Abstract
Determination of metanephrine (MN), normetanephrine (NMN) and 3-methoxytyramine (3-MT) in saliva could be of diagnostic value in patients with pheochromocytoma. This preliminary study was set out to determine metanephrine concentrations in saliva from healthy subjects compared to their simultaneously measured plasma levels. In addition, we studied the possible influence of pre-analytical conditions such as a collection device, awakening, position, and eating on the salivary metanephrine levels. We included 11 healthy volunteers. Fasting blood and saliva samples were collected in seated position and after 30 minutes of horizontal rest. Saliva samples 30 minutes after eating were also collected. Saliva was collected with and without the use of a polyethylene salivette. Plasma and salivary MN, NMN and 3-MT concentrations were determined using a High-Performance Liquid Chromatography tandem mass spectrometric technique (LC-MS/MS) with automated solid phase extraction sample preparation. Metanephrines were detectable in saliva from all participants both in seated and the supine position. We found no significant correlation between the MN, NMN and 3-MT concentrations in saliva and plasma in the seated or supine position. In addition, there was no difference between MN, NMN and 3-MT concentrations collected with or without a collection device. Plasma MN, NMN, 3-MT and salivary NMN concentrations collected in seated position were significantly higher compared concentrations of samples collected in supine position (all P<.05). In conclusion, salivary metanephrines can be detected with LC-MS/MS with sufficient sensitivity and precision. Our findings warrant evaluation of salivary metanephrine measurement in the work-up of patients who are suspected to harbor pheochromocytoma.
Keywords: saliva, metanephrines, pheochromocytoma, paraganglioma
Introduction
Sympathetic paragangliomas, either adrenal (i.e. pheochromocytoma or extra-adrenal (sympathetic paragangliomas)) are neuroendocrine tumors that are known for their overproduction of catecholamines, i.e. epinephrine, norepinephrine, and dopamine. The biochemical diagnosis of sympathetic paragangliomas is based on the demonstration of elevated metanephrine (MN), normetanephrine (NMN), or 3-methoxytyramine (3-MT) in plasma and/or urine. Measurement of plasma free metanephrines is currently considered to be the most accurate method for diagnosing these tumors (Lenders et al. 1995, Lenders et al. 2014). However, plasma metanephrines can be affected by several pre-analytical factors such as position (Lenders et al. 2007), age (Eisenhofer et al. 2013), coffee (Robertson et al. 1978, Deutschbein et al. 2010), diet (de Jong et al. 2009) and salt intake (Kerstens et al. 2012), certain drugs (depending on the method used) (e.g. mesalamine, sulfasalazine, and tricyclic antidepressants) (Bouhanick et al. 2010, Neary et al. 2011, Lenders et al. 2014), previous adrenalectomy (Osinga et al. 2013) and even the season of the year (Pamporaki et al. 2014). Plasma NMN and MN samples collected in seated position are 30% and 27% higher, respectively, compared to samples drawn after 30 minutes of supine rest (Lenders et al. 2007). Therefore, the United States Endocrine Society recommends that patients rest for 20–30 minutes in a supine position before blood sampling (Lenders et al. 2014). This requires reference values in supine position and moreover, increases costs for venipuncture and is more cumbersome (Chortis et al. 2014).
Catecholamines and metanephrines are biogenic amines that are easily transported over the salivary gland membrane, and should therefore be detectable in saliva. Assessment of late-night cortisol in saliva is now recommended part of routine diagnostics in diagnosing Cushing’s syndrome mostly for logistic reasons (Nieman et al. 2008, Guignat and Bertherat 2010). Salivary catecholamines and metanephrines are expected to reflect their respective concentrations in plasma. High-Performance Liquid Chromatography tandem mass spectrometric technique (LC-MS/MS) with automated solid phase extraction sample preparation is a highly sensitive technique that enables the measurement of very low concentrations of catecholamines and metanephrines in saliva. Free unconjugated metanephrines in plasma filtrate into saliva through gap junctions between cells of secretory units (Chiappin et al. 2007). Determination of metanephrines in saliva could for logistic reasons be useful in patients suspected of harboring a pheochromocytoma/paraganglioma. These patients could then collect saliva at home, which would enhance patient comfort and obviates the need of extra hospital facilities and costs.
This pilot study was initiated to determine metanephrines in saliva from healthy subjects. In addition, we tested the influence of several pre-analytical factors such as collection device, position, awakening and food on the salivary concentrations of metanephrines. Also the relation between salivary and plasma metanephrines concentrations was established.
Participants and Methods
Study population and design
In this single center study we examined 11 healthy nonsmoking volunteers older than 18 years. All healthy volunteers were seen at the Department of Endocrinology of the University Medical Center Groningen. Participants were not allowed to use medication known to interfere with metanephrines (Lenders et al. 2014).
The first saliva sample was collected at home directly after awakening (between 6.00 and 7.00 AM) in supine position (T0). Participants visited the outpatient clinic at 8.00 AM in a fasting state. Blood pressure was measured in seated and after 5 minutes in supine position using an automatic blood pressure measurement device. Blood and saliva samples were collected while participant were in seated position (T1). Thereafter, saliva samples were collected directly after changing into a supine position (T2). The second blood sample and third saliva sample were collected after 30 minutes of recumbency (T3). Ten minutes after blood sample collection at T3, saliva was again collected while remaining in supine position (T4). Thereafter, participants ate a standard breakfast, but were not allowed to smoke, drink caffeine containing products such as coffee or tea, or consume food products with a high (catechol)amine content such as walnuts, pineapple or bananas. Thirty minutes after finishing breakfast, participants were asked to collect saliva for the fifth time (T5).
Salivary and plasma samples were stored on ice until transportation to the department of laboratory medicine.
Approval of the study by the Medical Ethics Committee of the University Medical Center of Groningen in the Netherlands was requested but waved because the purpose of this study was to calibrate metanephrine values in saliva to the values in plasma and therefore according to the Dutch Medical Research Involving Human Subjects Act no further Institutional Review Board approval was required. All participants gave oral informed consent.
Saliva collection
Saliva was collected in two ways, either by direct spitting saliva into a collection tube (without a collection device) or by using a polyethylene swab (Salivettes®; Sarstedt, Nümbrecht, Germany), while participants were either in seated or supine position. Participants needed to chew or suck gently on the polyethylene swab for 2–3 minutes. Collected samples were immediately put on ice for transportation to the department of laboratory medicine and were subsequently stored at −80 °C until further processing.
Analytical methods
Blood samples were taken via venipuncture, with the participant either in the seated or supine position, using 4 ml Vacutainer Tubes (Becton Dickinson®) containing K2-EDTA as anticoagulant. Collected samples were immediately put on ice for transportation to the department of laboratory medicine. Blood samples were centrifuged for 12 minutes at 2500 g and saliva samples for 2 minutes at 1000 g. Samples were subsequently stored at −80 °C until processing.
Plasma free and saliva MN, NMN, and 3-MT were analyzed by HPLC-MS/MS with automated solid phase extraction sample preparation, exactly as described by de Jong et al. (de Jong et al. 2007). Established reference intervals for plasma free metanephrines were: MN 0.07–0.33 nmol/L, NMN 0.23–1.07 nmol/L, 3-MT<0.17 nmol/L (de Jong et al. 2007).
The intraassay and interassay analytical variation coefficients were 2.5% to 4.8% and 3.6% to 5.6% for free plasma MN, 5.1 to 6.2% and 4.2% to 7.1% for free plasma NMN, and 4.5% to 11.1% for free plasma 3-MT, respectively.
Statistical Analysis
Data are presented as mean ±standard deviation (SD) or as median with inter quartile range [IQR] where appropriate. Differences between salivary metanephrine samples collected with and without a collection device, in seated and supine position, before and after breakfast and after awakening were calculated with the Friedman’s two way ANOVA analysis.
Non-parametric correlation analysis (Spearmans ρ) was used to examine the relationship between blood and saliva samples. MN/NMN and NMN/3-MT ratios in plasma and saliva were calculated by dividing NMN by MN and 3-MT by NMN. A two-sided P<0.05 was considered statistically significant. Analyses were performed with SPSS statistics (version 22.0;IBM/SPSS, Armonk, New York) and Analyse-it Software Ltd (Ver.2.30, Leeds, United Kingdom).
Results
Participant characteristics
Four men and 7 women with a mean age (±SD) of 39±16 years participated. Mean blood pressure and pulse in seated and supine position were 126±17/80±8 mmHg, 70±9/min and 123±19/71±9 mmHg, 62±10/min, respectively.
Metanephrine concentrations simultaneously measured in saliva and plasma samples
Median concentrations of metanephrines in saliva and plasma at the different time points are shown in Table 1. Seated NMN/MN and 3-MT/NMN ratios were not significantly different between plasma and saliva, 1.94 [1.27–2.46] vs. 7.21 [5.33–8.16] (P=0.08) and 0.05 [0.04–0.06] vs. 0.12 [0.08–0.14] (P=0.08). Supine NMN/MN and 3-MT/NMN ratios were not significantly different between plasma and saliva, 1.49 [1.15–2.08] vs. 4.92 [3.62–5.25] (P=0.08) and 0.05 [0.04–0.10] vs. 0.11 [0.08–0.16] (P=0.17).
Table 1.
Median plasma and salivary concentrations of metanephrines in 11 healthy volunteers
| Plasma | Saliva | |||||
|---|---|---|---|---|---|---|
| Metanephrine | Normetanephrine | 3-Methoxytyramine | Metanephrine | Normetanephrine | 3-Methoxytyramine | |
| T0 (on awakening) | NA | NA | NA | 0.12 [0.07–0.14] | 0.47 [0.33–0.59] | 0.23 [0.10–0.35] |
| T1 (seated) | 0.18 [0.15–0.25] | 0.32 [0.26–0.55] | 0.019 [0.014–0.024] | 0.07 [0.06–0.09] | 0.50 [0.27–0.63] | 0.06 [0.03–0.10] |
| T2 (immediately after supine position) | NA | NA | NA | 0.08 [0.05–0.09] | 0.37 [0.25–0.54] | 0.04 [0.03–0.08] |
| T3 (30 minutes supine position) | 0.14 [0.12–0.20]* | 0.27 [0.18–0.37]* | 0.015 [0.013–0.021]* | 0.06 [0.05–0.09]¶ | 0.35 [0.19–0.38]*,¶ | 0.04 [0.03–0.05]¶ |
| T4 (10 minutes after T3) | NA | NA | NA | 0.07 [0.06–0.09] | 0.29 [0.22–0.38] | 0.04 [0.02–0.06] |
| T5 (30 minutes after breakfast) | NA | NA | NA | 0.05 [0.04–0.05]† | 0.26 [0.21–0.37]† | 0.05 [0.02–0.36]† |
Data in median with interquartile ranges between brackets. Concentrations of plasma and salivary metanephrine, normetanephrine and 3-methoxytyramine in nmol/L.
P< 0.05 seated vs. supine position;
P<0.05 before vs. after breakfast;
P<0.05 on awakening vs. after 30 minutes of supine rest in the hospital NA, not available.
Influence of collection device on the concentration of metanephrines
There were no differences in concentrations of metanephrines between samples collected with or without a saliva collection device in either seated position or recumbent position (data not shown). Therefore, all samples described in this study are calculated based on samples taken with a polyethylene swab as device (Salivettes®).
Correlations between salivary and plasma metanephrines
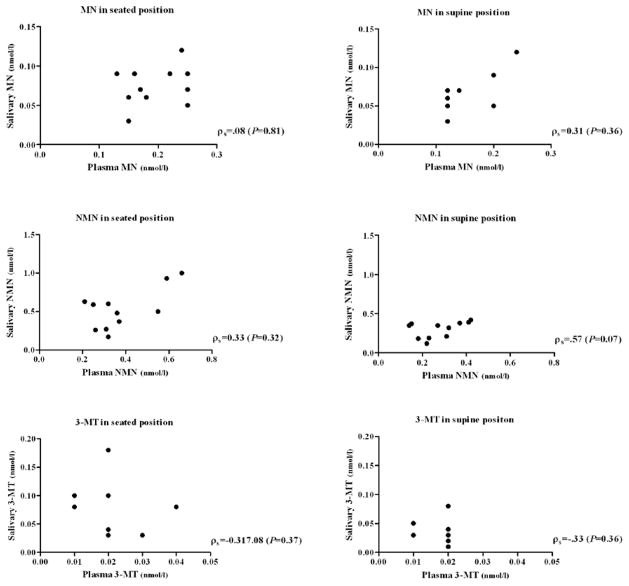
There was no significant correlation between the MN, NMN, and 3-MT concentrations in saliva and plasma in the seated position (T1), ρs= .08 (P=0.81), ρs=0.33 (P= 0.32) and ρs=−0.32 (P=0.37) respectively (Figure 1).
Figure 1.
Individual relationship between salivary and plasma metanephrines in seated and supine position
MN, metanephrine; NMN, normetanephrine; 3-MT, 3-methoxytyramine
There was also no significant relationship between MN, NMN, and 3-MT concentration in saliva and plasma in supine position (T3), ρs=0.31 (P=0.36), ρs=0.57 (P=0.07) and ρs=−0.33 (P=0.36) (Figure 1).
Influence of posture during sampling on salivary and plasma metanephrines
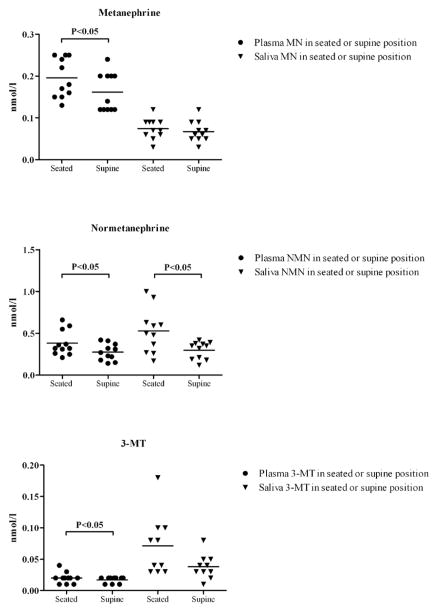
Plasma MN, NMN, and 3-MT concentrations collected in supine position were significantly lower compared to samples collected in seated position (MN −16.4%, NMN −27.5% and 3-MT −8.3% respectively, P<0.05; Table 1, Figure 2). Salivary NMN collected in supine position was also significantly lower compared to samples collected in seated position (NMN 38.4%, P<0.05). In contrast, there were no significant effects of posture during sampling on the salivary MN and 3-MT concentrations (P=0.53 and P=0.10; Table 1, Figure 2).
Figure 2.
Comparison between salivary and plasma metanephrines in seated and supine position
MN, metanephrine; NMN, normetanephrine; 3-MT, 3-methoxytyramine
Influence of eating on salivary metanephrines
Salivary MN and NMN concentrations were significantly lower after breakfast (T5) compared to samples collected fasting in seated position (T1) (both P<0.05) (Table 1). Only salivary MN was significantly lower after breakfast (T5) compared to samples collected fasting after 30 minutes of supine rest (T3) (P<0.05).
Awakening response
Salivary MN, NMN and 3-MT concentrations on awakening (T0) (around 6:00–7:00 AM) were significantly higher than after 30 minutes of recumbency in the hospital (around 9:00 AM) (T3) (all P<0.05) (Table 1).
Discussion
In the present study, we show that metanephrines can readily be measured in saliva of healthy volunteers using mass spectrometry based techniques. We investigated several pre-analytical conditions and found that both position during sample collection and eating are of importance, moreover, we found that there was only a weak correlation between saliva and plasma concentrations.
Whole saliva is a clear, slightly acidic (pH 6.0–7.0 or 5.5−6.0 when not stimulated) and complex biological fluid composed of secretions from salivary glands and a variety of enzymes, hormones, antibodies, antimicrobial constituents, and growth factors entering the saliva from the blood by either passive or active intracellular diffusion or extracellular ultrafiltration (Vining and McGinley 1986, Kaufman and Lamster 2002). Catecholamines and unconjugated metanephrines in plasma filtrate into saliva through gap junctions between cells of secretory units (Chiappin et al. 2007, Groschl 2008). The hormone concentration in saliva is 300–3000x lower than in plasma (Chiappin et al. 2007). We found average concentrations of free metanephrines to be comparable to concentrations measured in blood.
Stefanescu et al. measured salivary free MN and NMN levels in 30 patients with a pheochromocytoma and compared these to 70 normotensive healthy controls using an enzyme-linked immunosorbent assay (ELISA) (Stefanescu et al. 2011). The reported concentrations in healthy controls were similar to ours. Both salivary and plasma MN and NMN levels were increased in patients with pheochromocytoma when compared to healthy controls. In addition, they found a linear relationship between salivary and plasma MN and NMN levels (Stefanescu et al. 2011). In the present study, we used LC-MS/MS with automated solid phase extraction sample preparation to analyze both salivary and plasma samples. We could not reproduce this observation, as we only found a tendency towards a statistical significant correlation between plasma and saliva NMN in supine position, but there was no statistical significant correlation between MN, NMN in seated position, and 3-MT concentrations in saliva and plasma. Beside differences in group size, differences in techniques used for quantification of metanephrines could be an explanation. Differences in sample clean up could result in different recoveries from saliva, as it has been shown that binding proteins such as albumin and proline rich protein are present in saliva as well (Fiers et al. 2014).
The tendency towards difference in NMN/MN ratio between plasma and saliva can be an indication that there is a decreased filtration of the free MN fraction from plasma into saliva, or secretion of catecholamines by the salivary glands and subsequent metabolization of these catecholamines by COMT in saliva ((Uhlen et al. 2015), http://www.proteinatlas.org/ENSG00000093010-COMT/tissue, date: 05/26/2015). As we saw no difference between passive drewling and salivette collected saliva, it is unlikely that the use of a device (polyethylene Salivettes®) influenced the recovery of metanephrines. In addition, polyethylene Salivettes® have been found to be suitable for determining several (polar) hormones and therapeutic drugs in saliva (Groschl et al. 2008).
We found a decrease of salivary metanephrines 30 minutes after eating breakfast compared to the values during fasting. This could be explained by the stimulation of saliva production by chewing. Higashi et al. found a lower concentration of catecholamine end products (homovanillic acid (HVA) and 3-methoxy-4-hydroxyphenylglycol (MHGP)) after use of chewing gum (Higashi et al. 2012). Therefore, we believe it is appropriate to recommend that salivary samples should be obtained while fasting or at least 30 minutes after eating.
Comparable to salivary cortisol, we found elevated levels of metanephrines immediately after awaking. The cortisol awakening response was found to be decreased in patients with chronic pain or psychiatric conditions (posttraumatic stress disorders, chronic fatigue syndrome or sleep disorder), while healthy subjects can exhibit elevated cortisol response under some stress conditions (such as job stress) (Clow et al. 2004, Fries et al. 2009). Similar to cortisol, an increase of catecholamine levels directly after awakening has been described (Schofl et al. 1997). In addition, there is an indication that catecholamine show diurnal variation(Prinz et al. 1979). Concentrations of metanephrines were elevated immediately after awakening compared to values determined in supine position between 8.00 and 9.00 am. This suggests that salivary metanephrines could also represent an easy to collect biomarker to detect a diurnal rhythm or stress-related disorders to which an individual is exposed.
Assessment of salivary metanephrines might become a novel and clinically useful biochemical tool for the diagnosis of pheochromocytoma. Obviously, this requires determination of salivary metanephrines in a sufficient number of healthy controls and patients with a pheochromoyctoma in order to establish reference ranges for these metabolites.
Measurement of metanephrines in saliva instead of plasma could offer several advantages for clinical practice. It is noninvasive and free of harmful effects such as pain or hematoma formation and it gives the possibility to determine other hormones such as cortisol at the same time. This would make determination of salivary metanephrines very suitable for children as well as for periodic screening in patients with a hereditary pheochromocytoma/paraganglioma. In addition, it can readily be repeated at various intervals and patients can also collect the sample at home, which is likely to be more cost-effective compared to an in-hospital venipuncture (Chortis et al. 2014). We found no differences in concentration of metanephrines between saliva samples collected with or without a collection device. Taking into account that the collection of saliva with a device is more convenient for the patient, we would recommend the use of a polyethylene or polyester salivette® (Groschl et al. 2008).
In conclusion, LC-MS/MS enables to detect salivary MN, NMN and 3-MT. In order to become a new tool for the biochemical diagnosis of pheochromocytoma/paraganglioma, more samples need to be collected including those of pheochromocytoma/paraganglioma patients, taking into account the above described pre-analytical conditions.
Acknowledgments
Funding: no funding received.
Footnotes
This study or parts of it have not been presented elsewhere.
Declaration of interest: authors have nothing to disclose.
Author contribution statement: all authors contributed to the study conception, design and data interpretation, T.E. Osinga collected the data, M. van Faassen and I.P. Kema analyzing samples, all authors writing, editing and final proof.
References
- Bouhanick B, Fauvel J, Pont F. Biochemical misdiagnosis of pheochromocytoma in patients treated with sulfasalazine. Jama. 2010;304:1898–1901. doi: 10.1001/jama.2010.1563. [DOI] [PubMed] [Google Scholar]
- Chiappin S, Antonelli G, Gatti R, De Palo EF. Saliva specimen: a new laboratory tool for diagnostic and basic investigation. Clinica chimica acta; international journal of clinical chemistry. 2007;383:30–40. doi: 10.1016/j.cca.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Chortis V, Bancos I, Crowley RK, Arlt W. Supine or sitting? Economic considerations regarding patient position during plasma metanephrine analysis for the exclusion of chromaffin tumours. Clinical endocrinology. 2014;82:776–7. doi: 10.1111/cen.12587. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- de Jong WH, Eisenhofer G, Post WJ, Muskiet FA, de Vries EG, Kema IP. Dietary influences on plasma and urinary metanephrines: implications for diagnosis of catecholamine-producing tumors. The Journal of clinical endocrinology and metabolism. 2009;94:2841–2849. doi: 10.1210/jc.2009-0303. [DOI] [PubMed] [Google Scholar]
- de Jong WH, Graham KS, van der Molen JC, Links TP, Morris MR, Ross HA, de Vries EG, Kema IP. Plasma free metanephrine measurement using automated online solid-phase extraction HPLC tandem mass spectrometry. Clinical chemistry. 2007;53:1684–1693. doi: 10.1373/clinchem.2007.087114. [DOI] [PubMed] [Google Scholar]
- Deutschbein T, Unger N, Jaeger A, Broecker-Preuss M, Mann K, Petersenn S. Influence of various confounding variables and storage conditions on metanephrine and normetanephrine levels in plasma. Clinical endocrinology. 2010;73:153–160. doi: 10.1111/j.1365-2265.2009.03761.x. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Lattke P, Herberg M, Siegert G, Qin N, Darr R, Hoyer J, Villringer A, Prejbisz A, Januszewicz A, et al. Reference intervals for plasma free metanephrines with an age adjustment for normetanephrine for optimized laboratory testing of phaeochromocytoma. Annals of Clinical Biochemistry. 2013;50:62–69. doi: 10.1258/acb.2012.012066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiers T, Delanghe J, T’Sjoen G, Van Caenegem E, Wierckx K, Kaufman JM. A critical evaluation of salivary testosterone as a method for the assessment of 20 serum testosterone. Steroids. 2014;86:5–9. doi: 10.1016/j.steroids.2014.04.013. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): facts and future directions. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Groschl M. Current status of salivary hormone analysis. Clinical chemistry. 2008;54:1759–1769. doi: 10.1373/clinchem.2008.108910. [DOI] [PubMed] [Google Scholar]
- Groschl M, Kohler H, Topf HG, Rupprecht T, Rauh M. Evaluation of saliva collection devices for the analysis of steroids, peptides and therapeutic drugs. Journal of pharmaceutical and biomedical analysis. 2008;47:478–486. doi: 10.1016/j.jpba.2008.01.033. [DOI] [PubMed] [Google Scholar]
- Guignat L, Bertherat J. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline: commentary from a European perspective. European journal of endocrinology. 2010;163:9–13. doi: 10.1530/EJE-09-0627. [DOI] [PubMed] [Google Scholar]
- Higashi T, Hijikuro M, Yamagata K, Ogawa S. Influence of saliva flow rate stimulated by gum-chewing on salivary concentrations of catecholamine metabolites. Clinica chimica acta. 2012;414:248–252. doi: 10.1016/j.cca.2012.09.031. [DOI] [PubMed] [Google Scholar]
- Kaufman E, Lamster IB. The diagnostic applications of saliva-a review. Critical reviews in oral biology and medicine. 2002;13:197–212. doi: 10.1177/154411130201300209. [DOI] [PubMed] [Google Scholar]
- Kerstens MN, Kema IP, Dullaart RP. Plasma normetanephrine concentrations are affected by dietary sodium intake. Clinica chimica acta. 2012;413:1716–1717. doi: 10.1016/j.cca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Duh QY, Eisenhofer G, Gimenez-Roqueplo AP, Grebe SK, Murad MH, Naruse M, Pacak K, Young WF., Jr Pheochromocytoma and paraganglioma: an endocrine society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2014;99:1915–1942. doi: 10.1210/jc.2014-1498. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Keiser HR, Goldstein DS, Willemsen JJ, Friberg P, Jacobs MC, Kloppenborg PW, Thien T, Eisenhofer G. Plasma metanephrines in the diagnosis of pheochromocytoma. Annals of Internal Medicine. 1995;123:101–109. doi: 10.7326/0003-4819-123-2-199507150-00004. [DOI] [PubMed] [Google Scholar]
- Lenders JW, Willemsen JJ, Eisenhofer G, Ross HA, Pacak K, Timmers HJ, Sweep CG. Is supine rest necessary before blood sampling for plasma metanephrines? Clinical chemistry. 2007;53:352–354. doi: 10.1373/clinchem.2006.076489. [DOI] [PubMed] [Google Scholar]
- Neary NM, King KS, Pacak K. Drugs and pheochromocytoma--don’t be fooled by every elevated metanephrine. The New England journal of medicine. 2011;364:2268–2270. doi: 10.1056/NEJMc1101502#SA1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieman LK, Biller BM, Findling JW, Newell-Price J, Savage MO, Stewart PM, Montori VM. The diagnosis of Cushing’s syndrome: an Endocrine Society Clinical Practice Guideline. Journal of clinical endocrinology and metabolism. 2008;93:1526–1540. doi: 10.1210/jc.2008-0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osinga TE, van den Eijnden MH, Kema IP, Kerstens MN, Dullaart RP, de Jong WH, Sluiter WJ, Links TP, van der Horst-Schrivers AN. Unilateral and bilateral adrenalectomy for pheochromocytoma requires adjustment of urinary and plasma metanephrine reference ranges. Journal of clinical endocrinology and metabolism. 2013;98:1076–1083. doi: 10.1210/jc.2012-3418. [DOI] [PubMed] [Google Scholar]
- Pamporaki C, Bursztyn M, Reimann M, Ziemssen T, Bornstein SR, Sweep FC, Timmers H, Lenders JW, Eisenhofer G. Seasonal variation in plasma free normetanephrine concentrations: implications for biochemical diagnosis of pheochromocytoma. European journal of endocrinology. 2014;170:349–357. doi: 10.1530/EJE-13-0673. [DOI] [PubMed] [Google Scholar]
- Prinz PN, Halter J, Benedetti C, Raskind M. Circadian variation of plasma catecholamines in young and old men: relation to rapid eye movement and slow wave sleep. The Journal of clinical endocrinology and metabolism. 1979;49:300–304. doi: 10.1210/jcem-49-2-300. [DOI] [PubMed] [Google Scholar]
- Robertson D, Frolich JC, Carr RK, Watson JT, Hollifield JW, Shand DG, Oates JA. Effects of caffeine on plasma renin activity, catecholamines and blood pressure. The New England journal of medicine. 1978;298:181–186. doi: 10.1056/NEJM197801262980403. [DOI] [PubMed] [Google Scholar]
- Schofl C, Becker C, Prank K, von zur Muhlen A, Brabant G. Twenty-four-hour rhythms of plasma catecholamines and their relation to cardiovascular parameters in healthy young men. European journal of endocrinology / European Federation of Endocrine Societies. 1997;137:675–683. doi: 10.1530/eje.0.1370675. [DOI] [PubMed] [Google Scholar]
- Stefanescu AM, Schipor S, Paun DL, Dumitrache C, Badiu C. Salivary free catecholamines metabolites as possible biochemical markers in pheochromocytoma diagnosis. Acta Endocrinologica. 2011;7:431–442. [Google Scholar]
- Uhlen M, Fagerberg L, Hallstrom BM, Lindskog C, Oksvold P, Mardinoglu A, Sivertsson A, Kampf C, Sjostedt E, Asplund A, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347:1260419 1–9. doi: 10.1126/science.1260419. [DOI] [PubMed] [Google Scholar]
- Vining RF, McGinley RA. Hormones in saliva. Critical reviews in clinical laboratory sciences. 1986;23:95–146. doi: 10.3109/10408368609165797. [DOI] [PubMed] [Google Scholar]