Abstract
Background
Deep brain stimulation (DBS) is the chronic electrical stimulation of selected target sites in the brain through stereotactically implanted electrodes. More than 150 000 patients around the world have been treated to date with DBS for medically intractable conditions. The indications for DBS include movement disorders, epilepsy, and some types of mental illness.
Methods
This review is based on relevant publications retrieved by a selective search in PubMed and the Cochrane Library, and on the current guidelines of the German Neurological Society (Deutsche Gesellschaft für Neurologie, DGN).
Results
DBS is usually performed to treat neurological diseases, most often movement disorders and, in particular, Parkinson’s disease. Multiple randomized controlled trials (RCTs) have shown that DBS improves tremor, dyskinesia, and quality of life in patients with Parkinson’s disease by 25% to 50%, depending on the rating scales used. DBS for tremor usually involves stimulation in the cerebello-thalamo-cortical regulatory loop. In an RCT of DBS for the treatment of primary generalized dystonia, the patients who underwent DBS experienced a 39.3% improvement of dystonia, compared to only 4.9% in the control group. Two multicenter trials of DBS for depression were terminated early because of a lack of efficacy.
Conclusion
DBS is an established treatment for various neurological and psychiatric diseases. It has been incorporated in the DGN guidelines and is now considered a standard treatment for advanced Parkinson’s disease. The safety and efficacy of DBS can be expected to improve with the application of new technical developments in electrode geometry and new imaging techniques. Controlled trials would be helpful so that DBS could be extended to further indications, particularly psychiatric ones.
The earliest clinical application of chronic deep brain stimulation, as far as can be determined from the literature, was for the treatment of chronic pain in the 1970s. Deep brain stimulation (DBS) consists of the application of low-intensity electric impulses, typically at or near a frequency of 130 Hz, to strategic sites in the brain through permanently implanted electrodes. These impulses are thought to transiently activate nearby axons; the distant effects of stimulation depend on the function of the particular neurons stimulated, i.e., whether they are inhibitory or excitatory. Altered oscillation of network structures (e.g., altered beta-oscillation in Parkinson’s disease) leads to an improvement in the manifestations of disease.
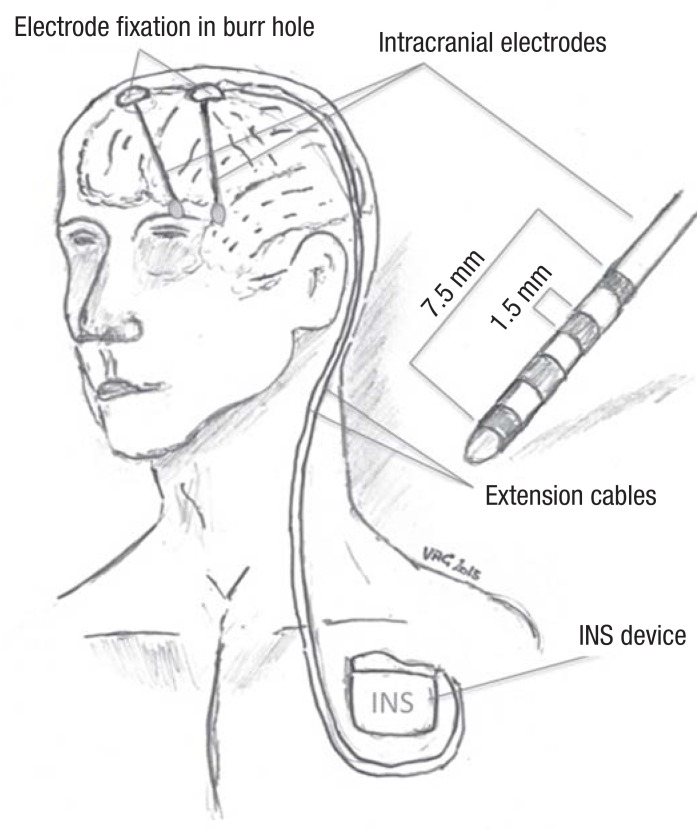
DBS is a purely symptomatic treatment that must be administered continuously and is therefore usually kept on 24 hours a day. In general, DBS is delivered in square-wave impulses of amplitude 1–5 V (or 0.5–10 mA) and duration 30–450 µs, with the precise values being determined by the indication and by clinically guided optimization in the individual patient. Intelligent (closed-loop) systems for DBS are under development but not yet available. DBS can be delivered in either a constant-voltage or a constant-current mode. The electrodes are implanted in target sites deep in the brain in a stereotactic operation that is usually performed under local anesthesia with, at most, light sedation. They are connected, by way of extension cables running down the subcutaneous tissue of the neck, to an impulse generator, also called an “internal neural stimulator” or “brain pacemaker,” that is subcutaneously implanted in the pectoral or abdominal area. Impulse generator implantation, the second and last part of the DBS operation, is performed under general anesthesia. (The battery inside the impulse generator is depleted in 3–5 years, at which time the impulse generator must be replaced in a short procedure under local anesthesia; impulse generators with externally rechargeable batteries are also available.) Once the entire system is in place, the physician can readjust the stimulation parameters telemetrically with a transcutaneous programming device (Figure 1).
Figure 1.
In deep brain stimulation (DBS), low-intensity electrical impulses at or near a frequency of 130 Hz are applied through stereotactically implanted electrodes to strategically chosen target sites in the brain. INS, internal neural stimulator (diagram by Volker A. Coenen)
Movement disorders
Parkinson’s disease
Deep brain stimulation (DBS) has been an established treatment for Parkinson’s disease for many years (1, 2). Because of the good evidence available from clinical studies, including six randomized controlled trials (RCTs) (3– 8), DBS is recommended for the treatment of Parkinson’s disease in the guidelines of the German Neurological Society (Deutsche Gesellschaft für Neurologie, DGN) (Table). Recent trials have shown that DBS is effective not only for patients in the advanced phase of the disease, but also for younger patients in its intermediate phase, to whom it provides a comparable improvement in quality of life (7, 9).
Table. Selected trials of deep brain stimulation for Parkinson’s disease.
| Trial | Evidence level | Target structure | Design | Patient characteristics | Result |
|---|---|---|---|---|---|
| Deuschl et al., 2006(3) ClinicalTrials.gov number, NCT00196911 |
Ib | STN | Multicenter, non-blinded, randomizedn n= 156 Endpoints: PDQ39; UPDRS III at 6 months |
n=78 DBS (60.5±7.4 yr, 64% m) vs. n=78 BMT (60.8±7.8 yr, 64% m) H&Y 2–5 (50% H&Y 4) Treatment with L-dopa: DBS: 13.0±5.8 yr BMT: 13.8±5.6 yr |
Superiority of DBS with respect to PDQ39 Summated score, compared at 6 months |
| EARLYSTIM Schuepbach et al. 2013 (7) ClinicalTrials.gov number, NCT00354133 |
Ib | STN | Multicenter, non-blinded, randomizedn = 251 Endpoints: PDQ39; UPDRS I–IV at 24 months |
n=124 DBS (52.9±6.6 yr, 66% m) vs n=127 BMT (52.2±6.1 yr, 77% m) Treatment with L-dopa: DBS: 4.8±3.3 yr BMT: 5.0±3.3 yr |
Superiority of DBS with respect to PDQ39 Summated score, compared at 2 years No significant difference in AE; in particular, no elevated incidence of suicide in the DBS group |
| Follet et al., 2010 (4) ClinicalTrials.gov numbers, NCT00056563 and NCT01076452 |
Ib | STN; GPi | Multicenter, randomized Complex design with randomization to BMT or DBS (STN, GPi); after 6 months of BMT, randomization to DBS in the STN or GPi n = 299 Endpoint: UPDRS III motor score at 24months |
n= 147 STN-DBS (61.9±8.7 yr, 79% m) vs. n=152 GPi-DBS (61.8±8.7 yr, 87.5% m) Treatment with L-dopa: STN-DBS: 11.1 ± 5 yr GPi-DBS: 11.5 ± 5.4 yr |
Significant improvement of motor scores in both groups compared to BMT No superiority of either STN- or GPi-DBS Improvement of PDQ39 iin both groups at 24 months Trend toward improvement of depression with GPi-DBS and worsening with STN-DBS (BDI) |
| NSTAPS Oderkerken et al., 2013 (5) www.controlled-trials.com, number ISRCTN85542074 |
Ib | STN; GPi | Multicenter, randomized n = 128 |
n= 63 STN-DBS (60.9 ± 7.6 yr) n= 65 GPi-DBS (59.1 ± 7.8 yr) H&Y 2.5 (0–4) Treatment with L-dopa: STN-DBS: 9.5 ± 5.6 yr GPi-DBS: 9.0 ± 3.9 yr |
No difference between groups in functional health assessed at 1 year STN-DBS lessens clinical disability and motor off-phases GPi-DBS reduces dyskinesia more effectively at 1 yr No difference between groups with respect to AE |
AE, adverse event(s); BDI, Beck Depression Inventory; BMT, best medical treatment; DBS, deep brain stimulation; GPi, globus pallidus internus; H&Y, Hoehn and Yahr disease stage (I–V); L-dopa, levodopa; m, male; PDQ39, Parkinson’s Disease Questionnaire; STN, subthalamic nucleus; UPDRS III, motor score of the Unified Parkinson’s Disease Rating Scale ; yr, years
Various brain structures can be used as targets for deep brain stimulation for the symptomatic treatment of Parkinson’s disease. In Europe, the subthalamic nucleus (STN) is the usual target for high-frequency stimulation, as STN stimulation has been shown to improve quality of life (with an increase of about 25% in the Parkinson`s Disease Questionnaire [PDQ]-39 score) and to consistently ameliorate parkinsonian rigidity, hypokinesia, and, in most cases, tremor (with an increase of 41% to 50% in the Unified Parkinson Disease Rating Scale [UPDRS] Motor Score) (3, 7). Moreover, STN stimulation enables a reduction of the dose of antiparkinsonian medication, with resulting improvement in drug-induced dyskinesia (54% improvement on a dyskinesia scale) and other drug side effects including hallucinations and impulse-control disorder (3, 10).
Outside Europe, the internal segment of the globus pallidus (GPi) is targeted as often as the STN in DBS for Parkinson’s disease. GPi stimulation combats dyskinesia even more effectively than STN stimulation does (89% vs. 62% in a comparative trial) (11), while relieving tremor to a comparable extent (12).
Pallidal stimulation, unlike subthalamic stimulation, generally does not enable a substantial reduction of the dose of dopaminergic medication (4, 6).
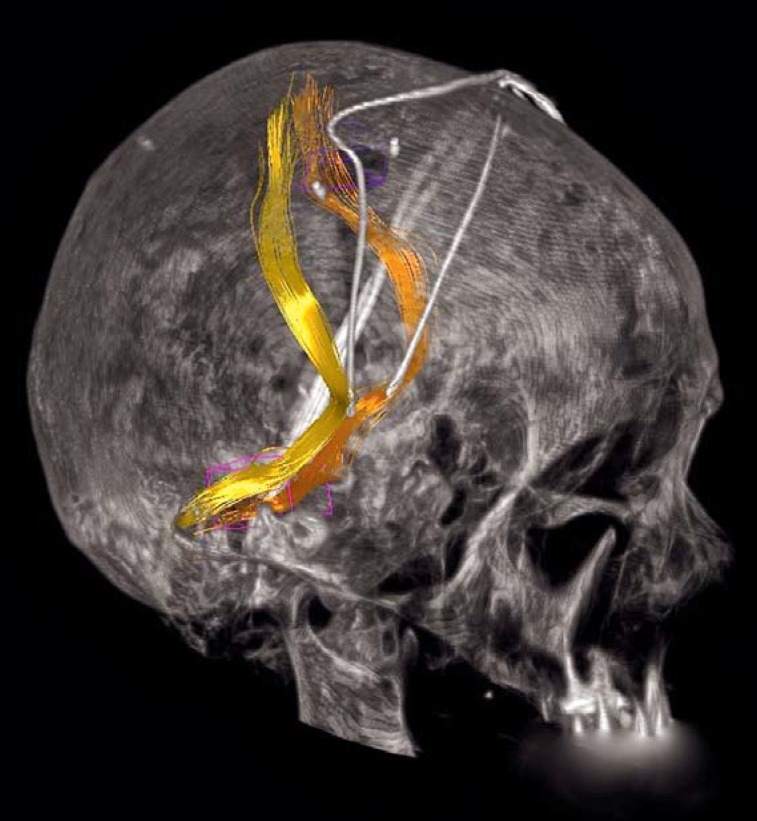
Comparative trials of pallidal versus subthalamic stimulation have revealed similar short-term benefits with respect to the cardinal manifestations of Parkinson’s disease, i.e., rigidity, tremor, and hypokinesia (4, 5), with a trend toward better improvement of akinesia under subthalamic stimulation. A lessening of the effect of pallidal stimulation over time has been seen in open long-term trials and has been effectively treated by conversion to subthalamic stimulation (13). These findings imply that the STN is the target of first choice, particularly for younger patients (7). A third potential DBS target is the nucleus ventralis intermedius of the thalamus (Vim), or the dentato-rubro-thalamic tract with which it is associated (14, 15), for patients whose main or only clinically significant parkinsonian manifestation is tremor (Figure 2).
Figure 2.
DBS of the dentato-rubro-thalamic tract (DRT, yellow and orange) for tremor (14, 15). Diffusion-tensor magnetic resonance imaging enables the selection of a target structure for the treatment of tremor that cannot be directly visualized by any other technique.
(Image: Volker A. Coenen)
The EarlyStim trial addressed the issue of the optimal timing of DBS in the treatment of Parkinson’s disease (7). Patients treated in previous trials (3, 4, 6, 16) had generally been over age 60 and had suffered from the disease for 11–13 years before treatment. The EarlyStim trial showed that younger patients (mean age, 52.5 years) with an average duration of illness of 7.5 years also benefit from STN-DBS, in comparison to best medical treatment. In this trial, STN-DBS brought about improvements in:
Quality of life (26% improvement on a quality of life questionnaire with STN-DBS, vs. –1% with best medical treatment)
Activities of daily living (30% vs. –12%)
Motor manifestations (54% vs. 4% improvement of the UPDRS motor score)
The duration of dyskinesia (20% vs. 2%).
The non-motor effects and side effects of STN stimulation are gradually being documented. STN stimulation has beneficial effects on urge incontinence (17), the duration and quality of sleep (18, 19), pain (20), and constipation (21). On the other hand, it can also cause adverse neuropsychiatric effects (cognitive changes [22, 23], depression, hypomania, apathy [8]) and weight gain.
The neuropsychiatric side effects of DBS arise through complex mechanisms, involving not only the underlying disease and the therapeutic stimulation per se, but also long-term sensitization due to the preceding years of dopaminergic substitution therapy, the trauma of electrode implantation, the reduction of medication enabled by DBS, and difficulties of emotional adaptation after life-altering surgery (24). Severe adverse effects were no more common in the surgical arm than in the non-surgical arm of the EarlyStim trial (123 vs. 128 events, respectively), probably because of improved patient selection with appropriate inclusion and exclusion criteria, and because the patients in both arms were treated by a multidisciplinary team that included psychologists and psychiatrists. Stimulation-induced motor side effects are a function of the particular target stimulated; the most common one is dysarthria (STN >> GPi > Vim), followed by dyskinesia (STN) and, rarely, lid apraxia (STN), which manifests itself as involuntary closure of the eyelids.
The improvement of the manifestations of Parkinson’s disease by deep brain stimulation in the subthalamic nucleus has been shown to persist without deterioration over follow-up intervals of five to ten years (25– 27).
Tremor
The earliest publication on DBS for the treatment of movement disorders, which appeared in 1987 (1), concerned the suppression of tremor with high-frequency stimulation in the nucleus ventralis intermedius of the thalamus (Vim). The clinical varieties of tremor include resting tremor, postural tremor, and action or intention tremor (28). All types of tremor are thought to be due to abnormal oscillatory activity in the cerebello-thalamo-cortical regulatory loop. DBS can now effectively control tremor due to any of several different underlying diseases, including parkinsonian tremor, essential tremor, and cerebellar tremor in multiple sclerosis. There have not been any large-scale, randomized, controlled trials comparing DBS with sham stimulation or best medical treatment for tremor. A trial comparing DBS with thalamotomy for tremor revealed better functional improvement with DBS (4.9 vs. 0.5 points on the Frenchay Activities Index) (29). Case reports indicate that DBS can also effectively treat post-traumatic (Holmes) tremor (30, 31), orthostatic tremor (32, 33), and neuropathic tremor (34). The efficacy of DBS for tremor depends mainly on the correct positioning of the stimulating electrode for modulation of the cerebello-thalamo-cortical loop, rather than on the underlying etiology (14, 35, 36).
The most common indication for DBS used specifically against tremor is certainly essential tremor, a disease with an overall prevalence of about 2.2% [37]. This heterogeneous tremor disorder, which can markedly impair patients’ everyday quality of life through a combination of postural, action, and intention tremor, has been found to respond to Vim-DBS for periods of ten years and more (38– 40). The success of DBS is, however, often limited by the development of tolerance, necessitating an enlargement of the volume of tissue activated (39, e1). Tolerance to stimulation may be less likely to develop if, instead of the Vim, the caudal portion of the zona incerta (e2, e3) or the dentato-rubro-thalamic tract (14) is taken as the stereotactic target. In the years to come, stereotactic surgery for tremor will probably more often be directed at these subthalamic structures, rather than the Vim. Comparative trials would be desirable but have not yet been performed.
DBS for cerebellar postural and intention tremor in multiple sclerosis is an off-label treatment whose indication must be decided upon on a case-to-case basis (e4). DBS does not improve cerebellar ataxia or other disabling motor manifestations of multiple sclerosis that often coexist with tremor.
Dystonia
Dystonia has been treated effectively with deep brain stimulation in the internal segment of the globus pallidus since the late 1990s. Initial trials conducted on individual patients with primary generalized dystonia (e5, e6) yielded promising results; these were then confirmed in a randomized controlled trial (e7) that included both a DBS group and a sham-stimulation group (20 patients each; mean improvement of 39.3% vs. 4.9% on the Burke-Fahn-Marsden Dystonia Rating Scale). After five years of follow-up, these patients experienced further improvement of their dystonia (mean, 57.8%, compared to preoperative scores) (e8). A particularly good response to DBS is seen in patients with autosomal dominant, early-onset, primary generalized dystonia due to a DYT1 mutation on chromosome 9q34, as long as DBS is performed early in the course of disease (e9). The evidence supporting DBS for primary cervical dystonia consisted, until recently, only of several non-sham-controlled trials that showed a beneficial effect (e10, e11). The first-ever sham-controlled trial has now been published (e12); it confirms the earlier findings (39.4% vs. 16.6% improvement on the Toronto Western Spasmodic Torticollis Rating Scale [TWSTRS]). Long-term follow-up is not yet available.
It is not yet clear whether the subthalamic nucleus might be a useful alternative target for DBS in dystonia without the bradykinetic side effects that have been described for GPi stimulation (e13). A pilot study has shown a comparable degree of improvement of dystonia with STN stimulation (ca. 63% improvement in TWSTRS) (e3), but there have not yet been any direct comparative trials of the two targets for the treatment of primary dystonia.
DBS is less effective against secondary types of dystonia (except tardive dyskinesia) than against primary dystonia. Case series are available on DBS for the treatment of two types of secondary neurodegenerative dystonia: pantothenate-kinase associated neurodegeneration (PKAN, a degenerative disorder involving iron deposition in the brain, formerly known as Hallervorden-Spatz disease) and dyskinetic childhood cerebral palsy (e14, e15). The benefit of DBS was variable and, on average, weak. On the other hand, a collection of published cases of DBS for dyskinesia due to neuroleptic use (tardive dyskinesia) revealed consistent, marked improvement (e16). A randomized, sham-controlled trial of DBS for tardive dyskinesia is now in progress in Germany.
Epilepsy
Deep brain stimulation in the anterior nucleus of the thalamus (ANT) for the treatment of epilepsy was approved for use in Europe (CE certified) in 2010 (e17). Further experimental techniques include centromedian and cerebellar stimulation. In the United States, ANT-DBS may currently be performed only in the setting of a clinical trial. ANT-DBS is a treatment option for patients with medically intractable epilepsy that is not amenable to resective surgery (e18); it is indicated in the treatment of epilepsy of focal onset with secondary generalization (e17, e19). Until the introduction of ANT-DBS, secondarily generalized epilepsy of focal onset that was not amenable to resection could only be treated with vagus nerve stimulation (e20). In the ANT-DBS approval study (the SANTE study), two types of adverse effect arose much more often in the stimulated group than in the control group: depression (14.8% vs. 1.8%) and subjective memory impairment (13% vs. 1.8%). A systematic Cochrane analysis yielded insufficient evidence from randomized controlled trials (RCTs) on cortical or deep brain stimulation to support the efficacy of centromedian (thalamic) or hippocampal DBS, or of cerebellar stimulation, for the treatment of epilepsy. In particular, the patients in the RCTs were not followed up long enough to demonstrate lasting efficacy (e21).
Mental illness
Obsessive-compulsive disorder
In a case report of a patient with obsessive-compulsive disorder (OCD) and comorbid depression, DBS in the nucleus accumbens and the caudate nucleus led to a remission (e22). In a case series of 14 patients with OCD, unilateral nucleus accumbens stimulation had a beneficial effect (e23). In other studies, stimulation of the ventral capsule/ventral striatum led to clinical improvement in 50% of patients; the side effects included transient hypomania and increased anxiety, which could be eliminated by changing the stimulation parameters (e23, e24). DBS for OCD has received CE certification and is thus permitted for use in the European Union. CE certification is largely based on safety, rather than efficacy. Notably, the German Association for Psychiatry, Psychotherapy and Psychosomatics (Deutsche Gesellschaft für Psychiatrie und Psychotherapie, Psychosomatik und Nervenheilkunde), in its pertinent S3 guideline, has stated that DBS “can” (rather than “should”) be used to treat OCD. Weak recommendations of this type are meant as an endorsement of the treatment in question only for severe, otherwise intractable cases, and for study in clinical trials. DBS is certainly not a standard treatment for OCD.
Further psychiatric indications
DBS has been used experimentally to treat several other types of mental illness, including depression, substance abuse, dementia, eating disorders, Gilles de la Tourette syndrome, and schizophrenia. We will briefly discuss DBS for the treatment of depression.
The target structures used to date in DBS for depression include the subgenual portion of the cingulate gyrus (Cg25) (e25), the ventral capsule/ventral striatum (VC/VS) (e26), the nucleus accumbens (NAcc) (e27), and the medial forebrain bundle (slMFB) (e28). Uncontrolled trials of DBS for refractory depression have yielded long-term clinical improvement in 50–60% of the roughly 100 patients treated in this way around the world (e29). Long-term follow-up of DBS in the Cg25 and NAcc revealed stable antidepressant effects (e30), in the sense that patients who responded to the treatment early on continued to be responders. These good results could not be replicated in multicenter trials. Two such trials of DBS for depression, using Cg25 and VC/VS as targets (respectively), were terminated early because of a lack of therapeutic benefit. The latter trial has been published (e31). The failure of these multicenter trials makes it difficult to justify the consumption of further resources for clinical trials of DBS for depression.
New technologies
Current steering
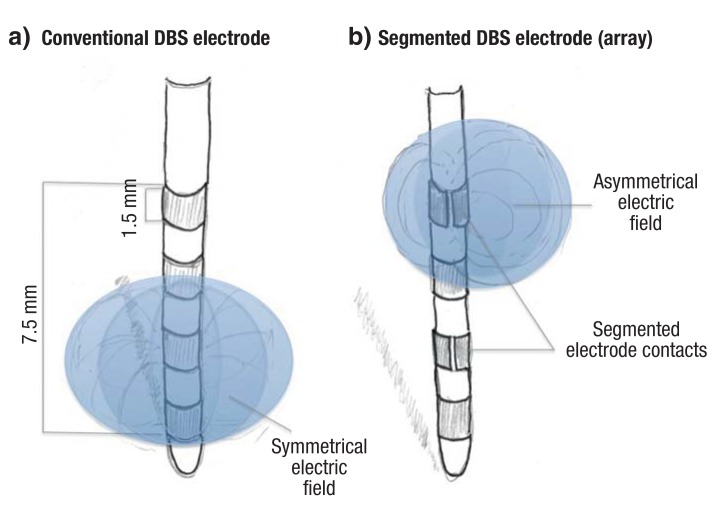
All DBS systems to date have used conventional ring-shaped electrodes to generate concentric (i.e., circularly symmetrical) electric fields for the high-frequency stimulation of brain tissue (Figure 3a). Longitudinal variation of the electric field to optimize the therapeutic effect is commercially available and is now being clinically tested. Recently, segmented, rather than ring-shaped, electrodes have been developed to enable three-dimensional shaping of the electric field, so that a more precisely selected region of brain tissue can be stimulated (Figure 3b). The goal of such methods is to make DBS more effective by broadening its therapeutic window, i.e., by enlarging the variety of parameter settings that can bring about a beneficial therapeutic effect without side effects (e32, e33).
Figure 3.
DIfferent varieties of DBS electrode (DBS, deep brain stimulation):
a) Ring-shaped stimulation, i.e., a concentric electric field, generated with an electrode of the conventional type.
b) An electrode with segmented contacts and laterally directed electric fields (array electrode). This technique carries the promise of enabling more precisely targeted stimulation of brain tissue
(Graphic by Volker A. Coenen)
New imaging methods
In magnetic resonance (MR) tractography, mathematical analysis of imaging data exploits the anisotropy of brain tissue to trace the fiber pathways of the brain noninvasively. The target region and its functional surroundings can be visualized as they lie anatomically in the individual patient (15, e34). Electric field simulation (EFS) (e34, e35) lets the physician preoperatively determine the optimal site of electrode implantation so that a therapeutic benefit with minimal side effects can be obtained. When applied separately or in combination, these techniques yield multiple advantages for the patient:
Guidance by the patient’s individual brain anatomy (which is variable from one patient to another), resulting in
A shorter duration of intraoperative testing and
Increased safety of the stereotactic procedure.
These techniques have been used to plan DBS interventions for the treatment of pain (e36) and tremor (14, 15) and can also be used to identify new DBS target sites (e28, e34). Their clinical benefit has not yet been confirmed in controlled trials.
Overview
Deep brain stimulation is now used in specialized centers to treat diseases that are refractory to medical treatment, or for which other treatment methods have failed. It has been approved in Europe for the treatment of movement disorders (Parkinson’s disease, tremor, dystonia), medically intractable epilepsy, and obsessive-compulsive disorder. In view of the established clinical efficacy of DBS and its endorsement in published guidelines, especially for the treatment of movement disorders, the underutilization of this technique by general practitioners and specialists (particularly those in private practice) is surprising.
Interdisciplinary teams are now investigating new modes of treatment for mental illness. Many diseases are becoming amenable to treatment with DBS; the new technologies described above are likely to expand the spectrum of indications for DBS and to increase the consistency of its therapeutic benefit.
The success of DBS depends largely on a well-functioning interdisciplinary team. Meticulous patient selection is important, as is the continued interdisciplinary treatment of patients with implanted systems over the long term, i.e., potentially for many years. The long-term treatment of these patients can now only be provided in a few specialized centers and will, in the future, generate high political and economic demands on the health-care system. As the number of patients with implanted DBS systems steadily grows, the outpatient sector must be prepared to meet the challenge, financially and otherwise. There is also a need for appropriate training of the physicians who will care for these patients over the long term, in order to raise their competence and self-confidence in providing treatments whose complexity is constantly increasing.
Key Messages.
Deep brain stimulation (DBS) is an established treatment for some neurological diseases.
DBS is a standard treatment of advanced Parkinson’s disease.
The safety and efficacy of DBS are steadily improving through ongoing technical refinement.
DBS has been used to treat epilepsy and various mental illnesses including dementia, depression, obsessive-compulsive disorder, substance abuse, and anorexia.
There is, as yet, insufficient evidence from controlled clinical trials to support the efficacy of DBS for psychiatric indications.
Acknowledgments
Translated from the original German by Ethan Taub, M.D.
Footnotes
Conflict of interest statement
Prof. Coenen has served as a paid consultant for Medtronic, Sapiens, and Precisis. He has received reimbursement of meeting participation fees and travel expenses from Medtronic, Boston Scientific, and Alvea. He has received lecture honoraria and research support from Medtronic and Boston Scientific.
Dr. Amtage has received reimbursement of meeting participation fees and travel expenses from Merz Pharmaceuticals, UCB Pharma, Medtronic, Orion Pharma, Actelion Pharma, and Ipsen Pharma and research support (payment into a third-party account) from Medtronic.
Prof. Volkmann has served as a paid consultant for Boston Scientific, Medtronic, and St. Jude Pharmaceuticals. He has received lecture honoraria from Teva, Allergan, Medtronic, St. Jude Pharmaceuticals, Boston Scientific, Abbvie, and Licher and research support from Boston Scientific, St. Jude Pharmaceuticals, Abbvie, Licher, UCB Pharma, and Teva.
Prof. Schläpfer has received reimbursement of travel expenses, lecture honoraria, and research support from Medtronic.
References
- 1.Benabid AL, Pollak P, Louveau A, Henry S, de Rougemont J. Combined (thalamotomy and stimulation) stereotactic surgery of the VIM thalamic nucleus for bilateral Parkinson disease. Appl Neurophysiol. 1987;50:344–346. doi: 10.1159/000100803. [DOI] [PubMed] [Google Scholar]
- 2.Benabid AL, Pollak P, Seigneuret E, Hoffmann D, Gay E, Perret J. Chronic VIM thalamic stimulation in Parkinson’s disease, essential tremor and extra-pyramidal dyskinesias. Acta Neurochir Suppl (Wien) 1993;58:39–44. doi: 10.1007/978-3-7091-9297-9_8. [DOI] [PubMed] [Google Scholar]
- 3.Deuschl G, Schade-Brittinger C, Krack P, et al. A randomized trial of deep-brain stimulation for Parkinson’s disease. NEJM. 2006;355:896–908. doi: 10.1056/NEJMoa060281. [DOI] [PubMed] [Google Scholar]
- 4.Follett KA, Weaver FM, Stern M, et al. Pallidal versus subthalamic deep-brain stimulation for Parkinson’s disease. NEJM. 2010;362:2077–2091. doi: 10.1056/NEJMoa0907083. [DOI] [PubMed] [Google Scholar]
- 5.Odekerken VJ, van Laar T, Staal MJ, et al. Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson’s disease (NSTAPS study): a randomised controlled trial. Lancet Neurol. 2013;12:37–44. doi: 10.1016/S1474-4422(12)70264-8. [DOI] [PubMed] [Google Scholar]
- 6.Okun MS, Gallo BV, Mandybur G, et al. Subthalamic deep brain stimulation with a constant-current device in Parkinson’s disease: an open-label randomised controlled trial. Lancet Neurol. 2012;11:140–149. doi: 10.1016/S1474-4422(11)70308-8. [DOI] [PubMed] [Google Scholar]
- 7.Schuepbach WM, Rau J, Knudsen K, et al. Neurostimulation for Parkinson’s disease with early motor complications. NEJM. 2013;368:610–622. doi: 10.1056/NEJMoa1205158. [DOI] [PubMed] [Google Scholar]
- 8.Thobois S, Ardouin C, Lhommee E, et al. Non-motor dopamine withdrawal syndrome after surgery for Parkinson’s disease: predictors and underlying mesolimbic denervation. Brain. 2010;133:1111–1127. doi: 10.1093/brain/awq032. [DOI] [PubMed] [Google Scholar]
- 9.Schupbach WM, Maltete D, Houeto JL, et al. Neurosurgery at an earlier stage of Parkinson disease: a randomized, controlled trial. Neurology. 2007;68:267–271. doi: 10.1212/01.wnl.0000250253.03919.fb. [DOI] [PubMed] [Google Scholar]
- 10.Amami P, Dekker I, Piacentini S, et al. Impulse control behaviours in patients with Parkinson’s disease after subthalamic deep brain stimulation: de novo cases and 3-year follow-up. J Neurol Neurosurg Psychiatry. 2015;86:562–564. doi: 10.1136/jnnp-2013-307214. [DOI] [PubMed] [Google Scholar]
- 11.Anderson VC, Burchiel KJ, Hogarth P, Favre J, Hammerstad JP. Pallidal vs subthalamic nucleus deep brain stimulation in Parkinson disease. Arch Neurol. 2005;62:554–560. doi: 10.1001/archneur.62.4.554. [DOI] [PubMed] [Google Scholar]
- 12.Weaver FM, Follett KA, Stern M, et al. Randomized trial of deep brain stimulation for Parkinson disease: thirty-six-month outcomes. Neurology. 2012;79:55–65. doi: 10.1212/WNL.0b013e31825dcdc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allert N, Lehrke R, Sturm V, Volkmann J. Secondary failure after ten years of pallidal neurostimulation in a patient with advanced Parkinson’s disease. J Neural Transm. 2010;117:349–351. doi: 10.1007/s00702-009-0363-1. [DOI] [PubMed] [Google Scholar]
- 14.Coenen VA, Allert N, Paus S, Kronenburger M, Urbach H, Madler B. Modulation of the cerebello-thalamo-cortical network in thalamic deep brain stimulation for tremor: a diffusion tensor imaging study. Neurosurgery. 2014 doi: 10.1227/NEU.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 15.Coenen VA, Madler B, Schiffbauer H, Urbach H, Allert N. Individual fiber anatomy of the subthalamic region revealed with diffusion tensor imaging: a concept to identify the deep brain stimulation target for tremor suppression. Neurosurgery. 2011;68:1069–1075. doi: 10.1227/NEU.0b013e31820a1a20. discussion 75-6. [DOI] [PubMed] [Google Scholar]
- 16.Williams A, Gill S, Varma T, et al. Deep brain stimulation plus best medical therapy versus best medical therapy alone for advanced Parkinson’s disease (PD SURG trial): a randomised, open-label trial. Lancet Neurol. 2010;9:581–591. doi: 10.1016/S1474-4422(10)70093-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herzog J, Weiss PH, Assmus A, et al. Improved sensory gating of urinary bladder afferents in Parkinson’s disease following subthalamic stimulation. Brain. 2008;131:132–145. doi: 10.1093/brain/awm254. [DOI] [PubMed] [Google Scholar]
- 18.Amara AW, Standaert DG, Guthrie S, Cutter G, Watts RL, Walker HC. Unilateral subthalamic nucleus deep brain stimulation improves sleep quality in Parkinson’s disease. Parkinsonism Relat Disord. 2012;18:63–68. doi: 10.1016/j.parkreldis.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chahine LM, Ahmed A, Sun Z. Effects of STN DBS for Parkinson’s disease on restless legs syndrome and other sleep-related measures. Parkinsonism Relat Disord. 2011;17:208–211. doi: 10.1016/j.parkreldis.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 20.Pellaprat J, Ory-Magne F, Canivet C, et al. Deep brain stimulation of the subthalamic nucleus improves pain in Parkinson’s disease. Parkinsonism Relat Disord. 2014;20:662–664. doi: 10.1016/j.parkreldis.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Zibetti M, Torre E, Cinquepalmi A, et al. Motor and nonmotor symptom follow-up in parkinsonian patients after deep brain stimulation of the subthalamic nucleus. Eur Neurol. 2007;58:218–223. doi: 10.1159/000107943. [DOI] [PubMed] [Google Scholar]
- 22.Witt K, Daniels C, Reiff J, et al. Neuropsychological and psychiatric changes after deep brain stimulation for Parkinson’s disease: a randomised, multicentre study. Lancet Neurol. 2008;7:605–614. doi: 10.1016/S1474-4422(08)70114-5. [DOI] [PubMed] [Google Scholar]
- 23.Witt K, Granert O, Daniels C, et al. Relation of lead trajectory and electrode position to neuropsychological outcomes of subthalamic neurostimulation in Parkinson’s disease: results from a randomized trial. Brain. 2013;136:2109–2119. doi: 10.1093/brain/awt151. [DOI] [PubMed] [Google Scholar]
- 24.Volkmann J, Daniels C, Witt K. Neuropsychiatric effects of subthalamic neurostimulation in Parkinson disease. Nat Rev Neurol. 2010;6:487–498. doi: 10.1038/nrneurol.2010.111. [DOI] [PubMed] [Google Scholar]
- 25.Rizzone MG, Fasano A, Daniele A, et al. Long-term outcome of subthalamic nucleus DBS in Parkinson’s disease: from the advanced phase towards the late stage of the disease? Parkinsonism Relat Disord. 2014;20:376–381. doi: 10.1016/j.parkreldis.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 26.Moro E, Lozano AM, Pollak P, et al. Long-term results of a multicenter study on subthalamic and pallidal stimulation in Parkinson’s disease. Mov Disord. 2010;25:578–586. doi: 10.1002/mds.22735. [DOI] [PubMed] [Google Scholar]
- 27.Rodriguez-Oroz MC, Moro E, Krack P. Long-term outcomes of surgical therapies for Parkinson’s disease. Mov Disord. 2012;27:1718–1728. doi: 10.1002/mds.25214. [DOI] [PubMed] [Google Scholar]
- 28.Botzel K, Tronnier V, Gasser T. The differential diagnosis and treatment of tremor. Dtsch Arztebl int. 2014;111:225–235. doi: 10.3238/arztebl.2014.0225. quiz 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuurman PR, Bosch DA, Bossuyt PM, et al. A comparison of continuous thalamic stimulation and thalamotomy for suppression of severe tremor. NEJM. 2000;342:461–468. doi: 10.1056/NEJM200002173420703. [DOI] [PubMed] [Google Scholar]
- 30.Nikkhah G, Prokop T, Hellwig B, Lucking CH, Ostertag CB. Deep brain stimulation of the nucleus ventralis intermedius for Holmes (rubral) tremor and associated dystonia caused by upper brainstem lesions. Report of two cases. J Neurosurg. 2004;100:1079–1083. doi: 10.3171/jns.2004.100.6.1079. [DOI] [PubMed] [Google Scholar]
- 31.Piette T, Mescola P, Henriet M, Cornil C, Jacquy J, Vanderkelen B. [A surgical approach to Holmes’ tremor associated with high-frequency synchronous bursts] Rev Neurol. 2004;160:707–711. doi: 10.1016/s0035-3787(04)71023-1. [DOI] [PubMed] [Google Scholar]
- 32.Espay AJ, Duker AP, Chen R, et al. Deep brain stimulation of the ventral intermediate nucleus of the thalamus in medically refractory orthostatic tremor: preliminary observations. Mov Disord. 2008;23:2357–2362. doi: 10.1002/mds.22271. [DOI] [PubMed] [Google Scholar]
- 33.Guridi J, Rodriguez-Oroz MC, Arbizu J, et al. Successful thalamic deep brain stimulation for orthostatic tremor. Mov Disord. 2008;23:1808–1811. doi: 10.1002/mds.22001. [DOI] [PubMed] [Google Scholar]
- 34.Weiss D, Govindan RB, Rilk A, et al. Central oscillators in a patient with neuropathic tremor: evidence from intraoperative local field potential recordings. Mov Disord. 2011;26:323–327. doi: 10.1002/mds.23374. [DOI] [PubMed] [Google Scholar]
- 35.Herzog J, Hamel W, Wenzelburger R, et al. Kinematic analysis of thalamic versus subthalamic neurostimulation in postural and intention tremor. Brain. 2007;130:1608–1625. doi: 10.1093/brain/awm077. [DOI] [PubMed] [Google Scholar]
- 36.Hamel W, Herzog J, Kopper F, et al. Deep brain stimulation in the subthalamic area is more effective than nucleus ventralis intermedius stimulation for bilateral intention tremor. Acta Neurochir. 2007;149:749–758. doi: 10.1007/s00701-007-1230-1. discussion 58. [DOI] [PubMed] [Google Scholar]
- 37.Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov. 2014;4 doi: 10.7916/D8TT4P4B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blomstedt P, Hariz GM, Hariz MI, Koskinen LO. Thalamic deep brain stimulation in the treatment of essential tremor: a long-term follow-up. Br J Neurosurg. 2007;21:504–509. doi: 10.1080/02688690701552278. [DOI] [PubMed] [Google Scholar]
- 39.Sydow O, Thobois S, Alesch F, Speelman JD. Multicentre European study of thalamic stimulation in essential tremor: a six year follow up. J Neurol Neurosurg Psychiatry. 2003;74:1387–1391. doi: 10.1136/jnnp.74.10.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Baizabal-Carvallo JF, Kagnoff MN, Jimenez-Shahed J, Fekete R, Jankovic J. The safety and efficacy of thalamic deep brain stimulation in essential tremor: 10 years and beyond. J Neurol Neurosurg Psychiatry. 2014;85:567–572. doi: 10.1136/jnnp-2013-304943. [DOI] [PubMed] [Google Scholar]
- e1.Barbe MT, Liebhart L, Runge M, et al. Deep brain stimulation in the nucleus ventralis intermedius in patients with essential tremor: habituation of tremor suppression. J Neurol. 2011;258:434–439. doi: 10.1007/s00415-010-5773-3. [DOI] [PubMed] [Google Scholar]
- e2.Blomstedt P, Fytagoridis A, Tisch S. Deep brain stimulation of the posterior subthalamic area in the treatment of tremor. Acta Neurochirur. 2009;151:31–36. doi: 10.1007/s00701-008-0163-7. [DOI] [PubMed] [Google Scholar]
- e3.Fytagoridis A, Sandvik U, Astrom M, Bergenheim T, Blomstedt P. Long term follow-up of deep brain stimulation of the caudal zona incerta for essential tremor. J Neurol Neurosurg Psychiatry. 2012;83:258–262. doi: 10.1136/jnnp-2011-300765. [DOI] [PubMed] [Google Scholar]
- e4.Timmermann L, Deuschl G, Fogel W, et al. [Deep brain stimulation for tremor in multiple sclerosis : consensus recommendations of the German Deep Brain Stimulation Association] Der Nervenarzt. 2009;80:673–677. doi: 10.1007/s00115-009-2697-1. [DOI] [PubMed] [Google Scholar]
- e5.Kumar R, Dagher A, Hutchison WD, Lang AE, Lozano AM. Globus pallidus deep brain stimulation for generalized dystonia: clinical and PET investigation. Neurology. 1999;53:871–874. doi: 10.1212/wnl.53.4.871. [DOI] [PubMed] [Google Scholar]
- e6.Vidailhet M, Vercueil L, Houeto JL, et al. Bilateral deep-brain stimulation of the globus pallidus in primary generalized dystonia. NEJM. 2005;352:459–467. doi: 10.1056/NEJMoa042187. [DOI] [PubMed] [Google Scholar]
- e7.Kupsch A, Benecke R, Muller J, et al. Pallidal deep-brain stimulation in primary generalized or segmental dystonia. NEJM. 2006;355:1978–1990. doi: 10.1056/NEJMoa063618. [DOI] [PubMed] [Google Scholar]
- e8.Volkmann J, Wolters A, Kupsch A, et al. Pallidal deep brain stimulation in patients with primary generalised or segmental dystonia: 5-year follow-up of a randomised trial. Lancet Neurol. 2012;11:1029–1038. doi: 10.1016/S1474-4422(12)70257-0. [DOI] [PubMed] [Google Scholar]
- e9.Isaias IU, Volkmann J, Kupsch A, et al. Factors predicting protracted improvement after pallidal DBS for primary dystonia: the role of age and disease duration. J Neurol. 2011;258:1469–1476. doi: 10.1007/s00415-011-5961-9. [DOI] [PubMed] [Google Scholar]
- e10.Kiss ZH, Doig-Beyaert K, Eliasziw M, et al. The Canadian multicentre study of deep brain stimulation for cervical dystonia. Brain. 2007;130:2879–2886. doi: 10.1093/brain/awm229. [DOI] [PubMed] [Google Scholar]
- e11.Skogseid IM, Ramm-Pettersen J, Volkmann J, Kerty E, Dietrichs E, Roste GK. Good long-term efficacy of pallidal stimulation in cervical dystonia: a prospective, observer-blinded study. Eur J Neurol. 2012;19:610–615. doi: 10.1111/j.1468-1331.2011.03591.x. [DOI] [PubMed] [Google Scholar]
- e12.Volkmann J, Mueller J, Deuschl G, et al. Pallidal neurostimulation in patients with medication-refractory cervical dystonia: a randomised, sham-controlled trial. Lancet Neurol. 2014;13:875–884. doi: 10.1016/S1474-4422(14)70143-7. [DOI] [PubMed] [Google Scholar]
- e13.Ostrem JL, Racine CA, Glass GA, et al. Subthalamic nucleus deep brain stimulation in primary cervical dystonia. Neurology. 2011;76:870–878. doi: 10.1212/WNL.0b013e31820f2e4f. [DOI] [PubMed] [Google Scholar]
- e14.Koy A, Hellmich M, Pauls KA. Effects of deep brain stimulation in dyskinetic cerebral palsy: a meta-analysis. Mov Disord. 2013;28:647–654. doi: 10.1002/mds.25339. [DOI] [PubMed] [Google Scholar]
- e15.et al. Timmermann L, Pauls KA, Wieland K, et al. Dystonia in neurodegeneration with brain iron accumulation: outcome of bilateral pallidal stimulation. Brain. 2010;133:701–712. doi: 10.1093/brain/awq022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e16.Mentzel CL, Tenback DE, Tijssen MA, Visser-Vandewalle VE, van Harten PN. Efficacy and safety of deep brain stimulation in patients with medication-induced tardive dyskinesia and/or dystonia: a systematic review. J Clin Psychiatry. 2012;73:1434–1438. doi: 10.4088/JCP.12r07643. [DOI] [PubMed] [Google Scholar]
- e17.Fisher R, Salanova V, Witt T, et al. Electrical stimulation of the anterior nucleus of thalamus for treatment of refractory epilepsy. Epilepsia. 2010;51:899–908. doi: 10.1111/j.1528-1167.2010.02536.x. [DOI] [PubMed] [Google Scholar]
- e18.Schulze-Bonhage A, Zentner J. The preoperative evaluation and surgical treatment of epilepsy. Dtsch Arztebl Int. 2014;111:313–319. doi: 10.3238/arztebl.2014.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e19.Hodaie M, Wennberg RA, Dostrovsky JO, Lozano AM. Chronic anterior thalamus stimulation for intractable epilepsy. Epilepsia. 2002;43:603–608. doi: 10.1046/j.1528-1157.2002.26001.x. [DOI] [PubMed] [Google Scholar]
- e20.Schulze-Bonhage A, Coenen V. [Treatment of epilepsy: peripheral and central stimulation techniques] Der Nervenarzt. 2013;84:517–528. doi: 10.1007/s00115-013-3749-0. quiz 29. [DOI] [PubMed] [Google Scholar]
- e21.Sprengers M, Vonck K, Carrette E, Marson AG, Boon P. Deep brain and cortical stimulation for epilepsy. The Cochrane Database of Systematic Reviews. 2014;6 doi: 10.1002/14651858.CD008497.pub2. CD008497. [DOI] [PubMed] [Google Scholar]
- e22.Aouizerate B, Cuny E, Martin-Guehl C, et al. Deep brain stimulation of the ventral caudate nucleus in the treatment of obsessive-compulsive disorder and major depression. Case report. J Neurosurg. 2004;101:682–686. doi: 10.3171/jns.2004.101.4.0682. [DOI] [PubMed] [Google Scholar]
- e23.Kuhn J, Grundler TO, Lenartz D, Sturm V, Klosterkotter J, Huff W. Deep brain stimulation for psychiatric disorders. Dtsch Arztebl Int. 2010;107:105–113. doi: 10.3238/arztebl.2010.0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e24.Greenberg BD, Malone DA, Friehs GM, et al. Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology. 2006;31 doi: 10.1038/sj.npp.1301165. [DOI] [PubMed] [Google Scholar]
- e25.Mayberg H, Lozano A, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- e26.Malone DA, Jr., Dougherty DD, Rezai AR, et al. Deep brain stimulation of the ventral capsule/ventral striatum for treatment-resistant depression. Biol Psychiatry. 2009;65:267–275. doi: 10.1016/j.biopsych.2008.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e27.Schlaepfer TE, Cohen MX, Frick C, et al. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- e28.Schlaepfer TE, Bewernick BH, Kayser S, Madler B, Coenen VA. Rapid effects of deep brain stimulation for treatment-resistant major depression. Biol Psychiatry. 2013;73:1204–1212. doi: 10.1016/j.biopsych.2013.01.034. [DOI] [PubMed] [Google Scholar]
- e29.Bewernick BH, Hurlemann R, Matusch A, et al. Nucleus accumbens deep brain stimulation decreases ratings of depression and anxiety in treatment-resistant depression. Biol Psychiatry. 2010;67:110–116. doi: 10.1016/j.biopsych.2009.09.013. [DOI] [PubMed] [Google Scholar]
- e30.Bewernick B, Kayser S, Sturm V, Schlaepfer TE. Long-term effects of nucleus accumbens deep brain stimulation in treatment-resistant depression: evidence for sustained efficacy. Neuropschopharmacology. 2012;37:1975–1985. doi: 10.1038/npp.2012.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e31.Dougherty DD, Rezai AR, Carpenter LL, et al. A randomized sham-controlled trial of deep brain stimulation of the ventral capsule/ventral striatum for chronic treatment-resistant depression. Biol Psychiatry. 2014 doi: 10.1016/j.biopsych.2014.11.023. doi: 10.1016/j.biopsych. 2014.11.023 (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- e32.Martens HC, Toader E, Decre MM, et al. Spatial steering of deep brain stimulation volumes using a novel lead design. Clin Neurophysiol. 2011;122:558–566. doi: 10.1016/j.clinph.2010.07.026. [DOI] [PubMed] [Google Scholar]
- e33.Pollo C, Kaelin-Lang A, Oertel MF, et al. Directional deep brain stimulation: an intraoperative double-blind pilot study. Brain. 2014;137:2015–2026. doi: 10.1093/brain/awu102. [DOI] [PubMed] [Google Scholar]
- e34.Coenen VA, Schlaepfer TE, Allert N, Madler B. Diffusion tensor imaging and neuromodulation: DTI as key technology for deep brain stimulation. Int Rev Neurobiol. 2012;107:207–234. doi: 10.1016/B978-0-12-404706-8.00011-5. [DOI] [PubMed] [Google Scholar]
- e35.Madler B, Coenen VA. Explaining clinical effects of deep brain stimulation through simplified target-specific modeling of the volume of activated tissue. AJNR. 2012;33:1072–1080. doi: 10.3174/ajnr.A2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- e36.Hunsche S, Sauner D, Runge MJ, et al. Tractography-guided stimulation of somatosensory fibers for thalamic pain relief. Stereotact Funct Neurosurg. 2013;91:328–334. doi: 10.1159/000350024. [DOI] [PubMed] [Google Scholar]