Abstract
Because pneumothorax is frequent in lymphangioleiomyomatosis, patients have expressed concerns regarding the risk of pneumothorax associated with pulmonary function or exercise testing. Indeed, pneumothorax has been reported in patients with lung disease after both of these tests. The aim of this study was to determine the incidence of pneumothorax in patients with lymphangioleiomyomatosis during admissions to the National Institutes of Health Clinical Research Center between 1995 and 2015. Medical records were reviewed to identify patients who had a pneumothorax during their stay at the National Institutes of Health. A total of 691 patients underwent 4,523 pulmonary function tests and 1,900 exercise tests. Three patients developed pneumothorax after pulmonary function tests and/or exercise tests. The incidence of pneumothorax associated with lung function testing was 0.14 to 0.29 of 100 patients or 0.02 to 0.04 of 100 tests. The incidence of pneumothorax in patients undergoing exercise testing was 0.14 to 0.28 of 100 patients or 0.05 to 0.10 of 100 tests. The risk of pneumothorax associated with pulmonary function or exercise testing in patients with lymphangioleiomyomatosis is low.
Key Words: exercise testing, lymphangioleiomyomatosis, pulmonary function test
Abbreviations: CPET, cardiopulmonary exercise testing; LAM, lymphangioleiomyomatosis; PFT, pulmonary function testing
Lymphangioleiomyomatosis (LAM) is a multisystem disease affecting primarily women, which is characterized by cystic lung destruction, abdominal angiomyolipomas, and lymphatic abnormalities consisting of lymphadenopathy, lymphangioleiomyomas, and chylous effusions.1, 2 Patients with LAM are at an increased risk of experiencing spontaneous pneumothoraces; in some series more than 50% of patients with LAM had pneumothoraces.3 Moreover, the recurrence rate of a pneumothorax in patients has been reported to be >80%.3 Changes in intrathoracic pressure, such as those associated with pulmonary function testing,4, 5, 6 air travel,7 incentive spirometry,8 and exercise testing,9 may increase the risk of pneumothorax, especially in patients with cystic lung diseases. Indeed, occurrences of pneumothorax or pneumomediastinum have been reported in patients with lung diseases and normal subjects undergoing pulmonary function tests and exercise studies.9, 10, 11 However, retrospective studies have shown that the actual risk of a pneumothorax in patients with LAM during air travel does not appear to exceed the risk of pneumothorax during other means of travel.7
The current study was prompted by concerns expressed by patients with LAM regarding the risk of pneumothorax during pulmonary function testing (PFT) and cardiopulmonary exercise testing (CPET). Furthermore, one patient experienced dyspnea during CPET, and although a chest radiograph taken afterward showed no evidence of pneumothorax, on the following day, after she returned home, she was found to have a pneumothorax that was confirmed by CT scan and required therapy. We questioned whether the CPET precipitated the pneumothorax. We hypothesized that because of the high incidence of spontaneous pneumothorax in LAM, patients with LAM may have an increased risk of pneumothorax after PFT or CPET. The aim of this study was to determine the incidence of pneumothorax associated with PFT and CPET in a cohort of 691 patients with LAM seen at the National Institutes of Health Clinical Center between 1995 and 2015.
Materials and Methods
Patients with LAM were enrolled in a Characterization of the Pathogenesis of Lymphangioleiomyomatosis protocol, approved by the National Heart, Lung, and Blood Institute Institutional Review Board (Protocol 95-H-0186), which began in 1995. All subjects gave written informed consent before enrollment. Clinical and functional data were obtained, including history and physical examination, laboratory tests, PFT, and CPET. Regardless of the mode of travel, patients had a chest radiograph on admission, before PFT or CPET. On the second day of admission they had PFT, and unless they were unable to exercise because of musculoskeletal problems they also had a CPET. A CT scan was not performed each visit, to avoid excess radiation exposure. On the visits when a CT scan was not performed per protocol, additional imaging studies of the lungs were not obtained after the admission chest radiograph, unless medically indicated.
Admission chest radiographs were evaluated for the presence of a pneumothorax.
We performed a retrospective review of the medical records of all patients with LAM seen between 1995 and 2015. In patients in whom we suspected the occurrence of a pneumothorax during admission that was temporally related to PFT or CPET, chest radiographs and/or CT scans were examined to confirm or exclude the presence of pneumothorax and its eventual resolution.
Results
Over 20 years, a total of 691 patients with LAM were seen at the National Institutes of Health Clinical Research Center, for a total of 4,523 visits. Six hundred patients were white, 43 were African American, 27 were Asian, and 21 were Hispanic. Mean age of patients at the time of the first visit was 43.2 ± 9.8 years. A total of 593 patients (86%) had sporadic LAM and 98 (14%) had tuberous sclerosis complex-LAM. Two hundred sixteen patients (31%) presented initially with pneumothorax, which was frequently recurrent, and 302 (44%) presented initially with dyspnea. The remaining patients presented with extrapulmonary signs or symptoms. Mean FEV1 and lung diffusion capacity for CO2 at the time of the first visit were 2.0% ± 0.7% (77% ± 27% predicted) and 16.0 ± 7.6 mL/min/mm Hg (74% ± 26% predicted), respectively. The 691 patients with LAM underwent a total of 4,523 PFTs and 1,900 CPETs (Table 1). Three patients (aged 43 ± 2 years; FEV1 = 96% ± 14% predicted and lung diffusion capacity for CO2 = 93% ± 8% predicted) had developed pneumothoraces after PFT or CPET (Table 1).
Table 1.
Demographic and Physiologic Features of Three Patients Who Developed a Pneumothorax After Pulmonary Function or Exercise Testing
| Patient | 1 | 2 | 3 |
|---|---|---|---|
| Age, y | 41 | 45 | 43 |
| Age of diagnosis, y | 40 | 44 | 43 |
| Race | White | White | White |
| Recurrent pneumothorax | Yes | No | Yes |
| History of chylothorax | No | Yes | No |
| History of pleurodesis | No | Yes | No |
| FEV1 (% predicted) | 105 | 104 | 80 |
| RV (% predicted) | 99 | 55 | 124 |
| RV/TLC ratio | 0.35 | 0.23 | 0.41 |
| Lung diffusion capacity CO2 (% predicted) | 102 | 91 | 86 |
| Peak VO2 (% predicted) | 76 | 83 | 170 |
| Peak Heart Rate (% predicted) | 97 | 93 | 96 |
| Peak SaO2 (%) | 99 | 94 | 95 |
| Pneumothorax after PFT | Yes | Possibly related | No |
| Pneumothorax after CPET | No | Possibly related | Yes |
| Treated with pleurodesis | Yes | No | Yes |
CPET = cardiopulmonary exercise testing; FEV1 = forced expiratory volume in the first second; PFT = pulmonary function testing; RV = residual volume; RV/TLC = ratio between RV and TLC; SaO2 = pulse oxygen saturation; TLC = total lung capacity; VO2 = oxygen uptake.
Case 1
A 41-year-old woman with LAM and recurrent pneumothorax was admitted for participation in the LAM protocol. Admission chest radiograph no evidence of pneumothorax. She reported mild dyspnea and chest discomfort after undergoing PFT. A subsequent chest radiograph showed a 60% pneumothorax, which was confirmed by chest CT scan. Since she had to travel home by airplane, the decision was to begin chest tube drainage and perform a mechanical and doxycycline pleurodesis. Total resolution of the pneumothorax was documented after surgery by chest radiographs. No complications or recurrence of the pneumothorax were reported after she returned home.
Case 2
A 45-year-old woman with LAM was admitted for participation in the LAM protocol. On admission, she did not give a history of chest pain or change in shortness of breath since the last visit approximately 7 months earlier. The admission chest radiograph showed no evidence of pneumothorax. The patient underwent PFT and CPET. On the following day, a CT scan showed a right pneumothorax. The patient was observed for the next 3 days after which a chest radiograph showed that the pneumothorax was small and had not increased in size. She was discharged in stable condition and was advised to follow up with her physician. Resolution and no recurrence of the pneumothorax were reported.
Case 3
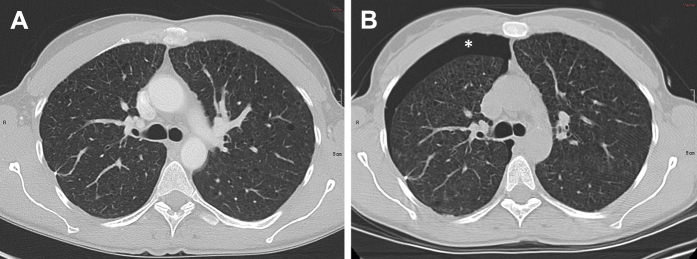
A 43-year-old woman with LAM and recurrent pneumothorax was admitted for participation in the LAM protocol. Chest radiograph on admission did not show a pneumothorax. PFT testing was done on the following day. A CT scan of the chest, abdomen, and pelvis performed on the next day showed no evidence of a pneumothorax (Fig 1A). Later that day, after she had a CPET, the patient reported mild chest discomfort. A CT scan performed in the morning on the following day showed a new right pneumothorax (Fig 1B). Video-assisted thoracoscopic surgery and talc pleurodesis were performed. She was discharged a week later in good condition. There was no recurrence of the pneumothorax.
Figure 1.
A 43-year-old woman with LAM and recurrent pneumothoraces. A, CT scan of the lungs shows no evidence of pneumothorax. On the same day as the imaging study, the patient had a cardiopulmonary exercise test. Subsequently, she reported right chest pain. B, CT scan demonstrating the presence of a right-sided pneumothorax (asterisk). She underwent a right-sided, video-assisted talc pleurodesis that resulted in complete resolution of the pneumothorax.
One patient experienced a symptomatic pneumothorax after PFT. Another patient (Patient 3) developed a pneumothorax after having undergone CPET. A third patient (Patient 2) had a pneumothorax after having had PFT and CPET, and because the patient had no symptoms, it was impossible to determine whether the pneumothorax was related to the PFT or CPET. Depending on whether this patient’s pneumothorax was related to PFT or not, we estimated that the incidence of pneumothorax associated with PFT in our cohort was 0.14 to 0.29 of 100 patients or 0.02 to 0.04 of 100 PFT. Similarly, depending on whether Patient 2 had a pneumothorax related to CPET or not, the incidence of pneumothorax in patients undergoing CPET was estimated at 0.14 to 0.28 of 100 patients or 0.05 to 0.10 of 100 CPET. Since in many of the patients in our study imaging studies were performed after PFT or CPET, and pneumothoraces were not observed, we assume that asymptomatic pneumothoraces did not occur.
Discussion
Although the frequency of spontaneous and recurrent pneumothorax in LAM is high, the incidence of pneumothorax associated with PFT or CPET in a large cohort of patients with LAM who underwent numerous tests was extremely low. The close temporal relationship between the tests, the occurrence of the pneumothorax, and the radiologic confirmation suggests that the effort associated with PFT or CPET may have caused the pneumothorax. The severity of lung disease did not appear to predict the risk of pneumothorax because the pneumothoraces occurred in patients with mild lung disease. However, two of the three patients had a recurrent pneumothoraces. It is therefore possible that the risk of pneumothorax associated with PFT or CPET may be even lower in patients who never had a pneumothorax. Indeed, pneumothoraces occur frequently in LAM and tend to recur. It is possible that in patients with LAM who never had a pneumothorax, pneumothorax would be even less likely to occur during PFT or exercise testing. Our data should reassure patients and physicians that PFT and CPET are safe tests that are not likely to cause pneumothorax.
Whether the patients in our study also developed asymptomatic pneumothoraces during PFT or CPET cannot be determined. We know that patients did not have a preexisting pneumothorax because patients who presented with a pneumothorax on admission were excluded from having PFT or CPET. Nevertheless, the occurrence of a small asymptomatic pneumothorax cannot be excluded. However, since many patients had CT scans either once a year or every 2 years, and these studies were done after PFT and exercise tests, we should have detected cases of asymptomatic pneumothorax. That we did not detect such cases makes it unlikely that asymptomatic pneumothoraces occurred in the patients in our study after PFT or exercise testing.
As our cases demonstrate, if a patient with LAM develops chest pain after undergoing either PFT or CPET, imaging studies must be obtained to rule out the presence of a pneumothorax and to initiate appropriate treatment.
Acknowledgments
Author contributions: A. M. T and J. M. are responsible for the study design, data analysis, and writing of the manuscript. A. M. J. and P. J. collected and reviewed clinical data.
Financial/nonfinancial disclosures: None declared.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Footnotes
FUNDING/SUPPORT: This study was supported by the Intramural Research Program, National Institutes of Health, National Heart, Lung, and Blood Institute [Grant 95-H-0186].
References
- 1.Ryu J.H., Moss J., Beck G.J. The NHLBI lymphangioleiomyomatosis registry: characteristics of 230 patients at enrollment. Am J Respir Crit Care Med. 2006;173(1):105–111. doi: 10.1164/rccm.200409-1298OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCormack F.X. Lymphangioleiomyomatosis: a clinical update. Chest. 2008;133(2):507–516. doi: 10.1378/chest.07-0898. [DOI] [PubMed] [Google Scholar]
- 3.Steagall W.K., Glasgow C.G., Hathaway O.M. Genetic and morphologic determinants of pneumothorax in lymphangioleiomyomatosis. Am J Physiol Lung Mol Physiol. 2007;293(3):L800–L808. doi: 10.1152/ajplung.00176.2007. [DOI] [PubMed] [Google Scholar]
- 4.Varkey B., Kory R. Mediastinal and subcutaneous emphysema following pulmonary function tests. Am Rev Respir Dis. 1973;108(6):1393–1396. doi: 10.1164/arrd.1973.108.6.1393. [DOI] [PubMed] [Google Scholar]
- 5.Krasnick J. Pneumomediastinum following spirometry. Chest. 2001;120(3):1043. doi: 10.1378/chest.120.3.1043. [DOI] [PubMed] [Google Scholar]
- 6.Manço J.C., Terra-Filho J., Silva G.A. Pneumomediastinum, pneumothorax and subcutaneous emphysema following the measurement of maximal expiratory pressure in a normal subject. Chest. 1990;98(6):1530–1532. doi: 10.1378/chest.98.6.1530. [DOI] [PubMed] [Google Scholar]
- 7.Taveira-DaSilva A.M., Burstein D., Hathaway O.M. Pneumothorax after air travel in lymphangioleiomyomatosis, idiopathic pulmonary fibrosis, and sarcoidosis. Chest. 2009;136(3):665–670. doi: 10.1378/chest.08-3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenny J.-E., Kuschner W.G. Pneumothorax caused by aggressive use of an incentive spirometer in a patient with emphysema. Respir Care. 2013;58(7):e77–e79. doi: 10.4187/respcare.02130. [DOI] [PubMed] [Google Scholar]
- 9.Ruff K., Winkler B., Hebestreit A., Gruber W., Hebestreit H. Risks associated with exercise testing and sports participation in cystic fibrosis. J Cyst Fibros. 2010;9(5):339–345. doi: 10.1016/j.jcf.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 10.Jun J.-B., Song S.-Y. The development of pneumomediastinum after pulmonary function testing in a patient with systemic sclerosis. Rheumatol Int. 2007;27(11):1097–1098. doi: 10.1007/s00296-007-0369-7. [DOI] [PubMed] [Google Scholar]
- 11.Araujo M.S., Arrabal Fernandes F.L., Kay F.U., Ribeiro Carvalho C.R. Pneumomediastinum, subcutaneous emphysema, and pneumothorax after a pulmonary function testing in a patient with bleomycin-induced interstitial pneumonitis. J Bras Pneumol. 2013;39(5):613–619. doi: 10.1590/S1806-37132013000500012. [DOI] [PMC free article] [PubMed] [Google Scholar]