Abstract
OBJECTIVES
Early-phase clinical trials play a pivotal role in drug development. However, limited data are available on outcomes of gastrointestinal (GI) cancer patients enrolled in phase I clinical trials. Here, we evaluated the characteristics associated with survival in GI cancer patients participating in phase I clinical trials and attempted to validate previously established prognostic models.
METHODS
All consecutive patients with advanced GI tumors who participated in phase I clinical trials at our institution from 1/2007 to 12/2013 and received at least one dose of the study drug were included. Cox regression models were used to estimate multivariable-adjusted hazard ratio (HR) and 95% confidence interval (CI).
RESULTS
In 243 study patients (median age 62 years [range 26–82]; 55% male), treatment included chemotherapy only (14%), targeted therapy (41%), chemotherapy+targeted therapy (42%), and others (2%) for the following disease types: pancreatic (42%), colorectal (34%), gastro-esophageal (10%), hepatobiliary (13%), and others (2%). Response rate was 4%, with 38% achieving stable disease and 42% having progressive disease. Median survival was 5.8 months (range: 0.2–52.4 months). Our multivariable Cox regression analyses included the following as predictors of survival: ECOG performance score ≥1 (HR:1.76), prior systemic therapies ≥2 (HR:1.63), LDH >618 IU/L (HR:1.85), sodium >135 mmol/L (HR:0.46), and white blood count >6×109/L (HR:1.5). Our dataset was consistent with previous prognostic scores.
CONCLUSIONS
This is the largest study to assess clinical outcomes in this patient population. Phase I trials provide clinical benefit to patients with advanced GI malignancies and should be recommended as a treatment option in appropriate patients.
Keywords: Clinical trial, Phase I, gastrointestinal neoplasms, clinical trial, survival, therapeutics
INTRODUCTION
Cancer is one of the leading causes of death in the United States, which has led to increased interest in the development of novel therapies.1,2,3 Phase I clinical trials are a key component to the development of new agents and are used to evaluate appropriate dosing and dosing schedules while monitoring toxicities in anticipation of future trials.4,5 Patient selection and prediction of patient survival are critical steps in the design of phase I clinical trials.4,5 Ideally, patients are selected who have a life expectancy of greater than 3 months. Although phase I clinical trials have been shown to be beneficial to certain patients, limited data are available on the clinical outcomes of enrolled patients.6–9 Due to this paucity of data, clinicians often have difficulties selecting appropriate patients and predicting which patients would benefit most.10,11 Despite the increased attention on developing methods to evaluate potential patients for enrollment, phase I trials continue to have 15–20% mortality in the first 90 days.10
Several studies have evaluated patient survival in clinical trials, and models have been developed to predict survival in these patient populations.7,12–14 One commonly used model is the Royal Marsden Hospital (RMH) score, which utilizes lactate dehydrogenase (LDH), albumin, and number of metastatic sites.10,15 Other models have since been developed, including a prognostic score proposed by MD Anderson Cancer Center (MDACC).7 This study validated the RMH score but also included patient characteristics such as Eastern Cooperative Oncology Group (ECOG) performance status and tumor type.7 However, these scoring systems have not been validated, making it difficult to extrapolate these results. More studies are required to adequately evaluate patient survival in phase I trials and patient characteristics that can assist in predicting outcomes.
In this study, our aim was to evaluate the clinical characteristics and survival of patients with GI malignancies enrolled in phase I clinical trials at Moffitt Cancer Center. We evaluated patient characteristics associated with survival and attempted to validate previously established prognostic models.
MATERIALS AND METHODS
Patients
This study included all patients with GI malignancies who were enrolled in phase I clinical trials from 1/2007 to 12/2013 at our institution. Only patients with advanced unresectable disease were included. Patients were excluded if they did not receive even a single dose of study drug or were enrolled in clinical trials for adjuvant treatment or supportive care. Patients who participated in more than one phase I clinical trial were considered for first trial on which they received treatment. All trials were registered and had received Institutional Review Board (IRB) approval. All patients had signed the informed consent for participation in the phase I clinical trials. IRB approval was obtained for this study.
Data Sources
The Oncore database, maintained by the Clinical Trials Office at Moffitt, was utilized to generate lists of phase I trials conducted at our institution and to extract trial-specific data, including dates of enrollment, study drug treatment, off-study date, trial type, the sponsor of the trial, and response. Information on survival was primarily extracted from Cancer Registry data. We also collected data from patient medical records, including demographics, disease status, ECOG performance status, initial diagnosis, staging, prior treatment (including surgery, radiation, and chemotherapy), medical history, site of metastases, and laboratory values. Laboratory values that were obtained included hemoglobin, white blood cell (WBC), neutrophils, platelets, aspartate aminotransferase (AST), alanine aminotransferase (ALT), bilirubin, LDH, sodium, glucose, protein, albumin, and international normalized ratio (INR). We used the Response Evaluation Criteria in Solid Tumors (RECIST) for response assessment.
Statistical Analysis
The primary endpoint of this study was overall survival (OS), which was analyzed using the Kaplan-Meier method from the start of first therapy until the time of death or censored at the most recent follow-up time. We also evaluated progression-free survival (PFS) of our patient population using the time to disease progression or time of death or censored at the most recent follow-up. A log-rank test was used to compare differences in survival among subgroups. Univariate and multivariate Cox proportional hazard models were used to assess associations between patient characteristics and clinical outcome. Associations were considered statistically significant if a Wald-test P value < 0.05 was achieved. We examined the predictive ability of prognostic factors for survival with the Harrell c-statistic; higher c-statistic indicates greater predictive ability.16 Harrell (concordance) C statistics or Somers’ D statistics was used to assess the prediction performance of RMH cancer risk scoring method and MDACC cancer risk scoring method. Somers’ D is a widely used concordance measure for the prediction of censored survival data, which differs from tau-b in that it uses a correction only for pairs that are tied on the independent variable. Statistical analyses were performed using SAS version 9.4 software (SAS Institute, Inc., Cary, NC) and open source statistical software R version 3.1.0.
Validation of RMH and MDACC Prognostic Score
The prognostic scoring methods developed by the RMH and MDACC groups were directly applied and validated with the Moffitt cohort. RMH prognostic scoring method was built by adding points with LDH >618 IU/L, albumin <3.5 mg/dL, and number of metastatic sites >2. Each variable was assigned one point with scores of 3 considered high risk and scores of 0 being lowest. For the MDACC prognostic score, an additional point was assigned for ECOG performance status ≥1, and GI tumor type with scores of 4 or 5 considered highest risk. Because we included only patients with GI cancers, all patients had at least one point.
RESULTS
Patient Characteristics
We included 243 patients with advanced GI tumors who participated in phase I clinical trials from 1/2007 to 12/2013 at Moffitt Cancer Center. The baseline characteristics are shown in Table 1 (median age was 62 years, range: 26–82 years, 55% male). Almost all patients (98%) had ECOG performance of 0 (28%) or 1 (70%). The majority of the patients were enrolled in industry-sponsored trials (81%), followed by institution-sponsored trials (11%) and externally peer-reviewed trials (8%). Of 243 patients, 143 (59%) received two or more prior systemic therapies. The primary site of malignancy included pancreas (42%), colon (34%), hepatobiliary (13%), and esophagus/gastric (10%). Other baseline characteristics include history of thromboembolism (12%), >2 sites of metastases (31%), hypoalbuminemia (27%), elevated AST (25%), elevated ALT (35%), hyperbilirubinemia (9%), hyperglycemia (44%), thrombocytopenia (23%), and elevated LDH (34%). In this study group, 84% of the patients received treatment that included targeted agents, with 42% receiving both a cytotoxic and a targeted agent as their phase I treatment.
Table 1.
Baseline Patient Characteristics
| Variable | N (%) |
|---|---|
|
| |
| Age (years) | |
| >60 years | 135/243 (55.56%) |
| ≤60 years | 108/243 (44.44%) |
|
| |
| Gender | |
| Male | 134/243 (55.1%) |
| Female | 109/243 (44.9%) |
|
| |
| History of DVT/PE | |
| No | 214/243 (88.1%) |
| Yes | 29/243 (11.9%) |
|
| |
| Disease site | |
| Pancreas | 101/243 (41.6%) |
| Colorectal | 83/243 (34.2%) |
| Gastro-esophageal | 24/243 (9.9%) |
| Hepatobiliary | 31/243 (12.8%) |
| Others | 4/243 (1.6%) |
|
| |
| Treatment type | |
| Chemo+targeted | 103/243 (42.4%) |
| Chemo only | 35/243 (14.4%) |
| Targeted Only | 100/243 (41.1%) |
| Other | 5/243 (2.1%) |
|
| |
| ECOG score | |
| 0 | 67/243 (27.6%) |
| ≥1 | 176/243 (72.4%) |
|
| |
| Number of metastatic sites | |
| ≤2 | 168/243 (69.1%) |
| >2 | 75/243 (30.9%) |
|
| |
| Prior systemic therapies | |
| ≤1 | 100/243 (41.1%) |
| >1 | 143/243 (58.9%) |
|
| |
| Presence of lung metastasis | |
| No | 146/243 (60.1%) |
| Yes | 97/243 (39.9%) |
|
| |
| Presence of liver metastasis | |
| No | 57/243 (23.5%) |
| Yes | 186/243 (76.5%) |
|
| |
| Prior radiation | |
| No | 170/243 (70.0%) |
| Yes | 73/243 (30.0%) |
|
| |
| Prior surgery | |
| No | 112/243 (46.1%) |
| Yes | 131/243 (53.9%) |
|
| |
| Albumin | |
| <3.5 g/dL (LLN) | 66/243 (27.2%) |
| >3.5 g/dL | 177/243 (72.8%) |
|
| |
| ALT | |
| ≤41 IU/dL (ULN) | 137/212 (64.6%) |
| >41 IU/dL | 75/212 (35.4%) |
|
| |
| AST | |
| ≤50 IU/dL (ULN) | 160/214 (74.8%) |
| >50 IU/dL | 54/214 (25.2%) |
|
| |
| Bilirubin | |
| ≤1.2 mg/dL (ULN) | 221/243 (90.9%) |
| >1.2 mg/dL | 22 (9.1%) |
|
| |
| Glucose | |
| ≤110 mg/dL | 136/243 (56.0%) |
| >110 mg/dL | 107/243 (44.0%) |
|
| |
| Hemoglobin | |
| <10 g/dL | 40/243 (16.5%) |
| ≥10 g/dL | 203/243 (83.5%) |
|
| |
| INR | |
| ≤1.1 | 167/229 (72.9%) |
| >1.1 | 62/229 (27.1%) |
|
| |
| LDH | |
| ≤618 IU/L | 125/190 (65.8%) |
| >618 IU/L | 65/190 (34.2%) |
|
| |
| Neutrophil | |
| ≤3.5 × 109/L (LLN) | 37/71 (52.1%) |
| >3.5 × 109/L | 34/71 (47.9%) |
|
| |
| Platelet | |
| <140 × 109/L | 55/243 (22.6%) |
| ≥140 × 109/L | 188/243(77.4%) |
|
| |
| Sodium | |
| ≤135 mmol/L (LLN) | 41/243 (16.9%) |
| >135 mmol/L | 202/243(83.1%) |
|
| |
| Serum protein, total | |
| <6.6 g/dL | 57/243 (23.5%) |
| ≥6.6 g/dL | 186 (76.5%) |
|
| |
| WBC | |
| ≤6 × 109/L | 123/243 (50.6%) |
| >6 × 109/L | 120/243 (49.4) |
ALT, alanine transaminase; AST, aspartate transaminase; DVT/PE, deep vein thrombosis/pulmonary embolism; ECOG, Eastern Cooperative Oncology Group; INR, International Normalized Ratio; LDH, lactate dehydrogenase; LLN, lower limits of normal; ULN, upper limits of normal; WBC, white blood cells.
Response
Our results showed that 42% of the patients progressed at the time of first staging scans. Partial responses (PR) were seen in 4% of the patients, with an additional 38% having stable disease. The clinical benefit rate was 42%. The clinical benefit rate was 57% (PR: 9%) in patients receiving chemotherapy and target therapy combination and 32% in patients receiving targeted therapy only. Response data were not available for 15% of the patients. Among the 10 responders, 9 patients received chemotherapy and targeted therapy combination and 1 patient received chemotherapy only. Seven patients with responses had pancreatic cancer and all of them received gemcitabine based regimen with somatostatin analogue as most common targeted therapy. There was 1 responder each with diagnosis of gastric cancer (carboplatin, paclitaxel and akt inhibitor), esophageal cancer (5-fluorouracil, irinotecan and somatostatin analogue) and cholangiocarcinoma (gemcitabine, cisplatin and pi3k inhibitor). The median PFS was 2 months (range: 0.2–30.8) for overall population. The median PFS among patients receiving targeted therapy, chemotherapy and combination therapy was 1.9 months, 1.9 months and 2.5 months respectively. The reason for patients coming off treatment included disease progression (64%), adverse events or complications (13%), patient withdrawal (11%), and others (11%).
Survival
The median OS for overall population was 5.8 months (range: 0.2–52.4 months). The median OS among patients receiving targeted therapy, chemotherapy and combination therapy was 4.4 months, 6.5 months and 6.3 months respectively. As shown in Table 2, variables associated with statistically significant worse survival on univariate analyses were ECOG performance status ≥1 (P=0.0003), ≥2 prior systemic therapies (P=0.0141), bilirubin >1.2 mg/dL (P=0.0267), LDH > 618 IU/L (P<0.0001), sodium ≤135 mmol/L (P=0.0033), and WBC count >6×109/L (P<0.0001). No statistical significance was seen for other laboratory values including albumin, ALT, AST, glucose, hemoglobin, INR, neutrophils, platelets, or total protein (Table 2). The 30-day and 90-day mortality rate was 0.8% (95% CI: 0.2%–3.3%) and 13.7% (95% CI: 19.0%–9.9%).
Table 2.
Univariate Analysis
| Variable | Reference | Level | HR (95% CI) | Overall P Value |
|---|---|---|---|---|
| Age | ≤60 years | >60 years | 0.89 (0.66,1.2) | 0.4485 |
| Gender | Male | Female | 0.85 (0.63,1.15) | 0.2894 |
| History of DVT/PE | No | Yes | 1.43 (0.89,2.3) | 0.1371 |
| Disease Site | Pancreas | Others | 1.15 (0.85,1.56) | 0.3663 |
| Sponsor Type | Industry | Externally peer reviewed | 1.72 (1.03,2.86) | 0.0551 |
| Industry | Institutional | 0.81 (0.52,1.26) | ||
| Treatment Class | Chemotherapy | Chemotherapy and targeted | 1.12 (0.72,1.75) | 0.3722 |
| Chemotherapy | Others | 5.21 (0.67,40.65) | ||
| Chemotherapy | Targeted therapy | 1.25 (0.79,1.97) | ||
| ECOG Score | 0 | ≥1 | 1.89 (0.34,2.69) | 0.0003 |
| Lung Metastases | No | Yes | 0.9 (0.66,1.22) | 0.4906 |
| Liver Metastases | No | Yes | 1.37 (0.96,1.96) | 0.0814 |
| Prior Radiation | No | Yes | 1.04 (0.74,1.45) | 0.8338 |
| Prior Surgery | No | Yes | 0.84 (0.62,1.14) | 0.2581 |
| Metastatic Sites | ≤2 | >2 | 1.12 (0.8,1.57) | 0.4986 |
| Prior Systemic | ≤1 | >1 | 1.48 (1.08,2.02) | 0.0141 |
| Therapies | ||||
| Albumin | <3.5 g/dL | ≥3.5 g/dL | 0.74 (0.54,1.03) | 0.0746 |
| ALT | ≤41 IU/dL | >41 IU/dL | 1.27 (0.89,1.82) | 0.1817 |
| AST | ≤50 IU/dL | >50 IU/dL | 1.44 (0.98,2.1) | 0.0629 |
| Bilirubin | ≤1.2 mg/dL | >1.2 mg/dL | 1.77 (1.07,2.94) | 0.0267 |
| Glucose | ≤110 mg/dL | >110 mg/dL | 1.2 (0.89,1.63) | 0.2293 |
| Hemoglobin | <10 g/dL | ≥10 g/dL | 0.82 (0.54,1.23) | 0.3354 |
| Hemoglobin_new | ≤11 g/dL | >11 g/dL | 0.86 (0.64,1.17) | 0.3392 |
| INR | <1.1 | >1.1 | 0.86 (0.64,1.17) | 0.3392 |
| LDH | ≤618 IU/L | >618 IU/L | 2.07 (1.44,2.96) | <0.0001 |
| Neutrophil | ≤3.5 × 109 | >3.5 × 109 | 1.64 (0.98,2.75) | 0.0602 |
| Platelet | <140 × 109/L | ≥140 × 109/L | 1.12 (0.78,1.61) | 0.5454 |
| Sodium | ≤135 mmol/L | >135 mmol/L | 0.56 (0.38,0.83) | 0.0033 |
| Total Serum Protein | <6.6 g/dL | ≥6.6 g/dL | 1.01 (0.7,1.44) | 0.9704 |
| WBC | ≤6 × 109 | >6 × 109 | 2.23 (1.63,3.06) | <0.0001 |
Multivariate survival analysis was also performed to evaluate patient factors that may be predictive of OS using stepwise Cox proportional hazard regression model (Table 3). The final model included ECOG performance score ≥1 (HR: 1.76, P=0.0049), prior systemic therapies ≥2 (HR: 1.63, P=0.0146), LDH >618 IU/L (HR: 1.85, P=0.0009), sodium >135 mmol/L (HR: 0.46, P=0.0021), and WBC >6 × 109 (HR: 1.50, P=0.0233).
Table 3.
Multivariate analysis
| Reference | Variable Level | Hazard Ratio (95% CI) | Overall P Value | |
|---|---|---|---|---|
| ECOG Score | 0 | ≥1 | 1.76 (1.19, 2.62) | 0.0049 |
| Prior Systemic Therapy | <2 | ≥2 | 1.63 (1.1, 2.4) | 0.0146 |
| LDH | ≤618 IU/L | >618 IU/L | 1.85 (1.29, 2.67) | 0.0009 |
| Sodium | ≤135 mmol/L | >135 mmol/L | 0.46 (0.28, 0.76) | 0.0021 |
| WBC | ≤6 × 109 | >6 × 109 | 1.5 (1.06, 2.14) | 0.0233 |
Application of RMH and MDACC Prognostic Score
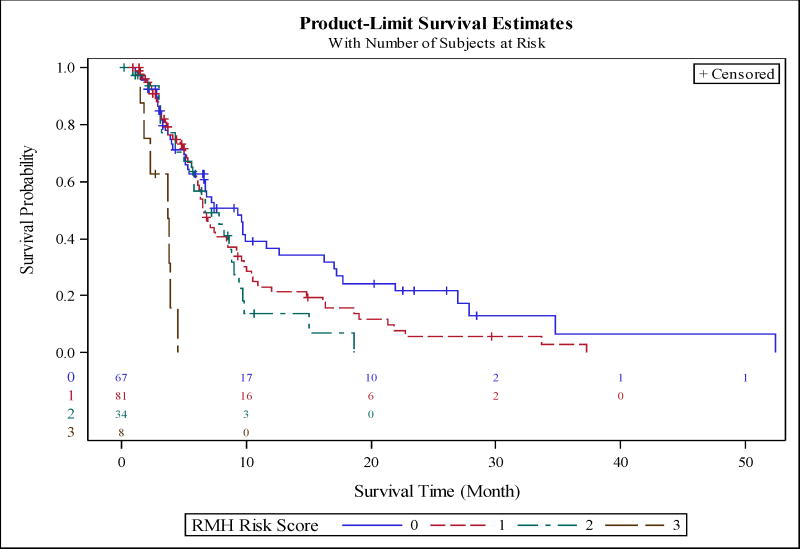
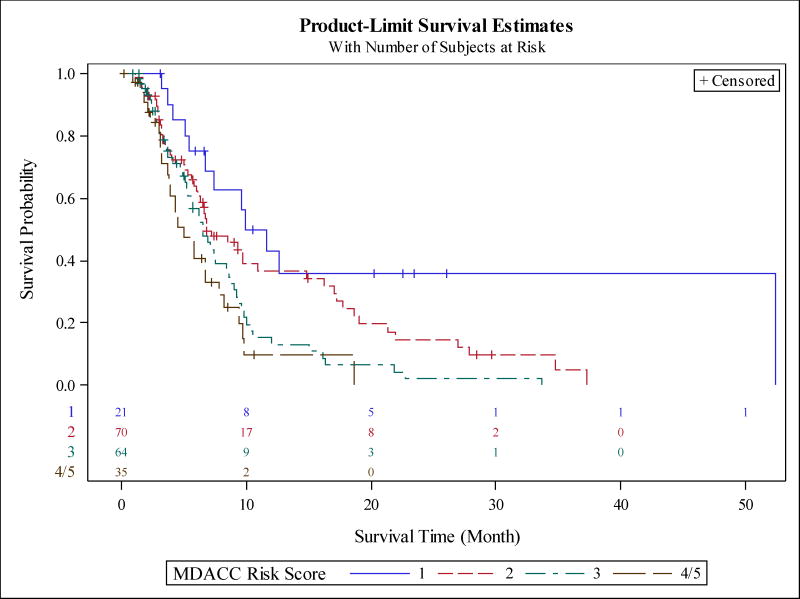
The median survival of subjects with RMH score of 0 (low risk), 1 (low-intermediate risk), 2 (intermediate risk), 3 (high risk) was 9.4, 6.6, 6.7 and 3.7 months, respectively (P<0.0001). Figure 1 shows the Kaplan-Meir survival curves by RMH score. The median survival of patients with MDACC score of 1 (low-intermediate risk), 2 (intermediate risk), 3 (high-intermediate risk), and 4/5 (high risk) was 10.8, 6.8, 6.5, and 5.1 months, respectively. Figure 2 shows the Kaplan-Meir survival curves by MDACC score. We also obtained 95% confidence intervals of c-statistics=0.444 (0.388, 0.498) with the RMH cancer risk scoring method and c-statistics=0.410 (0.359, 0.462) with the MDACC cancer risk scoring method.
Figure 1.
Application of Royal Marsden Hospital Prognostic score to our dataset. The model includes LDH >618 IU/L, albumin <3.5 mg/dL, and number of metastatic sites >2. Each variable was assigned one point with scores of 3 considered high risk and score of 0 being lowest. The median survival of subjects with score of 0 (low risk), 1 (low-intermediate risk), 2 (intermediate risk), and 3 (high risk) was 9.4, 6.6, 6.7, and 3.7 months, respectively (P<0.0001).
Figure 2.
Application of MD Anderson Cancer Center Prognostic score to our dataset. The model includes LDH >618, albumin <3.5, number of metastatic sites >2, ECOG performance status ≥1, and GI tumor type. Each variable was assigned one point, with 5 considered high risk and 0 being lowest. The median survival of patients with score of 1 (low-intermediate risk), 2 (intermediate risk), 3 (high-intermediate risk), and 4/5 (high risk) was 10.8, 6.8, 6.5, and 5.1 months, respectively (P=0.0004).
Discussion
In this study, we evaluated patient characteristics and OS for 243 consecutive patients with advanced GI tumors who participated in phase I clinical trials from 2007 to 2013 at Moffitt Cancer Center. This is the largest study of this patient population to date. Prior studies have evaluated patient characteristics and have proposed models for predicting patient survival but not in a specific population of patients with GI malignancies. In fact, very little data are available for this patient population, as well as in regard to which patient characteristics may influence survival. We also applied the RMH and MDACC prognostic scores that were developed using different disease types to our dataset to evaluate for consistency.
There have been many limitations of prior studies evaluating which patient factors are predictive of survival. In fact, in those investigations that have evaluated phase I trials, almost all have included patients with all cancer types. Only a limited number of studies have so far evaluated phase I patient outcomes and the corresponding patient characteristics in specific cancer populations including gastrointestinal (GI) malignancies.17,18 It is not understood whether differences in the pathophysiology of various malignancies can have a meaningful impact on prognostic factors. More studies are required within specific cancer populations to validate existing studies and evaluate for possible differences. Furthermore, treatments have become increasingly diverse, which can now include targeted therapies and immunomodulatory agents. Therefore, further studies are needed to evaluate patient populations in specific therapy classes. Because novel agents, including targeted and immunotherapy, have become more common, studies of prognostic models will need to adapt and incorporate a more representative population.19
Our multivariate analysis revealed improved survival associated with ECOG score ≥1, prior systemic therapies <2, LDH ≤618 IU/L, sodium >135 mmol/L, and WBC ≤6 × 109. ECOG Performance status, LDH levels, and number of prior therapies have been demonstrated to be prognostic factors in oncology patients participating in phase I clinical trials. In addition, we found hyponatremia and increased WBC count >6,000/μL to be a negative prognostic factor in our study. This is consistent with studies that have noted hyponatremia as a predictor of increased mortality in cancer patients as well as in the general population.20–23 However, these are small, retrospective studies that need further validation, especially in the setting of phase I clinical trials. Leukocytosis has also been associated with a worse prognosis in certain groups of cancer patients.24–27 Interestingly, previous studies have failed to show prognostic significance associated with increasing patient age.28,29 As we continue to evaluate these patient populations and their characteristics, we will improve our ability to predict survival.
In our GI patient group, we found median OS to be 5.8 months (0.2–52.4 months). We also noted a median PFS of 2 months (0.2–30.8 months). These findings are consistent with prior reports of phase I participants that included all malignancies, which showed OS between 5 and 10 months.8,14,30,31 It is expected that survival may improve in the future, especially as novel therapies including targeted therapies and immunotherapy become increasingly available. In our series, patients receiving combination of chemotherapy and targeted agents had better clinical outcomes than either treatment regimen alone. These findings need to be confirmed in a larger study. The patient populations evaluated in each study vary widely in type of malignancy, treatment type, institution, and many other variables, which may affect those patient characteristics predictive of survival.
We found that phase I clinical trials are a reasonable treatment option for patients with GI malignancies. The perception of phase I clinical trials as a valid treatment option will continue to improve as increasing evidence supports the role of clinical trials in patient care and guide patient selection into trials. The characteristics described here can potentially be used to evaluate patients for enrollment in phase I clinical trials. As more prognostic information becomes available regarding specific patient populations, it will remain crucial to evaluate each patient as an individual. It must be recognized that prognostic models cannot replace clinical judgment. Because there are limitations to the utility of prognostic models, there are also multiple limitations to our study. Although our sample size is the largest evaluation of all phase I GI cancer patients to date, a larger sample size would be required to more precisely translate our findings to broader populations.
In addition, because of the retrospective nature of our study, our results would need to be validated in future prospective studies. Our study also evaluated patients over a 6-year time span, which may be viewed as a limitation due to the development of newer therapeutic options. However, we did not find any differences in the survival by the year of enrollment. Our patients received a diverse range of therapeutic agents, with the majority receiving targeted therapy alone or a combination of chemo- and targeted therapy. This is consistent with the recent trend in drug development. This study includes patients from a single cancer center and therefore includes bias in patient selection, management decisions, malignancy types, and treatments that may not be reflective of the general patient population. We also recognize a selection bias because phase I study-related eligibility criteria typically excludes patients with poor performance status and organ dysfunction.
Clinical judgment continues to be the cornerstone for selecting patients for participation in early-phase clinical trials. It is unlikely that any prognostic model will be developed that can accurately predict outcomes for diverse groups of patients who may wish to enroll in phase I clinical trials. Despite this, the complexities and limitations of attempting to predict patient mortality in phase I studies should not deter the future evaluation of these patient populations. The models to predict outcome will continue to guide clinical decision making and are a key step in developing successful and efficient clinical trials in the future.
Acknowledgments
Funding: Our study received valuable assistance from the Clinical Trials Laboratory Core, Cancer Research Informatics Core, and Biostatistics Core at the H. Lee Moffitt Cancer Center and Research Institute, an NCI-designated Comprehensive Cancer Center, supported under NIH Grant P30-CA76292.
We thank Rasa Hamilton (Moffitt Cancer Center) for editorial assistance.
Footnotes
Conflicts of Interest: None
Presented in part at GI ASCO 2015
References
- 1.Society AC. Cancer Facts and Figures 2013. 2013. [Google Scholar]
- 2.Unger JM, Coltman CA, Crowley JJ, et al. Impact of the Year 2000 Medicare Policy Change on Older Patient Enrollment to Cancer Clinical Trials. Journal of Clinical Oncology. 2006;24(1):141–144. doi: 10.1200/JCO.2005.02.8928. [DOI] [PubMed] [Google Scholar]
- 3.Knickman JR, Snell EK. The 2030 problem: caring for aging baby boomers. Health services research. 2002;37(4):849–884. doi: 10.1034/j.1600-0560.2002.56.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Critical role of phase I clinical trials in cancer treatment. American Society of Clinical Oncology. Journal of Clinical Oncology. 1997;15(2):853–859. doi: 10.1200/JCO.1997.15.2.853. [DOI] [PubMed] [Google Scholar]
- 5.Eisenhauer EA, O’Dwyer PJ, Christian M, Humphrey JS. Phase I Clinical Trial Design in Cancer Drug Development. Journal of Clinical Oncology. 2000;18(3):684. doi: 10.1200/JCO.2000.18.3.684. [DOI] [PubMed] [Google Scholar]
- 6.Italiano A, Massard C, Bahleda R, et al. Treatment outcome and survival in participants of phase I oncology trials carried out from 2003 to 2006 at Institut Gustave Roussy. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2008;19(4):787–792. doi: 10.1093/annonc/mdm548. [DOI] [PubMed] [Google Scholar]
- 7.Wheler1 Jennifer, Tsimberidou1 Apostolia M, Hong1 David, et al. Survival of 1,181 Patients in a Phase I Clinic: The MD Anderson Clinical Center for Targeted Therapy Experience. Clin Cancer Research. 2012;18(10):2922–2929. doi: 10.1158/1078-0432.CCR-11-2217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wheler J, Tsimberidou AM, Hong D, et al. Survival of patients in a Phase 1 Clinic: the M. D. Anderson Cancer Center experience. Cancer. 2009;115(5):1091–1099. doi: 10.1002/cncr.24018. [DOI] [PubMed] [Google Scholar]
- 9.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. American journal of clinical oncology. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 10.Arkenau HT, Olmos D, Ang JE, et al. 90-Days mortality rate in patients treated within the context of a phase-I trial: how should we identify patients who should not go on trial? European journal of cancer (Oxford, England : 1990) 2008;44(11):1536–1540. doi: 10.1016/j.ejca.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Arkenau HT, Barriuso J, Olmos D, et al. Prospective validation of a prognostic score to improve patient selection for oncology phase I trials. J Clin Oncol. 2009;27(16):2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 12.Yourman Lindsey C, MSJL, MD, MAS, Schonberg Mara A, MD, MPH, Widera Eric W, MD, Smith Alexander K., MD, MS, MPH Prognostic Indices for Older Adults: A Systematic Review. JAMA: the journal of the American Medical Association. 2012;307(2):182–192. doi: 10.1001/jama.2011.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LoConte Noelle K, MD, 1,2, Smith Maureen, MD, MS, PhD,1,3, Alberti Dona, RN,1, Bozeman Jeffrey, BS,1, Cleary James F, MBBS,1, Setala Ashley N, 3, Wodtke Geoff, BS,3, Wilding George, MD,1,2, Holen Kyle D., MD1,2 Amongst eligible patients, age and comorbidity do not predict for dose limiting toxicity from phase I chemotherapy. Cancer Chemother Pharmacology. 2010;54(4):775–780. doi: 10.1007/s00280-009-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arkenau H-T, Barriuso J, Olmos D, et al. Prospective Validation of a Prognostic Score to Improve Patient Selection for Oncology Phase I Trials. Journal of Clinical Oncology. 2009;27(16):2692–2696. doi: 10.1200/JCO.2008.19.5081. [DOI] [PubMed] [Google Scholar]
- 15.Ignacio Garrido-Laguna MFJ, MD, PhD1, Vaklavas Christos, MD1, Falchook Gerald S, MD1, Fu Siqing, MD, PhD1, Hong David S, MAN, MD1, Tsimberidou Apostolia M, MD, PhD1, Wen Sijin, PhD2, Kurzrock Razelle., MD1 Validation of the Royal Marsden Hospital Prognostic Score in Patients Treated in the Phase I Clinical Trials Program at the MD Anderson Cancer Center. Cancer. 2012;118:1422–1428. doi: 10.1002/cncr.26413. [DOI] [PubMed] [Google Scholar]
- 16.Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statistics in medicine. 1996;15(4):361–387. doi: 10.1002/(SICI)1097-0258(19960229)15:4<361::AID-SIM168>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 17.Tsimberidou AM, Vaklavas C, Wen S, et al. Phase I clinical trials in 56 patients with thyroid cancer: the M. D. Anderson Cancer Center experience. The Journal of clinical endocrinology and metabolism. 2009;94(11):4423–4432. doi: 10.1210/jc.2009-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garrido-Laguna I, Janku F, Falchook GS, et al. Patients with advanced head and neck cancers have similar progression-free survival on phase I trials and their last food and drug administration-approved treatment. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(15):4031–4037. doi: 10.1158/1078-0432.CCR-10-0672. [DOI] [PubMed] [Google Scholar]
- 19.Fox E, Curt GA, Balis FM. Clinical Trial Design for Target-Based Therapy. The Oncologist. 2002;7(5):401–409. doi: 10.1634/theoncologist.7-5-401. [DOI] [PubMed] [Google Scholar]
- 20.Mohan S, Gu S, Parikh A, Radhakrishnan J. Prevalence of hyponatremia and association with mortality: results from NHANES. The American journal of medicine. 2013;126(12):1127–1137. e1121. doi: 10.1016/j.amjmed.2013.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doshi SM, Shah P, Lei X, Lahoti A, Salahudeen AK. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59(2):222–228. doi: 10.1053/j.ajkd.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Hermes A, Waschki B, Reck M. Hyponatremia as prognostic factor in small cell lung cancer--a retrospective single institution analysis. Respiratory medicine. 2012;106(6):900–904. doi: 10.1016/j.rmed.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Berardi R, Caramanti M, Fiordoliva I, et al. Hyponatraemia is a predictor of clinical outcome for malignant pleural mesothelioma. Supportive care in cancer : official journal of the Multinational Association of Supportive Care in Cancer. 2015;23(3):621–626. doi: 10.1007/s00520-014-2398-6. [DOI] [PubMed] [Google Scholar]
- 24.So KA, Hong JH, Jin HM, et al. The prognostic significance of preoperative leukocytosis in epithelial ovarian carcinoma: a retrospective cohort study. Gynecologic oncology. 2014;132(3):551–555. doi: 10.1016/j.ygyno.2014.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Njolstad TS, Engerud H, Werner HM, Salvesen HB, Trovik J. Preoperative anemia, leukocytosis and thrombocytosis identify aggressive endometrial carcinomas. Gynecologic oncology. 2013;131(2):410–415. doi: 10.1016/j.ygyno.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 26.Mabuchi S, Matsumoto Y, Hamasaki T, et al. Elevated white blood cell count at the time of recurrence diagnosis is an indicator of short survival in patients with recurrent cervical cancer. International journal of gynecological cancer : official journal of the International Gynecological Cancer Society. 2012;22(9):1545–1551. doi: 10.1097/IGC.0b013e31826ea0eb. [DOI] [PubMed] [Google Scholar]
- 27.Tomita M, Shimizu T, Hara M, Ayabe T, Onitsuka T. Preoperative leukocytosis, anemia and thrombocytosis are associated with poor survival in non-small cell lung cancer. Anticancer research. 2009;29(7):2687–2690. [PubMed] [Google Scholar]
- 28.Aapro MS, Köhne C-H, Cohen HJ, Extermann M. Never too old? Age should not be a barrier to enrollment in cancer clinical trials. The Oncologist. 2005;10(3):198–204. doi: 10.1634/theoncologist.10-3-198. [DOI] [PubMed] [Google Scholar]
- 29.Zafar SF, Heilbrun LK, Vishnu P, et al. Participation and survival of geriatric patients in Phase I clinical trials: the Karmanos Cancer Institute (KCI) experience. Journal of geriatric oncology. 2011;2(1):18–24. doi: 10.1016/j.jgo.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arkenau HT, Olmos D, Ang JE, de Bono J, Judson I, Kaye S. Clinical outcome and prognostic factors for patients treated within the context of a phase I study: the Royal Marsden Hospital experience. British journal of cancer. 98(6):1029–1033. doi: 10.1038/sj.bjc.6604218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bachelot T, Ray-Coquard I, Catimel G, et al. Multivariable analysis of prognostic factors for toxicity and survival for patients enrolled in phase I clinical trials. Annals of oncology : official journal of the European Society for Medical Oncology / ESMO. 2000;11(2):151–156. doi: 10.1023/a:1008368319526. [DOI] [PubMed] [Google Scholar]