Abstract
Purpose
Standard therapies for localized inoperable intrahepatic cholangiocarcinoma (IHCC) are ineffective. Advances in radiotherapy (RT) techniques and image guidance have enabled ablative doses to be delivered to large liver tumors. This study evaluated the effects of RT dose escalation in the treatment of IHCC.
Patients and Methods
Seventy-nine consecutive patients with inoperable IHCC were identified and treated with definitive RT from 2002 to 2014. At diagnosis, the median tumor size was 7.9 cm (range, 2.2 to 17 cm). Seventy patients (89%) received systemic chemotherapy before RT. RT doses were 35 to 100 Gy (median, 58.05 Gy) in three to 30 fractions for a median biologic equivalent dose (BED) of 80.5 Gy (range, 43.75 to 180 Gy).
Results
Median follow-up time for patients alive at time of analysis was 33 months (range, 11 to 93 months). Median overall survival (OS) time after diagnosis was 30 months; 3-year OS rate was 44%. Radiation dose was the single most important prognostic factor; higher doses correlated with an improved local control (LC) rate and OS. The 3-year OS rate for patients receiving BED greater than 80.5 Gy was 73% versus 38% for those receiving lower doses (P = .017); 3-year LC rate was significantly higher (78%) after a BED greater than 80.5 Gy than after lower doses (45%, P = .04). BED as a continuous variable significantly affected LC (P = .009) and OS (P = .004). There were no significant treatment-related toxicities.
Conclusion
Delivery of higher doses of RT improves LC and OS in inoperable IHCC. A BED greater than 80.5 Gy seems to be an ablative dose of RT for large IHCCs, with long-term survival rates that compare favorably with resection.
INTRODUCTION
Intrahepatic cholangiocarcinoma (IHCC) is an uncommon but lethal disease that arises from the epithelial lining of the intrahepatic biliary tree. Despite a rising incidence,1-3 the patterns of tumor recurrence and causes of disease-related death have not been well documented.
Surgery is considered the only potentially curative treatment for IHCC, but only approximately 30% of patients have operable disease.4,5 Nonoperative therapies have significant limitations, and the median survival for patients with inoperable disease is only 7 to 12 months.6 On the basis of the Advanced Biliary Cancer Trial,7 the current standard frontline therapy for locally advanced biliary tumors is systemic gemcitabine and cisplatin, often followed by hepatic-directed therapies, such as transarterial chemoembolization, thermal ablation, intra-arterial Y90 microspheres, and external radiotherapy (RT).
Prior studies have suggested that RT for inoperable IHCC can improve tumor local control (LC) and prolong survival.6,8,9 However, the role of RT in the definitive treatment of IHCC remains controversial. Conventional RT doses have been shown to be insufficient for disease control, with most patients experiencing local progression as the first site of disease after RT.10 During the past decade, technical advances in RT planning and delivery, such as intensity-modulated radiation therapy, respiratory gating, proton therapy, and image guidance with computed tomography (CT), have enabled the safe delivery of more than twice the radiation dose to large liver tumors. Since 2010, we have used daily diagnostic-quality CT image guidance11 coupled with inspiration breath-hold respiratory gating12 to control organ motion. More recently, we have used a simultaneous integrated boost (SIB) to the hypoxic centers of large tumors as well as simultaneous integrated protection to the abutting luminal organs. These techniques allow the delivery of ablative doses of RT to tumors located within millimeters of sensitive GI organs. Given this unique approach, we evaluated the influence of RT dose escalation on LC and overall survival (OS) in patients with inoperable IHCC, and determined whether a threshold RT dose is associated with a survival benefit.
PATIENTS AND METHODS
Patients
After institutional review board approval, we identified 79 patients with inoperable IHCC consecutively treated with definitive RT at The University of Texas MD Anderson Cancer Center from 2002 to 2014. This study was a single-institution retrospective design. All patients with inoperable IHCC treated with RT were included except for seven for whom treatment was with palliative intent. All patients completed the planned RT treatment. The diagnosis of IHCC was confirmed by histologic examination of biopsy specimens for all but two patients. All patients underwent multidisciplinary evaluation by medical, surgical, and radiation oncologists.
Treatment
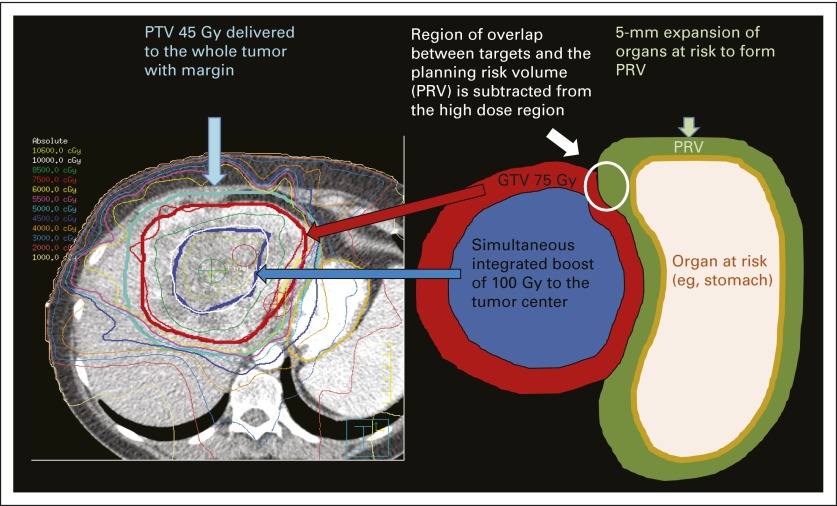
RT was delivered by three-dimensional conformal intensity-modulated radiation therapy with 6-MV photon beams or passive scatter proton beam techniques. Our practice pattern shifted to the delivery of higher doses of RT in 2010. All patients underwent planning CT scans in the treatment position with intravenous contrast unless medically contraindicated. For patients receiving therapy of 50.4 Gy or greater, a previously described CT- or fiducial-based kilovoltage image-guided inspiration breath-hold soft tissue alignment technique13 was used to minimize doses to the liver, bile duct, and GI mucosa. In other high-dose cases, an internal target volume was created, and patients were treated while free breathing with kilovoltage imaging alignment to bone. The gross tumor volume (GTV) was determined on the basis of all available imaging and included the primary tumor and radiographically involved lymph nodes. For microscopic disease, a 0- to 10-mm clinical target volume expansion was added to the GTV, and the planning target volume (PTV) included the clinical target volume with a 5-mm margin. For doses greater than 50.4 Gy, the GTV was treated with an SIB using a 0- to 5-mm PTV margin. A central SIB of 75 Gy in 15 fractions or 100 Gy in 25 fractions was used in selected larger tumors by constricting the GTV by 1 cm. Simultaneous integrated protection was accomplished with a 5-mm expansion of adjacent at-risk organs to create a planning risk volume that was then subtracted from the high-dose area of the GTV (Data Supplement and Fig 1).
Fig 1.
Radiation treatment plan illustrating the simultaneous integrated boost/simultaneous integrated protection (SIP) technique. A dose of 100 Gy in 25 fractions is delivered to the center of the tumor (dark blue contour) while the gross tumor volume (GTV) receives 75 Gy in 25 fractions (red contour). The GTV does not overlap with the planning risk volume (PRV) created by a 5-mm expansion of adjacent organs at risk for SIP. For treatment planning, the organ at risk is the priority constraint over the planning target volume (PTV). A microscopic dose of 45 Gy, which is within the tolerance of the gastric mucosa, is delivered to the whole tumor with margin as the PTV 45 Gy (light blue contour).
RESULTS
Patient and Treatment Characteristics
Patient characteristics are summarized in Table 1 and are separated by RT dose on the basis of treatment with doses greater or less than the median biologic equivalent dose (BED). To address potential differences between patients treated in the early versus more recent cohort, the characteristics are summarized by era of treatment in the Data Supplement; the only significant demographic difference by era is that more patients treated after 2010 had a worse Eastern Cooperative Oncology Group performance status (P = .03). Median follow-up time from diagnosis for all patients was 24 months (range, 4 to 133 months) and 33 months (range, 11 to 93 months) for patients alive at the time of analysis.
Table 1.
Characteristics of All Patients and by Treatment With BED ≤ 80.5 Gy Versus > 80.5 Gy
| Characteristic | No. of All Patients (% or range) | No. of Patients Treated With BED ≤ 80.5 Gy (% or range) | No. of Patients Treated With BED > 80.5 Gy (% or range) | P |
|---|---|---|---|---|
| No. of Patients | 79 | 60 | 19 | |
| Female | 46 (58) | 38 (63) | 8 (42) | .11 |
| Male | 33 (42) | 22 (37) | 11 (58) | |
| Median age, years | 63 (31-87) | 63 (31-84) | 63 (42-87) | .90* |
| Race/ethnicity | 1.0 | |||
| White | 66 (83) | 50 (84) | 16 (85) | |
| Hispanic | 7 (9) | 6 (10) | 1 (5) | |
| Black | 3 (4) | 2 (3) | 1 (5) | |
| Arab | 3 (4) | 2 (3) | 1 (5) | |
| ECOG performance status | .08 | |||
| 0 | 37 (47) | 32 (53) | 5 (26) | |
| 1 | 37 (47) | 25 (42) | 12 (63) | |
| 2 | 5 (6) | 3 (5) | 2 (11) | |
| 3 or 4 | 0 | 0 | 0 | |
| Tumor markers | ||||
| Median CA 19-9, U/mL | 97.6 (1-95,526) | 98.5 (1-95,526) | 49 (5.9-5,934) | .20* |
| Median CEA, μg/L | 2 (0.4-85) | 1.95 (1-83) | 2.3 (0.4-85) | .70* |
| Median size of primary tumor | ||||
| Maximum dimension, cm | 7.9 (2.2-17) | 8.75 (2.3-15) | 6.5 (2.2-17) | .28* |
| Radiation volume, cm3 | ||||
| Median GTV | 198 (12-966) | 232 (17-966) | 168 (12-711) | .21* |
| Median PTV | 548 (55-2,012) | 614 (55-2,012) | 415 (66-1,213) | .02* |
| Total No. of tumors, median | 1 (1-12) | 1 (1-12) | 2 (1-8) | .17* |
| Patients with satellite intrahepatic metastasis | 31 (39) | 21 (35) | 10 (53) | .19 |
| Tumor location | ||||
| Central | 44 (56) | 37 (62) | 7 (37) | .07 |
| Peripheral | 35 (44) | 23 (38) | 12 (63) | |
| T classification | .48 | |||
| 1 | 7 (9) | 6 (10) | 1 (5) | |
| 2 | 49 (62) | 34 (57) | 15 (79) | |
| 3 | 21 (26) | 18 (30) | 3 (16) | |
| 4 | 2 (3) | 2 (3) | 0 | |
| N classification | .30 | |||
| 0 | 33 (42) | 23 (38) | 10 (53) | |
| 1 | 46 (58) | 37 (62) | 9 (47) | |
| M classification | 1.0 | |||
| 0 | 63 (80) | 48 (80) | 15 (79) | |
| 1 | 16 (20) | 12 (20) | 4 (21) | |
| Overall disease stage | .15 | |||
| I | 4 (5) | 3 (5) | 1 (5) | |
| II | 17 (22) | 10 (17) | 7 (37) | |
| III | 11 (14) | 10 (17) | 1 (5) | |
| IV | 47 (59) | 37 (61) | 10 (53) |
NOTE. P values were calculated using Fisher's exact test unless otherwise noted.
Abbreviations: CEA, carcinoembryonic antigen; ECOG, Eastern Cooperative Oncology Group; GTV, gross tumor volume; PTV, planning target volume.
P value calculated by the nonparametric test of medians.
RT was individualized with the goal of achieving the highest minimum BED to the tumor while protecting at-risk organs. To achieve this goal, more fractions and smaller margins were used to treat tumors close to the bowel or when needed to protect normal liver and bile ducts (Data Supplement). The PTV was the only significant difference between patients treated with high versus low RT doses. Smaller PTVs were used for patients treated with BED greater than 80.5 Gy (Table 1) because the margins of treatment often were reduced to meet dose constraints when higher doses were delivered. There was no significant difference in the GTV depending on RT dose (Table 1) or era of treatment (Data Supplement). Treatment details are summarized in Table 2. The BED for the median RT dose and fractionation (58.05 Gy in 15 fractions) was 80.5 Gy (range, 43.75 to 180 Gy). The median RT dose delivered to central tumors was 58.05 Gy in 15 fractions compared with 60 Gy in 30 fractions for peripheral tumors. Almost all patients (n = 70; 89%) received chemotherapy before RT (Table 2).
Table 2.
Treatment Characteristics for All Patients and by Treatment With BED ≤ 80.5 Gy Versus > 80.5 Gy
| Treatment Characteristic | No. of All Patients (% or range) | No. of Patients Treated With BED ≤ 80.5 Gy (% or range) | No. of Patients Treated With BED > 80.5 Gy (% or range) |
|---|---|---|---|
| Median radiation dose, Gy | |||
| Total dose | 58.05 (35-100) | 54 (35-63) | 67.5 (60-100) |
| 90% GTV dose/fx | 2.5 (1.8-20) | 2 (1.8-3.87) | 4 (2.5-20) |
| PTV dose | 58.05 (35-75) | 50.4 (35-63) | 67.5 (58.05-75) |
| Minimum dose to GTV | 51.1 (27.2-68.8) | 50.5 (27.2-64) | 56.5 (32.1-68.8) |
| Minimum dose to PTV | 42.8 (2.9-61.1) | 40.8 (2.9-58.9) | 47.4 (13-61.1) |
| BED for all doses and fx* | 77 (43.75-180) | 63.72 (60-80.5) | 97.88 (87.5-180) |
| BED for median total RT dose* | 80.5 | 63.72 | 97.88 |
| Radiation technique | |||
| IMRT | 41 (52) | 30 (50) | 11 (58) |
| 3D proton beam | 25 (32) | 17 (28) | 8 (42) |
| Conventional 3D conformal | 13 (16) | 13 (22) | 0 |
| Higher central dose | 13 (16.5) | 5 (8) | 8 (42) |
| Breath-hold gating | 15 (19) | 6 (10) | 9 (47) |
| CT on rails image guidance | 12 (15) | 4 (7) | 8 (42) |
| Alloderm spacer placed | 3 (4) | 2 (3) | 1 (5) |
| Common fractionation regimens | |||
| 50.4 Gy in 28 fx | 19 (24) | 19 (32) | 0 |
| 58.05 Gy in 15 fx | 14 (18) | 14 (23) | 0 |
| 60 Gy in 30 fx | 4 (5) | 4 (7) | 0 |
| 67.5 Gy in 15 fx | 7 (9) | 0 | 7 (37) |
| 75 Gy in 25 fx | 5 (6) | 0 | 5 (26) |
| Chemotherapy treatment | |||
| Chemotherapy before RT | 70 (89) | 52 (87) | 18 (95) |
| Gemcitabine and cisplatin | 31 | ||
| Gemcitabine and cisplatin plus others† | 24 | ||
| Gemcitabine and capecitabine | 5 | ||
| Gemcitabine and oxaliplatin | 2 | ||
| Capecitabine and oxaliplatin | 2 | ||
| Capecitabine | 1 | ||
| Concurrent chemotherapy | 50 (63) | 39 (65) | 11 (58) |
| Capecitabine | 46 | ||
| Erlotinib | 2 | ||
| Bevacizumab | 2 | ||
| Chemotherapy after RT | 37 (47) | 28 (48) | 9 (47) |
| Irinotecan | 26 | ||
| Gemcitabine and cisplatin | 4 | ||
| Other | 7 |
Abbreviations: 3D, three dimensional; BED, biologic equivalent dose; CT, computed tomography; fx, fraction; GTV, gross tumor volume; IMRT, intensity-modulated radiation therapy; PTV, planning target volume; RT, radiotherapy.
BED delivered to the GTV.
Other chemotherapy, including capecitabine, oxaliplatin, carboplatin, fluorouracil, irinotecan, erolotinib, bevacizumab, and cetuximab.
Tumor Control and Patterns of Recurrence
Thirty-eight patients (48%) had radiographic evidence of primary tumor progression after completion of RT. Actuarial 1-, 2-, and 3-year LC rates from the start of RT were 81%, 45%, and 27%, respectively. The median duration of LC was 23 months after RT. The dominant pattern of recurrence was within the high-dose radiation region, which occurred in the majority of patients (n = 34; 89%); three patients (8%) had both in-field and marginal progression, and only one patient (3%) had a recurrence at the margin of the radiation dose. Local progression was isolated in 13 patients (34%) and synchronous with nodal, intrahepatic metastasis, or extrahepatic metastasis in 19 (51%); six patients (16%) developed intrahepatic metastasis as the first site of disease progression and ultimately progressed locally.
Evidence of new metastatic lesions in the liver developed in 40 patients (51%). At 1, 2, and 3 years, the actuarial rates of freedom from intrahepatic metastasis from the time of diagnosis were 91%, 69%, and 50%, respectively (median time, 37 months). Extrahepatic metastases developed in 32 patients (41%; median time, 38 months). One-, 2-, and 3-year actuarial rates of freedom from extrahepatic metastasis were 95%, 75%, and 56%, respectively.
Survival Outcomes and Causes of Death
The median survival time for the group as a whole was 30 months, with 1-, 2-, and 3-year OS rates of 87%, 61%, and 44%, respectively. Median progression-free survival (PFS) was 30 months, and 1-, 2-, and 3-year PFS rates were 88%, 61%, and 39%, respectively. At the time of analysis, 48 patients had died, of whom 32 died as a result of complications from primary or metastatic intrahepatic disease progression, including biliary complications (n = 16), vascular complications (n = 10), parenchymal liver failure from disease burden (n = 2), and a combination of these factors (n = 4; Table 3).
Table 3.
Causes of Death
| No. of All Patients (% of known deaths unless noted) | No. of Patients Treated With BED ≤ 80.5 Gy (% of known deaths unless noted) | No. of Patients Treated With BED > 80.5 Gy (% of known deaths unless noted) | |
|---|---|---|---|
| Total deaths | 48 (61*) | 43 (72*) | 5 (26*) |
| Known cause of death | 36 (75†) | 31 (72†) | 5 (100†) |
| Hepatic | |||
| Biliary complications | 16 (44) | 14 (45) | 2 (40) |
| Vascular complications | 10 (28) | 8 (26) | 2 (40) |
| Portal vein occlusion | 7 | 5 | 2 |
| IVC/hepatic vein occlusion | 3 | 3 | 0 |
| Parenchymal liver failure | 2 (6) | 2 (6) | 0 |
| Combination of above | 4 (11) | 4 (13) | 0 |
| Total | 32 (89) | 28 (90) | 4 (80) |
| Extrahepatic | |||
| Peritoneal carcinomatosis | 3 (8) | 2 (6) | 1 (20) |
| Lymphangitic disease in lungs | 1 (3) | 1 (3) | 0 |
| Total | 4 (11) | 3 (10) | 1 (20) |
Abbreviation: IVC, inferior vena cava.
Percentage of total patients in each group.
Percentage of all deaths in each group.
Prognostic Factors
RT dose was the single most important predictor of OS and LC. The significant prognostic effect of RT dose was seen when the total dose was analyzed as a continuous variable and divided into various dose groups. As a continuous variable, total RT dose was associated with improved LC (P = .03) and OS (P = .02). The median survival time of patients treated with doses higher than the conventional 50.4 Gy was 43 months versus 23 months for patients treated with 50.4 Gy or less (P = .01).
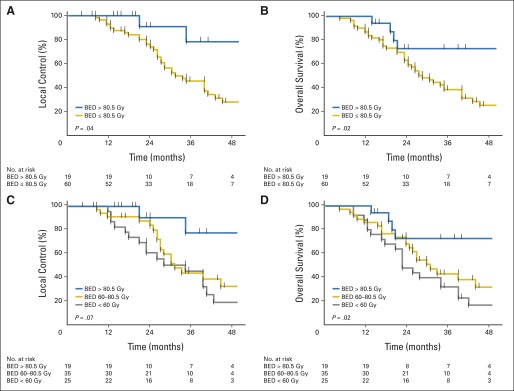
Total BED was also found to affect LC and OS. Patients treated with BED greater than 80.5 Gy (ie, more than the median dose/fraction) had a 3-year LC rate of 78% versus 45% for those treated with a total BED of 80.5 Gy or less (P = 0.04; Fig 2). The median OS for patients treated with BED greater than 80.5 Gy was not reached versus 27 months for those treated with BED 80.5 Gy or less (P = 0.02). Both the 2- and 3-year OS rates for patients treated with BED greater than 80.5 Gy were 73%, and for those treated with BED of 80.5 Gy or less, the OS rate was 58% at 2 years and 38% at 3 years. BED as a continuous variable also affected LC (P = 0.009) and OS (P = 0.004). The dose-response relationship of BED with LC and OS is shown in Figure 2.
Fig 2.
Effect of radiation dose of local control (LC) and overall survival (OS) from the time of diagnosis. Kaplan-Meier estimates of (A) LC and (B) OS according to a biologic equivalent dose (BED) less than 80.5 or 80.5 Gy or greater illustrate the superiority of the higher dose. BED analyzed as a continuous variable also had a dose-response effect on (C) LC and (D) OS.
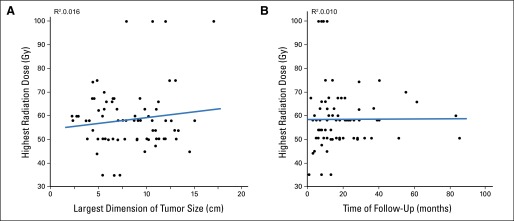
To evaluate the possibility that patients with smaller tumors had received higher RT doses, or that patients treated with higher doses (and presumably newer RT techniques) had shorter follow-up times, we analyzed RT dose versus tumor size and follow-up interval and found no correlations (Appendix Fig A1, online only). We further compared outcomes for patients treated before 2010 versus after 2010, when treatment policies shifted toward use of larger doses with CT image–guided RT; patients treated after 2010 had longer OS (50 months) than those treated before 2010 (24 months, P = .007).
Patient age, sex, race, and performance status did not influence OS, nor did baseline primary tumor size and presence of satellite intrahepatic, regional nodal, or extrahepatic metastasis at diagnosis. This latter finding is consistent with most patients having died as a result of liver failure related to progressive intrahepatic disease. Other than RT dose, CA 19-9 level was the only other variable associated with OS (P = .02).
Receipt of chemotherapy before RT did not affect OS (P = .06) or PFS (P = .84), but only nine patients did not receive chemotherapy before RT. Concurrent chemotherapy also did not affect OS (P = .12) or PFS (P = .34). Receipt of chemotherapy after RT did not affect OS (P = .83).
On multivariable analysis, radiation dose remained the only significant predictor of both LC and OS (Table 4). When BED was analyzed as a continuous variable with primary tumor size, presence or absence of satellite intrahepatic metastasis at diagnosis, and performance status, it was the only factor that correlated with LC (P = .004) and OS (P = .006). CA 19-9 was added to the multivariable analysis for OS because there were more events, and we found that BED remained significant (P = .024), but CA 19-9 was not (P = .063).
Table 4.
Effect of Radiation Dose on Local Control and Overall Survival in Multivariable Analysis
| Characteristic | Local Control |
Overall Survival |
||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| BED delivered* | 0.96 (0.94 to 0.98) | .004 | 0.97 (0.95 to 0.99) | .024 |
| Largest dimension of primary | 0.96 (0.86 to 1.08) | .510 | 1.05 (0.96 to 1.17) | .283 |
| Satellitosis at diagnosis | ||||
| Absent | Reference | Reference | ||
| Present | 1.89 (0.89 to 3.98) | .096 | 1.23 (0.59 to 2.57) | .564 |
| CA 19-9 | Not included in this model | 1.00 (1.00 to 1.00) | .063 | |
| ECOG performance status | ||||
| 0 | Reference | Reference | ||
| 1 | 1.28 (0.62 to 2.67) | .496 | 1.17 (0.59 to 2.31) | .659 |
| 2 | 0.81 (0.18 to 3.63) | .783 | 1.22 (0.27 to 5.49) | .793 |
Abbreviations: BED, biologic equivalent dose; ECOG, Eastern Cooperative Oncology Group; HR, hazard ratio.
BED as a continuous variable.
Toxicity
Overall, treatment was well tolerated. No cases of radiation-induced liver disease were documented. Despite the larger PTV used in the earlier treatment era and the higher doses delivered in the later era, no patients developed liver failure in the absence of intrahepatic tumor progression. Patients did not experience biliary obstruction or cholangitis during RT. However, three patients (4%) were hospitalized within 90 days of RT completion because of cholangitis related to stent failure (n = 2) or tumor progression (n = 1). Two other patients were hospitalized within 90 days of RT as a result of gastric bleeding and radiation pneumonitis after treatment for pulmonary metastases. Evidence of biliary stenosis developed in seven patients (9%), who subsequently had a stent placed, although it is difficult to attribute this to toxicity versus disease; four of the seven patients had primary tumor progression, and two experienced progression of intrahepatic satellite lesions. Biliary stenosis occurred at a median time of 10 months after RT (range, 2 to 33 months), and the maximum dose that the common bile duct received in these patients ranged from 34 Gy in 14 fractions to 75 Gy in 25 fractions.
DISCUSSION
This study demonstrates that using high radiation doses to treat inoperable IHCC improves LC, which translates into a major survival benefit for patients. The high doses delivered to large hepatic tumors, mere millimeters from luminal organs, were well tolerated, and the survival outcomes were comparable to those reported after surgical resection.14-16
Importantly, OS in this study is similar to that reported in patients with operable IHCC after resection with curative intent. Median survival time for patients who undergo at least a macroscopic total resection ranges from 25.5 to 37.4 months, with 3-year survival rates of 38% to 55%.14-16 In one such series, the median OS of 29 patients with an R0 resection was 45.9 months.14 Although the current results cannot be compared directly with the surgical series, the median survival time was not reached for patients treated with BED greater than 80.5 Gy, the median BED we delivered. The 3-year OS rate for the entire group was 44%, which is within the range reported in the surgical series, and the 3-year OS rate for patients treated with a BED greater than 80.5 Gy was 73%, higher even than what is reported in the surgical literature. Therefore, although surgery is considered the only curative option, dose-escalated RT achieved comparable results in patients with more advanced disease.
Previous prospective studies of hepatic tumors generally accrued a small subset of patients with cholangiocarcinoma; nevertheless, these trials demonstrated the feasibility of delivering definitive RT doses to liver tumors and suggested a dose-response relationship with OS.8,17,18 One of the first dose escalation studies involved 18 patients with IHCC treated with a median dose of 61.5 Gy (range, 28.5 to 90 Gy)8 and showed that higher RT doses were associated with improved survival for the group as a whole; the median survival times were 16.4 months or greater for patients treated with more than 70 Gy versus 11 months for those treated with less than 70 Gy. Similar to the current findings, the study found radiation dose to be the most important prognostic factor, and disease characteristics, such as primary liver tumor size or presence of extrahepatic disease, did not affect survival. The prospective study with the largest number of patients with IHCC involved 46 patients receiving RT (median dose, 60.75 Gy [range, 40 to 90 Gy] in twice-per-day fractions) with concurrent hepatic artery floxuridine.17 It too reported that RT dose was the only significant predictor of survival, with median survival time after doses greater than 75 Gy (BED > 71.88 Gy) being 23.9 versus 14.9 months for lower doses (P < .01). A phase I trial of 10 patients with IHCC receiving stereotactic body radiation therapy to a median dose of 36 Gy in six fractions (BED = 48 Gy) showed more modest outcomes, with a 65% 1-year LC rate and median survival of 15 months.18 Similar to the current study, toxicity after stereotactic body radiation therapy was often seen in the context of progressive disease; small bowel obstruction developed in one patient with extrahepatic disease progression, and two patients experienced decline in liver function. We used a higher median BED (80.5 Gy) than what was delivered in these studies, and the median survival times were longer at 30 months for the whole group and were not reached with BED greater than 80.5 Gy.
Most retrospective studies in patients with IHCC have reported poor survival outcomes after conventional RT doses.6,9,19 The largest study reported an analysis of SEER data in 3,839 patients from 1988 to 2003,6 including 396 patients who received RT only. The median survival was only 7 months versus 3 months for patients who received no treatment (P < .01). As a result of limitations of the database variables, no information was available on the RT dose delivered. Another group reported a series of 75 patients with IHCC, of whom 22 had received RT to a median dose of 50 Gy (range, 30 to 60 Gy) at 2 Gy per fraction.9 The 1- and 2-year survival rates were only 36.1% and 5.2%, respectively. Subsequently, Chen et al19 described a cohort of 84 patients with IHCC, of whom 35 received RT to a median dose of 50 Gy (range, 30 to 60 Gy) at 1.8 to 2 Gy per fraction, reporting a median survival time of 9.5 months versus 5.1 months for patients not treated with RT (P < .01). Although these studies demonstrated that RT improves survival over no treatment, they also revealed the limitations of conventional doses of RT compared with the present findings.
The current study represents a critical step toward understanding the potential curative role of high-dose RT in IHCC; however, it has some limitations inherent to any retrospective study. Although we addressed possible confounding factors in the RT dose analysis, we cannot fully account for all potential biases that would be addressed in a randomized study. Selection bias could have favored patients who received higher doses of RT, even though we did not find evidence to that effect. Furthermore, treatment-related toxicity may have been underestimated because it may not have been fully documented in the medical record. Finally, the variety of radiation dose/fractionation schedules used in this study may also be viewed as a limitation. However, this variation is common in the liver literature because different RT doses are needed to optimize tumor dose while respecting normal tissue constraints. We accounted for the various RT doses by analyzing the effect of both total RT dose and BED in the analysis. The results are consistent in that higher doses were all associated with improved outcomes.
A strength of this study is the inclusion of a relatively homogenous group of patients with inoperable IHCC—a unique cohort because all patients received definitive RT. Prior studies have included mixes of patients with operable and inoperable disease, intrahepatic with extrahepatic or hilar cholangiocarcinomas, other tumor histologies, or treatment with adjuvant and palliative intent. With 79 patients, the current study, to our knowledge, is the largest series of IHCC treated with definitive RT reported to date.
In summary, controlling the primary tumor with high-dose radiation produces a major survival benefit for patients with inoperable IHCC. Treatment with ablative doses of RT using high-quality daily CT image guidance with inspiration breath-hold gating can achieve survival times comparable to those achieved with resection. Higher total RT doses and higher doses delivered per fraction to achieve BED greater than 80.5 Gy should be considered for all patients undergoing definitive RT for IHCC if dose constraints to the liver, bile duct, stomach, and bowel can be met and image guidance is used to ensure that the dose is delivered safely. The findings support the use of 67.5 Gy in 15 fractions (BED, 97.88 Gy) in the current phase III, NRG-GI001 randomized trial evaluating whether the addition of RT to chemotherapy affects survival.
Supplementary Material
Acknowledgment
We thank Christine F. Wogan of MD Anderson's Department of Radiation Oncology for help with scientific editing.
Appendix
Fig A1.
No correlations were found between radiation dose and (A) tumor size and (B) duration of follow-up.
Footnotes
See accompanying editorial on page 203
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT02200042.
AUTHOR CONTRIBUTIONS
Conception and design: Randa Tao, Sunil Krishnan, Eugene J. Koay, Theodore S. Hong, Christopher H. Crane
Administrative support: Eugene J. Koay
Provision of study materials or patients: Eugene J. Koay, Christopher H. Crane
Collection and assembly of data: Randa Tao, Sunil Krishnan, Priya R. Bhosale, Andrew J. Bishop, Cameron W. Swanick, Prajnan Das, Christopher H. Crane
Data analysis and interpretation: Randa Tao, Sunil Krishnan, Priya R. Bhosale, Milind M. Javle, Thomas A. Aloia, Rachna T. Shroff, Ahmed O. Kaseb, Eugene J. Koay, Howard D. Thames, Theodore S. Hong, Prajnan Das, Christopher H. Crane
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Ablative Radiotherapy Doses Lead to a Substantial Prolongation of Survival in Patients With Inoperable Intrahepatic Cholangiocarcinoma: A Retrospective Dose Response Analysis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Randa Tao
No relationship to disclose
Sunil Krishnan
Research Funding: Shell Oil Company, Celgene, Malaysian Palm Oil Council, Elekta, Focused Ultrasound Foundation, John S. Dunn Foundation
Patents, Royalties, Other Intellectual Property: MD Anderson Cancer Center
Priya R. Bhosale
No relationship to disclose
Milind M. Javle
No relationship to disclose
Thomas A. Aloia
No relationship to disclose
Rachna T. Shroff
Stock or Other Ownership: Celgene
Consulting or Advisory Role: Clovis Oncology, Celgene
Research Funding: Eli Lilly, Celgene
Travel, Accommodations, Expenses: Celgene, Clovis Oncology
Ahmed O. Kaseb
No relationship to disclose
Andrew J. Bishop
No relationship to disclose
Cameron W. Swanick
No relationship to disclose
Eugene J. Koay
No relationship to disclose
Howard D. Thames
No relationship to disclose
Theodore S. Hong
Consulting or Advisory Role: Eisai
Research Funding: Novartis
Prajnan Das
Honoraria: Meniscus Health Care Communications, Potomac Center for Medical Education
Christopher H. Crane
Honoraria: Vertex Pharmaceuticals
Consulting or Advisory Role: Vertex Pharmaceuticals
REFERENCES
- 1.Patel T. Increasing incidence and mortality of primary intrahepatic cholangiocarcinoma in the United States. Hepatology. 2001;33:1353–1357. doi: 10.1053/jhep.2001.25087. [DOI] [PubMed] [Google Scholar]
- 2.Patel T. Worldwide trends in mortality from biliary tract malignancies. BMC Cancer. 2002;2:10. doi: 10.1186/1471-2407-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, McGlynn K, et al. Rising incidence of intrahepatic cholangiocarcinoma in the United States: A true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Vauthey JN, Blumgart LH. Recent advances in the management of cholangiocarcinomas. Semin Liver Dis. 1994;14:109–114. doi: 10.1055/s-2007-1007302. [DOI] [PubMed] [Google Scholar]
- 5.Chou FF, Sheen-Chen SM, Chen YS, et al. Surgical treatment of cholangiocarcinoma. Hepatogastroenterology. 1997;44:760–765. [PubMed] [Google Scholar]
- 6.Shinohara ET, Mitra N, Guo M, et al. Radiation therapy is associated with improved survival in the adjuvant and definitive treatment of intrahepatic cholangiocarcinoma. Int J Radiat Oncol Biol Phys. 2008;72:1495–1501. doi: 10.1016/j.ijrobp.2008.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 8.Dawson LA, McGinn CJ, Normolle D, et al. Escalated focal liver radiation and concurrent hepatic artery fluorodeoxyuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2000;18:2210–2218. doi: 10.1200/JCO.2000.18.11.2210. [DOI] [PubMed] [Google Scholar]
- 9.Zeng ZC, Tang ZY, Fan J, et al. Consideration of the role of radiotherapy for unresectable intrahepatic cholangiocarcinoma: A retrospective analysis of 75 patients. Cancer J. 2006;12:113–122. [PubMed] [Google Scholar]
- 10.Crane CH, Macdonald KO, Vauthey JN, et al. Limitations of conventional doses of chemoradiation for unresectable biliary cancer. Int J Radiat Oncol Biol Phys. 2002;53:969–974. doi: 10.1016/s0360-3016(02)02845-6. [DOI] [PubMed] [Google Scholar]
- 11.Shiu AS, Chang EL, Ye JS, et al. Near simultaneous computed tomography image-guided stereotactic spinal radiotherapy: An emerging paradigm for achieving true stereotaxy. Int J Radiat Oncol Biol Phys. 2003;57:605–613. doi: 10.1016/s0360-3016(03)00792-2. [DOI] [PubMed] [Google Scholar]
- 12.Wong JW, Sharpe MB, Jaffray DA, et al. The use of active breathing control (ABC) to reduce margin for breathing motion. Int J Radiat Oncol Biol Phys. 1999;44:911–919. doi: 10.1016/s0360-3016(99)00056-5. [DOI] [PubMed] [Google Scholar]
- 13.Peng Y, Vedam S, Chang JY, et al. Implementation of feedback-guided voluntary breath-hold gating for cone beam CT-based stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2011;80:909–917. doi: 10.1016/j.ijrobp.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 14.Weber SM, Jarnagin WR, Klimstra D, et al. Intrahepatic cholangiocarcinoma: Resectability, recurrence pattern, and outcomes. J Am Coll Surg. 2001;193:384–391. doi: 10.1016/s1072-7515(01)01016-x. [DOI] [PubMed] [Google Scholar]
- 15.Sulpice L, Rayar M, Boucher E, et al. Treatment of recurrent intrahepatic cholangiocarcinoma. Br J Surg. 2012;99:1711–1717. doi: 10.1002/bjs.8953. [DOI] [PubMed] [Google Scholar]
- 16.Ohtsuka M, Ito H, Kimura F, et al. Results of surgical treatment for intrahepatic cholangiocarcinoma and clinicopathological factors influencing survival. Br J Surg. 2002;89:1525–1531. doi: 10.1046/j.1365-2168.2002.02268.x. [DOI] [PubMed] [Google Scholar]
- 17.Ben-Josef E, Normolle D, Ensminger WD, et al. Phase II trial of high-dose conformal radiation therapy with concurrent hepatic artery floxuridine for unresectable intrahepatic malignancies. J Clin Oncol. 2005;23:8739–8747. doi: 10.1200/JCO.2005.01.5354. [DOI] [PubMed] [Google Scholar]
- 18.Tse RV, Hawkins M, Lockwood G, et al. Phase I study of individualized stereotactic body radiotherapy for hepatocellular carcinoma and intrahepatic cholangiocarcinoma. J Clin Oncol. 2008;26:657–664. doi: 10.1200/JCO.2007.14.3529. [DOI] [PubMed] [Google Scholar]
- 19.Chen YX, Zeng ZC, Tang ZY, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: A retrospective analysis of 84 patients. BMC Cancer. 2010;10:492. doi: 10.1186/1471-2407-10-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.