Abstract
Trichoderma-based formulations are applied as commercial biocontrol agents for soil-borne plant pathogens. Chlamydospores are active propagules in Trichoderma spp., but their production is currently limited due to a lack of optimal liquid fermentation technology. In this study, we explored response surface methodologies for optimizing fermentation technology in Trichoderma SH2303. Our initial studies, using the Plackett-Burman design, identified cornmeal, glycerol, and initial pH levels as the most significant factors (P<0.05) for enhancing the production of chlamydospores. Subsequently, we applied the Box-Behnken design to study the interactions between, and optimal levels of, a number of factors in chlamydospore production. These statistically predicted results indicated that the highest number of chlamydospores (3.6×108 spores/ml) would be obtained under the following condition: corn flour 62.86 g/L, glycerol 7.54 ml/L, pH 4.17, and 6-d incubation in liquid fermentation. We validated these predicted values via three repeated experiments using the optimal culture and achieved maximum chlamydospores of 4.5×108 spores/ml, which approximately a 8-fold increase in the number of chlamydospores produced by T. harzianum SH2303 compared with that before optimization. These optimized values could help make chlamydospore production cost-efficient in the future development of novel biocontrol agents.
Keywords: Trichoderma harzianum SH2303, Chlamydospore, Plackett-Burman screening, Box-Behnken design, Fermentation optimization
1. Introduction
Trichoderma are fast growing filamentous fungi found in many ecosystems (Marra et al., 2006) that kill other pathogenic fungi and consume them using hydrolytic enzymes (Zeilinger et al., 1999; Harman et al., 2004). This antagonistic behavior has led to their use (especially the mycoparasites T. atroviride, T. harzianum, and T. virens) as biological control agents (BCAs) for agricultural applications (Schuster and Schmoll, 2010; Carreras-Villasenor et al., 2011). Over the past decade, researchers have made significant progress towards understanding the genetic, developmental, and regulatory signaling elements of the secondary metabolites involved in the interaction of Trichoderma-plant-pathogens (Mach and Zeilinger, 2003; Harman, 2006; Woo et al., 2006).
Trichoderma species produce three major types of propagules (mycelia, conidia, and chlamydospores) (Verma et al., 2007) that possess distinct physiological characteristics in terms of production, stability, and BCA activity. For efficient BCA action, it is imperative to select the most suitable type of propagule. Most commercial Trichoderma-based formulations use conidiospores because they are affordable and can be propagated efficiently in standing liquid or solid form (Fravel, 2005). Chlamydospore-based formulations have other beneficial features, including resistance to drying and low temperatures, insensitivity to soil antibiotics, and extended preservation time, which in turn simplifies processing, storage, and transport of the BCA (Mishra et al., 2012). For instance, studies on the shelf life of the Trichoderma chlamydospore-based Tricoguard™ have shown that it can be safely stored for up to 270 d and can survive in the soil of natural ecosystems better than conidia (Jagtap and Bhatnagar, 2000; Mishra et al., 2012). However, a better understanding of the factors involved in the morphogenic switch from mycelia to chlamydospore production is still essential for its use in commercial formulations.
To date, researchers have focused primarily on the effect of environmental conditions, such as carbon (C) and nitrogen (N), C:N ratio, pH, light, and induction signals, on the initiation of conidiation in Trichoderma (Gao et al., 2007; Friedl et al., 2008; Steyaert et al., 2010b; Tisch and Schmoll, 2010). A few studies have reported the use of liquid-culture technology for the mass production and application of Trichoderma chlamydospores (Lewis and Papavizas, 1983; 1984), but there is still a barrier to their commercial use because of low fermentation yield and high cost. The objective of the present research, therefore, was to develop a cost-effective medium and optimal conditions for the production of chlamydospores in T. harzianum SH2303 (effective against corn stalk rot and Fusarium Wilt of Cucumber) (Lin et al., 2012) and thus to lay a solid foundation for the development of Trichoderma-based active chlamydospore formulations in agriculture.
2. Materials and methods
2.1. Strains and inoculation
We identified and isolated the T. harzianum strain SH2303 (CGMCC No. 4963) from corn fields in the Shanghai district based on morphology and internal transcribed spacer (ITS) sequence analysis; its NCBI accession number is KJ755188. The strain was subsequently grown on potato dextrose agar (PDA) plates, incubated at 28 °C for 5 d, and then the mature spores were harvested and adjusted to 1×107 conidia/ml with sterile distilled water. The conidial concentration was determined using a haemocytometer. A total of 100 μl homogeneous conidial suspension (about 1×106 conidia) was inoculated into 250 ml Erlenmeyer flasks containing 100 ml of sterilized medium and incubated in a rotary shaker at 28 °C and 200 r/min for 72 h. The initial pH value of the cultures was not controlled.
2.2. Medium and growth conditions
Six different kinds of medium were chosen for the initial screening experiment (Table 1): (1) potato dextrose (PD): potato extract 1000 ml and dextrose (glucose) 20 g/L; (2) potato sucrose (PS): potato extract 1 L and sucrose 20 g/L, pH 6.0–6.5; (3) Richard: sucrose 50 g/L, KNO3 10 g/L, K2HPO4 5 g/L, MgSO4 0.5 g/L, FeCl3 0.02 g/L; (4) Czapek: sucrose 30 g/L, KNO3 2 g/L, KH2PO4 1 g/L, FeSO4·7H2O 0.5 g/L, KCl 0.5 g/L, FeSO4 0.01 g/L; (5) Gorodkowa: peptone 10 g/L, beef extract 10 g/L, glucose 2.5 g/L, NaCl 5 g/L; (6) cornmeal medium: cornmeal and water (20:100 (w/v); corn flour 200 g/L). All prepared liquid media were sterilized at 115 °C for 15 min and stored at room temperature until further use. For the potato extracts we sliced the potatoes very thinly, immediately added 1000 ml water to prevent their oxidation, boiled them until soft for 10 min, and finally filtered them through a cotton-cloth.
Table 1.
Effects of different media on chlamydospore and conidiospore production by T. harzianum SH2303 in liquid culture
| No. | Medium | Main C/N source (g/L) | C:N (mol:mol) | ChlS (×106) | ConS (×106) |
| 1 | PD | Glucose (20) | (3–4):1 | 0.27±0.10 | 4.20±0.32 |
| 2 | PS | Sucrose (20) | (3–4):1 | 2.20±0.13 | 2.40±0.21 |
| 3 | Richard | Sucrose (50), KNO3 (10) | 17.5:1 | 0 | 3.40±0.20 |
| 4 | Czapek | Sucrose (30), KNO3 (2) | 52.5:1 | 0.62±0.05 | 1.20±0.18 |
| 5 | Gorodkowa | Glucose (2.5), peptone (10), beef extract (10) | 1:2 | 2.50±0.18 | 0 |
| 6 | Cornmeal | Corn flour (200) | 9:1 | 52.00±5.00 | 61.00±2.20 |
ChlS: chlamydospores; ConS: conidiospores; PD: potato dextrose; PS: potato sucrose. Carbon and nitrogen content in natural and synthetic ingredients. Peptone (12% nitrogen), beef extract (13% nitrogen), corn flour (8.7% nitrogen and 75.2% carbon)
The pH value of the fermentation media was adjusted by adding either 1 mol/L sodium hydroxide or 1 mol/L hydrochloric acid as required.
2.3. Experimental design and data analysis
The Plackett-Burman (PB) experimental design is a two-level, fractional, factorial design method that is based on the non-perfectly balanced block principle and intended to test for the dependence of some measured quantity on a number of independent variables (Lai et al., 2003). This design filters out the most important factors involved in the fermentation process in as few experiments as possible. We varied each factor (n=8) over 2 levels of specialized design (Table 2). The positive and negative effects of the various factors were determined according to the t-value test and displayed on a Pareto chart (Fig. 1).
Table 2.
Code and actual values of all 8 variables used in the Plackett-Burman design
| Variable | Culture factor | Code level | |
| Low (−1) | High (+1) | ||
| A | Inoculums (ml) | 2 | 8 |
| B | Peptone (g/L) | 0 | 4 |
| C | Initial PH | 3 | 7 |
| D | (NH)4SO4 (g/L) | 0 | 1 |
| E | KH2PO4 (g/L) | 0 | 3 |
| F | Corn flour (g/L) | 40 | 80 |
| G | MgSO4·7H2O (g/L) | 0 | 3 |
| H | Glycerol (g/L) | 0 | 5 |
Fig. 1.
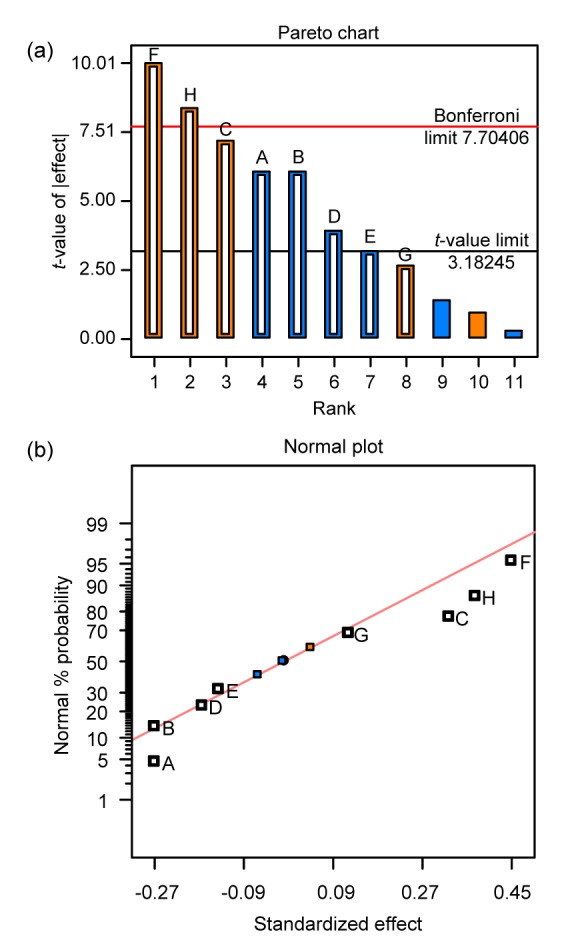
Pareto chart (a) and normal plot graph (b) identifying the screening factors
Hollow shapes represent the 8 factors of PB test, and among them, orange (F, H, C, G) denotes positive effects; blue (A, B, D, E) denotes negative effects; solid shapes represent 3 blank tests (Note: for interpretation of the references to color in this figure legend, the reader is referred to the web version of this article)
2.4. Box-Behnken design for chlamydospore optimization
The Box-Behnken design (BBD) is a statistical method that is used to study the maximum response corresponding to the best interactions in a multi-factor system (Bae and Shoda, 2005). Each factor, or independent variable, is placed at one of three equal spaced values, usually coded as −1, 0, +1. The quadratic regression equation was used to calculate the best fit of the main and interaction effects of the various factors.
We studied the optimization, interaction, and combined effects of the three major factors (corn flour, initial pH, and glycerol) on Trichoderma-derived chlamydospore production using 17 experimental runs corresponding to the Box-Behnken response surface method. This method was divided into two categories: (1) the factorial points of the variable values in X 1, X2, X 3 that constitute the three-dimensional vertex containing a total of 12 factorial points, and (2) zero (the center of the region); the zero experiment was repeated three times to estimate the experimental error. Subsequent to the regression analysis, the experimental factors (R 1) were expressed with the second order polynomial equation, as follows:
 . .
|
Optimization of the experimental data identified the model coefficient, while the simple multi-function trait analysis determined the extreme points and the maximum values of the corresponding independent variable.
2.5. Chlamydospore and conidiospore assay
The chlamydospores and conidiospores were assayed with a hemocytometer. Spore suspensions were harvested from 6-d cultures with 5 ml sterile water containing 0.01% Triton X-100. The suspensions were passed through four layers of cheese cloth to remove hyphal fragments, vortexed for 1 min to break up the clumps of cells, and counted microscopically (Olympus BX51TF, Japan). All experiments were carried out three times.
2.6. Statistical analysis
A logarithmic transformation was applied on the sporulation data before statistical analysis. Statistical analysis of all data was subjected to Design Expert 7.0 software (Stat-Ease, Inc., Minneapolis, MN, USA). The statistical significance of the regression coefficients was 95%. The optimum levels of variables were obtained by graphical analysis.
3. Results
3.1. Effect of the C:N ratio on chlamydospore formation
Five kinds of medium with different concentrations of C and N were chosen to identify the optimal C:N ratio (Table 1). Results showed that conidiospores, but not chlamydospores, were produced on the Richard medium (C:N=17.5:1) while the Gorodkowa medium (C:N=1:2) was able to produce chlamydospores only. Similarly, higher levels of glucose (PD) compared to sucrose (PS) promote conidiospore production. It is worth noting that the natural material cornmeal (C:N=9:1) is highly beneficial for the formation of both spores. Compared with synthetic media, cornmeal contains a variety of amino acids, trace elements, vitamins, and some unknown growth factors that are advantageous for the production of chlamydospores. As corn flour is cheap and easily acquired, we adopted corn flour as providing the optimal C and N for enhanced chlamydospore production.
3.2. Plackett-Burman screen of crucial factors
We also analyzed the following factors as possible candidates for improving chlamydospore formation: peptone, glycerol, (NH)4SO4, KH2PO4, MgSO4·7H2O, plus the inoculation volume and the initial pH of the medium. In Table 2, each row represents an experiment and each column represents an independent variable while the signs represent the two different levels (high and low) of the independent variable. The significant levels of each medium were determined by t-test; the predicted value was consistent with the real values as identified on the fitted first-order model (Table 3).
Table 3.
Experimental design of the Plackett-Burman method used for screening the culture
| Run | A | B | C | D | E | F | G | H | Y | Ŷ |
| 1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | 6.62 | 6.59 |
| 2 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | 6.32 | 6.30 |
| 3 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | 7.08 | 7.05 |
| 4 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | 6.45 | 6.48 |
| 5 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 6.56 | 6.57 |
| 6 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | +1 | 7.49 | 7.52 |
| 7 | +1 | +1 | −1 | +1 | +1 | +1 | −1 | −1 | 6.08 | 6.10 |
| 8 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | 6.38 | 6.41 |
| 9 | +1 | −1 | +1 | +1 | −1 | +1 | +1 | +1 | 7.38 | 7.39 |
| 10 | +1 | +1 | −1 | −1 | −1 | +1 | −1 | +1 | 6.87 | 6.86 |
| 11 | −1 | −1 | −1 | +1 | −1 | +1 | +1 | −1 | 6.92 | 6.91 |
| 12 | −1 | +1 | +1 | +1 | −1 | −1 | −1 | +1 | 6.79 | 6.76 |
A–H correspond to the different factors shown in Table 2, i.e., inoculums, peptone, initial pH, (NH)4SO4, KH2PO4, corn flour, MgSO4·7H2O, and glycerol in alphabetical order. Y, experimental responses; Ŷ, predicted values calculated from the fitted first-order model
Further analysis, analysis of variance (ANOVA), revealed that cornmeal, initial pH, and glycerol all had a significant positive influence on the formation of chlamydospores. Other factors such as inoculums and peptone were found to have negative effects. The calculated model F-value of 72.27 implies that the model is significant; there is only a 1.37% chance that such a large value could occur due to noise. Values of “Prob>F” that are less than 0.01 indicate that the model terms are extremely significant. In this case F, H, and C are significant (Table 4). The calculated “Pred R-Squared” of 0.8552 is in reasonable agreement with the “Adj R-Squared” of 0.9668. The “Adeq Precision” measures the signal to noise ratio; a ratio greater than 4 is desirable while the calculated ratio of 21.101 indicates an adequate signal. This model can be used to navigate the design space.
Table 4.
Analysis of variance for the Plackett-Burman design
| Factor | Sum of squares | df | Mean square | F-value | P-value (Prob>F) |
| Model | 2.005533 | 8 | 0.222837 | 72.27147 | 0.0137* |
| A-A | 0.224133 | 1 | 0.224133 | 72.69189 | 0.0135* |
| B-B | 0.224133 | 1 | 0.224133 | 72.69189 | 0.0135* |
| C-C | 0.313633 | 1 | 0.313633 | 101.71890 | 0.0097** |
| D-D | 0.093633 | 1 | 0.093633 | 30.36757 | 0.0314* |
| E-E | 0.061633 | 1 | 0.061633 | 19.98919 | 0.0466* |
| F-F | 0.607500 | 1 | 0.607500 | 197.02700 | 0.0050** |
| G-G | 0.043200 | 1 | 0.043200 | 14.01081 | 0.0645 |
| H-H | 0.425633 | 1 | 0.425633 | 138.04320 | 0.0072** |
| Residual | 0.006167 | 2 | 0.003083 | ||
| Cor total | 2.011700 | 10 |
P<0.01,
P<0.05
3.3. Box-Behnken design optimization
We next optimized the production of T. harzianum chlamydospores using corn flour (X 1), initial pH (X 2), and glycerol (X 3) as the crucial parameters in our Box-Behnken analysis (Table 5). The experimental results were fitted with a second order polynomial equation. The values of the regression coefficients were calculated and the fitted equations (in terms of the coded values) for predicting log chlamydospores (Y C) were as follows:
 . .
|
Table 5.
Results of the central composition experiment
| Run | Code variable level |
Real variable level |
log Chl |
|||||
| X 1 | X 2 | X 3 | Corn flour | Initial pH | Glycerol | Y | Ŷ | |
| 1 | 1 | 1 | 0 | 65 | 4.0 | 9.0 | 8.43 | 8.37 |
| 2 | 0 | 0 | 0 | 60 | 4.0 | 8.0 | 8.59 | 8.49 |
| 3 | 1 | 0 | −1 | 65 | 3.8 | 8.0 | 8.49 | 8.32 |
| 4 | 0 | 1 | −1 | 60 | 3.8 | 9.0 | 8.40 | 8.52 |
| 5 | −1 | −1 | 0 | 55 | 4.0 | 7.0 | 8.38 | 8.38 |
| 6 | 0 | 0 | 0 | 60 | 4.0 | 8.0 | 8.65 | 8.60 |
| 7 | 0 | 0 | 0 | 60 | 4.0 | 8.0 | 8.57 | 8.33 |
| 8 | 0 | −1 | −1 | 60 | 3.8 | 7.0 | 8.52 | 8.43 |
| 9 | −1 | 0 | −1 | 55 | 3.8 | 8.0 | 8.36 | 8.51 |
| 10 | −1 | 0 | 1 | 55 | 4.2 | 8.0 | 8.32 | 8.49 |
| 11 | 0 | −1 | 1 | 60 | 4.2 | 7.0 | 8.49 | 8.40 |
| 12 | 0 | 1 | 1 | 60 | 4.2 | 9.0 | 8.38 | 8.39 |
| 13 | 0 | 0 | 0 | 60 | 4.0 | 8.0 | 8.61 | 8.61 |
| 14 | 1 | 0 | 1 | 65 | 4.2 | 8.0 | 8.53 | 8.61 |
| 15 | 1 | −1 | 0 | 65 | 4.0 | 7.0 | 8.59 | 8.61 |
| 16 | 0 | 0 | 0 | 60 | 4.0 | 8.0 | 8.61 | 8.61 |
| 17 | −1 | 1 | 0 | 55 | 4.0 | 9.0 | 8.34 | 8.61 |
Y, experimental responses; Ŷ, predicted values calculated from the fitted second-order model; Chl: chlamydospore
Based on our results, the “Pred R-Squared” of 0.9172 is in reasonable agreement with the “Adj R-Squared” of 0.9494 and the “Adeq Precision” of 15.391 indicates an adequate signal. The analysis of variance for the refined model is summarized in Table 6. The model F-value of 34.35 implies that the model was significant; there is only a 0.01% chance that this value could occur due to noise. Values of “Prob>F” less than 0.05 indicate that the model terms are significant. For our experiments, X 1 (cornmeal) and X 3 (initial pH) were both significant. The calculated “Lack of Fit F-value” of 0.24 implies that it is not significantly relative to pure error. We found that there is a 86.69% chance that this value could occur due to noise. These results are consistent with the model. Chlamydospore production, as predicted by the final quadratic model together with the corresponding observed values, is shown in Table 5. Results show excellent agreement between the predicted value (Ŷ) and experimental data (Y). The optimum values for achieving the maximum level of chlamydospore production are X 1=62.86 g/L, X 2=4.17, and X 3=7.54 ml/L, and the predicted chlamydospore production corresponding to these values is 3.6×108 spores/ml. This concentration is the maximum value bounded by the experimental values.
Table 6.
ANOVA analysis of the regression equation
| Factor | Sum of squares | df | Mean square | F-value | P-value (Prob>F) |
| Model | 0.180000 | 9 | 0.020000 | 34.35000 | <0.0001** |
| X 1 | 0.051200 | 1 | 0.051200 | 86.46562 | <0.0001** |
| X 2 | 0.000312 | 1 | 0.000312 | 0.52774 | 0.4911 |
| X 3 | 0.023113 | 1 | 0.023113 | 39.03197 | 0.0004** |
| X 1 X 2 | 0.001600 | 1 | 0.001600 | 2.70205 | 0.1442 |
| X 1 X 3 | 0.003600 | 1 | 0.003600 | 6.07961 | 0.0431* |
| X 2 X 3 | 0.000025 | 1 | 0.000025 | 0.04222 | 0.8430 |
| X 1 2 | 0.039413 | 1 | 0.039413 | 66.55977 | <0.0001** |
| X 2 2 | 0.029887 | 1 | 0.029887 | 50.47191 | 0.0002** |
| X 3 2 | 0.023213 | 1 | 0.023213 | 39.20151 | 0.0004** |
| Residual | 0.004145 | 7 | 0.000592 | ||
| Lack of fit | 0.000625 | 3 | 0.000208 | 0.23674 | 0.8669 |
| Pure error | 0.003520 | 4 | 0.000880 | ||
| Cor total | 0.187212 | 16 |
P<0.01,
P<0.05
These experiments were performed in triplicate to confirm the accuracy of the model, yielding an average of 4.5×108 spores/ml. The agreement between the predicted and experimental results verifies the validity of the model and the existence of optimal conditions. For response contour plots (Fig. 2), based on the final model, we held two variables constant at their optimum levels while varying the other two within their experimental range. As shown in Fig. 2a, the maximum response occurs when cornmeal and initial pH are at approximately 62.86 g/L and 4.17, respectively. Moreover, these two factors were found to have synergistic effects on chlamydospore production. A 3D surface graph representation of the data corresponding to the contour plot of the predicted values at the optimal concentrations of corn flour and glycerol versus their desirability can be seen in Fig. 3.
Fig. 2.
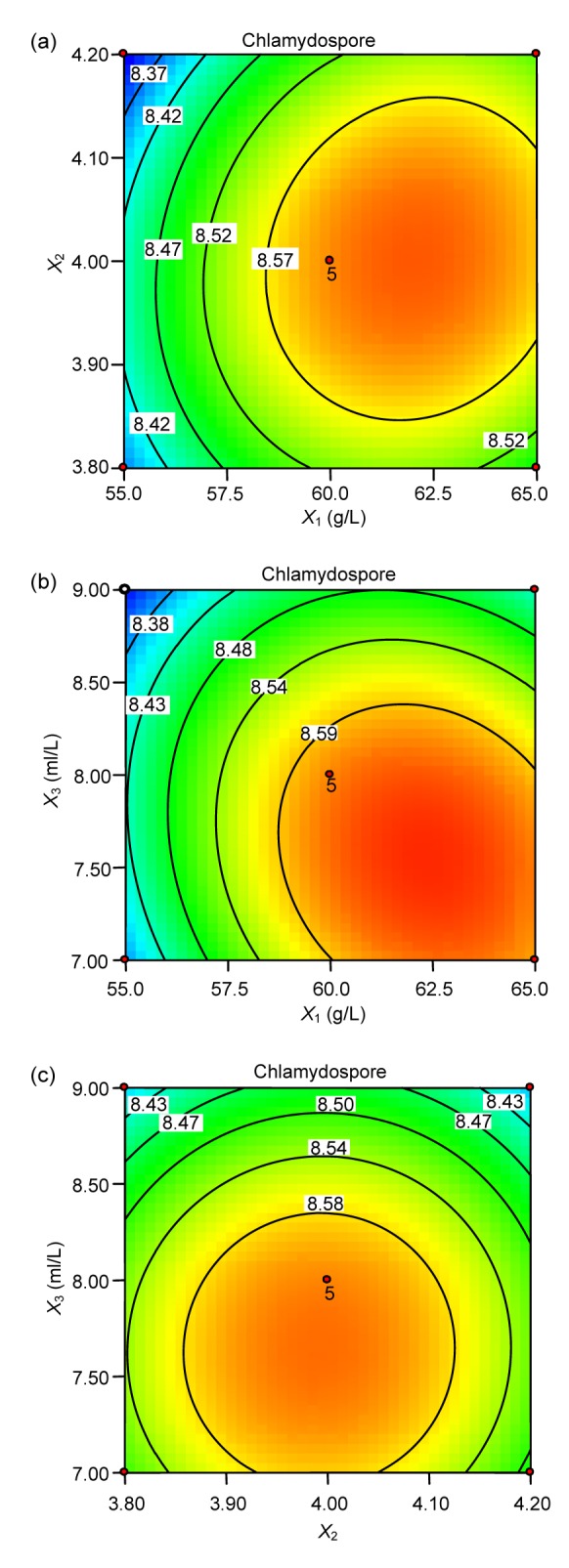
Contour plots of the interaction effect of two specific factors on chlamydospore production
(a) Effects of corn meal (X 1) and pH (X 2), and their mutual interaction; (b) Effects of corn meal (X 1) and glycerol (X 3), and their mutual interaction; (c) Effects of glycerol (X 3) and pH (X 2), and their mutual interaction
Fig. 3.
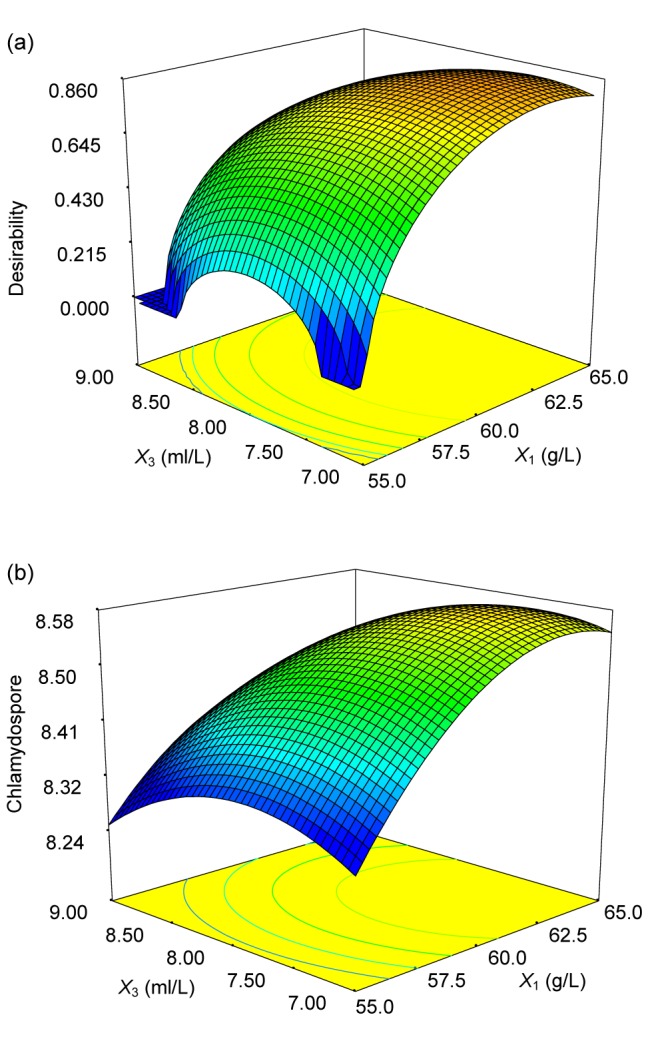
3D surface graph and its corresponding contour plot of prediction of chlamydospore production (b) and its desirability (a) at the optimal concentrations of corn flour (X 1) and glycerol (X 3)
4. Discussion
Trichoderma has the potential to produce chlamydospores cost-effectively and maintain long periods of vigorous vegetative growth during use (Fravel, 2005). We optimized statistically the fermentation conditions for the production of Trichoderma SH2303-derived chlamydospores in flask experiments using cornmeal as the basal medium. We also used a two-level Plackett-Burman and an optimal Box-Behnken design to determine the important factors and optimal concentrations for chlamydospore production. We were able to achieve maximum production of 4.5×108 chlamdospores/ml using an optimal medium consisting of cornmeal (62.86 g/L), glycerol (7.54 ml/L), and an initial pH of 4.17, in liquid culture. This is the first publication describing how to optimize the production of T. harizaiumm- derived chlamydospores using a Box-Behnken design with cornmeal as the primary nutrient.
Biotic factors (especially carbon and nitrogen status) influence conidiospore production in many species of Trichoderma (Gao et al., 2007). We found that the production of chlamydospores (thick-walled mitotic spores that generally act as “resting spores”) also relies heavily on different levels of nutrient (carbon and nitrogen) and environmental conditions (pH). Natural carbon and nitrogen sources, such as corn flour, were shown to benefit preferentially sporulation of both chlamydospores and conidiospores. These data disagree with a previous report which found that T. viride-derived conidiation is primarily dependent on the level of the carbon (Gao et al., 2007). Our data indicate that C:N ratio affects the production of T. harzianum SH2303-derived spores, while natural corn meal seems to maximize sporulation. The influence of C:N ratio on sporulation is strain-specific; however, hence consideration must be given to the complexity of nutrient requirements for improving spore yields for fungal biocontrol agents.
Several growth/production media using molasses, D-glucose, cellulose, and/or soluble starch have been shown to increase the production of Trichoderma spp. spores (Lewis and Papavizas, 1983). However, these substrates are not economically efficient, owing to high raw material costs and moderate sporulation (108 spores). In the current study, cornmeal, an important natural source of carbon and nitrogen with a relatively low cost, was shown to promote significantly the formation of SH2303-derived chlamydospores in liquid fermentation. Several other studies have reported on the use of alternative cheap raw materials for Trichoderma biomass production, including bran, and peanut hull meal (Singh et al., 2007; Maurya et al., 2012).
Glycerol is a rapid carbon source that benefits mycelial growth during the early stages of fermentation and has been found to increase the capacity of T. harzianum talc formulations to retain water, thus increasing their longevity (Sriram et al., 2011). Other environmental parameters such as light, temperature, and especially pH are also critical for chlamydospore production. Although conidial fungi can grow over a wide range of pH, maximum growth and sporulation occur near neutral pH. Our study clearly demonstrates that an acidic pH (4.17) maximizes chalamydospore formation. Similarly, a low ambient pH has been shown to favor conidiation in Trichoderma (Moreno-Mateos et al., 2007) and photoconidiation in T. pleuroticola, T. atroviride, and T. hamatum, suggesting that photoconidiation may also be regulated in the different species (Steyaert et al., 2010a). Recent research into the molecular regulation of conidiation by VELVET in T. virens, suggests that VeA acts as a positive regulator of conidiation and clamydospore production in a nutrient-rich liquid media, while the deletion of vel1 strongly promotes clamydospore production (Mukherjee and Kenerley, 2010). These data suggest how vel1 gene-mediated signaling may be manipulated to improve the production of chlamydospores.
We found that the efficient and effective use of Trichoderma to produce BCAs involves achieving a balance between total cost and the overall growth rate of the chlamydospores in a liquid medium. An understanding of liquid growth culture conditions will also be useful for future genome-wide analyses of gene expression changes during chlamydospore development. Future studies on other parameters, such as dissolved oxygen and carbon dioxide, and construction of vel1 over-expression stain, will further help maximize the production of chlamydospore-based BCAs during fermentation.
Footnotes
Project supported by the National Natural Science Foundation of China (Nos. 31201557 and 31270155), the Natural Science Foundation of Shanghai (No. 12ZR1414100), the Foundation of Basic Science and Technology of China (No. 2014FY20900), the Ministry of Education University Doctoral Foundation (No. 20120073120070), and the Shanghai Jiao Tong University Medical-Engineering Cross Research Fund (No. YG2015MS37), China
Compliance with ethics guidelines: Ya-qian LI, Kai SONG, Ya-chai LI, and Jie CHEN declare that they have no conflict of interest.
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Bae S, Shoda M. Statistical optimization of culture conditions for bacterial cellulose production using Box-Behnken design. Biotechnol Bioeng. 2005;90(1):20–28. doi: 10.1002/bit.20325. (Available from: http://dx.doi.org/10.1002/bit.20325) [DOI] [PubMed] [Google Scholar]
- 2.Carreras-Villasenor N, Sanchez-Arreguin JA, Herrera-Estrella AH. Trichoderma: sensing the environment for survival and dispersal. Microbiology. 2011;158(1):3–16. doi: 10.1099/mic.0.052688-0. (Available from: http://dx.doi.org/10.1099/mic.0.052688-0) [DOI] [PubMed] [Google Scholar]
- 3.Fravel DR. Commercialization and implementation of biocontrol. Annu Rev Phytopathol. 2005;43(1):337–359. doi: 10.1146/annurev.phyto.43.032904.092924. (Available from: http://dx.doi.org/10.1146/annurev.phyto.43.032904.092924) [DOI] [PubMed] [Google Scholar]
- 4.Friedl MA, Kubicek CP, Druzhinina IS. Carbon source dependence and photostimulation of conidiation in Hypocrea atroviridis . Appl Environ Microbiol. 2008;74(1):245–250. doi: 10.1128/AEM.02068-07. (Available from: http://dx.doi.org/10.1128/AEM.02068-07) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gao L, Sun MH, Liu XZ, et al. Effects of carbon concentration and carbon to nitrogen ratio on the growth and sporulation of several biocontrol fungi. Mycol Res. 2007;111(1):87–92. doi: 10.1016/j.mycres.2006.07.019. (Available from: http://dx.doi.org/10.1016/j.mycres.2006.07.019) [DOI] [PubMed] [Google Scholar]
- 6.Harman GE. Overview of mechanisms and uses of Trichoderma spp. Phytopathology. 2006;96(2):190–194. doi: 10.1094/PHYTO-96-0190. (Available from: http://dx.doi.org/10.1094/PHYTO-96-0190) [DOI] [PubMed] [Google Scholar]
- 7.Harman GE, Howell CR, Viterbo A, et al. Trichoderma species–opportunistic, avirulent plant symbionts. Nat Rev Microbiol. 2004;2(1):43–56. doi: 10.1038/nrmicro797. (Available from: http://dx.doi.org/10.1038/nrmicro797) [DOI] [PubMed] [Google Scholar]
- 8.Jagtap GP, Bhatnagar SS. Trichoderma chlamydospores-based formulation (Tricoguard™): impact on shelf life. Pestology. 2000;24(9):70–71. [Google Scholar]
- 9.Lai LST, Pan CC, Tzeng BK. The influence of medium design on lovastatin production and pellet formation with a high-producing mutant of Aspergillus terreus in submerged cultures. Process Biochem. 2003;38(9):1317–1326. (Available from: http://dx.doi.org/10.1016/S0032-9592(02)00330-8) [Google Scholar]
- 10.Lewis JA, Papavizas GC. Production of chlamydospores and conidia by Trichoderma spp. in liquid and solid growth media. Soil Biol Biochem. 1983;15(3):351–357. (Available from: http://dx.doi.org/10.1016/0038-0717(83)90083-4) [Google Scholar]
- 11.Lewis JA, Papavizas GC. Chlamydospore formation by Trichoderma spp. in natural substrates. Can J Microbiol. 1984;30(1):1–7. (Available from: http://dx.doi.org/10.1139/m84-001) [Google Scholar]
- 12.Lin Z, Ma J, Yi S, et al. Effect of salinity on the biocotrol Trichoderma strain SH2303. J Shanghai Jiaotong Univ (Agric Sci) 2012;30(5):51–53. (in Chinese) [Google Scholar]
- 13.Mach RL, Zeilinger S. Regulation of gene expression in industrial fungi: Trichoderma . Appl Microbiol Biotechnol. 2003;60(5):515–522. doi: 10.1007/s00253-002-1162-x. (Available from: http://dx.doi.org/10.1007/s00253-002-1162-x) [DOI] [PubMed] [Google Scholar]
- 14.Marra R, Ambrosino P, Carbone V, et al. Study of the three-way interaction between Trichoderma atroviride, plant and fungal pathogens by using a proteomic approach. Curr Genet. 2006;50(5):307–321. doi: 10.1007/s00294-006-0091-0. (Available from: http://dx.doi.org/10.1007/s00294-006-0091-0) [DOI] [PubMed] [Google Scholar]
- 15.Maurya DP, Singh D, Pratap D, et al. Optimization of solid state fermentation conditions for the production of cellulase by Trichoderma reesei . J Environ Biol. 2012;33(1):5–8. [PubMed] [Google Scholar]
- 16.Mishra DS, Prajapati CR, Gupta AK, et al. Relative bio-efficacy and shelf-life of mycelial fragments, conidia and chlamydospores of Trichoderma harzianum . Vegetos. 2012;25(1):225–232. [Google Scholar]
- 17.Moreno-Mateos MA, Delgado-Jarana J, Codón AC, et al. pH and Pac1 control development and antifungal activity in Trichoderma harzianum. Fungal Genet Biol. 2007;44(12):1355–1367. doi: 10.1016/j.fgb.2007.07.012. (Available from: http://dx.doi.org/10.1016/j.fgb.2007.07.012) [DOI] [PubMed] [Google Scholar]
- 18.Mukherjee PK, Kenerley CM. Regulation of morphogenesis and biocontrol properties in Trichoderma virens by a VELVET protein, Vel1. Appl Environ Microbiol. 2010;76(7):2345–2352. doi: 10.1128/AEM.02391-09. (Available from: http://dx.doi.org/10.1128/AEM.02391-09) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schuster A, Schmoll M. Biology and biotechnology of Trichoderma. Appl Microbiol Biotechnol. 2010;87(3):787–799. doi: 10.1007/s00253-010-2632-1. (Available from: http://dx.doi.org/10.1007/s00253-010-2632-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh A, Srivastava S, Singh HB. Effect of substrates on growth and shelf life of Trichoderma harzianum and its use in biocontrol of diseases. Bioresour Technol. 2007;98(2):470–473. doi: 10.1016/j.biortech.2006.01.002. (Available from: http://dx.doi.org/10.1016/j.biortech.2006.01.002) [DOI] [PubMed] [Google Scholar]
- 21.Sriram S, Roopa KP, Savitha MJ. Extended shelf-life of liquid fermentation derived talc formulations of Trichoderma harzianum with the addition of glycerol in the production medium. Crop Prot. 2011;30(10):1334–1339. (Available from: http://dx.doi.org/10.1016/j.cropro.2011.06.003) [Google Scholar]
- 22.Steyaert JM, Weld RJ, Stewart A. Ambient pH intrinsically influences Trichoderma conidiation and colony morphology. Fungal Biol. 2010;114(2-3):198–208. doi: 10.1016/j.funbio.2009.12.004. (Available from: http://dx.doi.org/10.1016/j.funbio.2009.12.004) [DOI] [PubMed] [Google Scholar]
- 23.Steyaert JM, Weld RJ, Stewart A. Isolate-specific conidiation in Trichoderma in response to different nitrogen sources. Fungal Biol. 2010;114(2-3):179–188. doi: 10.1016/j.funbio.2009.12.002. (Available from: http://dx.doi.org/10.1016/j.funbio.2009.12.002) [DOI] [PubMed] [Google Scholar]
- 24.Tisch D, Schmoll M. Light regulation of metabolic pathways in fungi. Appl Microbiol Biotechnol. 2010;85(5):1259–1277. doi: 10.1007/s00253-009-2320-1. (Available from: http://dx.doi.org/10.1007/s00253-009-2320-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verma M, Brar SK, Tyagi RD, et al. Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J. 2007;37(1):1–20. (Available from: http://dx.doi.org/10.1016/j.bej.2007.05.012) [Google Scholar]
- 26.Woo SL, Scala F, Ruocco M, et al. The molecular biology of the interactions between Trichoderma spp., phytopathogenic fungi, and plants. Phytopathology. 2006;96(2):181–185. doi: 10.1094/PHYTO-96-0181. (Available from: http://dx.doi.org/10.1094/PHYTO-96-0181) [DOI] [PubMed] [Google Scholar]
- 27.Zeilinger S, Galhaup C, Payer K, et al. Chitinase gene expression during mycoparasitic interaction of Trichoderma harzianum with its host. Fungal Genet Biol. 1999;26(2):131–140. doi: 10.1006/fgbi.1998.1111. (Available from: http://dx.doi.org/10.1006/fgbi.1998.1111) [DOI] [PubMed] [Google Scholar]


