Abstract
Background
Antiretroviral treatment (ART) for HIV-positive patients has expanded rapidly in Asia over the last ten years. Our study aimed to describe the time trends and risk factors for overall survival in patients receiving first-line ART in Asia.
Methods
We included HIV-positive adult patients who initiated ART between 2003–2013 (n=16 546), from seven sites across six Asia-Pacific countries. Patient follow-up was to May 2014. We compared survival for each country and overall by time period of ART initiation using Kaplan-Meier curves. Factors associated with mortality were assessed using Cox regression, stratified by site. We also summarized first-line ART regimens, CD4 count at ART initiation, and CD4 and HIV viral load testing frequencies.
Results
There were 880 deaths observed over 54 532 person-years of follow-up, a crude rate of 1.61 (1.51, 1.72) per 100 person-years. Survival significantly improved in more recent years of ART initiation. The survival probabilities at 4 years follow-up for those initiating ART in 2003–05 was 92.1%, 2006–09 was 94.3% and 2010–2013 was 94.5% (p<0.001). Factors associated with higher mortality risk included initiating ART in earlier time periods, older age, male sex, injecting drug use as HIV exposure and lower pre-ART CD4 count. Concurrent with improved survival was increased tenofovir use, ART initiation at higher CD4 counts, and greater monitoring of CD4 and HIV viral load.
Conclusions
Our results suggest that HIV-positive patients from Asia have improved survival in more recent years of ART initiation. This is likely a consequence of improvements in treatment and, patient management and monitoring over time.
Introduction
The introduction of combination antiretroviral treatment (ART) has substantially increased the survival of individuals living with HIV [1–4]. In developed countries, ample treatment options and accessibility have significantly improved overall survival and increased the life expectancy of HIV-positive individuals [5]. However, HIV-positive individuals from resource-limited settings (RLS) have faced significant barriers to accessing ART. Prior to 2005, it was estimated that only 7% of HIV-positive patients in need of treatment in low- and middle- income countries had access to ART [6]. The “3 by 5” initiative, launched by UNAIDS and the World Health Organization (WHO), aimed to provide a pathway to large-scale expansion of first-line ART for people living with HIV/AIDS in RLS [7]. In parallel to these global efforts, the number of eligible people accessing ART in the Asia-Pacific region has incrementally increased [8]. Yet, barriers to accessing treatment still remain for many HIV-positive patients in Asia [9, 10]. In 2012, the estimated treatment coverage rate for the Asia-Pacific was 51%, which was lower than the global average of 61% [8].
Over time, there have been changes to treatment guidelines and patient management to contribute to further improvements in survival. In 2006, the WHO guidelines recommended a move away from stavudine (d4T) use in first-line regimens, due to toxicities and side effects, and increased use of tenofovir (TDF), zidovudine (AZT) and abacavir (ABC) [11, 12]. The 2010 WHO guidelines recommended earlier ART initiation, increasing the suggested CD4 cell count threshold of when to initiate ART to 350 cells/mm3 in asymptomatic patients [13]. The 2013 WHO guidelines further increased this threshold to 500 cells/mm3 and, the 2015 guidelines now advise initiation of ART for all HIV-positive individuals, regardless of the CD4 cell count [14, 15]. Earlier initiation of ART has contributed to increased survival and longer life expectancies of HIV-positive people in the developed and RLS countries [16–20]. Countries in the Asia-Pacific region progressively aligned their national guidelines to the WHO guidelines to encourage earlier initiation of ART and the use of tolerable ART regimens [21, 22].
Although, there have been substantial improvements to the care of HIV-positive patients in the Asia-Pacific region, disparities in income and care infrastructures are important factors that influence a country’s ability to offer optimal care to HIV-positive patients, which impacts long-term outcomes and risks of drug resistance and mortality [23, 24]. Therefore, there is a distinct need to monitor and document the survival trends of HIV-positive patients in the Asia-Pacific.
This study aimed to analyze and describe the time trends in and factors affecting overall survival in HIV-positive patients receiving first-line ART enrolled in the TREAT Asia HIV Observational Database Low Intensity Transfer (TAHOD-LITE) cohort, and summarize other concurrent changes in treatment and patient management over the study period.
Methods
Data collection and participants
TAHOD-LITE is a sub-study of the TREAT Asia HIV Observational database (TAHOD). Whereas 21 HIV treatment centres participate in TAHOD and contribute detailed data on a subset of patients in care at the site [25], TAHOD-LITE currently involves seven sites from Cambodia, Hong Kong, India, Indonesia, Singapore and Vietnam, that contribute key data on all patients seen at the site. TAHOD-LITE began collecting retrospective data in 2014 and to date includes data from over 30 000 HIV-positive adult patients. As all patients seen at the sites are included in TAHOD-LITE, it allows robust and representative analyses of trends in ART use, response to treatment and other key patient care aspects at the sites. Patient data are collected during routine clinical care, and then anonymized before being electronically transferred to the data management and analysis centre at the Kirby institute, University of New South Wales, Sydney, Australia. The database closure date was in March–May 2014 for all of the participating sites. The current data transfer includes patient data through to May 2014. The core data variables collected include:
Demographics: Most recent clinic visit date, sex, date of birth, mode of HIV exposure, HIV diagnosis date, date of death and reason for death.
Hepatitis serology: HBV surface antigen test result and date, and HCV antibody test result and date.
Immunology and virology: CD4 and CD8 T lymphocyte counts and HIV viral load counts.
ART history.
Ethical approvals for TAHOD-LITE were obtained from Institutional Review Boards (IRB) at each participating site, the University of New South Wales, and the coordinating center at TREAT Asia/amfAR. Written informed consent for collection of study data is not obtained unless required by the site-specific IRBs.
This analysis included all patients aged over 18 years, who had been on a treatment regimen consisting of three or more drugs from 01 January 2003 to 31 December 2013 and who had at least one subsequent visit after the date of ART initiation. The primary study endpoint was mortality. Secondary analyses focused on temporal changes in the first-line ART regimen, CD4 cell count at ART initiation and, CD4 and HIV viral load testing frequencies.
Statistical analyses
Follow-up was from the start of ART initiation and censored at the date of death or most recent clinic visit, whichever occurred first. We used an intention-to-treat approach where any changes to treatment after ART initiation were ignored. Patients lost to follow-up (LTFU) were defined as those who had not been seen 12 months prior to 31 December 2013 and had not been transferred to another clinic or died. Sites ascertained deaths mainly through hospital records, although some sites further retrieved death records from death registries and/or tracing in cases where patients had died at home. We compared survival for each country and overall by time period of ART initiation using Kaplan-Meier curves. A log rank test was used to determine whether survival significantly differed between the study time periods.
Combination treatment regimens were categorized into three categories. The first treatment category consisted of nucleoside reverse transcriptase inhibitors (NRTIs) and a nonnucleoside reverse transcriptase inhibitor (NNRTI). The second treatment category consisted of NRTIs and a protease inhibitor (PI). The third treatment category was made of all other combinations of NRTIs, NNRTIs and PIs. The covariates considered were: time period of ART initiation (2003–05, 2006–09, 2010–13); age at ART initiation; mode of HIV exposure (heterosexual contact, homosexual contact, injecting drug use and other/unknown); pre-ART HIV viral load (≤100 000 copies/mL, >100 000 copies/mL, missing); pre-ART CD4 cell count (≤50 cells/μL, 51–100 cells/μL, 101–200 cells/μL, >200 cells/μL, missing); first ART regimen (NRTIs+NNRTI, NRTIs+PI, other); previous mono/dual therapy exposure (no, yes); HBV surface antigen (HBsAg: negative, positive, not tested); HCV antibody (HCV Ab: negative, positive, not tested). Cox proportional hazard models, stratified by clinical site, were used to evaluate the factors affecting mortality. Covariates were selected a priori for both univariate and multivariate analysis, with hazard ratios presented.
The CD4 cell count at ART initiation was considered as the most recent result within six months prior to ART initiation. The median CD4 cell count at ART initiation, with interquartile range (IQR), was then calculated by time period. The rate of CD4 and HIV viral load testing after ART initiation was calculated for each time period. CD4 and HIV viral load tests prior to ART initiation were excluded. CD4 and HIV viral load testing rates, and their 95% confidence intervals (95% CI), were presented per person-year of follow-up (per py).
Data were analysed using Stata version 12 (Stata Corporation, College Station, Texas, USA) and SAS Software (Version 9.4 for Windows).
Results
A total of 17 886 patients, aged 18 years or older at first clinic attendance, had initiated ART between 01 January 2003 and 31 December 2013. Of these, 1 340 patients did not have subsequent visits after ART initiation and were excluded. The remaining 16 546 patients were included in our analysis.
Baseline Characteristics
Overall, the majority of patients were male (68%), initiated ART in more recent time periods (2003–05: 17%; 2006–09: 37%; 2010–13: 46%) and had heterosexual contact as their mode of HIV exposure (82%). The median age at ART initiation was 35 years (IQR: 30–42). The median pre-ART HIV viral load was 122 409 copies/mL (IQR: 34 440 – 379 000) and CD4 cell count was 134 cells/μL (IQR: 50–229). Approximately 96% of the patients had initiated a first-line ART regimen consisting of NRTIs+NNRTI and about 4% of patients had previous mono/dual therapy. Over half of the patients had not been tested for HCV and/or HBV during care. Of those who were tested, 13% and 10% were positive for HCV and HBV, respectively. A summary of the patient demographics overall and by country is provided in Appendix 1.
Survival outcomes and survival probability
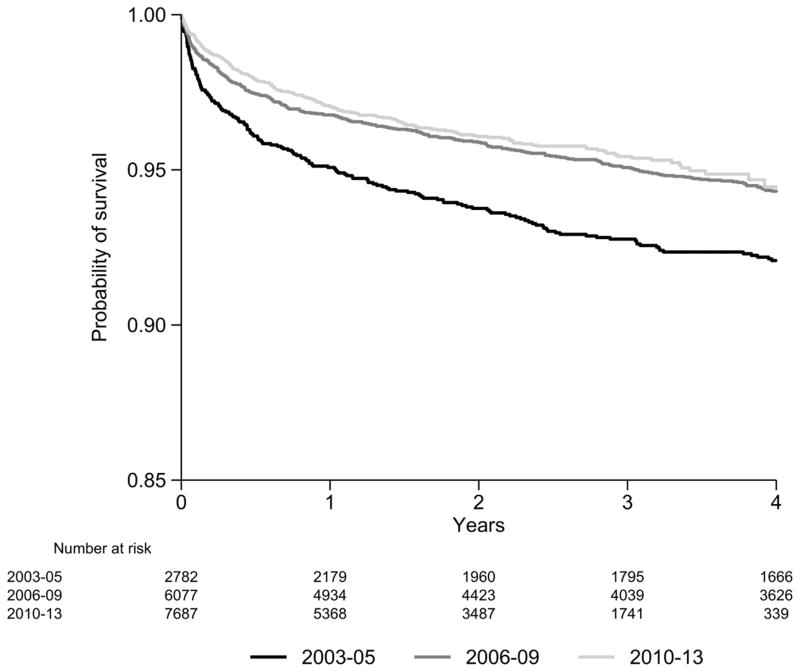
The mean duration of follow-up was approximately 40 months (95% CI: 39.36, 40.36). There were 880 deaths over 54 532 pys, for a crude death rate of 1.61 (95% CI: 1.51, 1.72) per 100 pys. Of the 880 deaths, there were 555 HIV-related deaths (63%), 191 non-HIV-related deaths (22%) and 134 deaths from unknown causes (15%). A total of 399 of the 880 deaths (39%) occurred in the first six months from ART initiation. The survival probabilities at one year follow-up for patients initiating ART in 2003–05, 2006–09 and 2010–13 was 95.1% (95% CI: 94.2, 95.9%), 96.8% (95% CI: 96.3, 97.2%) and 97.1% (95% CI: 96.6, 97.4%), respectively (Figure 1). The survival probabilities at four years of follow up for patients initiating ART in 2003–05, 2006–09 and 2010–13 was 92.1% (95% CI: 90.9, 93.1%), 94.3% (95% CI: 93.6, 94.9%) and 94.5% (95% CI: 93.5, 95.3%), respectively (Figure 1). The overall survival probabilities were significantly different between the three time periods (p< 0.001). Some differences were noted between countries (Appendix 2) and there was also an overall trend for a higher survival probability for those initiating in more recent years by country.
Figure 1.
Survival estimates across all countries by time period of ART initiation.
Predictors of mortality
The multivariate Cox regression model, stratified by clinical site, suggested that initiating ART in earlier time periods, older age, male sex, injecting drug use as the mode of HIV exposure and lower pre-ART CD4 cell count were significantly associated with a higher risk of mortality (Table 1). Patients initiating ART in 2006–09 and 2010–13 had a hazard ratio of 0.78 (95% CI: 0.65, 0.93) and 0.73 (95% CI: 0.59, 0.91) respectively, compared with those initiating ART in 2003–05, adjusting for other relevant covariates and stratified by clinical site.
Table 1.
Factors associated with mortality.
| Number of patients | Deaths | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | Adjusted HR | 95% CI | p | |||
| Total | 16546 | 880 | ||||||
|
| ||||||||
| Time period of ART Initiation | <0.001 | 0.005 | ||||||
| 2003–05 | 2782 | 257 | 1 | 1 | ||||
| 2006–09 | 6077 | 348 | 0.68 | (0.57, 0.81) | <0.001 | 0.78 | (0.65, 0.93) | 0.007 |
| 2010–13 | 7687 | 275 | 0.52 | (0.42, 0.64) | <0.001 | 0.73 | (0.59, 0.91) | 0.005 |
|
| ||||||||
| Age at ART initiation (years) | <0.001 | <0.001 | ||||||
| ≤30 | 4512 | 175 | 1 | 1 | ||||
| 31–40 | 7413 | 364 | 1.24 | (1.03, 1.48) | 0.021 | 1.11 | (0.92, 1.33) | 0.267 |
| 41–50 | 3129 | 182 | 1.47 | (1.19, 1.81) | <0.001 | 1.31 | (1.06, 1.63) | 0.013 |
| 51+ | 1492 | 159 | 2.62 | (2.09, 3.28) | <0.001 | 2.36 | (1.86, 2.99) | <0.001 |
|
| ||||||||
| Sex | ||||||||
| Male | 11253 | 702 | 1 | 1 | ||||
| Female | 5293 | 178 | 0.58 | (0.49, 0.69) | <0.001 | 0.74 | (0.62, 0.89) | 0.001 |
|
| ||||||||
| Mode of HIV Exposure | <0.001 | <0.001 | ||||||
| Heterosexual contact | 13512 | 684 | 1 | 1 | ||||
| Homosexual contact | 1085 | 44 | 0.54 | (0.38, 0.75) | <0.001 | 0.82 | (0.58, 1.15) | 0.255 |
| Injecting drug use | 773 | 87 | 2.30 | (1.75, 3.02) | <0.001 | 2.03 | (1.44, 2.87) | <0.001 |
| Other/unknown | 1176 | 65 | 1.00 | (0.77, 1.30) | 0.996 | 1.06 | (0.82, 1.38) | 0.641 |
|
| ||||||||
| Pre-ART HIV viral load (copies/mL) | ||||||||
| ≤100000 | 1515 | 53 | 1 | 1 | ||||
| >100000 | 1788 | 111 | 1.74 | (1.26, 2.42) | 0.001 | 1.21 | (0.87, 1.68) | 0.266 |
| Missing | 13243 | 716 | 2.51 | (1.85, 3.41) | <0.001 | 1.59 | (1.16, 2.19) | 0.004 |
|
| ||||||||
| Pre-ART CD4 cell count (cells/μL) | <0.001 | <0.001 | ||||||
| ≤50 | 3605 | 326 | 1 | 1 | ||||
| 51–100 | 2266 | 183 | 0.86 | (0.71, 1.03) | 0.110 | 0.88 | (0.73, 1.06) | 0.179 |
| 101–200 | 3796 | 172 | 0.46 | (0.38, 0.55) | <0.001 | 0.49 | (0.41, 0.60) | <0.001 |
| >200 | 4518 | 83 | 0.21 | (0.16, 0.27) | <0.001 | 0.26 | (0.20, 0.33) | <0.001 |
| Missing | 2361 | 116 | 0.53 | (0.42, 0.67) | <0.001 | 0.55 | (0.44, 0.69) | <0.001 |
|
| ||||||||
| First ART regimen | 0.526 | 0.190 | ||||||
| NRTI+NNRTI | 15911 | 835 | 1 | 1 | ||||
| NRTI+PI | 569 | 42 | 1.18 | (0.85, 1.63) | 0.320 | 1.36 | (0.98, 1.89) | 0.068 |
| Other/unknown | 66 | 3 | 0.74 | (0.24, 2.31) | 0.605 | 1.07 | (0.34, 3.36) | 0.903 |
|
| ||||||||
| Previous mono/duo exposure | ||||||||
| No | 15951 | 825 | 1 | 1 | ||||
| Yes | 595 | 55 | 1.41 | (1.07, 1.86) | 0.015 | 1.31 | (0.99, 1.75) | 0.060 |
|
| ||||||||
| HBV co-infection | ||||||||
| Negative | 5762 | 331 | 1 | 1 | ||||
| Positive | 646 | 48 | 1.23 | (0.91, 1.67) | 0.181 | 1.12 | (0.83, 1.53) | 0.451 |
| Not tested | 10138 | 501 | 1.58 | (1.19, 2.11) | 0.002 | 1.62 | (1.16, 2.26) | 0.004 |
|
| ||||||||
| HCV co-infection | ||||||||
| Negative | 7047 | 370 | 1 | 1 | ||||
| Positive | 1021 | 88 | 1.72 | (1.32, 2.24) | <0.001 | 1.07 | (0.77, 1.49) | 0.681 |
| Not tested | 8478 | 422 | 1.11 | (0.90, 1.38) | 0.326 | 0.87 | (0.69, 1.09) | 0.224 |
Note: Global p-values for year of ART initiation, age and pre-ART CD4 cell count are for tests for trend. Other global p-values are for tests for heterogeneity.
First-line ART regimen
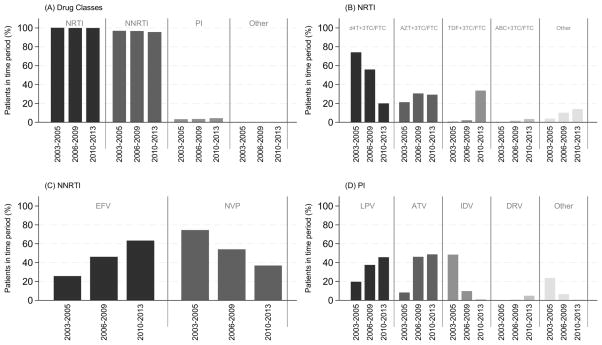
Figure 2A shows that NRTI and NNRTI use in the first-line ART regimen has remained high (>95%) over the study time periods of ART initiation, while PI use has been relatively uncommon (<10%) throughout. Nearly all NRTI drugs initiated were in combination with lamivudine (3TC) or emtricitabine (FTC). d4T + 3TC/FTC use decreased from 74.1% in 2003–05 to 20.0% in 2010–13, whilst TDF + 3TC/FTC use has increased for those initiating ART in more recent time periods, from 0.6% in 2003–05 to 33.5% in 2010–13. AZT + 3TC/FTC and abacavir (ABC) + 3TC/FTC use has remained relatively stable throughout the time periods of ART initiation (Figure 2B). Efavirenz (EFV) use increased, from 25.6% in 2003–05 to 63.1% in 2010–13 and nevirapine (NVP) use decreased, from 74.4% in 2003–05 to 36.8% in 2010–13 (Figure 2C). PI use has been dominated by lopinavir/ritonavir (LPV) and atazanavir/ritonavir (ATV) in more recent time periods, increasing from 19.6% and 8.2% in 2003–05 to 45.6% and 48.5% in 2010–13, respectively. Indinavir (IDV) use significantly decreased from 48.5% in 2003–05 to 0.6% in 2010–13 (Figure 2D). There were 66 patients that had initiated a first-line ART regimen consisting of other drug combinations. The majority of other regimens were NRTI+NNRTI+PI (n=42), while less common other drug regimens were NRTI only (n=6) or NRTI+ integrase inhibitor (n=7).
Figure 2. First-line ART regimen by time period of ART initiation.
(A) Proportion of patients by drug class. (B) Proportion of patients by NRTI drug or drug combination. Other NRTI drugs initiated included: Abacavir (0.1% overall time periods); Didanosine (0.7%); Stavudine (0.2%); Zazlcitabine (<0.1%); Zidovudine (0.1%); Emtricitabine (4.9%); Tenofovir (4.7%). (C) Proportion of patient by NNRTI drug. Not represented are Etravirine (<0.1%) and Rilpivirine (<0.1%). (D) Proportion of patients by PI Ddrug. Other PI drugs initiated included: Nelfinavir (2.1%); Ritonavir (0.4%); Saquinaavir (3.4%). NRTI = nucleoside reverse transcriptase inhibitor; NNRTI = nonnucleoside reverse transcriptase inhibitor; PI = protease inhibitor; 3TC/FTC = lamivudine/emtricitabine; d4T = stavudine; AZT = zidovudine; TDF = tenofovir; ABC = abacavir; EFV = efavirenze; NVP = nevirapine; LPV = lopinavir/ritonavir; ATV = atazanavir/ritonavir; IDV = indinavir; DRV = darunavir.
CD4 cell count at ART initiation
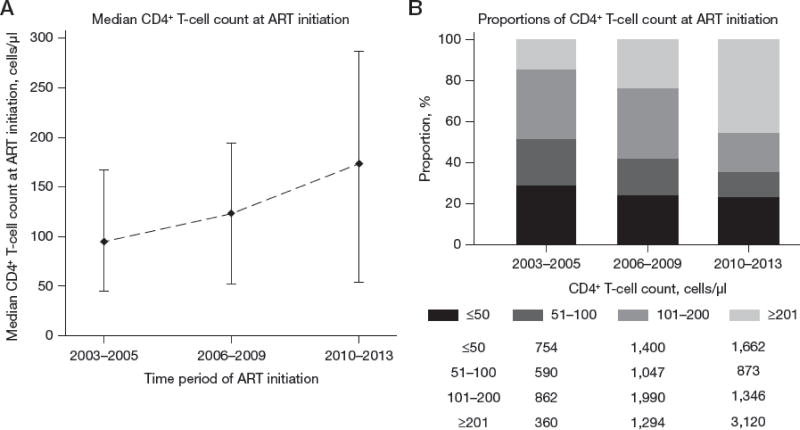
Of the 16 546 patients, 2 361 (14%) did not have a CD4 cell count within six months prior to ART initiation. Across all countries, there was an increasing trend in the median CD4 cell count at ART initiation, from 94.5 cells/μL (IQR: 45–167 cells/μL) in 2003–05 to 174 cells/μL (IQR: 54–286 cells/μL) in 2010–13 (Figure 3A). The proportion of patients with a CD4 cell count ≥201 cells/μL at ART initiation also increased with increasing year of ART initiation (Figure 3B). This increasing trend was also present when stratified by country, though less evident in sites from Indonesia and Vietnam.
Figure 3. Summary of the CD4 cell count (cells/μL) at ART initiation, across all countries.
(A) Median CD4 cell count (cells/μL) at ART initiation, by time period of ART initiation and with interquartile range shown. (B) Proportion of patients CD4 cell count (cells/μL) at ART initiation with absolute patient numbers below, by time period of ART initiation.
CD4 and HIV viral load testing rates
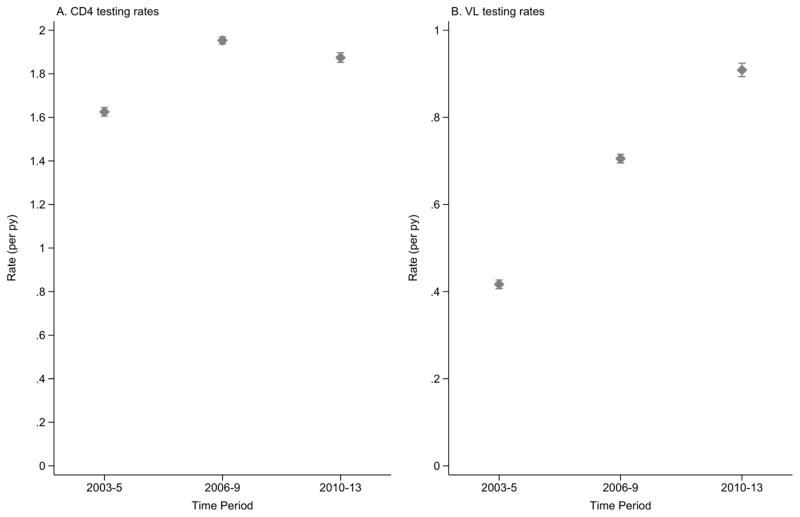
The crude rate of CD4 testing for all countries and all time periods was 1.84 tests per py (95% CI: 1.83, 1.85). The CD4 testing rate for all countries increased for those initiating ART in more recent time periods, from 1.63 tests per py (95% CI: 1.61, 1.64) in 2003–05 to 1.95 tests per py (95% CI: 1.94, 1.97) in 2006–09. The CD4 testing rate in 2010–13 was 1.87 tests per py (95% CI: 1.85, 1.90). Although this was slightly lower than the previous time period, it was still higher than the CD4 testing rate in 2003–05 (Figure 4A).
Figure 4. Rates per person-year (per py), with 95% confidence interval, of (A) CD4 testing and (B) HIV viral load (VL) testing, across all countries by time period of ART initiation.
The crude rate of HIV viral load (VL) testing for all countries and all time periods was 0.68 tests per py (95% CI: 0.67, 0.68). The VL testing rate for all countries steadily increased for those initiating in more recent time periods, from 0.42 tests per py (95% CI: 0.41, 0.43) in 2003–05 to 0.91 tests per py (95% CI: 0.89, 0.92) in 2010–13 (Figure 4B).
Discussion
In this large cohort of 16 546 HIV-positive patients receiving care in seven HIV treatment centres in Asia, the survival probability, at one year of follow-up, was about 2% higher for HIV-positive patients initiating a first-line ART in 2006–09 or 2010–13, as compared to 2003–05. At four years of follow-up, the survival probability for ART initiation in 2006–09 and 2010–13 remained 2–3% higher than for ART initiation in 2003–05. The multivariate analysis also suggested that those initiating ART in 2006–09 and 2010–13 were 22% and 27% less likely to die compared with those initiating in 2003–05.
The improved survival for those initiating in more recent times was probably due to a combination of changes to patient care in the Asia-Pacific region. First, there has been a move towards earlier initiation of ART at higher levels of CD4. Early initiation of ART is strongly associated with reduced mortality, morbidity and AIDS-related events [26, 27]. Since 2006, the WHO recommendations have increased the CD4 at ART initiation to encourage early ART initiation. More recently, WHO guidelines recommend initiating ART regardless of CD4 cell count [15]. Consequently, the median CD4 cell count at ART initiation has increased in more recent time periods in the Asia-Pacific region and other resource-limited countries. Second, there have been significant changes to the recommended first-line ART regimen. Most notable is the decreased use of d4T and NVP, and increased use of TDF and EFV to achieve more tolerable and convenient regimens. This is in agreement with the WHO recommendations to use alternatives instead of d4T and NVP due to the higher risk of drug toxicities and adverse events [11, 13, 28–32]. Third, there has been greater routine patient monitoring of CD4 and HIV viral load. CD4 testing has increased to nearly two tests per year per patient, while HIV viral load testing has steadily increased to almost one test per year per patient.
Studies in South Africa have shown similar improved survival after 12 months of ART for those initiating in 2006–07 compared to those initiating in 2002–05. There is also a clear linear trend of improved survival where the risk of mortality decreases for each successive year of ART initiation. Higher CD4 cell count at ART initiation was also strongly associated with reduced mortality in multivariate analysis [33, 34]. During this time, WHO guidelines recommended replacing d4T with TDF due to the high occurrence of toxicities [11]. A large South African cohort study found that 21% of patients substituted d4T with TDF in 2001–05 [35]. Similarly, studies in Uganda also reported increasing survival for those initiating in later years of ART initiation [36]. HIV-positive patients from developed countries have shown similar trend toward improved survival. Studies in British Columbia have reported decreased mortality rates and increased life expectancy with increasing year of ART initiation. The survival probability described had substantially increased for HIV-positive adults initiating ART from 64.4% (95% CI: 62.2–66.6%) in 1993–95 to 91.0% (95% CI: 89.5–92.5%) in 2002–04 at 24 months from ART initiation [37]. More recent estimates suggest further reductions in mortality rates for those initiating in 2008–12 [38]. There has been an increasing trend in the pre-ART median CD4 cell count, since 2004, due to the move towards earlier ART initiation at higher CD4 cell count levels [39]. Other developed countries have also shown a similar trend of reducing mortality rate with increasing year of ART initiation [40, 41]. . Clearly, improvements to patient care in other settings, including use of more tolerable regimens and earlier ART initiation, has also resulted in increased survival for those initiating in more recent time periods.
Despite the marked increase in survival in recent times, there was little evidence to suggest a significant difference in survival for those initiating in 2006–09 compared to 2010–13. This may be attributed to unmeasured factors relating to patient care, such as increased patient burden, poorer provider to patient ratios or differences in patient LTFU rates. The rate of patients LTFU was not reported as not all sites were able to distinguish between patients transferred to other clinics and patients not returning for care. However, for the sites that did provide patient transfer data, the rate of patients LTFU for those initiating in 2003–05 was 2.1 per 100 person-years (pys), in 2006–09 was 2.9 per 100 pys and in 2010–13 was 2.8 per 100 pys. The LTFU rate was 2.8 per 100 pys. Therefore, mortality estimates for those initiating in 2003–05 and 2006–09 may be underestimating the true mortality rate and contributing to the lack of difference in mortality rates between 2006–09 and 2010–13 [42].
There were limitations to our study. We used observational data from seven clinical sites to represent six countries in the Asia-Pacific region, where most countries had only one contributing clinical site. Therefore, our findings should not be interpreted as representative of the entire Asia-Pacific region nor the entire country but rather representative of the trends occurring within the clinical sites. Additionally, differences between the sites should be carefully interpreted. There are differences in when patients present for ART, and also in the type of ART and level of care that can be provided with local resourcing. In addition, there probably are unmeasurable confounders between the sites. However, our analysis does show a clear pattern of improving survival over time for treatment programs at all the sites. Also, the Cox regression model for mortality was stratified by site to account for the heterogeneity between the sites. The results are also heavily weighted by the Indian site that contributed a substantial proportion of patients to the cohort. Nonetheless, data are reasonably consistent across sites and use of stratification in the Cox regression analysis adjusts for site differences in patient numbers. Additionally, the limited number of data variables collected did not allow for investigation of survival for those receiving second-line ART, other reasons for treatment failure or the presence of AIDS-defining events.
Another limitation to our study is the potential bias in the recording of deaths. Deaths were ascertained mainly through hospital records, but, in some cases, further tracing through death registries or at home was conducted. It is possible that, with greater numbers of patients presenting in recent years, deaths were under-recorded for those initiating ART in recent years and contributed to an apparent reduction in mortality. However, the LTFU rate was consistently low for all year periods of ART initiation at 2.1 per 100 pys, 2.9 per 100 pys and 2.8 per 100 pys for those initiating in 2003–05, 2006–09 and 2010–13 respectively. Hence, it is unlikely that substantial under-recording of deaths occurred to lead to an apparent improvement in mortality for those initiating in more recent years.
Further advancements in treatment guidelines and patient management are likely to lead to continued improved survival in future years. Recent interim results from the START trial have reported that patients immediately initiating ART at CD4 cell count >500 cells/μL were 72% and 39% less likely to experience serious AIDS-related and serious non-AIDS-related events, respectively [26]. In our sample, the median CD4 cell count at ART initiation for those initiating in 2010–13 was below the recommended CD4 cell count threshold for ART initiation. There was also a wide range for CD4 cell count at ART initiation, suggesting that many patients presenting recently are still at advanced stages of HIV disease progression. Therefore in this setting, major improvements in survival could be possible through greater accessibility to care, earlier diagnosis and immediate ART initiation.
This analysis describes the survival trends for HIV-positive patients initiating a first-line regimen in an Asia-Pacific observational cohort between 2003 and 2013. Our findings suggest that the overall survival probabilities from first-line ART initiation for HIV-positive patients in the Asia-Pacific were significantly higher in more recent periods of ART. Other factors associated with improved survival include those aged ≤ 30 years at ART initiation, female sex and higher pre-ART CD4 cell counts. Simultaneously, there has been a move towards improved first-line ART regimens, initiation of ART at higher CD4 cell counts and, greater routine monitoring of CD4 and HIV viral load in more recent time periods. The combination of treatment and monitoring changes over time has likely contributed to the improvements in survival for HIV-positive patients initiating ART in recent years.
Supplementary Material
Acknowledgments
TAHOD-LITE (TREAT Asia HIV Observational Database Low-Intensity TransfEr) is an initiative of TREAT Asia, a program of amfAR, The Foundation for AIDS Research, with support from the U.S. National Institutes of Health’s National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development, and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907). The Kirby Institute is funded by the Australian Government Department of Health and Ageing, and is affiliated with the Faculty of Medicine, UNSW Australia. The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Footnotes
Disclosures
The authors do not have any competing interests to declare.
TAHOD-LITE study members:
PS Ly and V Khol, National Center for HIV/AIDS, Dermatology & STDs, Phnom Penh, Cambodia;
MP Lee, PCK Li, W Lam and YT Chan, Queen Elizabeth Hospital, Hong Kong, China;
N Kumarasamy, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site (CART CRS), YRGCARE Medical Centre, VHS, Chennai, India;
S Pujari, K Joshi, S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India;
TP Merati, DN Wirawan and F Yuliana, Faculty of Medicine Udayana University & Sanglah Hospital, Bali, Indonesia;
OT Ng, PL Lim, LS Lee and PS Ohnmar, Tan Tock Seng Hospital, Singapore;
JY Choi, Na S and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea;
TT Pham, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam;
KV Nguyen, HV Bui, DTH Nguyen and DT Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam;
AH Sohn, N Durier and B Petersen, TREAT Asia, amfAR - The Foundation for AIDS Research, Bangkok, Thailand;
NL De La Mata, A Jiamsakul, DC Boettiger and MG Law, The Kirby Institute, UNSW Australia, Sydney, Australia.
Authors’ contributions
NLD and ML contributed to the concept development. NK, VK, OTN, KVN, TPM, TTP and MPL contributed data for the analysis. NLD performed the statistical analysis and wrote the first draft of the manuscript. All authors commented on the draft manuscript and approved of the final manuscript.
References
- 1.Ma R. Early ART improves life expectancy in HIV patients. Practitioner. 2012 Jan;256(1747):5. [PubMed] [Google Scholar]
- 2.McManus H, O’Connor CC, Boyd M, Broom J, Russell D, Watson K, et al. Long-term survival in HIV positive patients with up to 15 Years of antiretroviral therapy. PLoS One. 2012;7(11):e48839. doi: 10.1371/journal.pone.0048839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabin CA. Do people with HIV infection have a normal life expectancy in the era of combination antiretroviral therapy? BMC Med. 2013;11:251. doi: 10.1186/1741-7015-11-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mocroft A, Ledergerber B, Katlama C, Kirk O, Reiss P, d’Arminio Monforte A, et al. Decline in the AIDS and death rates in the EuroSIDA study: an observational study. Lancet. 2003 Jul 5;362(9377):22–9. doi: 10.1016/s0140-6736(03)13802-0. [DOI] [PubMed] [Google Scholar]
- 5.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008 Jul 26;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organisation. Scaling up HIV/AIDS prevention, treatment and care: a report on WHO support to countries in implementing the “3 by 5” initiative 2004–2005. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- 7.World Health Organisation and UNAIDS. Treating 3 million by 2005: Making it happen. 2003 Available online at: http://www.who.int/3by5/publications/documents/isbn9241591129/en/. [verified April 2015]
- 8.Joint United Nations Programme on HIV/AIDS (UNAIDS) HIV in Asia and the Pacific: UNAIDS report 2013. Geneva: UNAIDS; 2013. Available online at: http://www.unaids.org/sites/default/files/media_asset/2013_HIV-Asia-Pacific_en_0.pdf. [verified April 2015] [Google Scholar]
- 9.Srikantiah P, Ghidinelli M, Bachani D, Chasombat S, Daoni E, Mustikawati DE, et al. Scale-up of national antiretroviral therapy programs: progress and challenges in the Asia Pacific region. AIDS. 2010 Sep;24( Suppl 3):S62–71. doi: 10.1097/01.aids.0000390091.45435.ea. [DOI] [PubMed] [Google Scholar]
- 10.Kazatchkine M, Atun R. HIV in Asia: universal access in sight. AIDS. 2010 Sep;24( Suppl 3):S1–2. doi: 10.1097/01.aids.0000390083.30188.d8. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organisation. ART Guidelines Committe Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach - 2006 revision. 2006 Available online at: http://www.who.int/hiv/pub/arv/adult/en/. [verified April 2015] [PubMed]
- 12.Makinson A, Moing VL, Kouanfack C, Laurent C, Delaporte E. Safety of stavudine in the treatment of HIV infection with a special focus on resource-limited settings. Expert Opin Drug Saf. 2008 May;7(3):283–93. doi: 10.1517/14740338.7.3.283. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organisation. ART Guidelines Committe Antiretroviral therapy for HIV infection in adults and adolescents: recommendations for a public health approach - 2010 revision. 2010 Available online at: http://www.who.int/hiv/pub/arv/adult2010/en/index.html. [verified April 2015] [PubMed]
- 14.World Health Organisation. Consolidated guidelines on the use of Antiretroviral Drugs for treating and preventing HIV infection: Recommendations for a Public Health approach. Geneva, Switzerland: 2013. Available online at: http://apps.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [verified October 2015] [PubMed] [Google Scholar]
- 15.World Health Organisation. Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV. 2015 Available online at: http://apps.who.int/iris/bitstream/10665/186275/1/9789241509565_eng.pdf. [verified January 2016] [PubMed]
- 16.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014 Apr;14(4):281–90. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Davis DH, Smith R, Brown A, Rice B, Yin Z, Delpech V. Early diagnosis and treatment of HIV infection: magnitude of benefit on short-term mortality is greatest in older adults. Age Ageing. 2013 Jul;42(4):520–6. doi: 10.1093/ageing/aft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goldman DP, Juday T, Seekins D, Linthicum MT, Romley JA. Early HIV treatment in the United States prevented nearly 13,500 infections per year during 1996–2009. Health Aff (Millwood) 2014 Mar;33(3):362–9. doi: 10.1377/hlthaff.2013.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romley JA, Juday T, Solomon MD, Seekins D, Brookmeyer R, Goldman DP. Early HIV treatment led to life expectancy gains valued at $80 billion for people infected in 1996–2009. Health Aff (Millwood) 2014 Mar;33(3):370–7. doi: 10.1377/hlthaff.2013.0623. [DOI] [PubMed] [Google Scholar]
- 20.Braitstein P, Brinkhof MW, Dabis F, Schechter M, Boulle A, Miotti P, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–24. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 21.National Committee for AIDS DaPPaCN. Vietnam AIDS response progress report 2012. Hanoi: NCADPPC; 2012. Available online at: http://www.unaids.org/sites/default/files/en/dataanalysis/knowyourresponse/countryprogressreports/2012countries/ce_VN_Narrative_Report.pdf. [verified April 2015] [Google Scholar]
- 22.Phuphuakrat A, Kiertiburanakul S, Sungkanuparph S. Current status of HIV treatment in Asia and the Pacific region. Sexual health. 2014 Jul;11(2):119–25. doi: 10.1071/SH13045. [DOI] [PubMed] [Google Scholar]
- 23.Kumarasamy N, Krishnan S. Beyond first-line HIV treatment regimens: the current state of antiretroviral regimens, viral load monitoring, and resistance testing in resource-limited settings. Current opinion in HIV and AIDS. 2013 Nov;8(6):586–90. doi: 10.1097/COH.0000000000000004. [DOI] [PubMed] [Google Scholar]
- 24.Kumarasamy N, Madhavan V, Venkatesh KK, Saravanan S, Kantor R, Balakrishnan P, et al. High frequency of clinically significant mutations after first-line generic highly active antiretroviral therapy failure: implications for second-line options in resource-limited settings. Clin Infect Dis. 2009 Jul 15;49(2):306–9. doi: 10.1086/600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005 Feb 1;38(2):174–9. doi: 10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Insight Start Study Group. Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, et al. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015 Aug 27;373(9):795–807. doi: 10.1056/NEJMoa1506816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, et al. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011 Jul;11(7):516–24. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 28.Van Griensven J, Zachariah R, Rasschaert F, Mugabo J, Atte EF, Reid T. Stavudine- and nevirapine-related drug toxicity while on generic fixed-dose antiretroviral treatment: incidence, timing and risk factors in a three-year cohort in Kigali, Rwanda. Trans R Soc Trop Med Hyg. 2010 Feb;104(2):148–53. doi: 10.1016/j.trstmh.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 29.Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, et al. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA. 2004 Jul 14;292(2):191–201. doi: 10.1001/jama.292.2.191. [DOI] [PubMed] [Google Scholar]
- 30.Ford N, Calmy A. Improving first-line antiretroviral therapy in resource-limited settings. Current opinion in HIV and AIDS. 2010 Jan;5(1):38–47. doi: 10.1097/COH.0b013e3283339b41. [DOI] [PubMed] [Google Scholar]
- 31.Shubber Z, Calmy A, Andrieux-Meyer I, Vitoria M, Renaud-Thery F, Shaffer N, et al. Adverse events associated with nevirapine and efavirenz-based first-line antiretroviral therapy: a systematic review and meta-analysis. AIDS. 2013 Jun 1;27(9):1403–12. doi: 10.1097/QAD.0b013e32835f1db0. [DOI] [PubMed] [Google Scholar]
- 32.Swaminathan S, Padmapriyadarsini C, Venkatesan P, Narendran G, Ramesh Kumar S, Iliayas S, et al. Efficacy and safety of once-daily nevirapine- or efavirenz-based antiretroviral therapy in HIV-associated tuberculosis: a randomized clinical trial. Clin Infect Dis. 2011 Oct;53(7):716–24. doi: 10.1093/cid/cir447. [DOI] [PubMed] [Google Scholar]
- 33.Cornell M, Grimsrud A, Fairall L, Fox MP, van Cutsem G, Giddy J, et al. Temporal changes in programme outcomes among adult patients initiating antiretroviral therapy across South Africa, 2002–2007. AIDS. 2010 Sep 10;24(14):2263–70. doi: 10.1097/QAD.0b013e32833d45c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boulle A, Van Cutsem G, Hilderbrand K, Cragg C, Abrahams M, Mathee S, et al. Seven-year experience of a primary care antiretroviral treatment programme in Khayelitsha, South Africa. AIDS. 2010 Feb 20;24(4):563–72. doi: 10.1097/QAD.0b013e328333bfb7. [DOI] [PubMed] [Google Scholar]
- 35.Boulle A, Orrel C, Kaplan R, Van Cutsem G, McNally M, Hilderbrand K, et al. Substitutions due to antiretroviral toxicity or contraindication in the first 3 years of antiretroviral therapy in a large South African cohort. Antivir Ther. 2007;12(5):753–60. doi: 10.1177/135965350701200508. [DOI] [PubMed] [Google Scholar]
- 36.Hermans SM, van Leth F, Manabe YC, Hoepelman AI, Lange JM, Kambugu A. Earlier initiation of antiretroviral therapy, increased tuberculosis case finding and reduced mortality in a setting of improved HIV care: a retrospective cohort study. HIV Med. 2012 Jul;13(6):337–44. doi: 10.1111/j.1468-1293.2011.00980.x. [DOI] [PubMed] [Google Scholar]
- 37.Lima VD, Hogg RS, Harrigan PR, Moore D, Yip B, Wood E, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007 Mar 30;21(6):685–92. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 38.Patterson S, Cescon A, Samji H, Chan K, Zhang W, Raboud J, et al. Life expectancy of HIV-positive individuals on combination antiretroviral therapy in Canada. BMC Infect Dis. 2015;15:274. doi: 10.1186/s12879-015-0969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Montaner JS, Lima VD, Barrios R, Yip B, Wood E, Kerr T, et al. Association of highly active antiretroviral therapy coverage, population viral load, and yearly new HIV diagnoses in British Columbia, Canada: a population-based study. Lancet. 2010 Aug 14;376(9740):532–9. doi: 10.1016/S0140-6736(10)60936-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Samji H, Cescon A, Hogg RS, Modur SP, Althoff KN, Buchacz K, et al. Closing the gap: increases in life expectancy among treated HIV-positive individuals in the United States and Canada. PLoS One. 2013;8(12):e81355. doi: 10.1371/journal.pone.0081355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antiretroviral Therapy Cohort C. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet. 2008 Jul 26;372(9635):293–9. doi: 10.1016/S0140-6736(08)61113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brinkhof MW, Pujades-Rodriguez M, Egger M. Mortality of patients lost to follow-up in antiretroviral treatment programmes in resource-limited settings: systematic review and meta-analysis. PLoS One. 2009;4(6):e5790. doi: 10.1371/journal.pone.0005790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.