Abstract
Background
Fatigue is a common symptom in patients with pulmonary arterial hypertension (PAH); however, the impact of fatigue on daily physical activity in PAH is unknown. Accelerometry is a validated measure for assessing physical activity. We hypothesized that patients with PAH reporting higher levels of fatigue would have lower daily physical activity measured by accelerometry.
Methods
We performed a prospective cohort study of 15 women with PAH. On day 1, subjects completed the Multidimensional Fatigue Inventory (MFI), the United States Cambridge Pulmonary Hypertension Outcome Review (US CAMPHOR), and a 6-min walk test. Subjects wore the accelerometer on their dominant hip and completed an activity diary for 7 days. On day 15, subjects repeated the MFI and the US CAMPHOR, and then wore the accelerometer and completed an activity diary for an additional 7 days. All multivariate analyses were adjusted for age, BMI, and PAH type.
Results
The mean age was 50.5 years, and 53% had idiopathic or heritable PAH. During the 2 weeks, subjects were mostly sedentary (85% of the time), although 10% of their time was spent performing low-level activity. Lower average daily counts were associated with worse self-reported energy levels, whereas less day-to-day physical activity variability was associated with more self-reported mental fatigue, physical fatigue, and total activity. Higher percentage of activity bouts was also associated with worse energy.
Conclusions
Women with PAH may spend most of their time being sedentary, and lower self-reported energy levels are associated with less daily activity. Interventions to improve symptoms such as fatigue may also increase physical activity levels in PAH.
Key Words: accelerometry, fatigue, physical activity, pulmonary arterial hypertension
Abbreviations: 6MWD, 6-min walk distance; HRQOL, health-related quality of life; ICC, intraclass correlation coefficient; MET, metabolic equivalent; MFI, Multidimensional Fatigue Inventory; PAH, pulmonary arterial hypertension; US CAMPHOR, United States Cambridge Pulmonary Hypertension Outcome Review; VM, vector magnitude
Pulmonary arterial hypertension (PAH) is a chronic disease affecting primarily middle age women.1 Patients with PAH report multiple symptoms including dyspnea, fatigue, and chest discomfort, along with exercise limitation.2 Fatigue is a multidimensional symptom defined as an overwhelming, debilitating, and sustained sense of exhaustion that decreases one’s ability to carry out daily activities, to work effectively, and to function.3 Fatigue contains overlapping components that are physical, cognitive, and affective.4 Physically, patients feel they do not have the energy to perform activities. Cognitively, patients may have difficulty concentrating and may lack motivation to participate in activities. More than 90% of patients with PAH report fatigue that interferes with their life.2 In addition, patients with PAH have diminished health-related quality of life (HRQOL) that is associated with fatigue.5 Not only is it commonly reported, but fatigue is also associated with dyspnea and psychological distress and can interfere with cognitive function6 and negatively affect functional status in PAH.5
Despite the pervasiveness in PAH, little is known about the impact of self-reported fatigue on physical activity. The 6-min walk distance (6MWD) is commonly used in the clinical setting as a measure of functional exercise capacity or the ability to perform activities of daily living that require sustained aerobic metabolism.7 The 6MWD reflects physical fitness but may not necessarily reflect physical activity in daily life, which may vary markedly day to day. The 6MWD is strongly associated with survival in PAH, but it is not an adequate surrogate end point.8
Physical activity is defined as body movements requiring more energy than resting that also enhances health.9 One of the quantitative metrics for activity levels is metabolic equivalents (METs). Activity levels range from minimal/sedentary (1.0-1.5 METs, eg, watching television); low intensity (1.6-3.0 METs, eg, folding laundry); moderate intensity (3.1-6.0 METs, eg, food shopping); vigorous (6.1- 9.0 METs, eg, jogging); and very vigorous (> 9.0 METs, eg, rollerblading).10 Accurate measurement of METs requires labor-intensive in-laboratory exercise testing, although accelerometry is a validated and objective measure for assessing physical activity in free-living environments.11, 12 In small studies of other chronic conditions, self-reported fatigue was inversely associated with decreased accelerometer-measured physical activity.13, 14 Investigators have found that patients with PAH are more sedentary15 compared with healthy individuals, but did not assess the association of physical activity with HRQOL or symptoms.16 We aimed to determine the reliability of measuring physical activity with an accelerometer, the association between self-reported fatigue and physical activity levels, and the association between physical activity levels and HRQOL in women with PAH.
Methods
Study Design and Subjects
We recruited a convenience sample of patients who were 18 years or older with PAH (idiopathic, heritable, or associated with connective tissue disease, congenital heart disease, drug/toxin use, or HIV) who were receiving targeted PAH therapy at a stable dose for at least 3 months. We excluded patients with chronic fatigue syndrome, sleep disorders, or major depression, or who were hospitalized or acutely ill. We also excluded subjects if they had gait limitations such as significant arthritis or excessive pain in joints, an orthopedic injury that limited mobility in the hip or knee, or neurologic conditions that affected balance. All patients were recruited from the Penn Medicine Pulmonary Hypertension/Pulmonary Vascular Disease Program. This study was approved by the University of Pennsylvania institutional review board (#817389) prior to subject enrollment.
Procedures
After providing informed consent, patients came to our center for a dedicated research visit. Sociodemographic information and medical history were collected. Transthoracic echocardiogram and N-terminal pro brain natriuretic peptide results were collected from the medical record if performed within the past 6 months. Subjects completed the Multidimensional Fatigue Inventory (MFI) and the United States Cambridge Pulmonary Hypertension Outcome Review (US CAMPHOR) questionnaires. Subjects also performed a 6MWD test. On the day of the research visit (after completing the other testing), subjects began wearing the accelerometer on their dominant hip. Subjects were instructed to wear the device during all waking hours and could remove the device for bathing/showering and sleeping. Subjects wore the accelerometer for days 1-7 (week 1) and completed an activity diary. They were instructed not to wear the accelerometer on days 8-14 (week 2). On day 15, subjects again completed the MFI and the US CAMPHOR and wore the accelerometer for an additional 7 days along with completing the activity diary (week 3). On day 21, the subjects returned the accelerometer, questionnaires, and the activity diary with a self-addressed, prepaid envelope. Other studies have found improved week-to-week reliability when accelerometry data are collected between 8 and 12 hours and 3 and 5 days.17
Accelerometry
Physical activity was measured by the ActiGraph wGT3X-BT monitor (ActiGraph Corp), a lightweight device (19 g) with dimensions of 4.6 cm × 3.3 cm × 1.5 cm that contains a rechargeable lithium battery with a recording capacity of approximately 25 days (Fig 1). The capacity for data storage is 120 days or 2 GB. The device is water resistant with sustained performance after submersion at 1 m for up to 30 min. The wGT3X-BT uses a solid-state triaxial accelerometer to collect data in three axes—vertical, anteroposterior, and mediolateral—at a frequency of 30 Hz. All three axes provide activity counts to compute a composite vector magnitude (VM).18, 19 Published validation studies of the wGT3X-BT indicate the device accurately measures physical activity.18 The intraclass correlation coefficient (ICC) across frequencies and all three axes combined in a previous study was 0.97 (P < .001).20
Figure 1.

Accelerometer.
All devices were initialized with the patient research identification number prior to the subject wearing the device. Initialization and data download were performed using the ActiGraph software. Subjects began wearing the device on the day of enrollment. Days with less than 2 hours of qualified wear time data identified by ActiLife software were discarded. The average (± SD) daily wear time was 10.72 ± 3.74 hours for week 1 and 11.88 ± 2.50 for week 3. The average intensity (sedentary, low, moderate, vigorous, or very vigorous) and standard deviation of the composite VM were the primary variables used in the analysis. We also analyzed bouts of being active, defined as episodes of continuous activity with VM levels greater than 40 (90th percentile).
Activity Diary
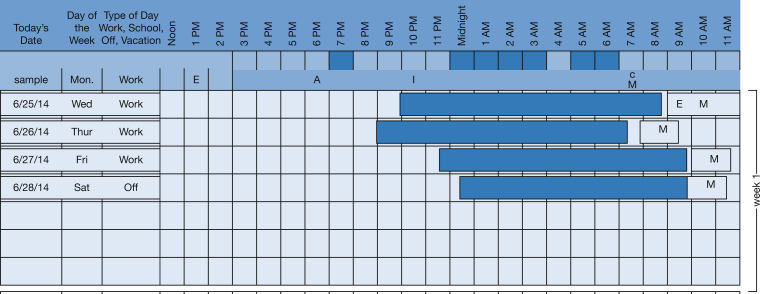
The activity diary included the date and day of each week with hours of the day. Subjects shaded in the areas when they were asleep. They also indicated consumption of caffeine, medication administration, and when they performed intentional exercise by marking this on the activity diary. Figure 2 presents a sample activity diary.
Figure 2.
Sample activity diary. Subjects shade in the corresponding blocks when they were sleeping. A= alcohol; E= exercise; M= medication.
Questionnaires
The MFI is a self-administered instrument containing 20 items that measures five subscales covering the dimensionality of fatigue: general fatigue, physical fatigue, reduced activity, reduced motivation, and mental fatigue.21 Each of the subscales contains four items. Responses are on a Likert scale ranging from “yes, that is true” to “no, that is not true.” Subjects mark the box that most closely relates to “how they have been feeling lately.” Each item in the subscale ranges from 1 to 5. The total subscale score ranges from 4 to 20, with a higher score indicating increased fatigue.
The HRQOL is defined as an individual’s perception of her physical and psychological well-being.22 The US CAMPHOR has three scales that measure total symptoms, activity, and quality of life along with three symptom subscales—energy, breathlessness, and mood.23 The US CAMPHOR is self-administered and takes approximately 10 to 15 min to complete. Higher scores indicate worse quality of life. The total summed symptom scale ranges from 0 to 25. The energy subscale scores range from 0 to 10; the breathlessness subscale scores range from 0 to 9; and the mood subscale scores range from 0 to 7. An example of an energy question is “I’m tired all the time.” The breathlessness subscales asks “I get out of breath when I walk,” and the mood subscale asks “I get very down.” The quality-of-life scale also contains 25 negatively weighted items that are scored the same, with scores ranging from 0 to 25. The activity scale contains 15 items. Activities are related to the ability of the person to perform “on your own without difficulty,” scored 0; “able to do on your own with difficulty,” scored 1; “unable to do on your own,” scored 2. Summed activity scores range from 0 to 30, higher scores indicate worse disability.
6-Min Walk Test
The 6-min walk test was performed indoors along a hard, flat corridor 30 m in length according to the American Thoracic Society guidelines to measure functional exercise capacity.24 Moderate to high relationships between the 6MWD and peak oxygen consumption via maximal exercise testing have been reported (r = 0.56-0.88).25, 26 Test-retest reliability is also high and has been reported as an ICC of 0.90.27 The technician was blinded to other subject information.
Data Analysis
Continuous variables are displayed as mean ± SD or median (interquartile range), and categorical variables are shown as number (percentage). Differences in physical activity scores (accelerometry) between the two weeks were analyzed using generalized estimating equation28 models with adjustment for age and total wear time. We used ICCs to assess the reliability of week 1 compared with week 3 physical activity levels. Paired t tests assessed the differences between week 1 and week 3 MFI and US CAMPHOR results.
Bivariate analysis included crude pairwise correlations between physical activity scores, self-reported fatigue (MFI), and HRQOL (US CAMPHOR) averaged across the two weeks. The physical activity scores included average counts per minute, the standard deviation of average daily counts, and the average proportion of active bouts. Generalized estimating equation models (accounting for the within-subject correlation of the two weeks of data) were used to evaluate the association of physical activity scores with self-reported fatigue (MFI: general, physical and mental subscales) and the US CAMPHOR total activity scores after adjusting for age, BMI, and PAH type (idiopathic/heritable vs associated). Probability values of less than .05 were considered statistically significant. For each of the physical activity measures, we conducted a two-step analysis. First, we conducted an initial screening by only including individual scales and basic demographics (age, BMI, and PAH type). We then built the multivariate model in a hierarchical order in which the most predictive questionnaire scores from the prescreening step were entered into the models first. The final model was selected to have the largest quasi-likelihood or the smallest quasi-likelihood under the independence model information criterion,29 while forcing age, BMI, and PAH type into the model.
Results
All 15 subjects completed the study. The mean age was 50.5 ±15.9 years, one third were non-Hispanic white, and 53% were diagnosed with idiopathic or heritable PAH (Table 1). Most subjects were taking phosphodiesterase type-5 inhibitors (60%) or endothelin receptor antagonists (53%), diuretics (53%), and anticoagulants (53%). Thirty-three percent required continuous intravenous prostacyclin analogue therapy (epoprostenol, n = 4; treprostinil, n = 1). Eighty percent of the sample was World Health Organization (WHO) functional class II, and the mean 6MWD was 412 ± 66.1 m.
Table 1.
Characteristics of Study Sample
| Characteristics | Value |
|---|---|
| Age, mean ± SD, y | 50.5 ± 15.9 |
| Female | 15 (100) |
| Race/ethnicity | |
| White (non-Hispanic) | 9 (60) |
| Black | 5 (33) |
| Other | 1 (7) |
| PAH etiology | |
| Idiopathic | 5 (33) |
| Connective tissue disease | 4 (27) |
| Heritable | 3 (20) |
| Congenital heart disease | 2 (13) |
| HIV | 1 (7) |
| Echocardiography (N = 14) | |
| Right ventricular systolic pressure, median (IQR), (n = 12), mm Hg | 78.5 (53.3-97.5) |
| Tricuspid annular plane systolic excursion, median (IQR), (n = 11), cm | 1.8 (1.6-2.6) |
| RV size | |
| Normal | 6 (43) |
| Mildly dilated | 1 (7) |
| Moderately dilated | 3 (21) |
| Severely dilated | 4 (29) |
| RV function | |
| Normal | 4 (29) |
| Mild dysfunction | 4 (29) |
| Moderate dysfunction | 6 (42) |
| Severe dysfunction | 0 |
| Tricuspid regurgitation | |
| None | 1 (7) |
| Mild | 5 (36) |
| Moderate | 7 (50) |
| Severe | 1 (7) |
| Right ventricular hypertrophy (n = 7) | |
| None | 4 (57) |
| Mild | 3 (43) |
| Pericardial effusion | |
| None | 12 (86) |
| Present | 2 (14) |
| Medications | |
| IV prostacyclin analogue | 5 (33) |
| Inhaled prostacyclin analogue | 2 (13) |
| Endothelin receptor antagonists | 8 (53) |
| PDE-5 inhibitors | 9 (60) |
| Diuretics | 8 (53) |
| Digoxin | 3 (20) |
| Anticoagulants | 8 (53) |
| WHO functional class | |
| Class II | 12 (80) |
| Class III | 3 (20) |
| BMI, mean ± SD, kg/m2 | 26.5 ± 4.2 |
| NT-pro BNP, mean ± SD, pg/mL (n = 11) | 216.0 (65.0-531.0) |
| 6 MWD, mean ± SD, m | 412.9 ± 66.1 |
Data are presented as No. (%) unless otherwise indicated. 6MWD = 6-min walk distance; IQR = interquartile range; NT-pro BNP = N-terminal pro brain natriuretic peptide; PAH = pulmonary arterial hypertension; PDE-5 = phosphodiesterase type 5; RV = right ventricular; WHO = World Health Organization.
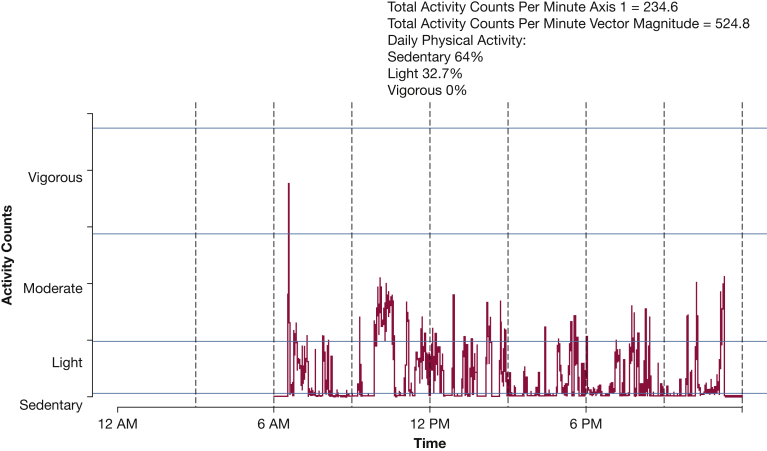
Subjects wore the accelerometer on average for 6.87 ± 1.1 days during week 1 and 6.55 ± 1.19 days during week 3. Figure 3 displays a sample of one subject’s daily accelerometry output. We first assessed the reliability of weekly physical activity (VM) between week 1 and week 3. The reliability of the axis 1 weekly average daily activity (ICC, 0.87; 95% CI, 0.66-0.95) was very high compared with good reliability of the VM weekly average daily activity (ICC, 0.70; 95% CI, 0.30-0.89). There were no significant differences in average counts per minute or in any other estimate of activity between week 1 and week 3 (Table 2). The average time spent being sedentary was 85%, and the average time spent performing moderate (or more intense) activity was only 2% to 3% for the sample. We did not observe an association between average daily temperature and physical activity (data not shown) during study enrollment between December 2013 and September 2014.
Figure 3.
Single day accelerometry data from a patient with World Health Organization functional class II pulmonary arterial hypertension.
Table 2.
Comparison of Week 1 and Week 3 Activity Levelsa and Wear Time
| Variable | Week 1 | Week 3 | P Valueb | ICC (95% CI) |
|---|---|---|---|---|
| Average counts/min (VM) | 379.3 ± 125.0 | 403.6 ± 165.8 | .91 | 0.70 (0.30-0.89) |
| Average counts/min (axis 1) | 172.9 ± 71.0 | 175.7 ± 63.3 | .90 | 0.87 (0.66-0.95) |
| % Time sedentary | 84.1 ± 9.7 | 85.3 ± 7.3 | .58 | 0.35 (−0.20 to 0.73) |
| % Low activity | 9.8 ± 5.1 | 10.8 ± 6.1 | .67 | 0.91 (0.74-0.97) |
| % Moderate activity | 2.4 ± 1.6 | 2.5 ± 1.3 | .82 | 0.90 (0.72-0.97) |
| % Vigorous activity | 0.2 ± 0.1 | 0.2 ± 0.1 | .83 | 0.89 (0.70-0.96) |
| % Very vigorous activity | 0.1 ± 0.05 | 0.05 ± 0.05 | .51 | 0.82 (0.53-0.94) |
| Sedentary time, min | 610.6 ± 120.6 | 639.7 ± 153.9 | .35 | 0.20 (−0.35 to 0.65) |
| Low activity time, min | 73.1 ± 48.7 | 76.3 ± 56.0 | .96 | 0.93 (0.79-9.98) |
| Moderate activity time, min | 17.7 ± 12.5 | 16.7 ± 9.9 | .49 | 0.64 (0.18-0.87) |
| Vigorous activity time, min | 1.3 ± 1.0 | 1.2 ± 1.0 | .70 | 0.96 (0.88-0.99) |
| Very vigorous activity time, min | 0.4 ± 0.4 | 0.5 ± 0.5 | .65 | 0.52 (0.01-0.82) |
| Total wear time, min/d | 643.3 ± 224.8 | 712.8 ± 150.2 | .94 | 0.23 (−0.32 to 0.67) |
Data are presented as mean ± SD. ICC = intraclass correlation coefficient; MET = metabolic equivalent; VM = vector magnitude.
Activity levels are defined as follows: sedentary = 1.0-1.5 METs; low intensity = 1.6-3.0 METs; moderate intensity = 3.1-6.0 METs; vigorous intensity = 6.0-9.0 METs; very vigorous intensity >9.0 METs.
Probability value for differences between week 1 and week 2 activity levels from generalized estimating equation model adjusted for age and total wear time.
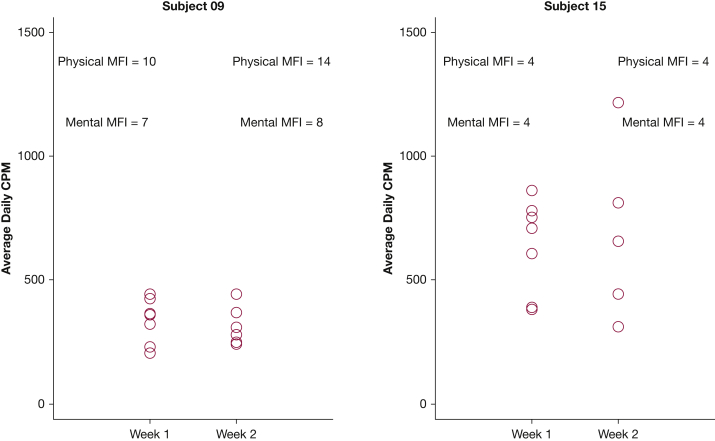
For the MFI subscales, the general fatigue and physical fatigue had the highest scores, indicating increased fatigue levels. The MFI subscales did not differ between the two weeks (Table 3). The US CAMPHOR total symptom scores, the total activity scores, and the quality of life scores were not significantly different between week 1 and week 3 (Table 3). The 6MWD was positively associated with physical activity bouts (r = 0.64; P = .011), and older age was significantly associated with reduced day-to-day variability in physical activity (r = −0.61; P = .015) and may have been associated with less daily physical activity (r = −0.46; P = .083) and fewer physical activity bouts (r = −0.48; P = .068), but these findings were not statistically significant. In multivariate analysis adjusted for age, BMI, and PAH type (idiopathic/heritable vs associated), worse energy scores (US CAMPHOR) were associated with lower average activity levels (estimate, −13.965; P = .013), whereas the fatigue scores (MFI) were not (Table 4). Total quality of life, symptoms, activity, and breathlessness by the US CAMPHOR were also not significantly associated with daily activity counts. Alternatively, greater self-reported mental fatigue (MFI) (estimate, −18.92; P < .001) and physical fatigue (MFI) (estimate, −7.65; P = .007) were independently associated with less variability in day-to-day physical activity. Worse total activity (US CAMPHOR) (estimate, 12.78; P < .001) was associated with increased day-to-day physical activity variability (Table 5), and worse energy (US CAMPHOR) was associated with fewer activity bouts (estimate, −0.24; P = .002) (Table 6). Figure 4 illustrates the daily average activity counts over the two weeks for two subjects. Subject 09 has lower variations in activity from one day to the next, whereas subject 15 has a much larger range of average daily activity (300 to 1200 counts/min). Their mental fatigue (MFI) and physical fatigue (MFI) show an inverse relationship with the activity variations as reported in the multivariate analysis.
Table 3.
MFI and US CAMPHOR Scores for Symptoms, Activity, and Quality of Life Scores and ICC
| Variable | Week 1 | Week 3 | P Valuea | ICC (95% CI) |
|---|---|---|---|---|
| MFIb | ||||
| General fatigue | 10.9 ± 3.6 | 11.3 ± 3.8 | .51 | 0.89 (0.68-0.96) |
| Physical fatigue | 10.5 ± 3.9 | 10.9 ± 4.1 | .51 | 0.91 (0.74-0.97) |
| Reduced activity | 9.9 ± 4.2 | 9.9 ± 4.6 | .94 | 0.82 (0.46-0.94) |
| Reduced motivation | 8.5 ± 3.2 | 8.7 ± 3.7 | .77 | 0.84 (0.53-0.96) |
| Mental fatigue | 7.3 ± 3.3 | 7.2 ± 2.9 | .78 | 0.90 (0.71-0.97) |
| US CAMPHORc | ||||
| Total symptom | 5.7 ± 4.9 | 6.6 ± 5.6 | .43 | 0.82 (0.46-0.94) |
| Energy | 2.3 ± 2.6 | 2.5 ± 3.2 | .72 | 0.86 (0.58-0.93) |
| Breathlessness | 2.2 ± 1.7 | 2.3 ± 1.6 | .85 | 0.81 (0.42-0.94) |
| Mood | 1.2 ± 1.4 | 1.8 ± 2.1 | .19 | 0.70 (0.12-0.90) |
| Total activity | 6.1 ± 4.5 | 7.3 ± 4.5 | .01 | 0.98 (0.94-0.99) |
| Quality of life | 3.7 ± 4.5 | 4.7 ± 6.0 | .32 | 0.88 (0.63-0.96) |
Data are presented as mean ± SD. MFI = Multidimensional Fatigue Inventory; US CAMPHOR = United States Cambridge Pulmonary Hypertension Outcome. See Table 2 legend for expansion of other abbreviation.
Probability value for differences between week 1 and week 3 MFI and US CAMPHOR scores.
MFI scores range 4-20; higher scores indicate increased fatigue.
US CAMPHOR total symptom and quality of life scores range 0-25; energy scores range 0-10; breathlessness scores range 0-8; mood scores range 0-7; total activity scores range 0-30; higher scores indicate worse symptoms, activity, and quality of life.
Table 4.
Association Between Average Daily Counts Per Minute and Self-Report Fatigue, Quality of Life, and Activity Levels
| Variable | Individual Scales |
Multiple Scales |
||
|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | |
| Physical fatigue (MFI) | −9.329 | .21 | … | … |
| Mental fatigue (MFI) | −4.541 | .45 | … | … |
| General fatigue (MFI) | −8.293 | .24 | … | … |
| Total quality of life (US CAMPHOR) | −1.578 | .75 | … | … |
| Total activity (US CAMPHOR) | −0.27 | .96 | … | … |
| Total symptom (US CAMPHOR) | −4.643 | .09 | … | … |
| Breathlessness (US CAMPHOR) | −9.266 | .29 | … | … |
| Mood (US CAMPHOR) | −1.297 | .91 | … | … |
| Energy (US CAMPHOR) | −13.965 | .013 | −13.965 | .013 |
All models are adjusted for age, BMI, and pulmonary arterial hypertension type (idiopathic/ heritable vs associated).
See Table 3 legend for expansion of abbreviations.
Table 5.
Association Between Day-to-Day Physical Activity Variability With Self-Report Fatigue, Quality of Life, and Activity Levels
| Variable | Individual Scales |
Multiple Scales |
||
|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | |
| Physical fatigue (MFI) | −2.945 | .441 | −7.645 | .007 |
| Mental fatigue (MFI) | −9.551 | .008 | −18.923 | < .001 |
| General fatigue (MFI) | −3.831 | .303 | … | … |
| Total quality of life (US CAMPHOR) | −1.42 | .584 | … | … |
| Total activity (US CAMPHOR) | −1.308 | .575 | 12.778 | < .001 |
| Total symptom (US CAMPHOR) | −1.677 | .416 | … | … |
| Breathlessness (US CAMPHOR) | −3.346 | .482 | … | … |
| Mood (US CAMPHOR) | −6.81 | .316 | … | … |
| Energy (US CAMPHOR) | −2.467 | .554 | … | … |
All models are adjusted for age, BMI, and pulmonary arterial hypertension type (idiopathic/ heritable vs associated).
See Table 3 legend for expansion of abbreviations.
Table 6.
Association Between Percentage of Activity Bouts With Self-Report Fatigue, Quality of Life, and Activity Levels
| Variable | Individual Scales |
Multiple Scales |
||
|---|---|---|---|---|
| Estimate | P Value | Estimate | P Value | |
| Physical fatigue (MFI) | −0.242 | .083 | … | … |
| Mental fatigue (MFI) | −0.115 | .325 | … | … |
| General fatigue (MFI) | −0.09 | .485 | … | … |
| Total quality of life (US CAMPHOR) | −0.021 | .808 | … | … |
| Total activity (US CAMPHOR) | −0.002 | .989 | … | … |
| Total symptom (US CAMPHOR) | −0.079 | .022 | … | … |
| Breathlessness (US CAMPHOR) | −0.157 | .286 | … | … |
| Mood (US CAMPHOR) | −0.032 | .874 | … | … |
| Energy (US CAMPHOR) | −0.239 | .002 | −0.239 | .002 |
All models are adjusted for age, BMI, and pulmonary arterial hypertension type (idiopathic/ heritable vs associated).
See Table 3 legend for expansion of abbreviations.
Figure 4.
Comparison of average accelerometry counts per minute (cpm) and physical fatigue and mental fatigue levels measured by the Multidimensional Fatigue Inventory (MFI) obtained from 2 subjects.
Discussion
This is the first study to investigate the association between physical activity levels measured by accelerometry, self-reported fatigue, and HRQOL in patients with PAH. In a sample of 15 women with PAH, weekly physical activity levels were reliably measured for a period of 3 weeks. The vast majority of time throughout the day was spent being sedentary, whereas only 2% to 3% was spent performing moderate activity (6 to 51 min), well below the recommended 150 min per week for healthy adults.9 Symptoms such as low energy (US CAMPHOR) and fatigue (MFI) were associated with lower activity and less day-to-day variability in activity, respectively.
A study of test-retest reliability of accelerometry in community-dwelling adults showed ICCs for various algorithms from 0.70 to 0.90, similar to our estimates.30 The reliability for axis 1 weekly average daily activity (ICC, 0.87; 95% CI, 0.66-0.95) was very high, and there was good reliability for the VM weekly average daily activity (ICC, 0.70; 95% CI, 0.30-0.89), suggesting that measurement periods of at least a week would provide reproducible data in clinical studies and clinical trials in PAH using accelerometry. In healthy adults, test-retest reliability for standing after only 2 days was an ICC of 0.56 and for walking, an ICC of 0.79 to 0.94.31 This demonstrates the feasibility of using accelerometry to reliably assess physical activity levels in patients with PAH.
Average VM activity counts per minute for the subjects during the two weeks of measurement were 391.5 ± 144.8 for the sample. Average vertical axis activity counts per minute for the subjects ranged between 172.9 ± 71.0 and 175.7 ± 63.3, similar to those seen in another published study of accelerometry in PAH.16 Normative data from the National Health and Nutritional Examination Survey showed average activity counts per minute for women 60 to 69 years of 251 counts/min and 170 counts/min for those 70 years and older.32 Our PAH sample was younger, but had much lower activity counts, suggesting that women with PAH have very limited activity levels even compared with individuals from the general population more than two decades older.
Our sample showed less sedentary time (85%) compared with 92% from the prior study in PAH,16 but only 0.3% of time in each group was spent on vigorous or very vigorous activity. A meta-analysis of adults determined that sedentary time was independently associated with all-cause mortality, cardiovascular disease incidence or mortality, cancer incidence or mortality (breast, colon, endometrial), and diabetes.33 Increased physical activity in COPD is associated with lower hospital admissions and lower mortality.34 After hospital discharge, COPD patients with lower physical activity levels were more likely to be rehospitalized.35 Pugh and colleagues16 found those patients with PAH who were categorized as WHO functional class III/IV had significantly lower activity counts than WHO functional class I/II, demonstrating lower activity levels with increased disease severity. It is possible that increasing physical activity and being less sedentary in PAH could decrease morbidity and mortality in PAH, but further studies are necessary.
The level of physical activity, whether it is depicted by average daily counts per minute or the duration of being active (proportion of active bouts), is negatively correlated with self-reported energy (US CAMPHOR). The day-to-day variability in physical activity, however, is more correlated with MFI mental fatigue scores. The MFI mental fatigue subscale asks questions about how well the subject can concentrate on tasks and if her thoughts easily wander.21 A study including patients with depression showed day-to-day physical activity variability that was associated with depressive symptoms36; as depressive symptoms increased, physical activity decreased. A study including patients with multiple sclerosis found mental fatigue to be a significant barrier to engaging in physical activity,37 and physical activity was associated with fatigue, depression, and HRQOL38 Perhaps there are modifiable factors or interventions to reduce mental fatigue, thereby promoting physical activity. Energy conservation39, 40 may be a possible intervention to promote sustained day-to-day activity to avoid days where patients with PAH are not mentally and physically fatigued.
Self-reported activity measured by the US CAMPHOR was actually inversely associated with physical activity measured by accelerometry. Overall, patients with PAH did not report greatly impaired activity levels even though much of their time was spent being sedentary. Patients may not be able to judge physical activity impairment or low levels of activity and may overestimate their activity levels. Alternatively, there may be a problem with the validity of either the US CAMPHOR subscale or the accelerometry metrics.
Average daily counts and activity bouts were associated with the 6MWD; however, the two different measurement approaches gauge distinct domains of physical activity and exercise. The 6MWD is a commonly used test to assess functional exercise capacity24 in response to therapy and clinical improvement or worsening, which is a different metric than that assessed by accelerometry (daily activity). The 6MWD assesses exercise capacity, a test that approximates maximal oxygen consumption in PAH. Physical activity varies from day to day, and such assessment of daily activity likely means more to a patient than the 6-min walk. Real-time activity measures allow us to assess the time-dependent activity patterns in real life conditions that might not be captured by the 6-min walk test. The maximal oxygen consumption during the 6-min walk test is actually higher than that achieved on a cardiopulmonary bicycle exercise test (CPET) in patients with PAH,41 and the distance walked has a strong correlation between peak oxygen consumption from both the 6-min walk test and CPET.42 This test is considered an intermediate end point by regulatory bodies and has been the basis for approval for almost all therapies for PAH. The 6MWD is also strongly associated with survival in PAH, but we have recently shown that it is not an adequate surrogate end point.8 Alternatively, accelerometry is not dependent on factors such as height and is considered a better indicator of physical activity in daily life than the 6MWD. Physical activity differs from day to day, whereas exercise capacity is relatively constant. Assessment of daily activity may be more clinically meaningful to a patient than the 6-min walk (however, this is not yet definitive) and could provide a more pertinent outcome in studies of treatment for PAH. The variability in physical activity that is not explained by the 6MWD shows that these are related, but distinct, domains.
There are several limitations to this study. First, we used a small sample size of women in this pilot study to determine the feasibility of using accelerometry. Larger studies need to be performed. Second, although our eligibility criteria did not exclude men, PAH primarily affects women (80%), and differences in the physical activity patterns between women and men would be important to study. Third, although we have shown reliability of activity measurement for a short time, there could be variability in physical activity levels related to season and such variability could depend on climate.15 In a study of patients with PAH, there was a negative association between heat and humidity and physical activity.15 Other studies have shown variation in higher minimum daily temperature and longer day length with higher physical activity levels.43 Fourth, depression or depressed mood are associated with fatigue. Although the US CAMPHOR mood subscale asks questions about “mood swings” and whether the subject is feeling “down,” a validated instrument to measure depression or depressed mood to account for the potential confounder of depressive symptoms that can be associated with fatigue is needed. Fifth, we did not perform echocardiograms or collect hemodynamics and N-terminal pro brain natriuretic peptide levels at enrollment. Future studies will include these data.
Conclusions
In conclusion, we have shown relationships between physical activity levels measured by accelerometry and self-report fatigue levels (MFI) and HRQOL in PAH. We found that physical activity measured by accelerometry was relatively stable from week to week. Average daily physical activity levels were negatively associated with energy (US CAMPHOR). Day-to-day physical activity variability levels were negatively associated with mental fatigue and physical fatigue (MFI). When examining physical activity bouts, results showed a negative association with energy (US CAMPHOR). Future research will include a larger sample size that includes both women and men. Additionally, studies of energy conservation or other interventions to increase physical activity are needed to assess their efficacy in improving patient-reported outcomes such as symptoms of fatigue, dyspnea, and HRQOL.
Acknowledgments
Author contributions: L. A. M. had full access to all the data in the study and takes responsibility for the integrity of the data. She contributed to the study design and conception, and data analysis and interpretation. She drafted the first draft of the manuscript and approved the final version. H. S. contributed to the study design, data analysis, and interpretation. J. S. F., K. A. S., and A. V. contributed to the study design, data interpretation, and drafting of the manuscript. D. P., C. A.-C., and D. D. contributed to the study design, patient recruitment, acquisition of data, and manuscript preparation. H. I. P. contributed to the study design, patient recruitment, data analysis, and manuscript preparation. M. S. S. contributed to the study design and conception, data interpretation, and manuscript preparation. S. M. K. contributed to the study design and conception, data analysis and acquisition, and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following: L. A. M. has received advisory board fees. J. S. F. has received advisory board fees and institutional funding for the conduct of multicenter clinical trials from Actelion. D. D. has received advisory board fees. S. M. K. has received advisory board fees. None of these were associated with the conduct of this study. None declared (H. S., K. A. S., A. V., D. P., C. A.-C., H. I. P., M. S. S.).
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript.
Other contributions: Thank you to William Russell, RT, for performing the 6-min walk tests.
Footnotes
FUNDING/SUPPORT: This study was partially funded by the National Institutes of Health [grants K23 NR014885 and K24 HL103844], University Research Foundation, University of Pennsylvania, and by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health [grant UL1TR000003].
References
- 1.Badesch D.B., Raskob G.E., Elliott C.G. Pulmonary arterial hypertension: baseline characteristics from the REVEAL Registry. Chest. 2010;137(2):376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 2.Matura L.A., McDonough A., Carroll D.L. Cluster analysis of symptoms in pulmonary arterial hypertension: a pilot study. Eur J Cardiovasc Nurs. 2012;11(1):51–61. doi: 10.1177/1474515111429649. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D., Krishnan E., DeWitt E.M., Khanna P.P., Spiegel B., Hays R.D. Patient-Reported Outcomes Measurement Information System (PROMIS®)—The future of measuring patient reported outcomes in rheumatology. Arthritis Care Res (Hoboken) 2011;63(suppl 11):S486–S490. doi: 10.1002/acr.20581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Echteld M.A., Passchier J., Teunissen S., Claessen S., de Wit R., van der Rijt C.C. Multidimensional fatigue and its correlates in hospitalised advanced cancer patients. Eur J Cancer. 2007;43(6):1030–1036. doi: 10.1016/j.ejca.2007.01.024. [DOI] [PubMed] [Google Scholar]
- 5.Matura L.A., McDonough A., Carroll D.L. Health-related quality of life and psychological states in patients with pulmonary arterial hypertension. J Cardiovasc Nurs. 2014;29(2):178–184. doi: 10.1097/JCN.0b013e318275330d. [DOI] [PubMed] [Google Scholar]
- 6.Falk K., Swedberg K., Gaston-Johansson F., Ekman I. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. Eur J Cardiovasc Nurs. 2007;6(2):99–104. doi: 10.1016/j.ejcnurse.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 7.Arena R., Myers J., Williams M.A., American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Nursing Assessment of functional capacity in clinical and research settings: a scientific statement from the American Heart Association Committee on Exercise, Rehabilitation, and Prevention of the Council on Clinical Cardiology and the Council on Cardiovascular Nursing. Circulation. 2007;116(3):329–343. doi: 10.1161/CIRCULATIONAHA.106.184461. [DOI] [PubMed] [Google Scholar]
- 8.Gabler N.B., French B., Strom B.L. Validation of 6-minute walk distance as a surrogate end point in pulmonary arterial hypertension trials. Circulation. 2012;126(3):349–356. doi: 10.1161/CIRCULATIONAHA.112.105890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Haskell W.L., Lee I.-M., Pate R.R., American Heart Association Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081. doi: 10.1161/CIRCULATIONAHA.107.185649. [DOI] [PubMed] [Google Scholar]
- 10.Ainsworth B.E., Haskell W.L., Leon A.S. Compendium of physical activities: classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25(1):71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 11.van der Werf S.P., Prins J.B., Vercoulen J.H., van der Meer J.W., Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. J Psychosom Res. 2000;49(5):373–379. doi: 10.1016/s0022-3999(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 12.Landis C.A., Frey C.A., Lentz M.J., Rothermel J., Buchwald D., Shaver J.L. Self-reported sleep quality and fatigue correlates with actigraphy in midlife women with fibromyalgia. Nurs Res. 2003;52(3):140–147. doi: 10.1097/00006199-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Roscoe J.A., Morrow G.R., Hickok J.T. Temporal interrelationships among fatigue, circadian rhythm and depression in breast cancer patients undergoing chemotherapy treatment. Support Care Cancer. 2002;10(4):329–336. doi: 10.1007/s00520-001-0317-0. [DOI] [PubMed] [Google Scholar]
- 14.van der Werf S.P., Prins J.B., Vercoulen J.H.M.M., van der Meer J.W.M., Bleijenberg G. Identifying physical activity patterns in chronic fatigue syndrome using actigraphic assessment. J Psychosom Res. 2000;49(5):373–379. doi: 10.1016/s0022-3999(00)00197-5. [DOI] [PubMed] [Google Scholar]
- 15.Jehn M., Gebhardt A., Liebers U. Heat stress is associated with reduced health status in pulmonary arterial hypertension: a prospective study cohort. Lung. 2014;192(4):619–624. doi: 10.1007/s00408-014-9587-4. [DOI] [PubMed] [Google Scholar]
- 16.Pugh M.E., Buchowski M.S., Robbins I.M., Newman J.H., Hemnes A.R. Physical activity limitation as measured by accelerometry in pulmonary arterial hypertension. Chest. 2012;142(6):1391–1398. doi: 10.1378/chest.12-0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aadland E., Ylvisåker E. Reliability of objectively measured sedentary time and physical activity in adults. PLoS One. 2015;10(7):e0133296. doi: 10.1371/journal.pone.0133296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelly L.A., McMillan D.G., Anderson A., Fippinger M., Fillerup G., Rider J. Validity of actigraphs uniaxial and triaxial accelerometers for assessment of physical activity in adults in laboratory conditions. BMC Med Phys. 2013;13(1):5. doi: 10.1186/1756-6649-13-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Howe C.A., Staudenmayer J.W., Freedson P.S. Accelerometer prediction of energy expenditure: vector magnitude versus vertical axis. Med Sci Sports Exerc. 2009;41(12):2199–2206. doi: 10.1249/MSS.0b013e3181aa3a0e. [DOI] [PubMed] [Google Scholar]
- 20.Santos-Lozano A., Marín P.J., Torres-Luque G., Ruiz J.R., Lucía A., Garatachea N. Technical variability of the GT3X accelerometer. Med Eng Phys. 2012;34(6):787–790. doi: 10.1016/j.medengphy.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 21.Smets E.M., Garssen B., Bonke B., De Haes J.C. The Multidimensional Fatigue Inventory (MFI) psychometric qualities of an instrument to assess fatigue. J Psychosom Res. 1995;39(3):315–325. doi: 10.1016/0022-3999(94)00125-o. [DOI] [PubMed] [Google Scholar]
- 22.The World Health Organization Quality of Life Assessment (WHOQOL): development and general psychometric properties. Soc Sci Med. 1998;46(12):1569–1585. doi: 10.1016/s0277-9536(98)00009-4. [DOI] [PubMed] [Google Scholar]
- 23.Gomberg-Maitland M., Thenappan T., Rizvi K., Chandra S., Meads D.M., McKenna S.P. United States validation of the Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR) J Heart Lung Transplant. 2008;27(1):124–130. doi: 10.1016/j.healun.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 24.ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 25.Faggiano P., D’Aloia A., Gualeni A., Giordano A. Assessment of oxygen uptake during the six-minute walk test in patients with heart failure. Chest. 1997;111(4) doi: 10.1378/chest.111.4.1146. 1146-1146. [DOI] [PubMed] [Google Scholar]
- 26.Guyatt G.H., Thompson P.J., Berman L.B. How should we measure function in patients with chronic heart and lung disease? J Chronic Dis. 1985;38(6):517–524. doi: 10.1016/0021-9681(85)90035-9. [DOI] [PubMed] [Google Scholar]
- 27.Demers C., McKelvie R.S., Negassa A., Yusuf S. RESOLVD Pilot Study Investigators. Reliability, validity, and responsiveness of the six-minute walk test in patients with heart failure. Am Heart J. 2001;142(4):698–703. doi: 10.1067/mhj.2001.118468. [DOI] [PubMed] [Google Scholar]
- 28.Liang K.-Y., Zeger S.L. Longitudinal data analysis using generalized linear models. Biometrika. 1986:13–22. [Google Scholar]
- 29.Pan W. Akaike’s information criterion in generalized estimating equations. Biometrics. 2001;57(1):120–125. doi: 10.1111/j.0006-341x.2001.00120.x. [DOI] [PubMed] [Google Scholar]
- 30.Sirard J.R., Forsyth A., Oakes J.M., Schmitz K.H. Accelerometer test-retest reliability by data processing algorithms: results from the Twin Cities Walking Study. J Phys Act Health. 2011;8(5):668–674. doi: 10.1123/jpah.8.5.668. [DOI] [PubMed] [Google Scholar]
- 31.Moe-Nilssen R. Test-retest reliability of trunk accelerometry during standing and walking. Arch Phys Med Rehabil. 1998;79(11):1377–1385. doi: 10.1016/s0003-9993(98)90231-3. [DOI] [PubMed] [Google Scholar]
- 32.Troiano R.P., Berrigan D., Dodd K.W., Mâsse L.C., Tilert T., McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40(1):181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 33.Biswas A., Oh P.I., Faulkner G.E. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Aymerich J., Lange P., Benet M., Schnohr P., Antó J.M. Regular physical activity reduces hospital admission and mortality in chronic obstructive pulmonary disease: a population based cohort study. Thorax. 2006;61(9):772–778. doi: 10.1136/thx.2006.060145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chawla H., Bulathsinghala C., Tejada J.P., Wakefield D., ZuWallack R. Physical activity as a predictor of thirty-day hospital readmission after a discharge for a clinical exacerbation of chronic obstructive pulmonary disease. Ann Am Thorac Soc. 2014;11(8):1203–1209. doi: 10.1513/AnnalsATS.201405-198OC. [DOI] [PubMed] [Google Scholar]
- 36.Burton C., McKinstry B., Szentagotai Tătar A., Serrano-Blanco A., Pagliari C., Wolters M. Activity monitoring in patients with depression: A systematic review. J Affect Disord. 2013;145(1):21–28. doi: 10.1016/j.jad.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 37.Kayes N.M., McPherson K.M., Schluter P., Taylor D., Leete M., Kolt G.S. Exploring the facilitators and barriers to engagement in physical activity for people with multiple sclerosis. Disabil Rehabil. 2011;33(12):1043–1053. doi: 10.3109/09638288.2010.520801. [DOI] [PubMed] [Google Scholar]
- 38.Motl R.W., McAuley E., Snook E.M., Gliottoni R.C. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14(1):111–124. doi: 10.1080/13548500802241902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barsevick A.M., Dudley W., Beck S., Sweeney C., Whitmer K., Nail L. A randomized clinical trial of energy conservation for patients with cancer-related fatigue. Cancer. 2004;100(6):1302–1310. doi: 10.1002/cncr.20111. [DOI] [PubMed] [Google Scholar]
- 40.Mathiowetz V., Matuska K.M., Murphy M.E. Efficacy of an energy conservation course for persons with multiple sclerosis. Arch Phys Med Rehabil. 2001;82(4):449–456. doi: 10.1053/apmr.2001.22192. [DOI] [PubMed] [Google Scholar]
- 41.Deboeck G., Niset G., Vachiery J.-L., Moraine J.-J., Naeije R. Physiological response to the six-minute walk test in pulmonary arterial hypertension. Eur Respir J. 2005;26(4):667–672. doi: 10.1183/09031936.05.00031505. [DOI] [PubMed] [Google Scholar]
- 42.Mainguy V., Malenfant S., Neyron A.-S. Alternatives to the six-minute walk test in pulmonary arterial hypertension. PLoS One. 2014;9(8):e103626. doi: 10.1371/journal.pone.0103626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Witham M.D., Donnan P.T., Vadiveloo T. Association of day length and weather conditions with physical activity levels in older community dwelling people. PLoS One. 2014;9(1):e85331. doi: 10.1371/journal.pone.0085331. [DOI] [PMC free article] [PubMed] [Google Scholar]