Abstract
Background
The aim of this study was to evaluate the evidence of low bone mineral density (BMD) in depression. Low BMD is a major risk factor for osteoporotic fractures and frailty.
Methods
The searched database was Pubmed, Meta‐analysis included human studies in men and women fulfilling the following criteria: (1) assessment of BMD in the lumbar spine, the femur or the total hip; (2) comparison of BMD between depressed individuals and the healthy control group; (3) measurement of BMD using dual‐energy X‐ray absorptiometry (DEXA); and (4) data on the mean, standard deviation, or standard error of BMD.
Results
Twenty‐one studies were identified, encompassing 1842 depressed and 17,401 nondepressed individuals. Significant negative composite weighted mean effect sizes were identified for the lumbar spine (d = −0.15, 95%CL −0.22 to −0.08), femur (d = −0.34, 95%CL −0.64 to −0.05), and total hip (d = −0.14, 95%CL −0.23 to −0.05) indicating low BMD in depression. Examining men and women shows low bone density in the lumbar spine and femur in women and low bone density in the hip in men. The differences between men and women with MDD and the comparison group tended to be higher when examined by expert interviewers. Low bone density was found in all age groups.
Conclusions
Bone mineral density is reduced in patients with depressive disorders. The studies provide little evidence for potential relevant mediating factors.
Keywords: Absorptiometry, bone density, depressive disorder, meta‐analysis, osteoporosis, photon
Background
Depression is a common chronic debilitating disease associated with mood and cognitive and physical symptoms (Pratt and Brody 2014). Major depressive disorder (MDD) is one of the leading causes of years lived with disability in most countries (Global Burden of Disease Study, Collaborators 2015). The risk of developing MDD is twice as high for women as for men (Kessler and Bromet 2013). Osteoporosis is a highly prevalent degenerative bone disease (Wade et al. 2014) and is characterized by low bone mineral density (BMD) and the deterioration of the skeletal structure (Golob and Laya 2015). It is associated with an increased risk of fracture. In 1994, the first observation that MDD is associated with low lumbar bone mineral density was published (Schweiger et al. 1994). In the following years, many studies that reexamined this effect were published. The results are heterogeneous depending on the study design, the evaluation of depression, and other characteristics of the included population.
There are many possible reasons why depression may be associated with low BMD. Patients with depression show dysregulation of the HPA system function. There is altered secretion of corticotropin‐releasing hormone, corticotropin and cortisol, and altered regulation of glucocorticoid and mineralocorticoid receptor signaling (Huang and Lin 2015). Cortisol is known to influence bone loss (Marques et al. 2009). Proinflammatory cytokines have been found to be associated with depressive disorders (Valkanova et al. 2013; Young et al. 2014). Studies have shown that elevated serum concentrations of IL‐1, IL‐6, TNF‐α, CRP, and MCP‐1 are present in depressed patients (Young et al. 2014). Recent studies have shown that cytokines and immune cells may have a direct osteoclastogenic effect (Jung et al. 2014) and also activate the HPA axis, with the resultant release of glucocorticoids. MDD may result in behavior changes, such as increased alcohol and nicotine intake, unhealthy nutrition, and decreased physical activity (Brot et al. 1999; Dorn et al. 2011; Cohn et al. 2014; Alghadir et al. 2015; Bailey and van Wijngaarden 2015; Kim et al. 2015; Villareal et al. 2016). The treatment of depression with antidepressants, such as tricyclic antidepressants (TCAs) or selective serotonin reuptake inhibitors (SSRIs), may also exert negative effects on bone health (Diem et al. 2013a; Sheu et al. 2015).
In particular, SSRIs were associated with an increased risk of fractures (Sheu et al. 2015). Whether SSRIs are associated with bone loss is controversial (Diem et al. 2013a). The specific biochemical nature of serotonergic pathways that influence bone metabolism remains still unclear. Serotonin receptors (5‐HT) and 5‐HT transporters (5‐HTT) were identified in many major bone cell types. A polymorphism in the promoter region of the serotonin transporter gene (5‐HTTLPR) is assumed to moderate the relationship between stress and depression (Karg et al. 2011). However, there are no data available on how this polymorphism affects BMD in the general population. SSRIs selectively and potently block 5‐HTT. This results in a higher concentration of free circulating 5‐HT, which may have negative effects on bone metabolism. In vitro studies showed that mice with a null mutation in the 5‐HTT gene or mice treated with SSRIs exhibit reduced BMD, altered skeletal architecture, and reduced bone mechanical properties (Bliziotes 2010; Warden et al. 2010).
An increased risk of osteoporosis is of high importance in patients with MDD because it may lead to an increased fracture rate and premature frailty. In its early stages, it is a symptom‐free disease and is undetectable in routine examinations. The knowledge of the association between depression and low BMD is important for several reasons. It affects counseling of patients with MDD with respect to exercise, nutrition, smoking, and alcohol consumption. It affects the determination of the necessity of diagnostic osteodensitometry and the need of treatment for osteoporosis.
This meta‐analysis examined the state of research on the relationship between MDD and BMD in humans. Earlier comprehensive reviews date from before 2010 (Mezuk et al. 2008; Yirmiya and Bab 2009). The aim was to perform a meta‐analysis on all relevant studies comparing patients with depression to an appropriate comparison population with respect to BMD.
Methods
Sample of studies
The meta‐analysis followed the frame provided by the PRISMA statement (Moher et al. 2009). Figure 1 provides the flowchart. Studies were identified using the computerized database MEDLINE, covering the period from its inception to May 15, 2015. A comprehensive literature search of MEDLINE was conducted without language restrictions and with the search terms bone and depression; results were restricted to human studies. We reviewed each title and abstract of articles to exclude obviously irrelevant publications. Relevant reports were also double checked with the references list of published articles, including several reviews, with no additional identified records. Inclusion criteria were as follows: (1) assessment of BMD in the lumbar spine, the femur, or the total hip, (2) comparison of BMD between depressed individuals and a healthy control group, (3) measurement of BMD using dual‐energy X‐ray absorptiometry (DEXA), and (4) data on the mean, standard deviation, or standard error of BMD. In total, 21 articles complied with these criteria (Michelson et al. 1996; Amsterdam and Hooper 1998; Whooley et al. 1999, 2004; Robbins et al. 2001; Kavuncu et al. 2002; Yazici et al. 2003, 2005; Jacka et al. 2005; Konstantynowicz et al. 2005; Ozsoy et al. 2005; Wong et al. 2005; Altindag et al. 2007; Diem et al. 2007, 2013b; Eskandari et al. 2007; Petronijevic et al. 2008; Charles et al. 2012; Cizza et al. 2012; Fazeli et al. 2013; Sommerhage et al. 2013).
Figure 1.
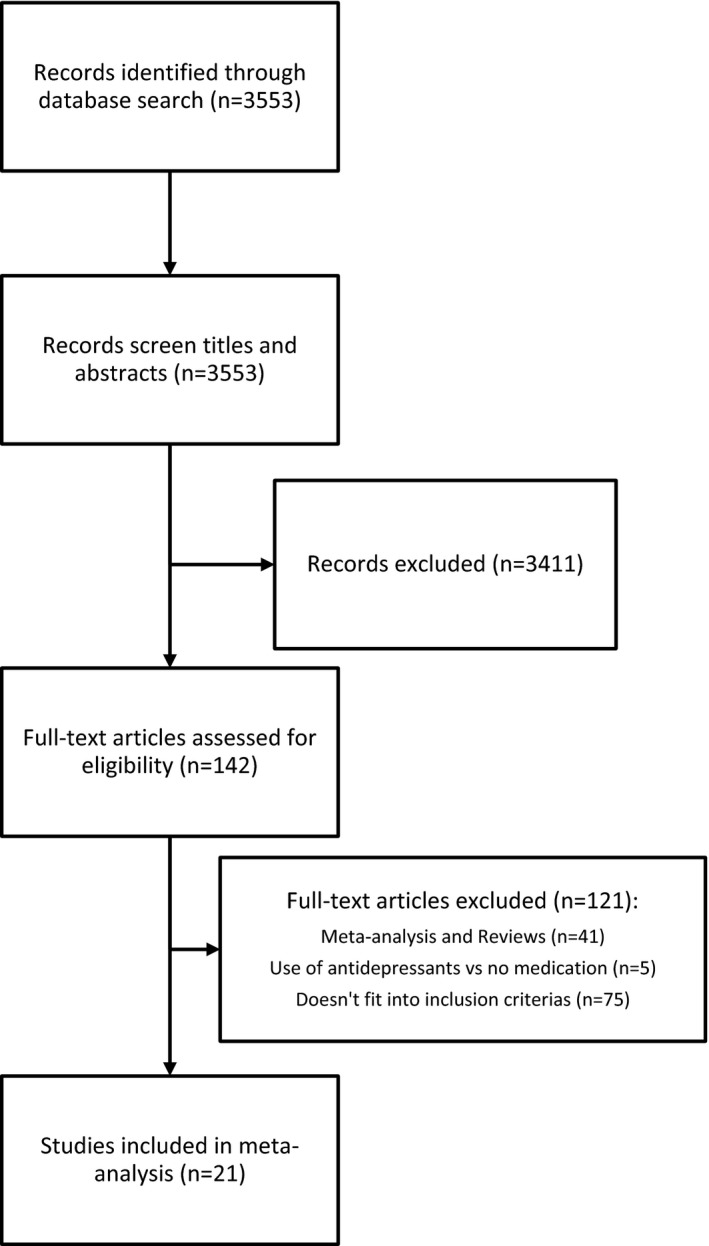
Diagram depicting the flow of information in the meta‐analysis according to PRISMA statement.
Data extraction
Data were extracted by two examiners (JUS and US) using standardized data abstraction forms. The extracted information included (1) the author's names, (2) year of publication, (3) country where the study was conducted, (4) sample size of the patient and the control groups, (5) gender, (6) age, (7) menopausal status, (8) medication use, (9) depression assessment tool, and (10) BMD, T score, and Z score of the lumbar spine, femur, and total hip. In two reports (Altindag et al. 2007; Cizza et al. 2012), the standard deviation was particularly small and the P‐value did not fit. We conservatively assumed that there was confusion of standard deviation and standard error, and we converted this value into standard deviation.
Statistical analyses
We carried out several meta‐analyses for BMD in depressed and nondepressed individuals. Analyses were performed with Comprehensive Meta‐Analysis (CMA) software (Englewood, New Jersey). In each meta‐analysis, standardized effect sizes derived from the individual studies were combined to determine a composite mean weighted effect size along with its 95% confidence interval (CI) and significance level (i.e., the effect size is significant if the CI does not include a zero). Greater weight is given to studies with larger samples; hence, this procedure corrects for bias with small sample sizes. Because the effects of depression on BMD were studied in different settings (e.g., depression diagnosed by an expert or self‐reported) and because participants’ demographic data differed greatly between studies, we assumed the presence of heterogeneity a priori – that the effect of individual trials would vary more than expected by chance alone. Therefore, the variance and statistical significance of differences were assessed with random‐effect calculations in all analyses. To determine the validity of the meta‐analysis, we employed funnel plots (i.e., plots of the standard difference in means [d] against the SEM). This was followed by quantitative evaluation of the degree of asymmetry. (Borenstein et al. 2009). The analyses were independently made for the following bones: lumbar spine, femur, and total hip. For each bone, all associated studies were pooled and individually analyzed for females and males.
Results
A total of 3553 records were identified through this search. Approximately 142 full‐text articles were assessed for eligibility. Of the 21 studies, five included females and males, 13 had only females, and three had only male participants. The studies encompassed 1842 depressed and 17,401 nondepressed individuals (Table 1).
Table 1.
Characteristics of all studies that compared bone mineral density in men and women with and without depression using dual‐energy X‐ray absorptiometry (DEXA)
| Study name | Year | Country | Depression/Control Subjects (n) | Gender | Age (Depression/Control) | Menopause status | Depression assessment tool | Bone site |
|---|---|---|---|---|---|---|---|---|
| S.J. Diem et al. | 2013b | US | 136/2328 | m | 76.7/75.7 | Self‐rating, GDS | Hip | |
| P.K. Fazeli et al. | 2013 | US | 17/16 | f | 16.6/16.3 | Pre | Expert opinion + CDRS‐R | Spine, femur, hip |
| P.K. Fazeli et al. | 2013 | US | 16/16 | m | 16.8/16.4 | Expert opinion + CDRS‐R | Spine, femur, hip | |
| V. Sommerhage et al. | 2013 | Estonia | 50/30 | f | 50/46 | Pre & post | Expert Interview MINI | Spine, femur |
| L.E. Charles et al. | 2012 | US | 14/13 | f | Unclear | Pre & post | Self‐rating, CES‐D | Spine, femur, hip |
| L.E. Charles et al. | 2012 | US | 17/20 | m | Unclear | Self‐rating, CES‐D | Spine, femur, hip | |
| G. Cizza et al. | 2012 | US | 92/44 | f | 36.0/35.3 | Pre | Expert Interview SCID | Spine, Femur, Hip |
| M. Petronijevic et al. | 2008 | Serbia | 73/47 | f | 40.7/40.5 | Pre | Expert interview, HAMD | Spine, femur |
| O. Altindag et al. | 2007 | Turkey | 36/41 | f | 39.8/42.8 | Pre | Expert interview, HAMD | Spine, femur |
| S. Diem et al. | 2007 | US | 200/3977 | f | 76.7/75.6 | Post | Self‐rating, GDS | Hip |
| F. Eskandari et al. | 2007 | US | 89/44 | f | 35/35 | Pre | Expert interview, HAMD | Spine, femur, hip |
| F.N. Jacka et al. | 2005 | Australia | 14/64 | f | 50.5/53.4 | Peri | Self‐rating | Spine, hip |
| J. Konstantynowicz et al. | 2005 | Poland | 14/31 | f | 17/16.5 | Pre | Expert interview, HAMD | Spine |
| S. Özsoy et al. | 2005 | Turkey | 21/23 | f | 37.57/33.37 | Pre & post | Expert interview, MADRS | Spine, femur |
| S. Özsoy et al. | 2005 | Turkey | 21/11 | m | 37.6/33.7 | Expert interview, MADRS | Spine, femur | |
| S.Y.S. Wong et al. | 2005 | Hong Kong | 169/1830 | m | 72.94/72.34 | Expert opinion | Spine, hip | |
| A.E. Yazici et al. | 2005 | Turkey | 35/30 | f | 44.8/46.2 | Pre | Expert interview, HAMD | Spine, femur |
| M. A. Whooley et al. | 2004 | US | 16/499 | m | 64.6/66.7 | Self‐rating, GDS | Spine, hip | |
| K.M. Yazici et al. | 2003 | Turkey | 25/15 | f | 30.8/31.2 | Pre | Expert interview, HAMD | Spine, femur |
| V. Kavuncu et al. | 2002 | Turkey | 42/42 | f | 35.4/36.7 | Pre | Expert interview, HAMD | Spine, femur |
| J. Robbins et al. | 2001 | US | 248/1304 | m/f | 74.9/74.2 | Self‐rating, CES‐D | Hip | |
| M.A. Whooley et al. | 1999 | US | 467/6947 | f | 74.5/73.3 | Post | Self‐rating, GDS | Spine, hip |
| J.D. Amsterdam et al. | 1998 | US | 4/3 | f | 38/37 | Pre & post | Expert interview, HAMD | Spine |
| J.D. Amsterdam et al. | 1998 | US | 2/2 | m | 48/39 | Expert interview, HAMD | Spine | |
| D. Michelson et al. | 1996 | US | 24/24 | f | 41/41 | Pre & post | Expert interview, SCID | Spine, femur |
Lumbar spine
Eighteen studies examined the lumbar spine; in four studies, data on females and males were shown separately. The effect sizes pooled for females and males, corresponding CI, P‐values, and relative weights for each study and a forest plot summarizing the association between depression and BMD are shown in Figure 2. The effect sizes ranged from −1.67 to 1.07, with 16 studies reporting either decreased or unchanged BMD and six studies showing increased BMD. The composite weighted mean effect size, d, was −0.30, and its CI was between −0.48 and −0.11; this implies that overall BMD is significantly lower in depressed than in nondepressed individuals (P = 0.001). Publication bias was assessed using the funnel plot procedure. There were nonsignificant rank correlation and regression intercept values. The fail‐safe number indicated that an additional 141 negative studies would be necessary for the present results to lose their significance.
Figure 2.
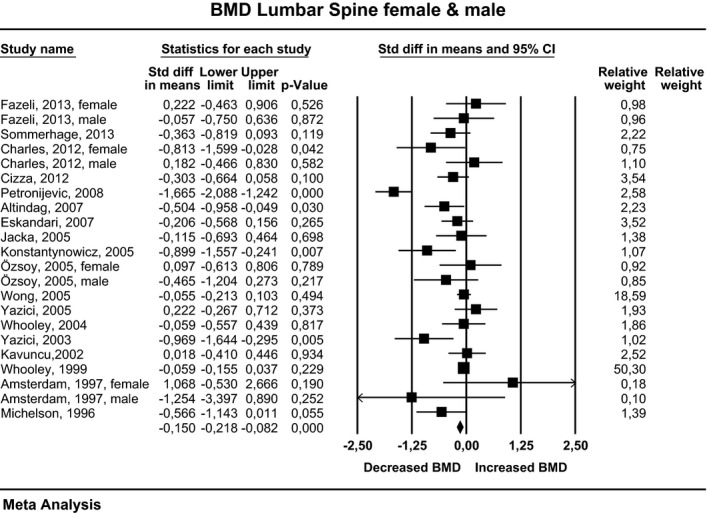
Estimates of all studies that compared bone density in the lumbar spine in men and women with and without depression. The diamond at the bottom of the graph denotes the overall estimate of the association between depression and lumbar spine bone density.
For females, the effect sizes ranged from −1.67 to 1.07, with 11 studies reporting either decreased or unchanged BMD and five studies showing increased BMD. The composite weighted mean effect size, d, was −0.36, and its CI was between −0.60 and −0.11. This implies that overall BMD was significantly lower in depressed than in nondepressed women (P = 0.005). For males, the effect sizes ranged from −0.47 to 0.18, with five studies reporting either decreased or unchanged BMD and one study showing increased BMD. However, the composite weighted mean effect size, d, was −0.06, and its CI was between −0.21 and 0.08. This implies that overall BMD was not significantly different in depressed and nondepressed men (P = 0.369).
In 10 studies, only premenopausal women were examined. The effect size ranged from −1.67 to 0.22, with seven studies reporting either decreased or unchanged BMD and three studies showing increased BMD. The composite weighted mean effect size, d, was −0.41, and its CI was between −0.80 and −0.042. This implies that overall BMD was significantly different in depressed and nondepressed premenopausal women (P = 0.030).
In 14 studies, depression was evaluated by expert interviews; in three studies, data on females and males were shown separately. When pooled for females and males, the effect sizes ranged from −1.67 to 1.07, with 12 studies reporting either decreased or unchanged BMD and five studies showing increased BMD. The composite weighted mean effect size, d, was −0.35, and its CI was between −0.60 and −0.09. This implies that overall BMD is significantly lower in depressed individuals evaluated by an expert than in nondepressed participants (P = 0.007). For females, the effect sizes ranged from −1.67 to 1.07, with eight studies reporting either decreased or unchanged BMD and five studies showing increased BMD. The composite weighted mean effect size, d, was −0.37, and its CI was between −0.69 and −0.048. This implies that overall BMD was significantly lower in depressed women evaluated by an expert than in nondepressed women (P = 0.024). For males, the effect size ranged from −1.25 to −0.05, with all four studies reporting either decreased or unchanged BMD. However, the composite weighted mean effect size, d, was −0.08, and its CI was between −0.22 and 0.07. This implies that the overall BMD was not significantly different in depressed men evaluated by an expert than in nondepressed men (P = 0.309).
In three studies, depression was self‐reported; in two studies, data for females and males were shown separately. When pooled for females and males, the effect sizes ranged from −0.81 to 0.18, with four studies reporting either decreased or unchanged BMD, and one study showing increased BMD. However, the composite weighted mean effect size, d, was −0.07, and its CI was between −0.18 and 0.04, implying that overall BMD was not significantly different in depressed patients evaluated by self‐reports and nondepressed participants (P = 0.217). For females, the effect sizes ranged from −0.81 to −0.06, with all three studies reporting either decreased or unchanged BMD. However, the composite weighted mean effect size, d, was −0.18, and its CI was between −0.52 and 0.16; this implies that the overall BMD was not significantly different in depressed women evaluated by a self‐reported test and nondepressed women (P = 0.295). For males, the effect sizes ranged from −0.06 to 0.18, with one study reporting either decreased or unchanged BMD and one study reporting an increase in BMD. However, the composite weighted mean effect size, d, was −0.03, and its CI was between −0.36 and 0.43. This implies that the overall BMD was not significantly different in depressed men evaluated by an expert and nondepressed men (P = 0.880).
Femur
Twelve studies examined the femur. In three studies, data on females and males were shown separately. Effect sizes pooled for females and males, corresponding CI, P‐values, and relative weights for each study and a forest plot summarizing the association between depression and BMD are shown in Figure 3. The effect sizes ranged from −1.67 to 0.50, with 12 studies reporting either decreased or unchanged BMD and three studies showing increased BMD. The composite weighted mean effect size, d, was −0.34, and its CI was between −0.64 and −0.05. This implies that the overall BMD was significantly lower in depressed than in nondepressed participants (P = 0.023). Publication bias was assessed using the funnel plot procedure. The rank correlation and regression intercept values were nonsignificant. The fail‐safe number indicates that an additional 85 negative studies would be necessary for the present results to lose their significance.
Figure 3.
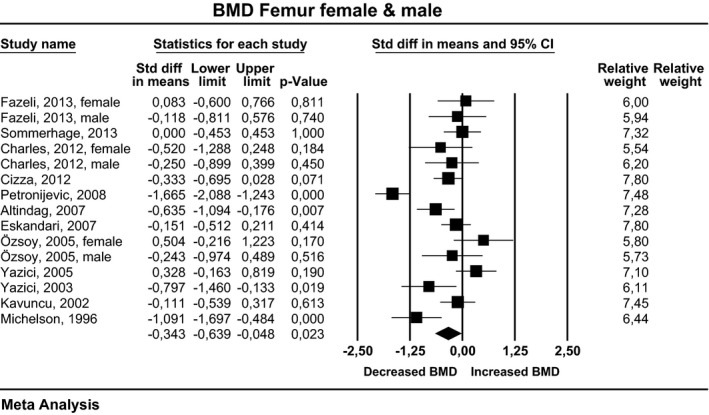
Estimates of all studies that compared bone density in the femur in men and women with and without depression. The diamond at the bottom of the graph denotes the overall estimate of the association between depression and femur bone density.
For females, the effect size ranged from −1.67 to 0.50, with nine studies reporting either decreased or unchanged BMD and three studies showing increased BMD. The composite weighted mean effect size, d, was −0.37, and its CI was between −0.72 and −0.023. This implies that the overall BMD was significantly lower in depressed than in nondepressed women (P = 0.037). For males, the effect size ranged from −0.25 to 0.12, with all three studies reporting either decreased or unchanged BMD. However, the composite weighted mean effect size, d, was −0.20, and its CI was between −0.60 and 0.19. This implies that the overall BMD was not significantly different in depressed and nondepressed men (P = 0.314).
In eight studies, only premenopausal women were examined. The effect sizes ranged from −1.67 to 0.328, with six studies reporting either decreased or unchanged BMD and two studies showing increased BMD. However, the composite weighted mean effect size, d, was −0.42, and its CI was between −0.85 and 0.22. This implies that the overall BMD was not significantly different in depressed and nondepressed premenopausal women (P = 0.063).
In 11 studies, depression was evaluated by an expert interview; in two studies, females and males were both examined and were shown individually. When pooled for females and males, the effect sizes ranged from −1.67 to 0.33, with 10 studies reporting either decreased or unchanged BMD and three studies showing increased BMD. The composite weighted mean effect size, d, was −0.34, and its CI was between −0.67 and −0.01. This implies that the overall BMD was significantly lower in depressed than in nondepressed participants (P = 0.045). For females, the effect size ranged from −1.67 to 0.33, with seven studies reporting either decreased or unchanged BMD and four studies showing increased BMD. However, the composite weighted mean effect size, d, was −0.36, and its CI was between −0.73 and 0.01. This implies that the overall BMD was not significantly different in depressed women evaluated by an expert and nondepressed women (P = 0.057). For males, the effect sizes ranged from −0.24 to −0.12, with both studies reporting either decreased or unchanged BMD. However, the composite weighted mean effect size, d, was −0.18, and its CI was between −0.68 and 0.33. This implies that overall BMD was not significantly different in depressed men evaluated by an expert than in nondepressed men (P = 0.491).
In one study, depression was evaluated by self‐reports. In this study, data on females and males were shown separately. When pooled for females and males, the effect sizes ranged from −0.52 to −0.25. However, the composite weighted mean effect size, d, was −0.36, and its CI was between −0.86 and 0.13. This implies that the overall BMD was not significantly different in depressed individuals evaluated by self‐reports and nondepressed participants (P = 0.152).
Total hip
Eleven studies examined the total hip. In two studies, data on females and males were shown separately. When pooled for females and males, effect sizes, corresponding CI, P‐values, and relative weights for each study, and a forest plot summarizing the association between depression and BMD are shown in Figure 4. The effect sizes ranged from −0.31 to 0.18, with 12 studies reporting either decreased or unchanged BMD and one study showing increased BMD. The composite weighted mean effect size, d, was −0.14, and its CI was between −0.23 and −0.05. This implies that the overall BMD was significantly lower in depressed than in nondepressed participants (P = 0.002). Publication bias was assessed using the funnel plot procedure. The rank correlation and regression intercept values were nonsignificant. The fail‐safe number indicates that an additional 34 negative studies would be necessary for the present results to lose their significance.
Figure 4.
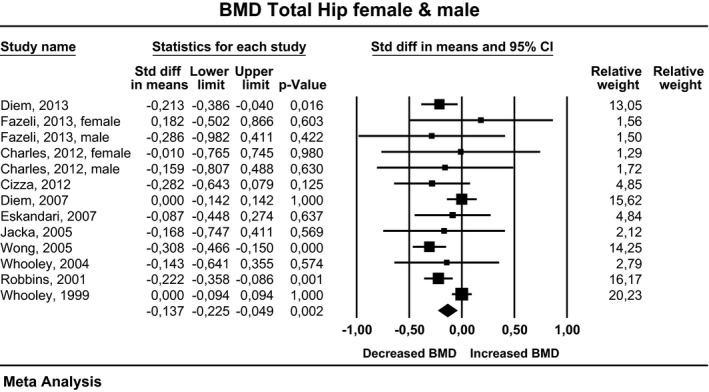
Estimates of all studies that compared bone density in the total hip in men and women with and without depression. The diamond at the bottom of the graph denotes the overall estimate of the association between depression and total hip bone density.
For females, the effect sizes ranged from −0.28 to 0.18, with six studies reporting either decreased or unchanged BMD and one study showing increased BMD. However, the composite weighted mean effect size, d, was −0.02, and its CI was between −0.09 and 0.06. This implies that overall BMD was not significantly different in depressed and nondepressed women (P = 0.669). For males, the effect size ranged from −0.31 to 0.14, with all five studies reporting either decreased or unchanged BMD. The composite weighted mean effect size, d, was −0.26, and its CI was between −0.37 and −0.15. This implies that overall BMD was significantly lower in depressed than in nondepressed men (P < 0.001).
In four studies, only premenopausal women were examined. The effect size ranged from −0.28 to 0.18, with three studies reporting either decreased or unchanged BMD and one study showing increased BMD. However, the composite weighted mean effect size, d, was −0.14, and its CI was between −0.37 and −0.08. This implies that overall BMD was not significantly different in depressed and nondepressed women (P = 0.202). In two studies, only postmenopausal women were examined. There were no differences in BMD between the depressed and the nondepressed participants.
In four studies, depression was evaluated by an expert interview, and in one study, data on females and males were shown separately. When pooled for females and males, the effect sizes ranged from −0.31 to 0.18, with four studies reporting either decreased or unchanged BMD, and one study showing increased BMD. The composite weighted mean effect size, d, was −0.262, and its CI was between −0.39 and −0.13. This implies that overall BMD was significantly lower in depressed than in nondepressed participants (P < 0.001). For females, the effect sizes ranged from −0.282 to 0.18, with two studies reporting either decreased or unchanged BMD and one study showing increased BMD. However, the composite weighted mean effect size, d, was −0.14, and its CI was between −0.38 and 0.10. This implies that overall BMD was not significantly different in depressed women evaluated by an expert and nondepressed women (P = 0.252). For males, the effect size ranged from −0.31 to −0.29, with both studies reporting either decreased or unchanged BMD. The composite weighted mean effect size, d, was −0.31, and its CI was between −0.46 and −0.15. This implies that the overall BMD was significantly lower in depressed men evaluated by an expert than in nondepressed men (P < 0.001).
In seven studies, depression was evaluated by self‐reports. In one study, data on females and males were shown separately. When pooled for females and males, the effect sizes ranged from −0.22 to 0.00, with all studies reporting either decreased or unchanged BMD. The composite weighted mean effect size, d, was −0.10, and its CI was between −0.19 and −0.01. This implies that the overall BMD was significantly lower in depressed individuals evaluated by self‐reports than in nondepressed participants (P = 0.037). For females, the effect sizes ranged from −0.17 to 0.00, with all four studies reporting either decreased or unchanged BMD. However, the composite weighted mean effect size, d, was 0.00, and its CI was between −0.08 and 0.07. This implies that overall BMD was not significantly different in depressed women evaluated by self‐reports and nondepressed women (P = 0.937). For males, the effect sizes ranged from −0.21 to 0.14, with all three studies reporting either decreased or unchanged BMD. The composite weighted mean effect size, d, was −0.20, and its CI was between −0.36 to −0.04. This implies that overall BMD was significantly lower in depressed men evaluated by self‐reports than in nondepressed men (P = 0.012).
Discussion
The meta‐analysis shows that major depressive disorder is associated with low bone density. Pooled data from men and women show that the bone density in all three examined locations (lumbar spine, femur, and total hip) was decreased with small‐to‐medium effect sizes. This result was similar to the conclusions made by the groups of Itai Bab and Brianna Mezuk (Mezuk et al. 2008; Yirmiya and Bab 2009). Of note, six new studies have been included since these reviews. No evidence for publication bias could be identified. The finding is of considerable importance, as osteoporosis and osteoporotic fractures may constitute a partial explanation for the increased mortality and decreased life expectancy in patients with severe mental disorders (Chang et al. 2011).
Examining men and women shows low BMD in the lumbar spine and femur in women and low BMD in the hip in men, which may correspond to a different pattern. However, the results may be an effect of the higher number of depressed women (1341) than depressed men (501) examined in the studies. The differences between men and women with MDD and the comparison group tended to be higher when examined by expert interviewers. Estimates of depression based on self‐ratings typically yield depression prevalence estimates that are considerably higher than the estimates based on expert interviews. This means that studies based on self‐rating may suffer from a high number of subjects false positively classified as depressed. There is no evidence that low BMD in depression is limited to any age group.
Although the phenomenon of decreased BMD in patients with depression is well established, the studies provide little evidence of the mediation or the genesis of osteoporosis. Only one of the included studies reported a significant correlation with cortisol (Altindag et al. 2007), one reported a significant effect of physical fitness (Diem et al. 2013b), two reported significant effects of smoking (Charles et al. 2012; Diem et al. 2013b), and none reported a significant effect of antidepressant medication on BMD. When evaluating cofactors, we must consider that most of the studies were pilot studies. In the 14 studies using expert interviews, the sample size of the depressed participants varied between 6 and 169. These sample sizes have the power to detect only medium‐to‐strong effect sizes and do not allow for multiple regression analysis with a high number of covariates.
Depression has an increased bidirectional association with a broad spectrum of medical disorders. Some of these disorders like thyroid disorders, diabetes, disorders with impaired vitamin D action, Cushing's syndrome, cancer and its treatment, chronic inflammatory disease, and its treatment with glucocorticoids or other substances, also directly affect bone. All studies included in this meta‐analysis report to have excluded men and women with comorbidity directly affecting bone. If we assume that this strategy was successful, the difference between healthy persons and men and women with depression in the general population may be underestimated. If we assume that it was incompletely effective, this may create a bias toward a higher difference. The latter possibility is not in accordance with the finding of similar BMD deficits in all age groups. All studies were controlled for antidepressant treatment. As discussed above the negative findings may be due to insufficient power.
The meta‐analysis was able to identify a substantial number of publications. We found no evidence for publication bias using the funnel plot technique. With respect to search bias, there was an excellent correspondence with earlier meta‐analyses. The main limitation is the pilot character of most studies included. A methodological limitation is the use of DEXA in all studies. Standard DEXA technique has only a limited sensitivity for changes in trabecular bone which are characteristic for the effects of glucocorticoids (Leib and Winzenrieth 2015).
Conclusion
This meta‐analysis points to the following research needs. Longitudinal studies with a sufficient power to evaluate potential mediators of the effect of depression on BMD are needed. These studies should use expert interviews to reduce the high number of subjects with a false‐positive diagnosis of depression that occurs in studies using self‐reports and carefully assess the relevant covariates. Such studies may guide the future development of prophylactic or therapeutic strategies for low BMD in patients with depression.
Conflict of Interest
None declared.
Schweiger J. U., Schweiger U., Hüppe M., Kahl K. G., Greggersen W., Fassbinder E., Bone density and depressive disorder: a meta‐analysis, Brain and Behavior, 2016; 6(8), e00489, doi: 10.1002/brb3.489
References
- Alghadir, A. H. , Gabr S. A., and Al‐Eisa E.. 2015. Physical activity and lifestyle effects on bone mineral density among young adults: sociodemographic and biochemical analysis. J. Phys. Ther. Sci. 27:2261–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altindag, O. , Altindag A., Asoglu M., Gunes M., Soran N., and Deveci Z.. 2007. Relation of cortisol levels and bone mineral density among premenopausal women with major depression. Int. J. Clin. Pract. 61:416–420. [DOI] [PubMed] [Google Scholar]
- Amsterdam, J. D. , and Hooper M. B.. 1998. Bone density measurement in major depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 22:267–277. [DOI] [PubMed] [Google Scholar]
- Bailey, R. L. , and van Wijngaarden J. P.. 2015. The role of B‐vitamins in bone health and disease in older adults. Curr. Osteoporos. Rep. 13:256–261. [DOI] [PubMed] [Google Scholar]
- Bliziotes, M. 2010. Update in serotonin and bone. J. Clin. Endocrinol. Metab. 95:4124–4132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein, M. , Hedges L. V., Higgins J. P., and Rothstein H. R.. 2009. Introduction to meta‐analysis. Wiley, Chichester. [Google Scholar]
- Brot, C. , Jorgensen N. R., and Sorensen O. H.. 1999. The influence of smoking on vitamin D status and calcium metabolism. Eur. J. Clin. Nutr. 53:920–926. [DOI] [PubMed] [Google Scholar]
- Chang, C. K. , Hayes R. D., Perera G., Broadbent M. T., Fernandes A. C., Lee W. E., et al. 2011. Life expectancy at birth for people with serious mental illness and other major disorders from a secondary mental health care case register in London. PLoS One 6:e19590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles, L. E. , Fekedulegn D., Miller D. B., Wactawski‐Wende J., Violanti J. M., Andrew M. E., et al. 2012. Depressive symptoms and bone mineral density among police officers in a northeastern US City. Glob. J. Health Sci. 4:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cizza, G. , Mistry S., Nguyen V. T., Eskandari F., Martinez P., Torvik S., et al. 2012. Do premenopausal women with major depression have low bone mineral density? A 36‐month prospective study. PLoS One 7:e40894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn, A. M. , Cobb C., Hagman B. T., Cameron A., Ehlke S., and Mitchell J. N.. 2014. Implicit alcohol cognitions in risky drinking nicotine users with and without co‐morbid major depressive disorder. Addict. Behav. 39:797–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem, S. J. , Blackwell T. L., Stone K. L., Yaffe K., Haney E. M., Bliziotes M. M., et al. 2007. Use of antidepressants and rates of hip bone loss in older women: the study of osteoporotic fractures. Arch. Intern. Med. 167:1240–1245. [DOI] [PubMed] [Google Scholar]
- Diem, S. J. , Ruppert K., Cauley J. A., Lian Y., Bromberger J. T., Finkelstein J. S., et al. 2013a. Rates of bone loss among women initiating antidepressant medication use in midlife. J. Clin. Endocrinol. Metab. 98:4355–4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diem, S. J. , Harrison S. L., Haney E., Cauley J. A., Stone K. L., Orwoll E., et al. 2013b. Depressive symptoms and rates of bone loss at the hip in older men. Osteoporos. Int. 24:111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn, L. D. , Pabst S., Sontag L. M., Kalkwarf H. J., Hillman J. B., and Susman E. J.. 2011. Bone mass, depressive, and anxiety symptoms in adolescent girls: variation by smoking and alcohol use. J. Adolesc. Health 49:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskandari, F. , Martinez P. E., Torvik S., Phillips T. M., Sternberg E. M., Mistry S., et al. 2007. Low bone mass in premenopausal women with depression. Arch. Intern. Med. 167:2329–2336. [DOI] [PubMed] [Google Scholar]
- Fazeli, P. K. , Mendes N., Russell M., Herzog D. B., Klibanski A., and Misra M.. 2013. Bone density characteristics and major depressive disorder in adolescents. Psychosom. Med. 75:117–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Burden of Disease Study, Collaborators . 2015. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 386:743–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golob, A. L. , and Laya M. B.. 2015. Osteoporosis: screening, prevention, and management. Med. Clin. North Am. 99:587–606. [DOI] [PubMed] [Google Scholar]
- Huang, T. L. , and Lin C. C.. 2015. Advances in biomarkers of major depressive disorder. Adv. Clin. Chem. 68:177–204. [DOI] [PubMed] [Google Scholar]
- Jacka, F. N. , Pasco J. A., Henry M. J., Kotowicz M. A., Dodd S., Nicholson G. C., et al. 2005. Depression and bone mineral density in a community sample of perimenopausal women: Geelong Osteoporosis Study. Menopause 12:88–91. [DOI] [PubMed] [Google Scholar]
- Jung, S. M. , Kim K. W., Yang C. W., Park S. H., and Ju J. H.. 2014. Cytokine‐mediated bone destruction in rheumatoid arthritis. J. Immunol. Res. 2014:263625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karg, K. , Burmeister M., Shedden K., and Sen S.. 2011. The serotonin transporter promoter variant (5‐HTTLPR), stress, and depression meta‐analysis revisited: evidence of genetic moderation. Arch. Gen. Psychiatry 68:444–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavuncu, V. , Kuloglu M., Kaya A., Sahin S., Atmaca M., and Firidin B.. 2002. Bone metabolism and bone mineral density in premenopausal women with mild depression. Yonsei Med. J. 43:101–108. [DOI] [PubMed] [Google Scholar]
- Kessler, R. C. , and Bromet E. J.. 2013. The epidemiology of depression across cultures. Annu. Rev. Public Health 34:119–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, J. , Nakamura T., Kikuchi H., Yoshiuchi K., Sasaki T., and Yamamoto Y.. 2015. Covariation of depressive mood and spontaneous physical activity in major depressive disorder: toward continuous monitoring of depressive mood. IEEE J. Biomed. Health Inform. 19:1347–1355. [DOI] [PubMed] [Google Scholar]
- Konstantynowicz, J. , Kadziela‐Olech H., Kaczmarski M., Zebaze R. M., Iuliano‐Burns S., Piotrowska‐Jastrzebska J., et al. 2005. Depression in anorexia nervosa: a risk factor for osteoporosis. J. Clin. Endocrinol. Metab. 90:5382–5385. [DOI] [PubMed] [Google Scholar]
- Leib, E. S. , and Winzenrieth R.. 2015. Bone status in glucocorticoid‐treated men and women. Osteoporos. Int. 27:39–48. [DOI] [PubMed] [Google Scholar]
- Marques, A. H. , Silverman M. N., and Sternberg E. M.. 2009. Glucocorticoid dysregulations and their clinical correlates. From receptors to therapeutics. Ann. N. Y. Acad. Sci. 1179:1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezuk, B. , Eaton W. W., and Golden S. H.. 2008. Depression and osteoporosis: epidemiology and potential mediating pathways. Osteoporos. Int. 19:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michelson, D. , Stratakis C., Hill L., Reynolds J., Galliven E., Chrousos G., et al. 1996. Bone mineral density in women with depression. N. Engl. J. Med. 335:1176–1181. [DOI] [PubMed] [Google Scholar]
- Moher, D. , Liberati A., Tetzlaff J., and Altman D. G.. 2009. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med. 6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozsoy, S. , Esel E., Turan M. T., Kula M., Demir H., Kartalci S., et al. 2005. [Is there any alteration in bone mineral density in patients with depression?]. Turk Psikiyatri Derg. 16:77–82. [PubMed] [Google Scholar]
- Petronijevic, M. , Petronijevic N., Ivkovic M., Stefanovic D., Radonjic N., Glisic B., et al. 2008. Low bone mineral density and high bone metabolism turnover in premenopausal women with unipolar depression. Bone 42:582–590. [DOI] [PubMed] [Google Scholar]
- Pratt, L. A. , and Brody D. J.. 2014. Depression in the U.S. household population, 2009–2012. NCHS Data Brief. 172:1–8. [PubMed] [Google Scholar]
- Robbins, J. , Hirsch C., Whitmer R., Cauley J., and Harris T.. 2001. The association of bone mineral density and depression in an older population. J. Am. Geriatr. Soc. 49:732–736. [DOI] [PubMed] [Google Scholar]
- Schweiger, U. , Deuschle M., Korner A., Lammers C. H., Schmider J., Gotthardt U., et al. 1994. Low lumbar bone mineral density in patients with major depression. Am. J. Psychiatry 151:1691–1693. [DOI] [PubMed] [Google Scholar]
- Sheu, Y. H. , Lanteigne A., Sturmer T., Pate V., Azrael D., and Miller M.. 2015. SSRI use and risk of fractures among perimenopausal women without mental disorders. Inj. Prev. 21:397–403. [DOI] [PubMed] [Google Scholar]
- Sommerhage, V. , Kull I., Schweiger U., and Rudolf S.. 2013. Bone mineral density in a cohort of Estonian women with major depression. Arch. Osteoporos. 8:163. [DOI] [PubMed] [Google Scholar]
- Valkanova, V. , Ebmeier K. P., and Allan C. L.. 2013. CRP, IL‐6 and depression: a systematic review and meta‐analysis of longitudinal studies. J. Affect. Disord. 150:736–744. [DOI] [PubMed] [Google Scholar]
- Villareal, D. T. , Fontana L., Das S. K., Redman L., Smith S. R., Saltzman E., et al. 2016. Effect of two‐year caloric restriction on bone metabolism and bone mineral density in non‐obese younger adults: a randomized clinical trial. J. Bone Miner. Res. 31:40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade, S. W. , Strader C., Fitzpatrick L. A., Anthony M. S., and O'Malley C. D.. 2014. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch. Osteoporos. 9:182. [DOI] [PubMed] [Google Scholar]
- Warden, S. J. , Robling A. G., Haney E. M., Turner C. H., and Bliziotes M. M.. 2010. The emerging role of serotonin (5‐hydroxytryptamine) in the skeleton and its mediation of the skeletal effects of low‐density lipoprotein receptor‐related protein 5 (LRP5). Bone 46:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whooley, M. A. , Kip K. E., Cauley J. A., Ensrud K. E., Nevitt M. C., and Browner W. S.. 1999. Depression, falls, and risk of fracture in older women. Study of Osteoporotic Fractures Research Group. Arch. Intern. Med. 159:484–490. [DOI] [PubMed] [Google Scholar]
- Whooley, M. A. , Cauley J. A., Zmuda J. M., Haney E. M., and Glynn N. W.. 2004. Depressive symptoms and bone mineral density in older men. J. Geriatr. Psychiatry Neurol. 17:88–92. [DOI] [PubMed] [Google Scholar]
- Wong, S. Y. , Lau E. M., Lynn H., Leung P. C., Woo J., Cummings S. R., et al. 2005. Depression and bone mineral density: is there a relationship in elderly Asian men? Results from Mr. Os (Hong Kong). Osteoporos. Int. 16:610–615. [DOI] [PubMed] [Google Scholar]
- Yazici, K. M. , Akinci A., Sutcu A., and Ozcakar L.. 2003. Bone mineral density in premenopausal women with major depressive disorder. Psychiatry Res. 117:271–275. [DOI] [PubMed] [Google Scholar]
- Yazici, A. E. , Bagis S., Tot S., Sahin G., Yazici K., and Erdogan C.. 2005. Bone mineral density in premenopausal women with major depression. Joint Bone Spine 72:540–543. [DOI] [PubMed] [Google Scholar]
- Yirmiya, R. , and Bab I.. 2009. Major depression is a risk factor for low bone mineral density: a meta‐analysis. Biol. Psychiatry 66:423–432. [DOI] [PubMed] [Google Scholar]
- Young, J. J. , Bruno D., and Pomara N.. 2014. A review of the relationship between proinflammatory cytokines and major depressive disorder. J. Affect. Disord. 169:15–20. [DOI] [PubMed] [Google Scholar]


