Myeloproliferative neoplasms (MPNs) are diseases characterized by the pathologic expansion of myeloid cells of the hematopoietic lineage. The three ‘classical' MPNs include polycythemia vera (PV, increase in erythrocytes), essential thrombocythemia (ET, increase in platelets) and primary myelofibrosis (PMF, usually elevated platelet counts associated with fibrotic deposition in the bone marrow).1 MPNs are essentially clonal diseases driven by somatic mutations in hematopoietic stem and progenitor cells. So far, three genes have been identified that can drive the disease phenotype when mutated.2 Activating mutations in Janus Kinase 2 (JAK2) and the thrombopoietin receptor (MPL) have been known for close to a decade and their mechanism of action has been extensively studied.3, 4, 5, 6, 7, 8 Recently, we and others identified somatic mutations in the CALR gene in 25–35% of ET and PMF patients.9, 10
CALR encodes the calreticulin protein that functions as a chaperone in the endoplasmic reticulum (ER).11 Calreticulin performs critical quality control functions by binding the sugar residues of N-glycosylated, immature and unfolded proteins, preventing their trafficking to the Golgi and allowing folding mechanisms to operate.12 Moreover, the negatively charged C-terminal end of calreticulin allows it to bind calcium ions and act as a calcium buffer in the ER, thereby playing an important role in calcium-mediated intracellular signaling.11
The CALR mutations associated with MPNs occur exclusively in the last exon of the gene (exon 9). These mutations are insertions and/or deletions that result in a ‘frameshift' to a specific alternative reading frame, leading to the synthesis of a novel C-terminal peptide in the mutants that consists predominantly of positively charged amino acids. Despite the considerable heterogeneity of CALR mutations at the deoxyribonucleic acid (DNA) level, the translation from the alternative reading frame results in a relatively uniform C-terminal amino-acid sequence of the mutant CALR protein. We have previously shown that expression of the most prevalent mutant CALR (del52) can induce cytokine independence in Ba/F3 cells. This is associated with JAK2-mediated constitutive activation of the signal transducer and activator of transcription 5 (STAT5), which is the same signaling pathway activated by the other mutated genes driving the MPN phenotype—JAK2 and MPL.9
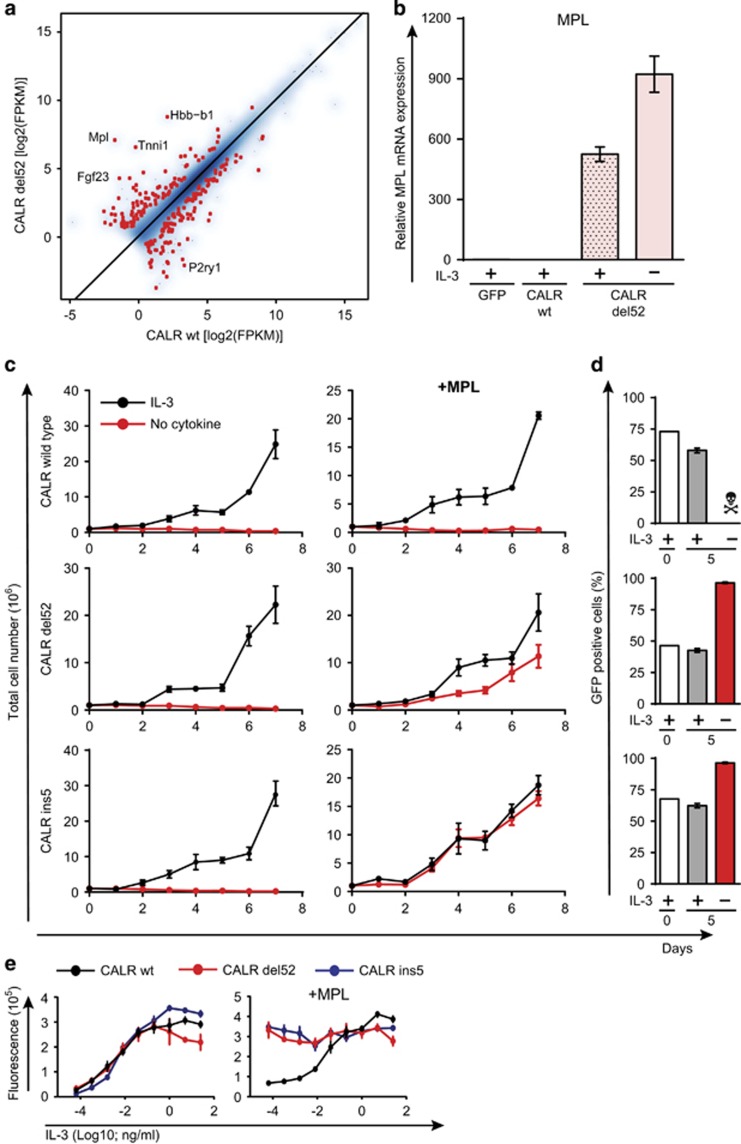
To understand the mechanism of action of mutant CALR and to identify the most differentially expressed genes, we performed gene expression analysis by transcriptome sequencing of the previously published parental Ba/F3 cell lines.9 Strikingly, parental Ba/F3 cells transformed by the expression of CALR-del52 showed high levels of murine endogenous c-mpl mRNA expression (Figure 1a). This was confirmed by quantitative polymerase chain reaction (PCR) analysis of c-mpl expression in these cells (Figure 1b). However, in further experiments, transduction of parental Ba/F3 cells with the mutant CALR did not lead to upregulation of c-mpl expression or cytokine independence. This implied that the CALR mutant does not induce the transcription of the thrombopoietin receptor and the upregulation observed previously was purely a stochastic event. Therefore, we hypothesized that the oncogenic activity of the mutant CALR is dependent on the thrombopoietin receptor (MPL). Indeed, cotransduction of cells with retroviruses expressing mutant CALR (Type 1 and Type 2) and human MPL resulted in consistent transformation of the cells. Overexpression of wild-type CALR did not induce any cytokine independence in the cells even in the presence of MPL (Figure 1c). Moreover, cells expressing both MPL (retrovirus with green fluorescent protein marker) and a mutant CALR had a clear selective advantage over cells expressing only the mutant CALR, when cultured in IL-3-free medium (Figure 1d). This is also evident in dose−response curves of the double-transduced cells to increasing concentrations of IL-3 (Figure 1e).
Figure 1.
Presence of MPL is required for Ba/F3 transformation by CALR mutants. (a) Correlation of expression values (FPKM) between Ba/F3 cells retrovirally transduced with CALR wild type (wt) and del52 mutant. Differentially expressed genes are highlighted in red. The top five differentially expressed genes are labeled. (b) qPCR analysis of c-mpl expression levels in Ba/F3 cells retrovirally transduced with CALR wt and del52 mutant. A total of 106 cells (transduced and selected with puromycin) were seeded, in triplicates, in medium with or without IL-3 (1 ng/ml). The total number of live cells was counted everyday for 1 week. (c) Growth curves of Ba/F3 cells retrovirally transduced with CALR wt and mutants, individually or with MPL, in the presence and absence of IL-3 (1 ng/ml). (d) Flow cytometric analysis of the percentage of GFP (MPL)-positive cells after transduction in the bulk culture (day 0) and after 5 days in medium with or without IL-3 (1 ng/ml). (e) Dose−response curve, to increasing concentrations of IL-3, of Ba/F3 cells retrovirally transduced with CALR wt and mutants, individually or with MPL.
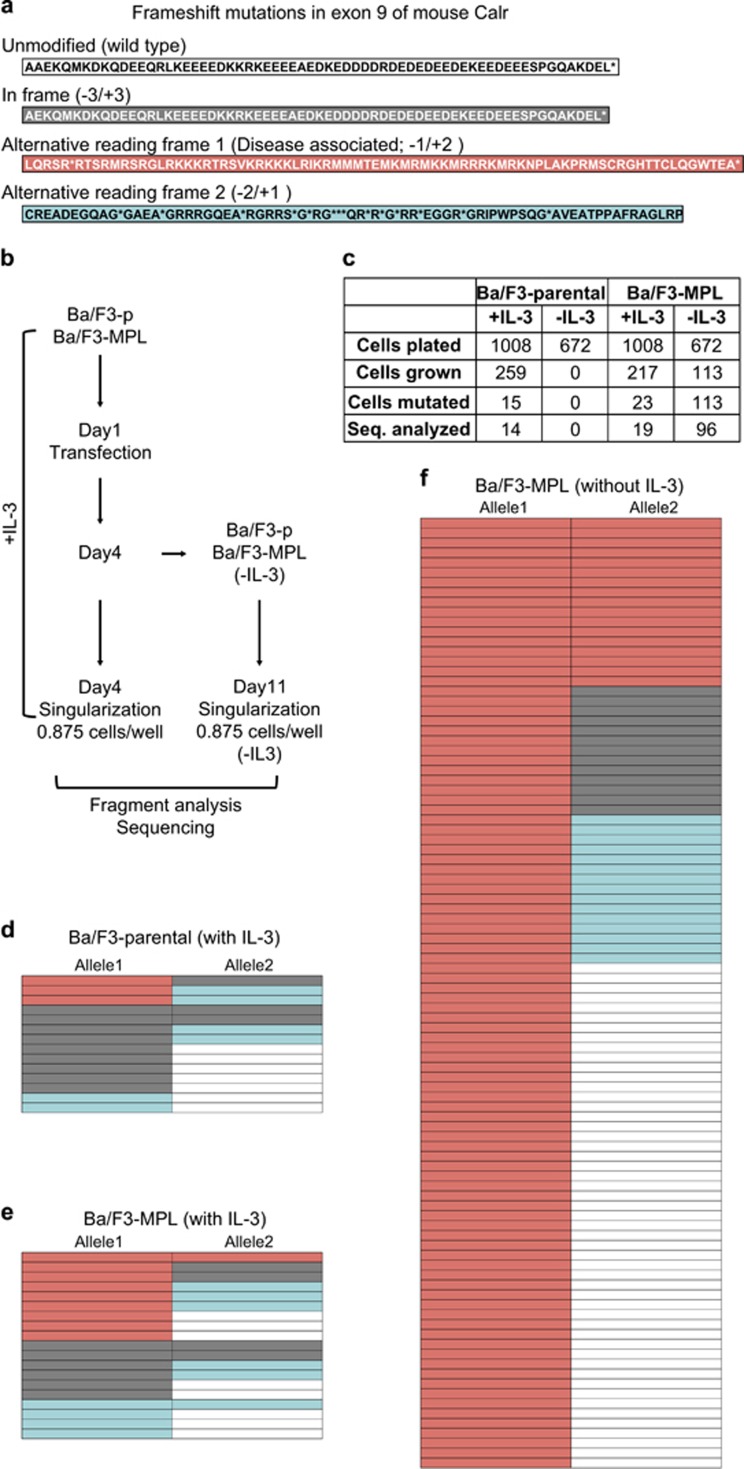
We also performed CRISPR/Cas9-mediated modification of the murine endogenous Calr locus in Ba/F3 cells. The Ba/F3 parental and Ba/F3-MPL cells were transfected with plasmids expressing the Cas9 protein and a guide RNA targeting the exon 9 of murine Calr. The cells were singularized by serial dilution and cultured with and without IL-3 (Figure 2b). DNA was obtained from the colonies growing out of the single cells and a fragment size assay was performed to assess the mutational status of the endogenous Calr gene. Colonies carrying a mutation in the Calr gene were sequenced (Supplementary Table 1). Those colonies showing multiple peaks in the PCR product-sizing assay by fragment analysis were not analyzed further by sequencing, as they probably did not arise from a single cell (Figure 2c). In many cases, mutations were seen in both alleles of the Calr gene. In the presence of IL-3, frameshift mutations were detected in Calr gene in all three frames (in variable combinations within the two alleles) in both Ba/F3 parental (Figure 2d) and Ba/F3-MPL (Figure 2e) cells. However, in the absence of IL-3, not a single colony grew in Ba/F3 parental cells. Importantly, in Ba/F3-MPL cells, every colony had at least one allele mutated to the disease-associated reading frame when cultured in the absence of IL-3 (Figure 2f and Supplementary Table 1), underlining the absolute requirement of MPL for the ability of mutant CALR to transform Ba/F3 cells. Although the novel peptide at the C-terminal end of mutant mouse Calr is not completely identical to the human ortholog, it is still able to induce Ba/F3 transformation in the presence of MPL.
Figure 2.
Only Ba/F3-MPL cells with Calr frameshift mutations to disease-associated reading frame can grow in the absence of IL-3. (a) Amino-acid sequence of exon 9 of mouse Calr upon mutation to different reading frames. (b) Schematic representation of the experimental workflow. (c) Number of cells plated and colonies analyzed under different conditions. (d) Reading frames of the two alleles of endogenous Calr in Ba/F3 parental cells with IL-3. (e) Ba/F3-MPL cells with IL-3. (f) Ba/F3-MPL cells cultured in the absence of IL-3. Color code as in (a).
These data are in complete accordance with two recent studies that have shown that mutant CALR induces JAK-STAT activation downstream of MPL receptor. Marty et al.13 show that the CALR-del52 induces thrombocytosis, leading to PMF, in a bone marrow transplantation assay, and that this is dependent on MPL-mediated JAK2 activation. Chachou et al.14 demonstrate that the CALR mutants directly interact with MPL and induce activation of the receptor. Moreover, both studies show that the expression of MPL is required for the transformation of Ba/F3 cells by CALR mutants.
Our data also imply that MPL is indispensable for the transformation of Ba/F3 cells by the CALR mutants. In an exceptional case, where the parental Ba/F3 cells could be transformed by mutant CALR, this was only possible by a stochastic event leading to selection of those cells that strongly upregulated the expression of endogenous MPL. In fact, this rare stochastic event led us to identify the mechanism of action by which CALR mutants can induce ligand-independent activation of the JAK-STAT signaling pathway in a completely unbiased approach. This ability of mutant CALR to activate MPL would explain the occurrence of CALR mutations specifically in ET and PMF. Both these diseases manifest as increase in thrombocyte numbers, and thrombocyte differentiation is induced by the activation of the thrombopoietin receptor (MPL). Furthermore, we performed CRISPR/Cas9-induced mutagenesis of the murine endogenous Calr locus. Our data showed that mutant Calr can likely mediate clonal advantage in only those hematopoietic progenitors that express MPL—hematopoietic stem cells and megakaryocytic progenitors. The bone marrow transplantation experiments reported by Marty et al.13 demonstrated the same concept in vivo. Moreover, activation of MPL leads to further downstream activation of the receptor-associated JAK2. This explains the ability of mutant CALR to activate JAK-STAT signaling as we had proposed previously and the efficacy of JAK2 inhibitors seen in two PMF patients.15
Acknowledgments
HN and RK acknowledge the support received by Austrian Science Fund (FWF: project numbers F2812-B20 and F4702-B20). BK was supported by MH CZ - DRO (FNBr, 65269705) and MUNI/A/1028/2015. WV and CM are supported by a grant from la Ligue Nationale contre le Cancer (équipe labellisée Hana Raslova 2013, 2016). Support for SNC and CP from Fondation contre le cancer, Salus Sanguinis, ARC and IAP MEGEI Belgium is acknowledged. RJ is supported by the Austrian Science Fund-FWF P29018-B30.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on the Leukemia website (http://www.nature.com/leu)
Supplementary Material
References
- Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. IARC: Lyon, 2008. [Google Scholar]
- Cazzola M, Kralovics R. From Janus kinase 2 to calreticulin: the clinically relevant genomic landscape of myeloproliferative neoplasms. Blood 2014; 123: 3714–3719. [DOI] [PubMed] [Google Scholar]
- Baxter EJ, Scott LM, Campbell PJ, East C, Fourouclas N, Swanton S et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet 2005; 365: 1054–1061. [DOI] [PubMed] [Google Scholar]
- James C, Ugo V, Le Couedic JP, Staerk J, Delhommeau F, Lacout C et al. A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera. Nature 2005; 434: 1144–1148. [DOI] [PubMed] [Google Scholar]
- Kralovics R, Passamonti F, Buser AS, Teo SS, Tiedt R, Passweg JR et al. A gain-of-function mutation of JAK2 in myeloproliferative disorders. N Engl J Med 2005; 352: 1779–1790. [DOI] [PubMed] [Google Scholar]
- Levine RL, Wadleigh M, Cools J, Ebert BL, Wernig G, Huntly BJ et al. Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis. Cancer Cell 2005; 7: 387–397. [DOI] [PubMed] [Google Scholar]
- Pikman Y, Lee BH, Mercher T, McDowell E, Ebert BL, Gozo M et al. MPLW515L is a novel somatic activating mutation in myelofibrosis with myeloid metaplasia. PLoS Med 2006; 3: e270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott LM, Tong W, Levine RL, Scott MA, Beer PA, Stratton MR et al. JAK2 exon 12 mutations in polycythemia vera and idiopathic erythrocytosis. N Engl J Med 2007; 356: 459–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med 2013; 369: 2379–2390. [DOI] [PubMed] [Google Scholar]
- Nangalia J, Massie CE, Baxter EJ, Nice FL, Gundem G, Wedge DC et al. Somatic CALR mutations in myeloproliferative neoplasms with nonmutated JAK2. N Engl J Med 2013; 369: 2391–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak M, Groenendyk J, Szabo E, Gold LI, Opas M. Calreticulin, a multi-process calcium-buffering chaperone of the endoplasmic reticulum. Biochem J 2009; 417: 651–666. [DOI] [PubMed] [Google Scholar]
- Molinari M, Eriksson KK, Calanca V, Galli C, Cresswell P, Michalak M et al. Contrasting functions of calreticulin and calnexin in glycoprotein folding and ER quality control. Mol Cell 2004; 13: 125–135. [DOI] [PubMed] [Google Scholar]
- Marty C, Pecquet C, Nivarthi H, Elkhoury M, Chachoua I, Tulliez M et al. Calreticulin mutants in mice induce an MPL-dependent thrombocytosis with frequent progression to myelofibrosis. Blood 2015; published ahead of print 25 November 2015 doi:10.1182/blood-2015-11-679571. [DOI] [PubMed]
- Chachoua IPC, El-Khoury M, Nivarthi H, Albu RI, Marty C, Gryshkova V et al. Thrombopoietin receptor activation by myeloproliferative neoplasm associated calreticulin mutants. Blood 2015; published ahead of print 14 December 2015 doi:10.1182/blood-2015-11-681932. [DOI] [PubMed]
- Passamonti F, Caramazza D, Maffioli M. JAK inhibitor in CALR-mutant myelofibrosis. N Engl J Med 2014; 370: 1168–1169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.