Abstract
Background
Although existing research highlights the relationship of OSA and cardiovascular disease, the effect of OSA treatment on cardiovascular biomarkers remains unclear. We evaluated the effect of OSA treatment on oxidative stress/inflammation measures.
Methods
We conducted a parallel, randomized controlled trial in moderate to severe OSA (apnea-hypopnea index ≥ 15) patients to examine effects of 2-month CPAP vs sham-CPAP on the primary outcome of oxidative stress/inflammation (F2-isoprostanes: ng/mg) and myeloperoxidase: pmol/L) and secondary oxidative stress measures. Exploratory secondary analyses included vascular and systemic inflammation markers. Linear models adjusted for baseline values examined effect of CPAP on biomarker change (least squares means, 95% CI) including secondary stratified analyses examining CPAP adherence and degree of hypoxia.
Results
Of 153 participants, 76 were randomized to CPAP and 77 to sham-CPAP. In an intent-to-treat analyses, no significant change was observed in the sham and CPAP groups respectively: F2-isoprostanes (−0.02 [−0.12 to 0.10] vs −0.08 [−0.18 to 0.03]) or myeloperoxidase (−3.33 [−17.02 to 10.37] vs −5.15 [−18.65 to 8.35]), nor other oxidative markers; findings that persisted in analyses stratified by adherence and hypoxia. Exploratory analyses revealed percentage reduction of soluble IL-6 receptor (ng/mL) levels (−0.04 [−0.08 to −0.01] vs 0.02 [−0.02 to 0.06], P = .019) and augmentation index (%) (−6.49 [−9.32 to −3.65] vs 0.44 [−2.22 to 3.10], P < .001) with CPAP compared with sham, respectively.
Conclusions
In moderate to severe OSA, 2-month CPAP vs sham did not reduce oxidative stress despite consideration of a broad range of measures, positive airway pressure adherence, and hypoxia burden. These findings suggest that nonoxidative stress pathways primarily modulate OSA-related cardiovascular consequences.
Trial Registration
Key Words: CPAP, inflammation, obstructive sleep apnea
Abbreviations: AHI, apnea-hypopnea index; AIx, augmentation index; CVD, cardiovascular disease; DBP, diastolic blood pressure; IRB, institutional review board; SBP, systolic BP; sIL-6R, soluble interleukin-6
OSA, characterized by repetitive episodes of complete or partial upper airway obstruction, is common and increasingly prevalent.1 Data generated from several longitudinal cohort studies provide strong evidence for an increased risk of hypertension and cardiovascular disease (CVD) in individuals with OSA.2, 3, 4 OSA represents an independent risk factor for cardiovascular morbidity and cardiovascular-specific mortality.5 Physiologic perturbations including surges in sympathetic activation, intermittent hypoxia/reoxygenation, intrathoracic pressure alterations, and sleep fragmentation have been implicated.6, 7, 8 Recently, efforts have focused on inter-related domains of oxidative stress, systemic inflammation, and endothelial dysfunction as potential mechanistic facilitators of cardiovascular risk in OSA.
Oxidative stress, in particular, has engendered interest given the rationale that OSA morbidity results from intermittent hypoxia—oftentimes quite profound—and reoxygenation, resulting in flux of reactive oxygen species and end-organ injury (eg, endothelial damage and alterations in chemoreception).7, 9 Although experimental intermittent hypoxia models clearly demonstrate increased vascular production of reactive oxygen species,10, 11, 12 activation of nicotinamide adenine dinucleotide phosphate oxidase, and enhanced lipid peroxidation,13, 14, 15 clinical results in OSA are less conclusive.16, 17, 18, 19, 20 However, some signal has been observed with F-2 isoprostanes, an established stable marker of lipid peroxidation recognized to play a role in atherogenesis.16, 21
The effect of OSA treatment with CPAP on cardiovascular biomarkers, particularly oxidative stress, remains largely unknown. Prior attempts have been hampered by heterogeneity of methodologies used, limited consideration of confounding influences, use of a nonrandomized study design, relatively small sample sizes, and inconsistency in findings.22, 23, 24, 25, 26, 27 In the present study, we sought to examine the effect of CPAP use in moderate to severe OSA on markers of oxidative stress/inflammation (primary outcomes: F2-isoprostanes and myeloperoxidase). Secondarily, vascular measures and markers of inflammation were examined. We postulate that reversal of OSA pathophysiology with CPAP results in improvement in levels of oxidative stress and, secondarily, improvement in systemic inflammation and measures of arterial stiffness. Some of the results of this study have been previously reported in the form of abstracts.28, 29
Materials and Methods
Study Design
The Sleep Apnea Stress Study (National Institutes of Health clinical trials registry NCT00607893) is a single-center, parallel-group, randomized, double-blind, sham-controlled trial involving recruitment from the sleep programs of two hospital systems of Case Western Reserve University (ie, University Hospitals Case Medical Center and MetroHealth Medical Center; additional details can be found in the e-Appendix).
The study was designed to investigate the effect of CPAP treatment compared with control (sham CPAP) for improvement in cardiovascular measures in patients with moderate to severe OSA (apnea hypopnea index ≥ 15) naïve to OSA therapy. Cardiovascular measures were obtained at the baseline assessment and after 8 weeks of treatment. A 2-week run-in period on CPAP with a 1-month washout period was implemented to ascertain acceptable CPAP adherence as an entry criterion into the trial. The study was funded by the National Heart, Lung, and Blood Institute (K23 HL079114) and the CPAP machines were donated by the Philips Respironics Corporation. A Data Safety Monitoring Board was assembled to provide oversight of study progress, including recruitment and safety monitoring according to a prespecified Data Safety Monitoring Plan. A blinded physician observer (safety monitor) was responsible for assessing participant safety. Participants were contacted at regular intervals to assess for adverse events. The research protocol was approved by the institutional review board (IRB; University Hospitals IRB 07-06-25, MetroHealth Medical Center IRB 10-00370, and Cleveland Clinic IRB 13-716) and all participants provided written informed consent. The authors had sole access to the data with direct supervision of data collection, performed the statistical analyses, and manuscript writing without input or review by the study sponsor or the Philips Respironics Corporation. All authors vouch for the completeness and accuracy of the data.
Participants
Inclusion criteria were those 20 to 75 years of age with apnea-hypopnea index (AHI) ≥ 15 based upon in-laboratory clinical polysomnography. The optimal pressure to address OSA with the goal of AHI < 5 was identified in the clinical sleep laboratory. Those with CPAP adherence of ≥ 4 h for ≥ 70% of the time monitored during a 2-week run-in period were eligible to proceed to randomization. Exclusion criteria were current or planned use of OSA treatment outside of the clinical trial, use of supplemental oxygen, non-OSA primary sleep disorder, drowsy driving, use or anticipated use of corticosteroids or potent anti-inflammatory/immunosuppressant medications, and/or unstable medical conditions.
Randomization and Masking
A computerized permuted block design with varying block size and stratification according to age, sex, and sleep apnea severity was used to randomize 149 participants to active vs sham CPAP (REMstar Pro, Philips Respironics, Inc.) for a 2-month period. CPAP was equipped with heated humidification and expiratory pressure relief as needed and sham CPAP was delivered using a customized Philips Respironics device and masks with increased expiratory ports designed to deliver negligible pressure (0.4 ± 1 SD cm H2O pressure). Participants and personnel were blinded to treatment assignments with the exception specific members of the database management team and research staff who provided the CPAP units and education to the participants. All participants received standardized sleep hygiene and healthy lifestyle education.
Outcomes
Participants underwent overnight polysomnography, anthropometry, morning venipuncture, overnight urine collection, evening saliva collection, resting morning and evening BP measurement, and collection of measures of vascular stiffness at baseline and 8 weeks at the Dahms Clinical Research Unit. The primary outcome was oxidative stress/inflammation measures urinary F2-isoprostanes adjusted for creatinine16, 21 and the leukocyte oxidant generating enzyme myeloperoxidase30 given biologic plausibility and results of prior studies. Additional measures of oxidative stress considered include: lipoprotein (a), oxidized low-density lipoprotein, paraoxonase-1, and aryl esterase. Secondary outcomes included arterial stiffness measures: augmentation index (AIx), pulse wave velocity, and the additional inflammation-related markers soluble IL-6R (sIL-6R) and IL-6. Exploratory outcomes included averaged BP measures (morning and evening systolic, diastolic, and mean arterial BP) and additional vascular measures (augmentation pressure, pulse pressure). Details of data collection are provided in the e-Appendix.
Statistical Analyses
Results are expressed as mean ± SD or median [25th, 75th percentiles] for continuous variables and N (%) for categorical variables. Spearman correlation was used to assess the association between baseline AHI and outcomes (see e-Appendix). For comparison of baseline characteristics between the groups (CPAP vs sham-CPAP), t test or Wilcoxon rank sum test was used for continuous variables, Pearson’s χ2 test or Fisher’s exact test for nominal categorical variables, and Mantel-Haenszel test for ordinal categorical variables. The changes from baseline to week 8 for outcomes were calculated as the absolute difference between two visits and percent change from baseline. Linear regression was used to compare the change between sham and CPAP groups, adjusted for baseline values. Log transformation was performed for sIL-6R and F2-isoprostanes/creatinine analyses to satisfy the normal distribution assumption.
Secondary analyses were performed to take into consideration CPAP adherence (defined as ≥ 4 h per night for 70% of the nights monitored). Exploratory stratified analyses were performed to evaluate for differences in the effect of CPAP on outcomes in relation to OSA severity and obesity as well as hypoxic exposure defined by the baseline oxygen desaturation index (≥ 3% and alternatively ≥ 4%).
A P value < .05 was the threshold for a difference statistically significant. SAS, version 9.3 (The SAS Institute), was used to perform all analyses.
Results
Study Participants
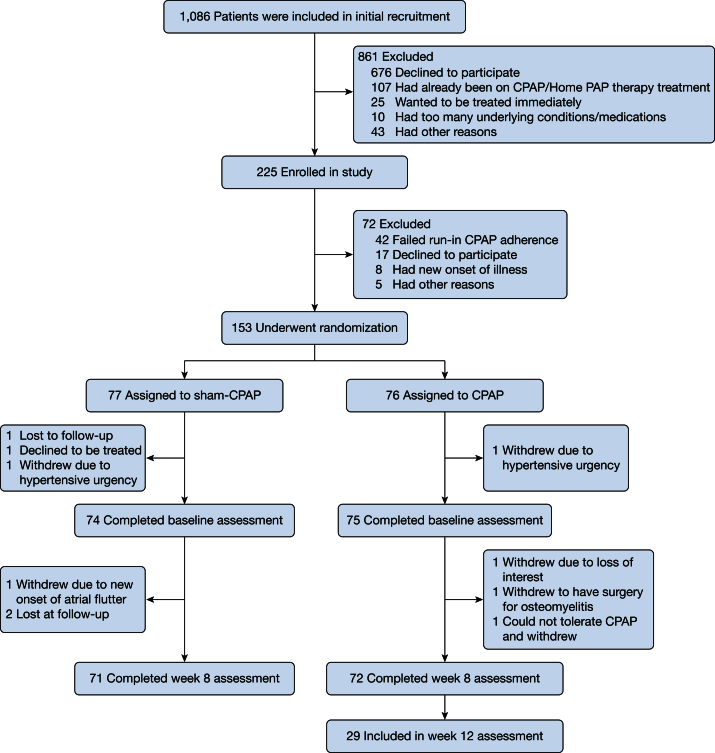
Among 1,086 subjects who were approached for initial recruitment, 262 were interested in participating and were screened for enrollment. Of 225 individuals who met eligibility, 153 underwent randomization: 76 were randomized to CPAP and 77 to sham-CPAP. Ten participants (6.5%) were excluded after randomization and did not complete the 8 weeks of the study (Fig 1). The baseline characteristics of those who completed the 8-week follow-up examination revealed a middle-aged, obese sample with moderate to severe OSA. Overall, approximately one-half were women and one-half were African Americans with similar baseline characteristics (Table 1). Cardiac comorbidities and the use of antihypertensive, anti-inflammatory, cholesterol-lowering medications and aspirin were balanced across the groups. Both groups had well-controlled BP at baseline and were balanced in terms of type of antihypertensive medication per group. As expected, the AHI was significantly reduced from baseline to 8-week follow-up in the CPAP group (19.1 [12.5, 35.1] to 2.5 [0.66, 5.6]) compared with the sham group (20.5 [11.7, 37.7] to 16.7 [9.7, 36.7]), P < .001. Overall, there was no significant change in BMI from baseline to follow-up (37.3 ± 8.1 at baseline; 37.4 ± 7.9 at follow-up). CPAP adherence data revealed longer than average use in the active CPAP group (median hour use was 4.5 [2.6, 5.7] vs 2.5 [0.95, 4.7] h in the sham group), P = .003.
Figure 1.
Numbers of patients who were screened, randomly assigned to a study group, and included in analysis.
Table 1.
Baseline Characteristics of the Study Participants
| Overall (N = 149) | Sham (n = 74) | CPAP (n = 75) | |
|---|---|---|---|
| Age, y | 51.0 ± 11.7 | 51.7 ± 11.8 | 50.3 ± 11.7 |
| Male sex, No. (%) | 80 (53.7) | 39 (52.7) | 41 (54.7) |
| Race, No. (%)a | |||
| Caucasian | 78 (52.3) | 41 (55.4) | 37 (49.3) |
| African American | 67 (45.0) | 31 (41.9) | 36 (48.0) |
| Other | 4 (2.7) | 2 (2.7) | 2 (2.7) |
| BMI, kg/mb | 37.3 ± 8.1 | 37.3 ± 8.3 | 37.3 ± 8.1 |
| Cardiovascular disease, No. (%) | 25 (17.0) | 15 (20.5) | 10 (13.5) |
| Hypertension No. (%) | 94 (63.9) | 44 (60.3) | 50 (67.6) |
| Diabetes mellitus, No. (%) | 26 (17.5) | 14 (18.9) | 12 (16.0) |
| Smoking status, No. (%) | |||
| Current smoker | 18 (12.2) | 7 (9.6) | 11 (14.9) |
| Prior smoker | 68 (46.3) | 31 (42.5) | 37 (50.0) |
| Never smoker | 61 (41.5) | 35 (47.9) | 26 (35.1) |
| Medication use, No. (%) | |||
| Aspirin | 49 (33.1) | 28 (38.4) | 21 (28.0) |
| Anti-inflammatory analgesic | 20 (13.5) | 7 (9.6) | 13 (17.3) |
| Cholesterol-lowering medication | 60 (40.5) | 31 (42.5) | 29 (38.7) |
| Antihypertensive medication | 74 (50.0) | 36 (49.3) | 38 (50.7) |
| Alpha-adrenergic blocker | 3 (4.6) | 2 (6.3) | 1 (3.0) |
| Beta-adrenergic blocker | 24 (16.2) | 12 (16.4) | 12 (16.0) |
| Calcium channel blocker | 28 (18.9) | 12 (16.4) | 16 (21.3) |
| ACE inhibitors | 32 (21.6) | 16 (21.9) | 16 (21.3) |
| Thiazide diuretic | 39 (26.4) | 15 (20.5) | 24 (32.0) |
| AHI, events/h | 19.3 [12.5,36.6] | 20.6 [11.7, 37.7] | 19.1 [12.5, 35.1] |
| Time < 90% oxygen saturation, % of sleep time | 2.0 [0, 7.0] | 2.5 [0, 8.0] | 2.0 [0, 6.0] |
| Arousal index, events/h | 17.6 [12.0, 24.0] | 17.6 [12.5, 23.8] | 17.6 [11.8, 24.2] |
| ESS score | 10.0 [7.0, 13.0] | 10.0 [8.0, 13.0] | 10.0 [6.0, 14.0] |
| Self-reported sleep duration, min | 458.6 [411.4, 518.6] | 475.7 [420.0, 518.6] | 445.7 [405.0, 518.6] |
| F2-isoprostanes/Cr, ng/mg | 0.44 [0.28, 0.72] | 0.47 [0.28, 0.78] | 0.40 [0.27, 0.60] |
| Myeloperoxidase, pmol/L | 290.0 [235.0, 391.0] | 301.5 [254.0, 403.0] | 283.0 [230.0, 370.0] |
| IL-6, pg/mL | 3.0 [1.9, 4.3] | 2.9 [2.0, 4.7] | 3.0 [1.7, 4.2] |
| sIL-6R, ng/mL | 40,554.0 [34,152.0, 50,592.0] | 42,455.5 [34,899.0, 50,592.0] | 38,484.0 [33709.0, 51565.0] |
| SBP, evening, mm Hg | 125.5 ± 15.0 | 125.6 ± 15.5 | 125.3 ± 14.5 |
| SBP, morning, mm Hg | 126.3 ± 15.8 | 125.7 ± 17.1 | 126.9 ± 14.5 |
| DBP, evening, mm Hg | 70.2 ± 10.9 | 69.2 ± 10.9 | 71.3 ± 10.8 |
| DBP, morning, mm Hg | 77.9 ± 9.7 | 76.3 ± 10.1 | 79.6 ± 9.1 |
| Mean arterial BP, evening, mm Hg | 87.8 [80.0, 95.6] | 87.3 [78.7, 93.6] | 88.2 [80.0, 97.6] |
| Mean arterial BP, morning, mm Hg | 93.8 [87.3, 100.2] | 92.1 [84.4, 99.6] | 94.4 [88.9, 102.9] |
| AIx, evening, % | 74.5 [62.0, 83.0] | 74.5 [62.0, 83.5] | 73.8 [62.0, 82.5] |
| AIx, morning, % | 77.5 [67.5, 89.0] | 77.3 [69.5, 88.0] | 78.0 [66.5, 91.0] |
| Pulse wave velocity, evening, cm/s | 9.1 [7.8, 10.6] | 9.3 [7.8, 10.8] | 9.0 [7.7, 10.2] |
| Pulse wave velocity, morning, cm/s | 9.0 [7.7, 10.8] | 9.3 [7.6, 11.4] | 8.9 [7.8, 10.3] |
Soluble values are presented as mean ± SD, median [25th, 75th percentiles], or No. (%). AHI = apnea hypopnea index; AIx = augmentation index; Cr = creatinine; DBP = diastolic BP; ESS = Epworth Sleepiness Scale; sIL-6R = soluble IL-6 receptor; SBP = systolic BP.
Race was self-reported.
BMI is the weight in kilograms divided by the square of the height in meters.
Study Outcomes
In the intent-to-treat adjusted analyses, the absolute change from baseline to 8-week follow-up between the two groups showed no statistically significant difference in the primary outcome: F2-isoprostanes and myeloperoxidase (Table 2). There were no significant differences between the two groups of change in other oxidative stress markers (lipoprotein (a), oxidized low-density lipoprotein paraoxonase, and aryl esterase levels).
Table 2.
Effect of CPAP Treatment on Cardiovascular Biomarkers, Intent to Treat (Change of Biomarker From Baseline)
| Outcome | Sham (n = 71) | CPAP (n = 72) | P Value |
|---|---|---|---|
| Oxidative stress/inflammation biomarkers | |||
| F2-isoprostanes/Cra | −0.02 (−0.12 to 0.10) | −0.08 (−0.18 to 0.03) | .38 |
| Myeloperoxidase, pmol/L | −3.33 (−17.02 to 10.37) | −5.15 (−18.65 to 8.35) | .85 |
| Lipoprotein (a), mg/dL | 0.00 (−0.01 to 0.02) | 0.00 (−0.01 to 0.02) | .99 |
| Oxidized LDL, U/L | −1.38 (−2.69 to −0.07) | −1.34 (−2.64 to −0.03) | .96 |
| Paraoxonase, U/mL | 1.37 (−55.91 to 58.64) | −17.02 (−73.03 to 38.99) | .65 |
| Aryl esterase, nmol/min/mL | −0.92 (−6.47 to 4.63) | −5.74 (−11.16 to −0.31) | .22 |
| sIL-6Ra | 0.02 (−0.02 to 0.06) | −0.04 (−0.08 to −0.01) | .019 |
| IL-6 (pg/mL) | 0.29 (−0.10 to 0.68) | −0.08 (−0.46 to 0.29) | .17 |
| Arterial stiffness measures | |||
| AIx, evening, % | −0.43 (−3.87 to 3.00) | −1.79 (−5.36 to 1.78) | .59 |
| AIx, morning, % | 0.44 (−2.22 to 3.10) | −6.49 (−9.32 to −3.65) | < .001 |
| Augmentation pressure, evening, mm Hg | 0.36 (−0.90 to 1.63) | −0.18 (−1.52 to 1.15) | .56 |
| Augmentation pressure, morning, mm Hg | 0.34 (−1.03 to 1.72) | −1.46 (−2.98 to 0.07) | .085 |
| Pulse wave velocity, evening, cm/s | −0.08 (−0.57 to 0.40) | −0.30 (−0.80 to 0.20) | .55 |
| Pulse wave velocity, morning, cm/s | −0.25 (−0.82 to 0.32) | −0.42 (−1.01 to 0.16) | .68 |
| BP | |||
| SBP, evening, mm Hg | −0.95 (−3.59 to 1.69) | −0.41 (−3.03 to 2.21) | .78 |
| SBP, morning, mm Hg | 0.17 (−2.44 to 2.78) | −3.44 (−6.03 to −0.85) | .054 |
| DBP, evening, mm Hg | −0.45 (−2.52 to 1.61) | −0.83 (−2.88 to 1.22) | .80 |
| DBP, morning, mm Hg | 0.11 (−1.73 to 1.94) | −1.37 (−3.19 to 0.46) | .27 |
| Mean arterial BP, evening, mm Hg | −0.59 (−2.53 to 1.35) | −0.72 (−2.65 to 1.20) | .92 |
| Mean arterial BP, morning, mm Hg | 0.24 (−1.64 to 2.12) | −2.17 (−4.03 to −0.30) | .076 |
Results are least square means and 95% CI of absolute change from baseline, with adjustment of baseline. See Table 1 legend for expansion of abbreviations.
Data was log-transformed for analysis and transformed back for presentation. The findings reflect the percentage change (ie, the percent of change at week 8 to baseline values).
Analysis of the secondary vascular outcomes demonstrated significant reduction of morning AIx values in the CPAP compared with the sham group. A trend favoring a decrease in the morning systolic BP in the active CPAP group was observed, but not statistically significant. No other differences were found in other exploratory measures of BP or vascular stiffness.
In terms of other secondary outcome measures, sIL-6R values were log-transformed for analyses and then back-transformed for interpretation, thereby the difference represents the percent change of week 8 to baseline. sIL-6R levels demonstrated a 4% reduction in the CPAP group (−0.04, −0.08, −0.01) compared with a 2% increase in the sham group (0.02, −0.02, 0.06), P = .019 at 8 weeks. No significant differences were observed in IL-6 levels from baseline to follow-up between the CPAP and sham CPAP groups.
Secondary Analyses
To examine treatment effect, we performed stratified analysis among adherent CPAP (n = 29), nonadherent CPAP (n = 38), and sham groups (n = 71). Similar to primary analyses, morning AIx differed significantly among groups (absolute difference: −7.16% [−11.76 to −2.56], P = .003) in the CPAP adherent group) (Table 3). Unlike the primary analysis, the significant difference of sIL-6R between CPAP and sham did not persist after examined by adherence (−0.06 [−0.12 to 0.00] vs −0.02 [−0.08 to 0.03] vs 0.02 [−0.02 to 0.06], respectively, P = .066). Lipoprotein (a) differed across groups with a significant reduction among groups: −0.02 [−0.04 to 0.01] vs 0.02 [0.00-0.04] vs 0.00 [−0.01 to 0.02], respectively, P = .040). Other biomarkers did not demonstrate appreciable changes. No statistically significant interactions were observed between study arm and baseline hypoxia.
Table 3.
Absolute Change From Baseline: Adjusted for Baseline, Group Split by Compliant Days (≥ 4 h) ≥ 70%a
| Outcome | No. | Sham (n = 71) | CPAP Nonadherent (n = 38) | CPAP Adherent (n = 29) | P Value |
|---|---|---|---|---|---|
| Oxidative stress/inflammation markers | |||||
| F2-isoprostanes/Crb | 125 | −0.01 (−0.12 to 0.11) | −0.09 (−0.23 to 0.07) | −0.08 (−0.24 to 0.11) | .65 |
| Myeloperoxidase, pmol/L | 132 | −3.26 (−17.25 to 10.73) | −0.53 (−19.75 to 18.69) | −9.79 (−31.66 to 12.08) | .81 |
| Lipoprotein (a), mg/dL | 133 | 0.00 (−0.01 to 0.02) | 0.02 (0.00 to 0.04) | −0.02 (−0.04 to 0.01) | .040 |
| Oxidized LDL, U/L | 133 | −1.38 (−2.70 to −0.05) | −1.02 (−2.85 to 0.81) | −1.38 (−3.46 to 0.70) | .95 |
| Paraoxonase, U/mL | 129 | 1.92 (−56.46 to 60.30) | −5.89 (−85.25 to 73.46) | −33.30 (−124.9 to 58.33) | .81 |
| Aryl esterase, nmol/min/mL | 129 | −1.00 (−6.64 to 4.63) | −2.65 (−10.27 to 4.96) | −9.62 (−18.46 to −0.79) | .27 |
| sIL-6Rb | 123 | 0.02 (−0.02 to 0.06) | −0.02 (−0.08 to 0.03) | −0.06 (−0.12 to 0.00) | .066 |
| IL-6 (pg/mL) | 123 | 0.30 (−0.10 to 0.70) | −0.12 (−0.64 to 0.41) | −0.03 (−0.64 to 0.58) | .40 |
| Arterial stiffness measures | |||||
| AIx, evening | 98 | −0.38 (−3.85 to 3.09) | −1.60 (−6.25 to 3.05) | −2.78 (−8.85 to 3.30) | .77 |
| AIx, morning | 93 | 0.46 (−2.23 to 3.14)c | −6.25 (−9.97 to −2.53)d | −7.16 (−11.76 to −2.56) | .003 |
| Augmentation pressure, evening, mm Hg | 102 | 0.37 (−0.91 to 1.64) | 0.19 (−1.57 to 1.95) | −0.79 (−3.02 to 1.45) | .67 |
| Augmentation pressure, morning, mm Hg | 95 | 0.35 (−1.02 to 1.73) | −1.24 (−3.24 to 0.77) | −2.13 (−4.56 to 0.29) | .15 |
| Pulse wave velocity, evening | 87 | −0.10 (−0.59 to 0.40) | −0.31 (−1.00 to 0.37) | −0.31 (−1.22 to 0.60) | .85 |
| Pulse wave velocity, morning | 104 | −0.26 (−0.84 to 0.32) | −0.39 (−1.14 to 0.35) | −0.23 (−1.33 to 0.86) | .95 |
| BP | |||||
| SBP, evening, mm Hg | 136 | −0.88 (−3.56 to 1.80) | −0.47 (−4.25 to 3.31) | 0.80 (−3.43 to 5.03) | .80 |
| SBP, morning, mm Hg | 136 | 0.19 (−2.45 to 2.83) | −2.48 (−6.19 to 1.24) | −3.65 (−7.78 to 0.48) | .24 |
| DBP, evening, mm Hg | 136 | −0.35 (−2.39 to 1.70) | −0.45 (−3.33 to 2.42) | 0.47 (−2.72 to 3.66) | .90 |
| DBP, morning, mm Hg | 136 | 0.25 (−1.57 to 2.08) | −0.51 (−3.08 to 2.06) | −1.70 (−4.53 to 1.14) | .52 |
| Mean arterial BP, evening, mm Hg | 136 | −0.49 (−2.42 to 1.43) | −0.44 (−3.15 to 2.28) | 0.47 (−2.55 to 3.49) | .86 |
| Mean arterial BP, morning, mm Hg | 136 | 0.33 (−1.54 to 2.21) | −1.29 (−3.94 to 1.35) | −2.43 (−5.35 to 0.49) | .26 |
Results are least square means and 95% CI. See Table 1 legend for expansion of abbreviations.
Pairwise comparison: cSham differed from CPAP adherent, dCPAP nonadherent differed from CPAP adherent.
Groups split by compliant days (≥ 4 h) ≥ 70%.
Data were log-transformed for analysis, and transformed back for presentation.
Subgroup Exploratory Analyses
In those with less OSA severity (AHI < 30), the CPAP group demonstrated reduction of morning AIx in less severe OSA (−6.70 [−10.25 to −3.15] vs 0.27 [−3.20 to 3.75] P = .006) and those with severe OSA (−6.07 [−11.02 to −1.13] vs 0.69 [−3.57 to 4.95], P = .042) and reduced sIL-6R compared with the sham group, respectively (0.95 [0.91-0.99] vs 1.02 [−0.98 to 1.08], P = .029). In both the less obese and more obese groups, a reduction in AIx was observed in CPAP compared with sham; however, this reduction was more pronounced in the more obese compared with the less obese, respectively (more obese group: −7.91 [−11.89 to 3.93] vs −2.09 [−6.14 to 1.96], P = .04 and less obese group: (−5.05 [−9.04 to −1.07] vs 2.42 [−1.15 to 5.98], P = .007). In those with normal weight or lesser degree of obesity (BMI ≤ 36.3), CPAP group reduced sIL-6R compared with sham (0.97, 0.92-1.02 vs 1.04, 0.99-1.10, P = .04) (e-Tables 1-5).
Discussion
The results of the current randomized controlled trial demonstrate that in a group enriched with African Americans and women with previously unrecognized moderate to severe OSA, 2 months of CPAP compared with sham CPAP did not result in appreciable improvement in the primary outcome of urinary F2-Isoprostanes, an established global marker of oxidant stress most studied to date,31 and myeloperoxidase. Moreover, improvement was not observed in other markers of oxidative stress. Alternatively, some of the secondary markers demonstrated improvement; however, these findings should be viewed as exploratory. Morning vascular function characterized by the AIx, a measure of arterial stiffness and predictor of increased cardiovascular events,32, 33 was improved with CPAP compared with sham CPAP. Furthermore, sIL-6R, a marker of systemic inflammation previously identified to be associated with OSA, was significantly reduced in the intervention group compared to sham CPAP. Additional analyses revealed that the findings of sIL-6R and AIx improvement appeared to be most pronounced in those with lesser degree of OSA and less obese. In light of conflicting existing data, findings from this larger clinical trial suggest that oxidative stress is not a central mechanism by which OSA confers increased CVD risk.
The lack of a statistically significant improvement in F2-isoprostanes, a measure of oxidative stress and myeloperoxidase, an enzymatic source of reactive oxidant species, in response to CPAP in moderate to severe OSA is unanticipated, but corroborates findings from smaller clinical trials.34, 35 We focused on markers with either sufficient available data supporting an association with OSA such as F2-isoprostanes16, 21 or markers firmly implicated in CVD such as myeloperoxidase.30 There are several potential reasons for these findings. First, it is possible that the duration of OSA treatment was insufficient or the overall degree of OSA (mean AHI = 20) and hypoxia (average 2% sleep time < 90% oxygen saturation) was not sufficient to result in oxidative stress up-regulation to allow for observation of CPAP responsiveness. In a prior smaller randomized trial involving a severe degree of OSA and hypoxia (6% sleep time < 90% oxygen saturation), a significant reduction in F2-isoprostane levels was observed,21 suggesting a certain threshold of hypoxia and/or duration of therapy may be required. Conversely, smaller studies linking F2-isoprostanes and OSA may be confounded by unrecognized associations of F2-isoprostanes and other CVD comorbidities that our randomization design was able to overcome. Second, it is plausible that at lesser degrees of hypoxia, counterregulatory antioxidant mechanisms are at play, and greater hypoxia burden may be overcome by pro-oxidant stress, at which point treatment amenability is realized. Third, given that cellular and molecular studies have identified protein expression markers involved in oxidative stress signaling pathways in OSA, it is possible that measurement of oxidant-modified products such as those measured in this trial may not accurately reflect processes at a cellular level.17, 36
Experimental physiologic data have identified wave reflections such as measured by the AIx in the pathogenesis of left ventricular failure37, 38 and in epidemiologic studies as a predictor of major adverse cardiovascular events.32, 33 Our findings support a reduction in morning and not evening AIx, suggesting immediate responsiveness to PAP-related reversal of overnight OSA-induced physiologic stress. These results are somewhat anticipated given the known elevations in morning arterial stiffness (with normal peripheral BP) in patients with OSA.39 These findings are also consistent with the diurnal variability of AIx observed in a smaller trial,40 albeit more pronounced in our trial potentially because of inclusion of a higher percentage of African Americans, a group vulnerable to impaired microvascular function.41 The magnitude of CPAP effect on morning AIx (−6.49 [−9.32 to −3.65]) is within range of antihypertensive medication effect (5.4 ± 2.5 reduction).42 Our findings confirm interventional trial data involving smaller samples that demonstrated improvement in AIx.43, 44 In contrast, use of CPAP in those with OSA without history of CVD or minimally symptomatic OSA did not result in significant reductions of AIx.45, 46 The lack of improvement in pulse wave velocity with CPAP may result from longstanding, irreversible OSA-induced vascular damage.
The role of sIL-6R as a pro-inflammatory marker in development of cardiovascular disease,47 conversion of fibroblasts to myofibroblasts,48 and adverse ventricular remodeling after myocardial ischemia49, 50 has garnered much attention. Our findings of improvement of sIL-6R levels with CPAP compared with sham CPAP must be interpreted cautiously given the small magnitude of change and because the results did not persist when taking into consideration PAP adherence, which also may be attributable to more limited power. The direction of our findings is consistent with prior work demonstrating monotonic relationships of sIL-6R and OSA severity independent of obesity and other confounders.51 Unlike the current study, results from the Icelandic Sleep Apnea Cohort did not support a difference in PAP users vs nonusers in 2-year follow-up sIL-6R levels after adjusting for confounders.52 Potential reasons for differences include interventional vs observational study designs as well as race/ethnicity and sex differences between the study samples. The lack of significant reduction in IL-6 in response to CPAP in our study has been corroborated by prior trials including the MOSAIC [Multicenter International Study of Oxaliplatin] trial.53, 54, 55
The strengths of the study include the randomized design, ethnic diversity, blinding of key personnel, use of standardized measures, degree of PAP adherence in accordance with clinical standards, and low dropout rates. Limitations of the study include the possibility that the hypoxia exposure was not sufficiently profound and the follow-up period was not long enough to observe an effect on treatment. Importantly, the examination of secondary outcomes in the present study should be interpreted with caution and need to be confirmed in future studies.
In conclusion, in a diverse group with moderate to severe OSA, CPAP did not result in improvement in the primary outcome of urinary F2-isoprostane and circulating myeloperoxidase levels nor other oxidative stress measures. Improvement in secondary measures of morning AIx and sIL-6R was observed. These data support that CPAP exerts cardiovascular benefits via select pathways and suggest that treatment benefits may be mediated less by oxidative stress and perhaps more so by improvement in the vascular dysfunction intrinsic to OSA and potentially the systemic inflammatory state. We cannot, however, exclude the possibility that a higher hypoxia or apnea burden may be more amenable to CPAP responsiveness resulting in oxidative stress improvement.
Acknowledgments
Author contributions: All authors have contributed sufficiently to the project to be included as authors and they agree with the submission. R. M. is the guarantor and takes responsibility for the content of the manuscript, including the data and analysis. H. L. P. M. and H. K. W. participated in manuscript preparation and writing. S. L. H. participated in the study design, manuscript preparation, and biochemical analyses. R. P. T. was involved in manuscript preparation and biochemical analyses. K. P. S. participated in patient enrollment, safety monitoring, and manuscript preparation. D. A. participated in manuscript preparation and patient enrollment. J. B. and L. W. contributed to data collection and analysis. S. R. P. was involved in data analysis and manuscript preparation. R. M. was involved in conception of the study design, patient enrollment, project management, data analysis and monitoring, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: R. M. has received National Institutes of Health (NIH) funding for which she has served as Principal Investigator (National Heart, Lung, and Blood Institute RO1 1 R01 HL 109493, R21 HL108226); her institution has received positive airway pressure machines and equipment from Philips Respironics for use in NIH-funded research; she has received honorarium from the American Academy of Sleep Medicine for speaking; she serves as the Associate Editor for the journal CHEST; and she has received royalties from Up to Date. S. L. H. has been named as coinventor on issued and pending patents held by the Cleveland Clinic relating to cardiovascular and inflammation diagnostics; has received consultancy fees from the following companies: Cleveland Heart Lab, Esperion, Lilly, Liposcience Inc., P&G, and Pfizer Inc; received research funds from Abbott, Astra Zeneca, Cleveland Heart Lab, Liposcience Inc., P&G, Pfizer Inc., and Takeda; and reports having the right to receive royalty payments for inventions or discoveries related to cardiovascular diagnostics or therapeutics from Cleveland Heart Lab., Siemens, Esperion, and Frantz Biomarkers, LLC. S.R.P. have received grant funding from the American Sleep Medicine Foundation and the ResMed Foundation. None declared (H. L. P. M., R. P. T., K. P. S., D. A., J. B., L. W., H. K. W.).
Role of sponsors: The sponsors had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: We thank Sandra Stennis, MS, Joan Aylor, BA, Kathryn Clark, Rawan Salem, RPSGT, Heather Rogers, BA, the Case Western Reserve University Clinical Research Unit team, and Hong Li, MS, who contributed to data extraction and study coordination.
Additional information: The e-Appendix and e-Tables can be found in the Supplemental Materials section of the online article.
Footnotes
Some of the results of this study have been previously reported in the form of abstracts at the 28th Annual Meeting of Associated Professional Sleep Societies, June 2014, Minneapolis, MN.
FUNDING/SUPPORT: This study was supported by the National Heart, Lung and Blood Institute [Grant K23HL079114] and National Institutes of Health (NIH) National Center for Research Resources [Grant UL1 RR024989]. Dr Hazen was supported by NIH [Grant P01 HL076491]. Mass spectrometry studies were performed on instruments housed within a core partially supported by an AB SCIEX Center of Innovation grant.
Supplementary Data
References
- 1.Peppard P.E., Young T., Barnet J.H., Palta M., Hagen E.W., Hla K.M. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin J.M., Carrizo S.J., Vicente E., Agusti A.G.N. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 3.Nieto F.J., Young T.B., Lind B.K. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. Sleep Heart Health Study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 4.Young T., Peppard P., Palta M. Population-based study of sleep-disordered breathing as a risk factor for hypertension. Arch Intern Med. 1997;157(15):1746–1752. [PubMed] [Google Scholar]
- 5.Punjabi N.M., Caffo B.S., Goodwin J.L. Sleep-disordered breathing and mortality: a prospective cohort study. PLoS Med. 2009;6(8):e1000132. doi: 10.1371/journal.pmed.1000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elmasry A., Lindberg E., Hedner J., Janson C., Boman G. Obstructive sleep apnoea and urine catecholamines in hypertensive males: a population-based study. Eur Respir J. 2002;19(3):511–517. doi: 10.1183/09031936.02.00106402. [DOI] [PubMed] [Google Scholar]
- 7.Lavie L. Obstructive sleep apnoea syndrome – an oxidative stress disorder. Sleep Med Rev. 2003;7(1):35–51. doi: 10.1053/smrv.2002.0261. [DOI] [PubMed] [Google Scholar]
- 8.Weiss J.W., Remsburg S., Garpestad E., Ringler J., Sparrow D., Parker J.A. Hemodynamic consequences of obstructive sleep apnea. Sleep. 1996;19(5):388–397. doi: 10.1093/sleep/19.5.388. [DOI] [PubMed] [Google Scholar]
- 9.Iturriaga R., Andrade D.C., Rio R Del. Enhanced carotid body chemosensory activity and the cardiovascular alterations induced by intermittent hypoxia. Front Physiol. 2014;5:468. doi: 10.3389/fphys.2014.00468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen L., Einbinder E., Zhang Q., Hasday J., Balke C.W., Scharf S.M. Oxidative stress and left ventricular function with chronic intermittent hypoxia in rats. Am J Respir Crit Care Med. 2005;172(7):915–920. doi: 10.1164/rccm.200504-560OC. [DOI] [PubMed] [Google Scholar]
- 11.Troncoso Brindeiro C.M., Silva AQ da, Allahdadi K.J., Youngblood V., Kanagy N.L. Reactive oxygen species contribute to sleep apnea-induced hypertension in rats. Am J Physiol Heart Circ Physiol. 2007;293(5):H2971–H2976. doi: 10.1152/ajpheart.00219.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou W., Li S., Wan N., Zhang Z., Guo R., Chen B. Effects of various degrees of oxidative stress induced by intermittent hypoxia in rat myocardial tissues. Respirol Carlton Vic. 2012;17(5):821–829. doi: 10.1111/j.1440-1843.2012.02157.x. [DOI] [PubMed] [Google Scholar]
- 13.Jun J., Savransky V., Nanayakkara A. Intermittent hypoxia has organ-specific effects on oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2008;295(4):R1274–R1281. doi: 10.1152/ajpregu.90346.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin Q.-C., Chen L.-D., Chen G.-P. Association between nocturnal hypoxia and liver injury in the setting of nonalcoholic fatty liver disease. Sleep Breath Schlaf Atm. 2015;19(1):273–280. doi: 10.1007/s11325-014-1008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peng Y.-J., Nanduri J., Yuan G. NADPH oxidase is required for the sensory plasticity of the carotid body by chronic intermittent hypoxia. J Neurosci Off J Soc Neurosci. 2009;29(15):4903–4910. doi: 10.1523/JNEUROSCI.4768-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ben M Del, Fabiani M., Loffredo L. Oxidative stress mediated arterial dysfunction in patients with obstructive sleep apnoea and the effect of continuous positive airway pressure treatment. BMC Pulm Med. 2012;12:36. doi: 10.1186/1471-2466-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyugovskaya L., Lavie P., Lavie L. Increased adhesion molecules expression and production of reactive oxygen species in leukocytes of sleep apnea patients. Am J Respir Crit Care Med. 2002;165(7):934–939. doi: 10.1164/ajrccm.165.7.2104126. [DOI] [PubMed] [Google Scholar]
- 18.Oyama J., Yamamoto H., Maeda T., Ito A., Node K., Makino N. Continuous positive airway pressure therapy improves vascular dysfunction and decreases oxidative stress in patients with the metabolic syndrome and obstructive sleep apnea syndrome. Clin Cardiol. 2012;35(4):231–236. doi: 10.1002/clc.21010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schulz R., Mahmoudi S., Hattar K. Enhanced release of superoxide from polymorphonuclear neutrophils in obstructive sleep apnea. Impact of continuous positive airway pressure therapy. Am J Respir Crit Care Med. 2000;162(2 Pt 1):566–570. doi: 10.1164/ajrccm.162.2.9908091. [DOI] [PubMed] [Google Scholar]
- 20.Svatikova A., Wolk R., Lerman L.O. Oxidative stress in obstructive sleep apnoea. Eur Heart J. 2005;26(22):2435–2439. doi: 10.1093/eurheartj/ehi440. [DOI] [PubMed] [Google Scholar]
- 21.Alonso-Fernández A., García-Río F., Arias M.A. Effects of CPAP on oxidative stress and nitrate efficiency in sleep apnoea: a randomised trial. Thorax. 2009;64(7):581–586. doi: 10.1136/thx.2008.100537. [DOI] [PubMed] [Google Scholar]
- 22.Vlachantoni I.-T., Dikaiakou E., Antonopoulos C.N., Stefanadis C., Daskalopoulou S.S., Petridou E.T. Effects of continuous positive airway pressure (CPAP) treatment for obstructive sleep apnea in arterial stiffness: a meta-analysis. Sleep Med Rev. 2013;17(1):19–28. doi: 10.1016/j.smrv.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Lima AMJ de, Franco C.M.R., Castro CMMB de, Bezerra A. de A., Ataíde L., Halpern A. Effects of nasal continuous positive airway pressure treatment on oxidative stress and adiponectin levels in obese patients with obstructive sleep apnea. Respir Int Rev Thorac Dis. 2010;79(5):370–376. doi: 10.1159/000227800. [DOI] [PubMed] [Google Scholar]
- 24.Kartali N., Daskalopoulou E., Geleris P. The effect of continuous positive airway pressure therapy on blood pressure and arterial stiffness in hypertensive patients with obstructive sleep apnea. Sleep Breath Schlaf Atm. 2014;18(3):635–640. doi: 10.1007/s11325-013-0926-0. [DOI] [PubMed] [Google Scholar]
- 25.Christou K., Kostikas K., Pastaka C., Tanou K., Antoniadou I., Gourgoulianis K.I. Nasal continuous positive airway pressure treatment reduces systemic oxidative stress in patients with severe obstructive sleep apnea syndrome. Sleep Med. 2009;10(1):87–94. doi: 10.1016/j.sleep.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Bakker J.P., Campbell A.J., Neill A.M. Pulse wave analysis in a pilot randomised controlled trial of auto-adjusting and continuous positive airway pressure for obstructive sleep apnoea. Sleep Breath Schlaf Atm. 2011;15(3):325–332. doi: 10.1007/s11325-010-0385-9. [DOI] [PubMed] [Google Scholar]
- 27.Drager L.F., Bortolotto L.A., Figueiredo A.C., Krieger E.M., Lorenzi G.F. Effects of continuous positive airway pressure on early signs of atherosclerosis in obstructive sleep apnea. Am J Respir Crit Care Med. 2007;176(7):706–712. doi: 10.1164/rccm.200703-500OC. [DOI] [PubMed] [Google Scholar]
- 28.Ashraf F., Tracy R., Li H., Auckley D., Patel S., Walia H., Mehra R. Effects of obstructive sleep apnea treatment on systemic inflammation: results of the sleep apnea stress study (SASS) randomized controlled trial (oral presentation) Annual Meeting of Associated Professional Sleep Societies. 2014 [Google Scholar]
- 29.Paz y Mar H., Li H., Auckley D., Patel S., Walia H., Strohl K., Mehra R. Effects of continuous positive airway pressure on measures of arterial stiffness in obstructive sleep apnea: Results of the sleep apnea stress study randomized controlled trial (oral presentation) Annual Meeting of Associated Professional Sleep Societies. 2014 [Google Scholar]
- 30.Brennan M.-L., Penn M.S., Van Lente F. Prognostic value of myeloperoxidase in patients with chest pain. N Engl J Med. 2003;349(17):1595–1604. doi: 10.1056/NEJMoa035003. [DOI] [PubMed] [Google Scholar]
- 31.Oxidative Stress/Inflammation and Heart, Lung, Blood, and Sleep Disorders. 2011. National Heart, Lung, and Blood Institute website. http://www.nhlbi.nih.gov/research/reports/2004-oxidative-stress. Accessed April 12, 2016.
- 32.Chirinos J.A., Kips J.G., Jacobs D.R. Arterial wave reflections and incident cardiovascular events and heart failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60(21):2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chirinos J.A., Zambrano J.P., Chakko S. Aortic pressure augmentation predicts adverse cardiovascular events in patients with established coronary artery disease. Hypertension. 2005;45(5):980–985. doi: 10.1161/01.HYP.0000165025.16381.44. [DOI] [PubMed] [Google Scholar]
- 34.Sivam S., Witting P.K., Hoyos C.M. Effects of 8 weeks of CPAP on lipid-based oxidative markers in obstructive sleep apnea: a randomized trial. J Sleep Res. 2015;24(3):339–345. doi: 10.1111/jsr.12271. [DOI] [PubMed] [Google Scholar]
- 35.Stradling J.R., Schwarz E.I., Schlatzer C. Biomarkers of oxidative stress following continuous positive airway pressure withdrawal: data from two randomised trials. Eur Respir J. 2015;46(4):1065–1071. doi: 10.1183/09031936.00023215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoffmann M.S., Singh P., Wolk R., Narkiewicz K., Somers V.K. Obstructive sleep apnea and intermittent hypoxia increase expression of dual specificity phosphatase 1. Atherosclerosis. 2013;231(2):378–383. doi: 10.1016/j.atherosclerosis.2013.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gillebert T.C., Lew W.Y. Influence of systolic pressure profile on rate of left ventricular pressure fall. Am J Physiol. 1991;261(3 Pt 2):H805–H813. doi: 10.1152/ajpheart.1991.261.3.H805. [DOI] [PubMed] [Google Scholar]
- 38.Kobayashi S., Yano M., Kohno M. Influence of aortic impedance on the development of pressure-overload left ventricular hypertrophy in rats. Circulation. 1996;94(12):3362–3368. doi: 10.1161/01.cir.94.12.3362. [DOI] [PubMed] [Google Scholar]
- 39.Phillips C., Hedner J., Berend N., Grunstein R. Diurnal and obstructive sleep apnea influences on arterial stiffness and central blood pressure in men. Sleep. 2005;28(5):604–609. doi: 10.1093/sleep/28.5.604. [DOI] [PubMed] [Google Scholar]
- 40.Hoyos C.M., Yee B.J., Wong K.K., Grunstein R.R., Phillips C.L. Treatment of Sleep Apnea With CPAP Lowers Central and Peripheral Blood Pressure Independent of the Time-of-Day: a randomized controlled study. Am J Hypertens. 2015;28(10):1222–1228. doi: 10.1093/ajh/hpv023. [DOI] [PubMed] [Google Scholar]
- 41.Morris A.A., Patel R.S., Binongo J.N.G. Racial differences in arterial stiffness and microcirculatory function between Black and White Americans. J Am Heart Assoc. 2013;2(2):e002154. doi: 10.1161/JAHA.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Park J.B., Ha J.-W., Jung H.-O., Rhee M.-Y. FOCUS Investigators. Randomized trial comparing the effects of a low-dose combination of nifedipine GITS and valsartan versus high-dose monotherapy on central hemodynamics in patients with inadequately controlled hypertension: FOCUS study. Blood Press Monit. 2014;19(5):294–301. doi: 10.1097/MBP.0000000000000061. [DOI] [PubMed] [Google Scholar]
- 43.Kohler M., Pepperell J.C.T., Casadei B. CPAP and measures of cardiovascular risk in males with OSAS. Eur Respir J. 2008;32(6):1488–1496. doi: 10.1183/09031936.00026608. [DOI] [PubMed] [Google Scholar]
- 44.Litvin A.Y., Sukmarova Z.N., Elfimova E.M. Effects of CPAP on “vascular” risk factors in patients with obstructive sleep apnea and arterial hypertension. Vasc Health Risk Manag. 2013;9:229–235. doi: 10.2147/VHRM.S40231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jones A., Vennelle M., Connell M. The effect of continuous positive airway pressure therapy on arterial stiffness and endothelial function in obstructive sleep apnea: a randomized controlled trial in patients without cardiovascular disease. Sleep Med. 2013;14(12):1260–1265. doi: 10.1016/j.sleep.2013.08.786. [DOI] [PubMed] [Google Scholar]
- 46.Kohler M., Craig S., Pepperell J.C.T. CPAP improves endothelial function in patients with minimally symptomatic OSA: results from a subset study of the MOSAIC trial. Chest. 2013;144(3):896–902. doi: 10.1378/chest.13-0179. [DOI] [PubMed] [Google Scholar]
- 47.Sarwar N., Butterworth A.S. IL6R Genetics Consortium Emerging Risk Factors Collaboration. Interleukin-6 receptor pathways in coronary heart disease: a collaborative meta-analysis of 82 studies. Lancet. 2012;379(9822):1205–1213. doi: 10.1016/S0140-6736(11)61931-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meléndez G.C., McLarty J.L., Levick S.P., Du Y., Janicki J.S., Brower G.L. Interleukin 6 mediates myocardial fibrosis, concentric hypertrophy, and diastolic dysfunction in rats. Hypertension. 2010;56(2):225–231. doi: 10.1161/HYPERTENSIONAHA.109.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kobara M., Noda K., Kitamura M. Antibody against interleukin-6 receptor attenuates left ventricular remodelling after myocardial infarction in mice. Cardiovasc Res. 2010;87(3):424–430. doi: 10.1093/cvr/cvq078. [DOI] [PubMed] [Google Scholar]
- 50.Nian M., Lee P., Khaper N., Liu P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ Res. 2004;94(12):1543–1553. doi: 10.1161/01.RES.0000130526.20854.fa. [DOI] [PubMed] [Google Scholar]
- 51.Mehra R., Storfer-Isser A., Kirchner H.L. Soluble interleukin 6 receptor: A novel marker of moderate to severe sleep-related breathing disorder. Arch Intern Med. 2006;166(16):1725–1731. doi: 10.1001/archinte.166.16.1725. [DOI] [PubMed] [Google Scholar]
- 52.Arnardottir E.S., Lim D.C., Keenan B.T. Effects of obesity on the association between long-term sleep apnea treatment and changes in interleukin-6 levels: the Icelandic Sleep Apnea Cohort. J Sleep Res. 2015;24(2):148–159. doi: 10.1111/jsr.12252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kritikou I., Basta M., Vgontzas A.N. Sleep apnoea, sleepiness, inflammation and insulin resistance in middle-aged males and females. Eur Respir J. 2014;43(1):145–155. doi: 10.1183/09031936.00126712. [DOI] [PubMed] [Google Scholar]
- 54.Arias M.A., García-Río F., Alonso-Fernández A. CPAP decreases plasma levels of soluble tumour necrosis factor-alpha receptor 1 in obstructive sleep apnoea. Eur Respir J. 2008;32(4):1009–1015. doi: 10.1183/09031936.00007008. [DOI] [PubMed] [Google Scholar]
- 55.Stradling J.R., Craig S.E., Kohler M. Markers of inflammation: data from the MOSAIC randomised trial of CPAP for minimally symptomatic OSA. Thorax. 2015;70(2):181–182. doi: 10.1136/thoraxjnl-2014-205958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.