10 and PM2.5 levels including nearby monitors that are not co-located monitors, and taking the difference (11, 16). Both approaches have strengths and limitations. The number of co-located monitor varies by time and location, which impacts the former approach. Spatial heterogeneity of PM10 and PM10-2.5, especially PM10-2.5, is a potential concern in the latter approach (19-21). This could be particularly problematic in the western U.S., where the size of counties can be large compared to the east, and air monitor networks are far more disperse.
We explored associations between PM10-2.5 gestational exposure and birth weight. We investigated the robustness of associations by different exposure approaches for PM10-2.5 and by adjustment for PM2.5 exposure. Analysis stratified by geographical region was conducted. Previous studies found heterogeneous associations of PM on health outcomes (3, 16). Birth weight is an important predictor for mortality and morbidity during childhood (1), therefore it is important to investigate whether exposure to PM10-2.5 is associated with birth weight. As scientific evidence of linking PM10-2.5 with adverse health, particularly birth weight, is limited, our study will inform further understanding of PM10-2.5. Further, as direct measurement of PM10-2.5 is not available nationwide, our sensitivity analysis of different PM10-2.5 exposure estimations adds important insights on exposure approaches.
2. Material and methods
2.1 Birth data
Birth certificate data of the U.S. mainland from January 1999 to December 2007 were obtained from the National Center for Health Statistics. Data included variables relating to mothers and infants, such as maternal residential county at delivery, gestational length, and birth weight. We also obtained variables relating county socio-economic status (SES) from 2000 Census and 2006 American Community Survey to represent neighborhood SES: percentage of educational attainment less than a high school degree for population ≥25 years, and percentage of households with income below the poverty line as defined by family income and size and price index (22).
Delivery date was estimated based on last menstrual period (LMP) and gestational length, assuming conception two weeks after LMP. If the estimated delivery date was >30 days from the midday of the birth month reported on the birth certificate, that birth was excluded from analysis. Furthermore, births were excluded from analysis if gestational age was <37 or >44 weeks, plural deliveries, or impossible gestational age and birth weight combinations (23). These selection criteria were used in previous studies, and further description is available elsewhere (24, 25).
2.2 Exposure assessment
PM2.5 and PM10 monitor values from January 1998 to December 2007 were obtained from the U.S. EPA Air Quality Systems networks. We excluded monitors if their observation frequency is less than 120 days within the study period or if the observation period (time between first and last observation within the study period) is less than one year, to avoid seasonal bias. PM10-2.5 level was estimated by subtracting the PM2.5 value from PM10 value at the co-located monitor on the same day. PM10-2.5 values were removed from data if calculated values were negative (6.7%).
Each day, PM10-2.5 exposure levels were assigned to counties, based on the nearest co-located PM10 and PM2.5 monitors within 35 km of the county’s population weighted centroid, which was calculated using the Census 2010 population. The 35 km buffer distance was based on assessments of spatial correlation across PM10 concentrations calculated with semivariograms. We found that for more than 75% of days, the minimum semivariance among monitor pairs fell within a 0 to 40km distance. Because our exposure of interest is PM10-2.5, ideally the initial buffer size for analyses would be determined based on PM10-2.5 concentrations. However, due to the sparse number of co-located monitors, we lack the power to calculate spatial dependence using semivariograms. Therefore, we determined the buffer size using estimated semivariance of PM10, which is more heterogeneous compared to PM2.5 (26).
Gestational and trimester exposures of PM10-2.5 were estimated based on residential county of the mother at time of birth. First, second, and third trimester were defined as 1-13 weeks, 14-26 weeks, and week 27 to delivery, respectively. We first estimated weekly-averaged PM10-2.5 levels, which were calculated to avoid observational frequency bias (1, 24). Gestational and trimester PM10-2.5 levels were estimated by averaging weekly levels. Births were excluded if more than 25% of weekly-averaged PM10-2.5 levels were unavailable in any trimester. This will prevent seasonally biased air pollution sampling (1). Counties were excluded if the available length of data (time between first and last daily PM10-2.5 estimate in that county) was less than 4 years or the number of births within that county during the study period is less than 100, so that we exclude counties with a short observation period. To control for overall weather discomfort, apparent temperature for each trimester was calculated following same approach used to estimate trimester PM10-2.5 exposures. Daily apparent temperature was estimated by combing daily temperature and dew point, which were obtained from closest weather station from county’s population-weighted centroid (27).
2.3 Statistical analysis
A linear model with birth weight as a continuous variable was used. We explored separate models of 1) PM10-2.5 gestational exposure, and 2) PM10-2.5 trimester exposures: all three trimester exposures included simultaneously. Adjusted variables include sex of infant; gestational length; maternal age, race, educational attainment, and marital status; birth order; the trimester in which prenatal care began; alcohol and/or tobacco consumption during pregnancy; indicator variables of birth year, season, and state; neighborhood SES (% less than high school education and % of households below poverty line); and apparent temperature for each trimester. When these variables were missing on birth certificate, we labeled as unknown and included in the analyses. The statistical model and covariates are chosen following our earlier studies (1, 24). For the trimester exposures model, we conducted a trimester residual model to address correlation of exposures among trimesters. In brief, a specific trimester exposure level was used to predict the remaining trimester exposures. We took residuals of actual exposure levels from predicted values, and included them in the model in addition to the referenced trimester. Detail explanations of the trimester residual model can be found elsewhere (1, 28).
A number of sensitivity analyses were conducted. First, we applied two alternative methods of estimating PM10-2.5 (PM10-2.5_alt1 and PM10-2.5_alt2). For daily PM10-2.5alt1 values, we averaged available co-located monitors within the county; an approach that has been applied in many previous births outcome studies for estimating gestational exposures for air pollutants (29, 30). As another approach, PM10-2.5_alt2, we subtracted the daily county average PM2.5 from the daily county average PM10, regardless of whether the monitors were co-located. Several studies used this definition to explore relationships with adverse health outcomes (11, 16). Second, we explored whether the gestational PM10-2.5 association, using our original PM10-2.5 exposure estimate, is robust to adjustment by gestational exposure of PM2.5 by adding this variable to the main model. Gestational exposure of PM2.5 was estimated in the same way as PM10-2.5 exposure. Furthermore, we stratified the analysis by region (East, South, North, and West), based on U.S. EPA regions (Supplementary Figure 1) (31). In addition to the 35 km buffer, we performed analyses with two other buffer sizes (20 and 50 km). Moreover, instead of removing negative values of PM10-2.5, we retained these negative values and included them in the analyses. For comparison purpose, all results of PM10-2.5 associations were expressed in birth weight change (g) per interquartile range (IQR) increase in gestational PM10-2.5 exposures using the original PM10-2.5 estimation.
3. Results
There were 12,066,006 births, who had at least one PM10-2.5 estimation. Among them, 102,844 births (0.8%) were excluded, because their estimated delivery date was >30 days from the midday of the birth month on the birth certificate. Birth exclusion criteria (e.g. plural, preterm births, etc.) omitted 1,951,164 births (14.3%), and 2,245,421 births (16.5%) did not meet air pollution criteria (i.e. missing > 25% PM10-2.5 observations in any trimester). More than one exclusion criteria could be applied for some births. After applying these exclusion criteria, analysis included 8,017,865 births from 224 counties. Average birth weight was 3,394.0 g (standard deviation (SD): 467.9), and mean PM10-2.5 and PM2.5 levels during gestation were 13.7 μg/m3 (SD: 5.7, IQR: 7.8) and 12.9 μg/m3 (SD: 3.6, IQR: 5.3), respectively. Correlation between gestational exposures of PM10-2.5 and PM2.5 was 0.11. Distribution of pregnancy-related variables is summarized in Table 1. The population included 720,670 births from the East (43 counties), 2,054,373 from the North (73 counties), 1,992,041 from the South (58 counties), and 3,250,781 from the West (50 counties) region. Supplementary Table 1 shows summary statistics, including PM10-2.5 and PM2.5 gestational exposure, stratified by region.
Table 1.
Summary Statistics of Population and Exposures
| Total Number of Births | 8,017,865 | |
|
| ||
| PM Gestational Exposures | ||
|
| ||
| PM10-2.5 Gestational Exposurea | mean (SD) | 13.72 μg (5.67) |
|
| ||
| PM2.5 Gestational Exposure | mean (SD) | 12.87 μg (3.58) |
|
| ||
| Mother and Infant Characteristics | ||
|
| ||
| Birth Weight | mean (SD) | 3,394.0 g (467.9) |
|
| ||
| 37-38 weeks | 2,421,380 (30.2%) | |
| Gestational Length | 39-40 | 4,185,847 (52.2%) |
| 41-44 | 1,410,638 (17.6%) | |
|
| ||
| <20 years | 841,790 (10.5%) | |
| 20-24 | 1,976,411 (24.7%) | |
| Maternal Age | 25-29 | 2,175,054 (27.1%) |
| 30-34 | 1,884,789 (23.5%) | |
| 35-39 | 934,974 (11.7%) | |
| >40 | 204,847 (2.6%) | |
|
| ||
| White | 6,149,190 (76.7%) | |
| Maternal Race | Black | 1,221,968 (15.2%) |
| Other | 646,707 (8.1%) | |
|
| ||
| Less than High School | 1,980,837 (24.7%) | |
| High School | 2,195,200 (27.4%) | |
| Maternal Educational Attainment | Some College | 1,671,202 (20.8%) |
| College | 2,037,459 (25.4%) | |
| Unknown | 133,167 (1.7%) | |
|
| ||
| Maternal Marital Status | Married | 5,050,811 (63.0%) |
| Unmarried | 2,967,054 (37.0%) | |
|
| ||
| Infant's Sex | Male | 4,079,206 (50.9%) |
| Female | 3,938,659 (49.1%) | |
|
| ||
| First Birth | 2,749,952 (34.3%) | |
| Parity | Second or Later Birth | 5,243,384 (65.4%) |
| Unknown | 24,529 (0.3%) | |
|
| ||
| First | 6,498,200 (81.0%) | |
| Second | 1,088,782 (13.6%) | |
| Trimester Prenatal Care Begin | Third | 227,415 (2.8%) |
| Never | 71,847 (0.9%) | |
| Unknown | 131,621 (1.6%) | |
|
| ||
| Yes | 489,034 (6.1%) | |
| Tobacco Consumption During Pregnancy | No | 5,177,739 (64.6%) |
| Unknown | 2,351,092 (29.3%) | |
|
| ||
| Alcohol Consumption During Pregnancy | Yes | 32,248 (0.4%) |
| No | 4,154,003 (51.8%) | |
| Unknown | 3,831,614 (47.8%) | |
|
| ||
| SES Levels of Residential County | ||
|
| ||
| Less than High School Education (%) | mean (SD) | 18.5 % (6.4) |
|
| ||
| Below Poverty Line (%) | mean (SD) | 13.8 % (4.3) |
PM10-2.5 is based on original estimation.
Our main model showed association between gestational PM10-2. 5 exposure and birth weight. Birth weight decreased 6.6 g (95% Confidence Interval (CI): 5.9, 7.2) per IQR increase (7.8 μg/m3) of PM10-2.5 gestational exposure (Table 2). Sensitivity analysis using alternative estimations of PM10-2.5 also exhibited associations, although with different magnitudes. An IQR increase of PM10-2.5_alt1 and PM10-2.5_alt2 were associated with lowering birth weight by 10.4g (95% CI: 9.6, 11.2) and 3.4 g (95% CI: 2.8, 4.1), respectively. Correlation between original PM10-2.5 estimation and PM10-2.5_alt1, and original PM10-2.5 estimation and PM10-2.5_alt2 were 0.90 and 0.76, respectively. The association between PM10-2.5 and birth weight was robust after adjusting for PM2.5 gestational exposure: 5.4 g (95% CI: 4.7, 6.1). In same model, exposure to PM2.5 also showed an association with birth weight: 4.4 g (95% CI: 3.6, 5.2) decrease of birth weight per IQR increase (5.3 μg/m3) in PM2.5 gestational exposure. We also observed an association for PM2.5 gestational exposure in a model without PM10-2.5: 6.4 g (95% CI: 5.6, 7.1).
Table 2.
Change in Birth Weight (95% CI) per IQR Increase of PM10-2.5
| Pollutant | N | Exposure Period | Change in Birth Weight (g)a |
|---|---|---|---|
| Whole Gestation | −6.6 (−7.2, −5.9) | ||
| PM10-2.5 | 8,017,865 | 1st trimester | −2.0 (−2.6, −1.3) |
| 2nd trimester | −2.1 (−2.8, −1.4) | ||
| 3rd trimester | −2.6 (−3.3, −2.0) | ||
|
| |||
| PM10-2.5_alt1b | 8,799,256 | Whole Gestation | −10.4 (−11.2, −9.6) |
|
| |||
| PM10-2.5_alt2c | 10,597,927 | Whole Gestation | −3.4 (−4.1, −2.8) |
|
| |||
| Whole Gestation | −5.4 (−6.1, −4.7) | ||
| PM10-2.5 (adjusted by PM2.5) | 8,017,865 | 1st trimester | −1.5 (−2.2, −0.8) |
| 2nd trimester | −1.8 (−2.5, −1.1) | ||
| 3rd trimester | −2.1 (−2.8, −1.5) | ||
IQRs are 7.8 μg/m3 for PM10-2.5; for comparison purpose, the same IQR values were applied for PM10-2.5, PM10-2.5_alt1, and PM10-2.5_alt2 estimates. Included adjusted variables were sex of infant, gestational length, maternal age, race, educational attainment, and marital status, birth order, the trimester in which prenatal care began, alcohol and/or tobacco consumption during pregnancy, indicator variable of birth year, season, and state, neighborhood SES (% less than high school education and % of households below poverty line), and apparent temperature for each trimester.
PM10-2.5_alt1 is estimated by averaging the PM10-2.5 values estimated only from co-located monitors within a county.
PM10-2.5_alt2 is based on daily estimates based on using all PM monitors to estimate county levels of PM10 and PM2.5 separately and then subtracting them.
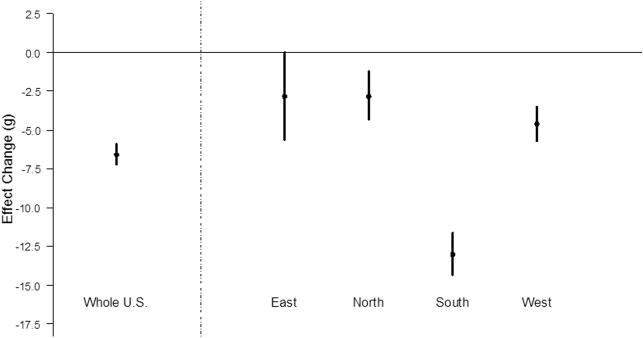
Associations were found in all three trimesters (Table 2) with a 2.0 g (95% CI: 1.3, 2.6), 2.1 g (95% CI: 1.4, 2.8), and 2.6 g (95% CI: 2.0, 3.2) decrease in birth weight per IQR increase (7.8 μg/m3) for the first, second, and third trimester PM10-2.5 exposures, respectively. Correlations among trimester exposures are 0.61 to 0.74. Results were consistent under the trimester residual model (results not shown). Results of the trimester model were robust after controlling for PM2.5 trimester exposures: the birth weight decrease per IQR increase in PM10-2.5 exposure was 1.5 g (95% CI: 0.8, 2.2), 1.8 g (95% CI: 1.1, 2.5), and 2.1 g (95% CI: 1.5,2.8) for the first, second, and third trimesters, respectively (Table 2). Figure 1 shows results stratified by region. All four regions indicated reductions in birth weight by PM10-2.5 gestational exposure, and estimates were particularly high in South (14.3g (95% CI: 13.0, 15.7)). Applying different buffer sizes showed similar results: 4.9g (95% CI: 5.6, 6.3) for the 20 km buffer, and 6.5g (95% CI: 5.8, 7.2) for the 50 km buffer. Similarly, replacing negative values to zero did not impact our results: 5.8g (95% CI: 5.3, 6.3).
Figure 1.
Associations between PM10-2.5 and Birth Weight: Overall and Stratified by Region. Change in birth weight (g) per IQR increase (7.8 μg/m3) of PM10-2.5 gestational exposure. For comparison purpose, the same IQR values were applied to estimates in all regions. The points represent the central estimated effects, and the vertical lines the 95% CIs.
4. Discussion
While evidence of PM10 and PM2.5 impacts on birth outcomes have been frequently reported (1, 15), far less research is known regarding the potential health burden of PM10-2.5 on birth weight. To our knowledge, this research is the largest national study investigating the association between PM10-2.5 and birth outcomes to date. Our results indicated that gestational exposure to PM10-2.5, as well as exposure in all three trimesters, was associated with lower birth weight. The association was retained after adjusting for PM2.5. Notably, PM2.5 was also associated with birth weight in a two-pollutant model, suggesting that PM10-2.5 and PM2.5 are independent risk factors for lower birth weight. While PM10 and PM2.5 are regulated by many government agencies, most of them, including EPA, do not set a health-based standard for PM10-2.5. Our findings indicate that mothers living in high PM10-2.5 area may have higher risk of experiencing adverse birth outcomes.
In this study, heterogeneous effect estimates were found by region, with particularly high estimates in the Southern U.S. (Figure 1). This might be explained by exposure measurement error (32), or differences in populations by region, such as BMI (33). Alternatively, this may result from differences in PM10-2.5 chemical constituents, which relates to the sources. Other work has demonstrated that the distribution of PM2.5 chemical constituents differ by region (34), which is linked to heterogeneous health effect estimates for PM2.5 (11, 24, 30). A large portion of PM10-2.5 chemical constituents is crustal elements (i.e. aluminum, silicon etc.) and organic carbon (OC) (35-38). However, PM2.5’s detailed spatial-temporal distribution throughout the U.S. remains unknown, because a U.S. national monitoring network for PM10-2.5 chemical constituents’ concentrations or for PM10-2.5 total mass does not exist. There are a few studies that have explored the spatial-temporal distribution of PM10-2.5 in selected cities. For instance, a recent EPA pilot study revealed the distribution for selected locations (8). The study measured PM10-2.5 and selected PM10-2.5 chemical constituents in Phoenix, Arizona, and East St. Louis, Illinois, from June 2010 to May 2011. The annual average of PM10-2.5 total mass value was much higher in Phoenix (18.7 μg/m3) than East St. Louis (9.4 μg/m3). Local environments and sources could contribute to difference in PM10-2.5 composition with higher silicon concentrations observed in Phoenix and higher OC in East St. Louis. Phoenix is surrounded by desert, while emissions from traffic and industrial plants, sources of OC, play a major role in East St. Louis (34). Heterogeneous PM10-2.5 levels in 20 European areas were also observed in ESCAPE project (39). Furthermore, variability of PM10-2.5 and its chemical constituents was observed across cities and within cities according to a study conducted in three U.S. cities (40). Further data collection across U.S. is needed to better understanding for seasonal variations within a city or between cities.
A U.S. national study by Parker et al. explored associations between gestational/trimester exposures to PM10-2.5 and PM10 with birth weight (16). Birth weight was associated with PM10-2.5 for gestational and all three trimester exposures. For instance, their result showed that gestational exposure to PM10-2.5 was associated with 9.9 g (95% CI: 5.8, 14.0) lower birth weight per our IQR increase, which is higher than our estimate (6.6 g (95% CI: 5.9, 7.2)). These associations were robust after controlling for PM2.5, although their PM2.5 estimate showed protective associations with birth weight. The study by Parker et al. also conducted regional stratification analyses, but findings differed from ours. They did not find associations in some regions, and the association was the strongest in Northwest, which corresponds to the northern part of our West region plus the western part of our North region, whereas we observed associations in all regions with the highest estimate in the South region. Several factors likely contributed to the varying results. We only used monitors with observation frequency ≥120 days and observation period ≥1 years to avoid seasonally biased sampling. Our study period was longer than the Parker et al. study (9 years vs. 3 years), leading to more subjects and counties available for analyses. Counties included in analyses were different, which could lead to a different distribution of PM10-2.5 chemical constituents. Finally, adjusted variables were different: we included gestational length, neighborhood SES variables, etc.
A few other studies have explored the relationship between PM10-2.5 and birth outcomes (13-16). Gestational exposure to PM10-2.5 was linked to increased risk of low birth weight in Florida (13). A study in Atlanta found that third trimester exposure to PM10-2.5 was associated with lower birth weight for non-Hispanic Blacks and Hispanics, but not for non-Hispanic Whites (14). They did not investigate the association with gestational, first trimester, or second trimester exposures. Dadvand et al. conducted a study in Barcelona, Spain, and found an association between third trimester exposure to PM10-2.5 and low birth weight (15); however, this association became null after adjusting for proximity to major roads.
Attention should be paid to the estimation of PM10-2.5, as the methods differ by studies. Our original estimation was calculated by subtracting daily PM2.5 values from PM10 values at the nearest co-located monitor within 35 km from the residential county’s population weighted centroid. In Parker et al., PM10-2.5 was based on daily values calculated as the difference between county averaged PM10 and PM2.5, which corresponds to our PM10-2.5_alt2. Both PM10-2.5 estimations showed associations with lower birth weight in our analyses, but with different estimates (Table 2). Our method of PM10-2.5_alt1, similar to PM10-2.5_alt2 but only using co-located monitors, was applied in handful studies (9, 10). Results using PM10-2.5_alt1 supported associations, but magnitudes differed. Both PM10-2.5_alt1 and PM10-2.5_alt2 have limitations; exposure misclassification is a problematic in large counties for PM10-2.5_alt1, and inaccurate measurement for PM10-2.5 due to different sampling locations for PM10 and PM2.5 is an issue for PM10-2.5_alt2. EPA recommends direct measurement of PM10-2.5 using dichotomous sampling method, which minimizes the measurement error by segregating fine and coarse particles onto separate filters (8, 41). EPA regards the direct measurements of PM10-2.5 as preferable to estimations based on PM10 and PM2.5 measurements due to uncertainties (8, 42). Nevertheless, most of current PM10-2.5 literatures rely on PM10-2.5 estimation due to limited availability of PM10-2.5 dichotomous sampling (9, 11).
Our original PM10-2.5 estimation, based on co-located monitor, avoids spatial exposure misclassification. The spatial heterogeneity of pollutants can vary by pollutant (e.g., PM10, PM2.5, and PM10-2.5) (19, 25, 40). Supplementary Figure 2 presents correlations of PM2.5 or PM10 monitor pairs against distances between monitors. The plot indicated more homogeneous levels of PM2.5 over long distances compared to PM10, which exhibited higher heterogeneity over short distances. Although the spatial heterogeneity of PM10-2.5 is not fully understood due to the lack of a large ambient monitoring network, this study only assigned PM10-2.5 exposure estimates to counties with population weighted centroid ≤ 35 km from the co-located monitor to minimize exposure misclassification. Using other buffer sizes (20 and 50 km) showed similar results, though results should be interpreted with caution since population characteristics could differ by different buffer sizes (25). Nevertheless, some exposure misclassification may exist due to the spatial heterogeneity of PM10-2.5, measurement error, and relying on to ambient monitors, which do not account for individual behavior patterns. Additional research is needed to better understand the implications of methods to estimate PM10-2.5 exposure and their implications for exposure misclassification, health effects, etc.
The lack of direct measurements for PM10-2.5 contributes to key limitations for all large studies on PM10-2.5 including this study. For instance, calculated PM10-2.5 values were occasionally negative as mentioned above, indicating measurement error. However, according to EPA committee, negative values for PM10-PM2.5 are not a serious concern if they constitute a small fraction of data and are spatially unbiased (43). In our analysis, we removed negative PM10-2.5 dates from data, and results were similar when these negative values were included in analysis. A further challenge is that the number of U.S. air quality monitors has declined over recent years for several states (e.g. New York), particularly for PM10, hindering the estimation of PM10-2.5: PM10 monitors in U.S. decreased from 1,055 in 1999 to 872 in 2007. In fact, available monitor numbers were less in East region compared to other regions (Supplementary Table 1), resulting in less subjects in this region.
These challenges in assessing exposure likely contribute to the lack of studies on this topic, which also highlights the need for such research. We addressed these limitations by investigating multiple exposure estimations, and identified associations under all methods. Still, there is a possibility that PM10-2.5 served as surrogates for other unmeasured pollutants. Our study also faces limitations that are typical of national studies. For example, we could only access to limited maternal information; we cannot adjust maternal BMI or neighborhood SES levels in census tract unit. We also cannot access maternal residential history; some mothers could move during pregnancy, leading to exposure misclassification. A recent study, however, found that changes in effect estimates were negligible even after taking account for maternal mobility (44). Second, attention should be paid to some variables (e.g. tobacco and alcohol consumption) on birth certificate for their reliability and validity (45). The reliability and validity of these variables are not fully known, but one study concluded that variables on birth certificate are adequate to use for adjustment purpose (46). Indeed, associations between PM10-2.5 and birth weight were retained after excluding mothers with smoking and/or alcohol consumption status during pregnancy (results now shown). Another limitation is that the monitors used in this study are not equally distributed and tend to be located in urban areas (47). Therefore, mothers who are exposed to higher pollutants than others are more likely to be included in our analyses. In addition, average birth weight for births within the 35km buffer (3394.0g) is slightly lower than for those who lived outside the buffer (3,403.4g), and maternal characteristics and SES are also different (25). Depending on the population patterns, selection of buffer size can introduce selection bias; however, we present analyses with two other buffer sizes (20 and 50 km), and results were robust.
Several plausible biological mechanisms have been proposed for an impact of PM10-2.5 on birth weight. Animal experiments found that exposure to PM10-2.5 evokes lung inflammation (48, 49), and the estimate of PM10-2.5 was stronger than that of PM2.5 per mass (50). Inflammation may change blood viscosity, which eventually alters placenta vascular function (51). An alternative explanation is that nutrition transfer and/or placenta development is prevented by placental mitochondrial alterations, which could be caused by PM10-2.5 exposure (52). Exposure to PM10-2.5 induces oxidative stress, which may contribute to lower birth weight (53). Similar to PM2.5 chemical constituents, various combinations of PM10-2.5 chemical constituents may involve different biological pathway in different trimesters (24, 25). Further investigations are necessary to investigate potential biological mechanism for PM10-2.5 exposure and birth outcomes.
A handful of studies explored associations of PM10-2.5 with mortality and morbidity outcomes (10, 54), but studies linking to adverse birth outcomes remain scarce and inconclusive (14, 16). Our study found that exposures to PM10-2.5 during gestation and all three trimesters are associated with lower birth weight among term birth infants, in addition to the association of PM2.5 and birth weight. Heterogeneous results by region could relate to region-specific emission patterns and different PM10-2.5 chemical constituents, as well as exposure measurement error and differences in populations. PM10-2.5 is not directly regulated, but our findings indicate potentially important health effects of PM10-2.5. Since every pregnant woman is at risk, exposure to PM10-2.5 is a public health concern. Research on PM10-2.5 has been hindered by the lack of widespread direct measurement of PM10-2.5. Future studies may apply further alternative estimation (e.g. combining satellite imagery) or sophisticated statistical modeling, such as generalized linear mixed models. As the U.S. EPA prepares to expand the PM10-2.5 monitoring network, which measure PM10-2.5 total mass and chemical constituents (8), additional research could evaluate the results identified here. This will shed light on spatio-temporal distribution of PM10-2.5, and aid identification of the regions and populations with higher health risks.
Supplementary Material
Highlights.
Only a few studies have explored birth outcomes in association with PM10-2.5
We explored associations between PM10-2.5 gestational exposure and birth weight
PM10-2.5 is associated with birth weight in addition to PM2.5
Our findings indicate potentially important health effects of PM10-2.5
Acknowledgement
Grants/ Financial Support: This work was supported by U.S. Environmental Protection Agency (EPA RD 83479801); and National Institute for Environmental Health Sciences at the National Institute of Health (NIEHS R01ES019560, R01ES019587)
Abbreviation
- EPA
U.S. Environmental Protection Agency
- IQR
interquartile range
- LMP
last menstrual period
- NAAQS
National Ambient Air Quality Standards
- PM
particulate matter
- PM10
particulate matter with aerodynamic diameter ≤ 10 μm
- PM10-2.5
coarse particulate matter
- PM2.5
particulate matter with aerodynamic diameter ≤ 2.5 μm
- OC
organic carbon
- SES
socio-economic status
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: None declared.
Contributor Information
Keita Ebisu, Email: keita.ebisu@aya.yale.edu.
Jesse D. Berman, Email: jesse.berman@yale.edu.
Michelle L. Bell, Email: michelle.bell@yale.edu.
References
- 1.Bell ML, Ebisu K, Belanger K. Ambient air pollution and low birth weight in Connecticut and Massachusetts. Environ Health Perspect. 2007;115(7):1118–24. doi: 10.1289/ehp.9759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pedersen M, Giorgis-Allemand L, Bernard C, et al. Ambient air pollution and low birthweight: a European cohort study (ESCAPE) The Lancet Respiratory medicine. 2013;1(9):695–704. doi: 10.1016/S2213-2600(13)70192-9. [DOI] [PubMed] [Google Scholar]
- 3.Peng RD, Dominici F, Pastor-Barriuso R, et al. Seasonal analyses of air pollution and mortality in 100 US cities. American journal of epidemiology. 2005;161(6):585–94. doi: 10.1093/aje/kwi075. [DOI] [PubMed] [Google Scholar]
- 4.Stafoggia M, Samoli E, Alessandrini E, et al. Short-term associations between fine and coarse particulate matter and hospitalizations in Southern Europe: results from the MED-PARTICLES project. Environmental health perspectives. 2013;121(9):1026–33. doi: 10.1289/ehp.1206151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adar SD, Filigrana PA, Clements N, et al. Ambient Coarse Particulate Matter and Human Health: A Systematic Review and Meta-Analysis. Curr Environ Health Rep. 2014;1:258–74. doi: 10.1007/s40572-014-0022-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atkinson RW, Kang S, Anderson HR, et al. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69(7):660–5. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lamichhane DK, Leem JH, Lee JY, et al. A meta-analysis of exposure to particulate matter and adverse birth outcomes. Environ Health Toxicol. 2015;30:e2015011. doi: 10.5620/eht.e2015011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. EPA . Pilot Study on Coarse PM Monitoring. US Environmental Protection Agency; Washington, DC: 2015. EPA-454/R-15-001. [Google Scholar]
- 9.Peng RD, Chang HH, Bell ML, et al. Coarse particulate matter air pollution and hospital admissions for cardiovascular and respiratory diseases among Medicare patients. Jama. 2008;299(18):2172–9. doi: 10.1001/jama.299.18.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powell H, Krall JR, Wang Y, et al. Ambient Coarse Particulate Matter and Hospital Admissions in the Medicare Cohort Air Pollution Study, 1999-2010. Environmental health perspectives. 2015 doi: 10.1289/ehp.1408720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zanobetti A, Schwartz J. The effect of fine and coarse particulate air pollution on mortality: a national analysis. Environmental health perspectives. 2009;117(6):898–903. doi: 10.1289/ehp.0800108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samoli E, Stafoggia M, Rodopoulou S, et al. Associations between fine and coarse particles and mortality in Mediterranean cities: results from the MED-PARTICLES project. Environmental health perspectives. 2013;121(8):932–8. doi: 10.1289/ehp.1206124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salihu HM, Ghaji N, Mbah AK, et al. Particulate pollutants and racial/ethnic disparity in feto-infant morbidity outcomes. Maternal and child health journal. 2012;16(8):1679–87. doi: 10.1007/s10995-011-0868-8. [DOI] [PubMed] [Google Scholar]
- 14.Darrow LA, Klein M, Strickland MJ, et al. Ambient air pollution and birth weight in full-term infants in Atlanta, 1994-2004. Environmental health perspectives. 2011;119(5):731–7. doi: 10.1289/ehp.1002785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dadvand P, Ostro B, Figueras F, et al. Residential proximity to major roads and term low birth weight: the roles of air pollution, heat, noise, and road-adjacent trees. Epidemiology. 2014;25(4):518–25. doi: 10.1097/EDE.0000000000000107. [DOI] [PubMed] [Google Scholar]
- 16.Parker JD, Woodruff TJ. Influences of study design and location on the relationship between particulate matter air pollution and birthweight. Paediatric and perinatal epidemiology. 2008;22(3):214–27. doi: 10.1111/j.1365-3016.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 17.Villeneuve PJ, Burnett RT, Shi Y, et al. A time-series study of air pollution, socioeconomic status, and mortality in Vancouver, Canada. Journal of exposure analysis and environmental epidemiology. 2003;13(6):427–35. doi: 10.1038/sj.jea.7500292. [DOI] [PubMed] [Google Scholar]
- 18.Klemm RJ, Lipfert FW, Wyzga RE, et al. Daily mortality and air pollution in Atlanta: two years of data from ARIES. Inhalation toxicology. 2004;16(Suppl 1):131–41. doi: 10.1080/08958370490443213. [DOI] [PubMed] [Google Scholar]
- 19.Bell ML, Ebisu K, Peng RD. Community-level spatial heterogeneity of chemical constituent levels of fine particulates and implications for epidemiological research. Journal of exposure science & environmental epidemiology. 2011;21(4):372–84. doi: 10.1038/jes.2010.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sawvel EJ, Willis R, West RR, et al. Passive sampling to capture the spatial variability of coarse particles by composition in Cleveland, OH. Atmospheric environment. 2015;105:61–9. [Google Scholar]
- 21.Ito K, De Leon S, Thurston GD, et al. Monitor-to-monitor temporal correlation of air pollution in the contiguous US. Journal of exposure analysis and environmental epidemiology. 2005;15(2):172–84. doi: 10.1038/sj.jea.7500386. [DOI] [PubMed] [Google Scholar]
- 22.US Census Bureau . American Community Survey 2009 5-year estimates. US Census Bureau; Washington, DC: 2009. [Google Scholar]
- 23.Alexander GR, Himes JH, Kaufman RB, et al. A United States national reference for fetal growth. Obstet Gynecol. 1996;87(2):163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 24.Ebisu K, Bell ML. Airborne PM2.5 chemical components and low birth weight in the northeastern and mid-Atlantic regions of the United States. Environmental health perspectives. 2012;120(12):1746–52. doi: 10.1289/ehp.1104763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ebisu K, Belanger K, Bell ML. Association between airborne PM2.5 chemical constituents and birth weight-implication of buffer exposure assignment. Environ Res Lett. 2014;9(8) doi: 10.1088/1748-9326/9/8/084007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson WE, Suh HH. Fine particles and coarse particles: concentration relationships relevant to epidemiologic studies. Journal of the Air & Waste Management Association. 1997;47(12):1238–49. doi: 10.1080/10473289.1997.10464074. [DOI] [PubMed] [Google Scholar]
- 27.Kalkstein LS, Valimont KM. An Evaluation of Summer Discomfort in the United-States Using a Relative Climatological Index. B Am Meteorol Soc. 1986;67(7):842–8. [Google Scholar]
- 28.Mostofsky E, Schwartz J, Coull BA, et al. Modeling the association between particle constituents of air pollution and health outcomes. American journal of epidemiology. 2012;176(4):317–26. doi: 10.1093/aje/kws018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geer LA, Weedon J, Bell ML. Ambient air pollution and term birth weight in Texas from 1998 to 2004. Journal of the Air & Waste Management Association. 2012;62(11):1285–95. doi: 10.1080/10962247.2012.707632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bell ML, Belanger K, Ebisu K, et al. Relationship between birth weight and exposure to airborne fine particulate potassium and titanium during gestation. Environmental research. 2012;117:83–9. doi: 10.1016/j.envres.2012.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. EPA EPA Regional Contacts. 2015 ( http://www2.epa.gov/lead/epa-regional-contacts). (Accessed June 22, 2015)
- 32.Zeger SL, Thomas D, Dominici F, et al. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environmental health perspectives. 2000;108(5):419–26. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu Z, Han S, Zhu J, et al. Pre-pregnancy body mass index in relation to infant birth weight and offspring overweight/obesity: a systematic review and meta-analysis. PLoS One. 2013;8(4):e61627. doi: 10.1371/journal.pone.0061627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bell ML, Dominici F, Ebisu K, et al. Spatial and temporal variation in PM(2.5) chemical composition in the United States for health effects studies. Environmental health perspectives. 2007;115(7):989–95. doi: 10.1289/ehp.9621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Almeida SM, Pio CA, Freitas MC, et al. Approaching PM(2.5) and PM(2.5-10) source apportionment by mass balance analysis, principal component analysis and particle size distribution. The Science of the total environment. 2006;368(2-3):663–74. doi: 10.1016/j.scitotenv.2006.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Malm WC, Pitchford ML, McDade C, et al. Coarse particle speciation at selected locations in the rural continental United States. Atmospheric environment. 2007;41(10):2225–39. [Google Scholar]
- 37.Cheung K, Daher N, Kam W, et al. Spatial and temporal variation of chemical composition and mass closure of ambient coarse particulate matter (PM10-2.5) in the Los Angeles area. Atmospheric environment. 2011;45(16):2651–62. [Google Scholar]
- 38.Clements N, Eav J, Xie M, et al. Concentrations and source insights for trace elements in fine and coarse particulate matter. Atmospheric environment. 2014;89:373–81. [Google Scholar]
- 39.Eeftens M, Tsai M-Y, Ampe C, et al. Spatial variation of PM 2.5, PM 10, PM 2.5 absorbance and PM coarse concentrations between and within 20 European study areas and the relationship with NO 2–results of the ESCAPE project. Atmospheric environment. 2012;62:303–17. [Google Scholar]
- 40.Zhang K, Larson TV, Gassett A, et al. Characterizing spatial patterns of airborne coarse particulate (PM10–2.5) mass and chemical components in three cities: the Multi-Ethnic Study of Atherosclerosis. 2014 doi: 10.1289/ehp.1307287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.U.S. EPA . Consultation on Coarse Particle Speciation. Environmental Protection Agency; Research Triangle Park, NC: 2009. [Google Scholar]
- 42.U.S. EPA . Integrated science assessment for particulate matter. US Environmental Protection Agency; Washington, DC: 2009. EPA/600/R-08/139B. [PubMed] [Google Scholar]
- 43.U.S. EPA . Clean Air Scientific Advisory Committee (CASAC) Ambient Air Monitoring and Methods (AAMM) Subcommittee consultation on methods for measuring coarse-fraction particulate matter (PMc) in ambient air (July 2004) US Environmental Protection Agency; Washington, DC: 2004. EPA-SAB-CASAC-CON-04-005. [Google Scholar]
- 44.Pereira G, Bracken MB, Bell ML. Particulate air pollution, fetal growth and gestational length: The influence of residential mobility in pregnancy. Environmental research. 2016;147:269–74. doi: 10.1016/j.envres.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Northam S, Knapp TR. The reliability and validity of birth certificates. J Obstet Gynecol Neonatal Nurs. 2006;35(1):3–12. doi: 10.1111/j.1552-6909.2006.00016.x. [DOI] [PubMed] [Google Scholar]
- 46.Honein MA, Paulozzi LJ, Watkins ML. Maternal smoking and birth defects: validity of birth certificate data for effect estimation. Public Health Rep. 2001;116(4):327–35. doi: 10.1016/S0033-3549(04)50054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bell ML, Ebisu K. Environmental inequality in exposures to airborne particulate matter components in the United States. Environmental health perspectives. 2012;120(12):1699–704. doi: 10.1289/ehp.1205201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Happo MS, Salonen RO, Halinen AI, et al. Inflammation and tissue damage in mouse lung by single and repeated dosing of urban air coarse and fine particles collected from six European cities. Inhalation toxicology. 2010;22(5):402–16. doi: 10.3109/08958370903527908. [DOI] [PubMed] [Google Scholar]
- 49.Wegesser TC, Last JA. Lung response to coarse PM: bioassay in mice. Toxicol Appl Pharmacol. 2008;230(2):159–66. doi: 10.1016/j.taap.2008.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gerlofs-Nijland ME, Rummelhard M, Boere AJ, et al. Particle induced toxicity in relation to transition metal and polycyclic aromatic hydrocarbon contents. Environ Sci Technol. 2009;43(13):4729–36. doi: 10.1021/es803176k. [DOI] [PubMed] [Google Scholar]
- 51.Delfino RJ, Staimer N, Tjoa T, et al. Associations of primary and secondary organic aerosols with airway and systemic inflammation in an elderly panel cohort. Epidemiology. 2010;21(6):892–902. doi: 10.1097/EDE.0b013e3181f20e6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janssen BG, Munters E, Pieters N, et al. Placental mitochondrial DNA content and particulate air pollution during in utero life. Environmental health perspectives. 2012;120(9):1346–52. doi: 10.1289/ehp.1104458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lauer FT, Mitchell LA, Bedrick E, et al. Temporal-spatial analysis of U.S.-Mexico border environmental fine and coarse PM air sample extract activity in human bronchial epithelial cells. Toxicol Appl Pharmacol. 2009;238(1):1–10. doi: 10.1016/j.taap.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. doi: 10.1136/bmj.f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.