Abstract
Purpose
Significant improvement in survival outcomes has been established with the addition of trastuzumab to adjuvant chemotherapy for human epidermal growth factor receptor 2 (HER2) –positive early breast cancer treatment. However, trastuzumab may increase the risk of cardiac toxicity, and long-term evaluation of its incidence and risk factors are warranted.
Methods
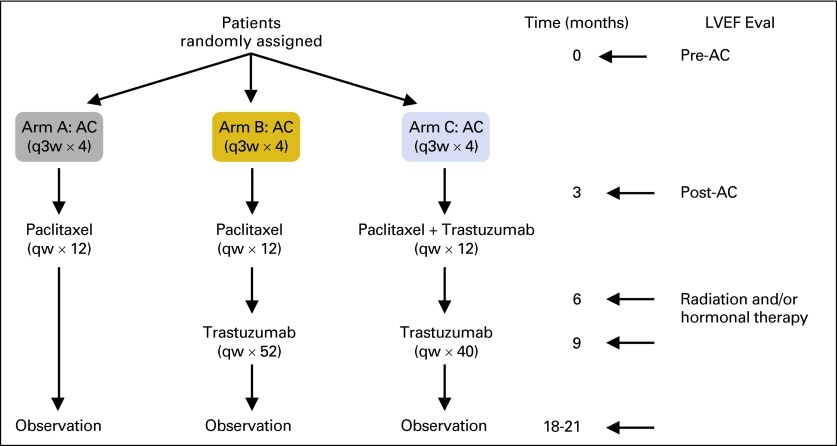
NCCTG (Alliance) N9831 trial compared adjuvant doxorubicin and cyclophosphamide (AC) followed by either weekly paclitaxel (arm A); paclitaxel then trastuzumab (arm B); or paclitaxel plus trastuzumab followed by trastuzumab alone (arm C) in patients with HER2-positive breast cancer. Cumulative incidence of cardiac events (CE) and left ventricular ejection fraction (LVEF) were evaluated in 1,944 women who proceeded to post-AC therapy. Risk factors for trastuzumab-induced cardiac toxicity were identified by Cox regression models.
Results
The 6-year cumulative incidence of CE was 0.6% in arm A, 2.8% in arm B, and 3.4% in arm C. At a median follow-up of 9.2 years, only two additional CHF diagnoses (of 1,046 patients) occurred beyond our previously reported follow-up time of 3.75 years. LVEF recovered in the majority of the patients who developed CHF. There were two cardiac deaths in arm A and one each in arms B and C. Age of 60 years or older, registration LVEF less than 65%, and use of antihypertensive medications were associated with an increased risk of CE in arms B and C.
Conclusion
The cumulative incidence of CE at 6 years was slightly higher with the addition of trastuzumab; however, the late development of CE is infrequent. Trastuzumab (in the context of anthracycline- and taxane-based therapy) continues to have a favorable benefit-risk ratio.
INTRODUCTION
Approximately 20% of primary invasive breast carcinomas overexpress HER2.1 The addition of trastuzumab (Herceptin; Genentech, San Francisco, CA) to adjuvant chemotherapy has been shown to significantly improve disease-free survival (DFS) and overall survival (OS) and forms the backbone of HER2-positive breast cancer treatment.2-6 A joint analysis of two pivotal adjuvant trials—NCCTG (North Central Cancer Treatment Group) N9831 (Alliance) and NSABP (National Surgical Adjuvant Breast and Bowel Project) B-31 (NRG)—demonstrated that the addition of 1 year of trastuzumab to doxorubicin and cyclophosphamide (AC) followed by paclitaxel improved DFS and OS; the relative risk reduction was 40% and 37%, respectively, at a median follow-up of 8.4 years.7
Cardiac toxicity is the most concerning adverse effect of trastuzumab. This usually manifests as an asymptomatic decline in left ventricular ejection fraction (LVEF) rather than symptomatic congestive heart failure (CHF).8 The overall incidence of cardiac toxicity is variable, depends on the definition used in various clinical trials, and has been reported to be 2% to 7% for trastuzumab monotherapy, 2% to 13% for trastuzumab and paclitaxel, and as high as 27% in combination with anthracyclines (typically with cumulative doses greater than 300 mg/m2).9
We have previously reported the short-term cardiac safety analysis observed in the N9831 adjuvant trial after a median follow-up of 3.75 years.10 This trial randomly assigned patients with early-stage, operable, HER2-positive breast cancer to receive four cycles of AC followed by either paclitaxel alone (arm A), or paclitaxel with trastuzumab either sequentially (arm B) or concurrently (arm C). The 3-year cumulative incidence of cardiac events (CEs) reported was 0.3% in arm A, 2.8% in arm B, and 3.3% in arm C.10 In this analysis, factors associated with an increased risk of CE were older age (60 years or older), lower LVEF at registration (less than 55% but greater than the lower limit of normal [LLN]), and use of antihypertensive medications.
Given the convincing efficacy of trastuzumab, an ongoing evaluation of the incidence and risk factors for trastuzumab-associated cardiac toxicity is imperative. Here, we update the cardiac safety analysis of the N9831 trial after a median follow-up time of 9.2 years for patients who proceeded to post-AC therapy. The investigators of the B-31 trial developed a cardiac risk score (CRS) to incorporate the patient's age and baseline LVEF to predict the absolute risk of CEs in individual patients.11 Because the treatment arms in the B-31 trial were comparable to arms A and C of N9831, we also sought to validate the cardiac risk score in our patients.
METHODS
We have previously described the N9831 treatment protocol, eligibility criteria, and cardiac monitoring procedures2,5 (AppendixFig A1, online only). Briefly, patients with primary, operable, HER2-positive (by immunohistochemistry [IHC], 3+, or fluorescent in situ hybridization [FISH] ratio, ≥ 2), lymph node–positive or high-risk lymph node–negative breast cancer, with no evidence of metastases were enrolled on this study. At registration, all patients were required to have an LVEF within the institutional normal range as assessed by multigated acquisition scan (MUGA) or echocardiography (ECHO). Patients with active cardiac disease; history of CHF, arrhythmia, or valvular heart disease; myocardial infarction; uncontrolled hypertension; or other cardiovascular disorders were excluded. Written informed consent was obtained from all patients. MUGA or ECHO to evaluate LVEF was performed at baseline (within 3 weeks before registration), at 3, 6, and 9 months after registration, and after completion of chemotherapy (18 months for arms A and C and 21 months for arm B). Thereafter, LVEF evaluation by MUGA or ECHO was performed at 6 years. Moreover, patients were observed for clinical cardiac events yearly after completion of the assigned treatment.
In this study, initiation of trastuzumab was not permitted in patients whose one-time post-AC LVEF decreased by 15 percentage points or more to a value of less than or equal to 15 percentage points below the institutional LLN or in those who developed symptoms related to left ventricular dysfunction, cardiac ischemia, or arrhythmia while they received AC. Hence, this cardiac safety analysis is restricted to patients who proceeded to post-AC therapy and received at least one dose of post-AC therapy. During treatment with trastuzumab or paclitaxel and trastuzumab, if LVEF decreased by greater than 15% points or to 10% to 15% points below the LLN (compared with registration LVEF), trastuzumab treatment was held. After repeat evaluation at 4 weeks, if the LVEF recovered, trastuzumab was resumed; if no recovery was noted, trastuzumab was discontinued. LVEF recovery was defined as the return of LVEF to at least the institutional LLN.
Cardiac events were defined as symptomatic CHF, definite cardiac death (CD; as a result of myocardial infarction, CHF, or arrhythmia), or probable CD (patient death without documented etiology). Three cardiologists independently investigated all CEs, and an event was confirmed as a CE if agreement was reached between at least two cardiologists.
Statistical Analyses
The cumulative incidence of CEs after starting post-AC treatment was estimated nonparametrically, in which patients were considered at risk from day 1 of cycle 5 until recurrence, second primary cancer (including contralateral breast disease), noncardiac deaths, or last follow-up.12 In the subset of patients randomly assigned to trastuzumab-containing regimens, log-rank tests were used to assess potential risk factors for CEs, in which the time for patients who did not develop a cardiac adverse event was censored at the last follow-up date. Point and interval estimates of associated hazard ratios (HRs) were obtained from univariable Cox models by using registration risk factors such as age, LVEF at registration, use of antihypertensive medications, ethnicity, body mass index (BMI), and radiation therapy as predictors. The CRS was calculated by using the formula from the B-31 trial11 and was also included as a univariable risk factor in a Cox model. The predicted probability of a CE was also plotted as a function of the CRS. Mean levels of LVEF at 6-years after registration and the mean change from baseline were compared between each trastuzumab-containing arm and the non–trastuzumab-containing arm by using two-sample t tests or the Wilcoxon signed-rank test, if the normality assumption was not met. The significance level for all tests was designated as .05. Two-sided tests were used for all analyses.
RESULTS
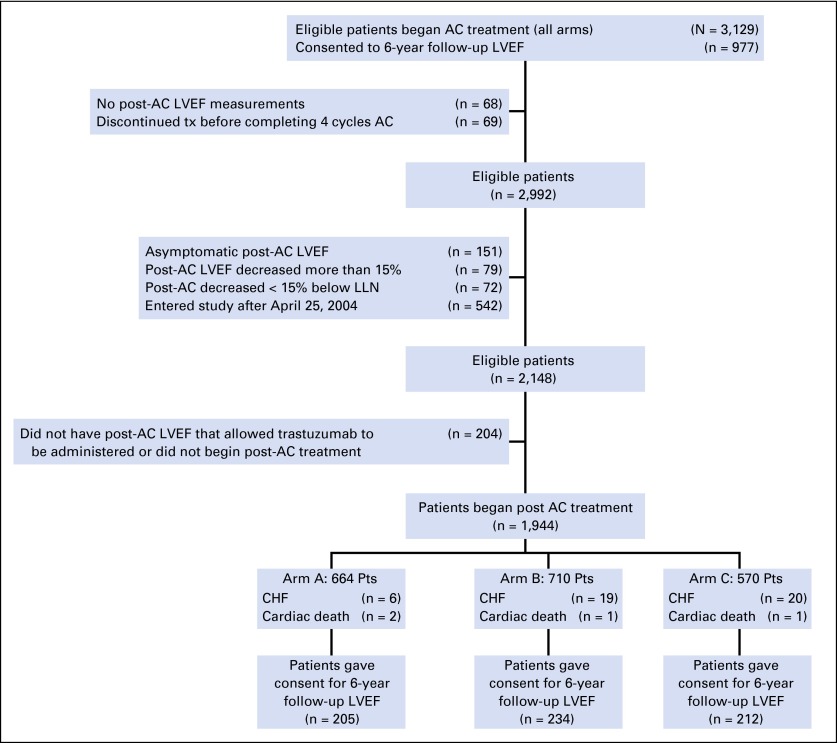
Of the 3,129 eligible patients who began AC treatment, 1,944 patients proceeded to post-AC therapy (n = 1,876 patients with satisfactory post-AC LVEF that allowed trastuzumab administration; n = 68 patients who had no LVEF evaluation) and represented the primary evaluable cohort for assessment of cardiac events (arm A, n = 664; arm B, n = 710; arm C, n = 570; Fig 1). The median follow-up time was 9.2 years (min, 0 years; max, 12.9 years). Baseline characteristics of the patients were similar across all three arms.5,10 The median age of patients was 49 years (range, 19 to 79 years), and 86% of patients were white. Approximately 17% of patients (n = 331) reported use of antihypertensive medications at baseline (arm A, n = 114; arm B, n = 115; arm C, n = 102), and 75% of patients (n = 1,451) received radiation therapy (arm A, n = 494; arm B, n = 535; arm C, n = 422). The median baseline LVEF was 63% (min, 45%; max, 87%). Of the 1,944 patients, 651 patients consented to a 6-year follow-up LVEF evaluation.
Fig 1.
CONSORT diagram for follow-up and cardiac events for NCCTG N9831 adjuvant trastuzumab trial. AC, doxorubicin plus cyclophosphamide; CHF, congestive heart failure; LLN, lower limit of normal; LVEF, left ventricular ejection fraction.
LVEF in the Post-AC Treatment Patients at 6 Years
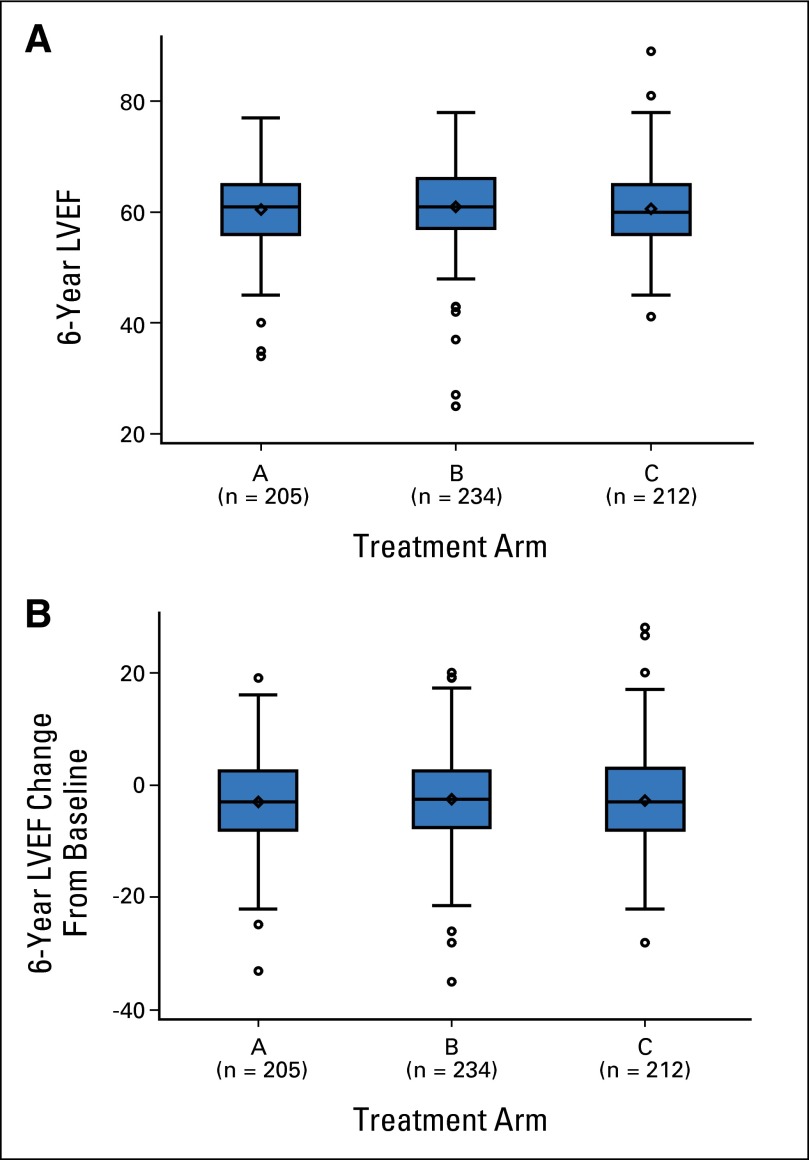
The median LVEF at 6 years was 61% in arm A (range, 34% to 77%), 61% in arm B (range, 25% to 78%), and 60% in arm C (range, 41.2% to 89%; Fig 2A). The absolute median LVEF change from baseline at 6 years was −3.0% in arm A (range, −33% to +19%), −2.5% in arm B (range, −34.9% to +20%), and −3.0% in arm C (range, −28% to +28%; Fig 2B). Table 1 summarizes the changes in LVEF in patients who proceeded to post-AC therapy and those who consented to a 6-year LVEF evaluation.
Fig 2.
(A) The 6-year median (range) left ventricular ejection fraction (LVEF) values in treatment arms A, B, and C. Arm A, 61% (34% to 77%); arm B, 61% (25% to 78%); and arm C, 60% (41.2% to 89%). (B) The 6-year median (range) LVEF change from baseline in treatment arms A, B, and C. Arm A, −3.0% (−33% to 19%); arm B, −2.5% (−34.9% to 20%); and arm C, −3.0% (−28% to 28%).
Table 1.
Change in LVEF in Patients Who Proceeded to Post-AC Therapy and Who Consented to LVEF Evaluation at 6 Years
| LVEF Variable | No. (%) of Patients by Arm |
||
|---|---|---|---|
| A (n = 664) | B (n = 710) | C (n = 570) | |
| All patients on the N9831 cohort (N = 1944) with LVEF decrease* | |||
| By ≥ 10 points | 174 (26.2) | 251 (35.3) | 227 (39.8) |
| By ≥ 15 points | 64 (9.6) | 104 (14.6) | 107 (18.7) |
| To below the LLN | 80 (12) | 119 (16.7) | 136 (23.8) |
| Patients on the N9831 cohort who had a 6-year LVEF evaluation (n = 651) | 205 | 234 | 212 |
| With LVEF decrease by ≥ 10 points | 42 (20.5) | 46 (19.6) | 48 (22.6) |
| With LVEF decrease by ≥ 15 points | 19 (9.2) | 17 (7.3) | 19 (8.9) |
| With LVEF decrease to below the LLN | 13 (6.3) | 11 (4.7) | 10 (4.7) |
Abbreviations: AC, doxorubicin plus cyclophosphamide; LLN, lower limit of normal; LVEF, left ventricular ejection fraction.
Multigated acquisition scan or echocardiography to evaluate LVEF was performed at baseline (within 3 weeks before registration), at 3, 6, and 9 months after registration, and after completion of chemotherapy (18 months for arms A and C and 21 months for arm B).
Clinically Significant Cardiac Events During Post-AC Treatment Among Patients Who Received Trastuzumab
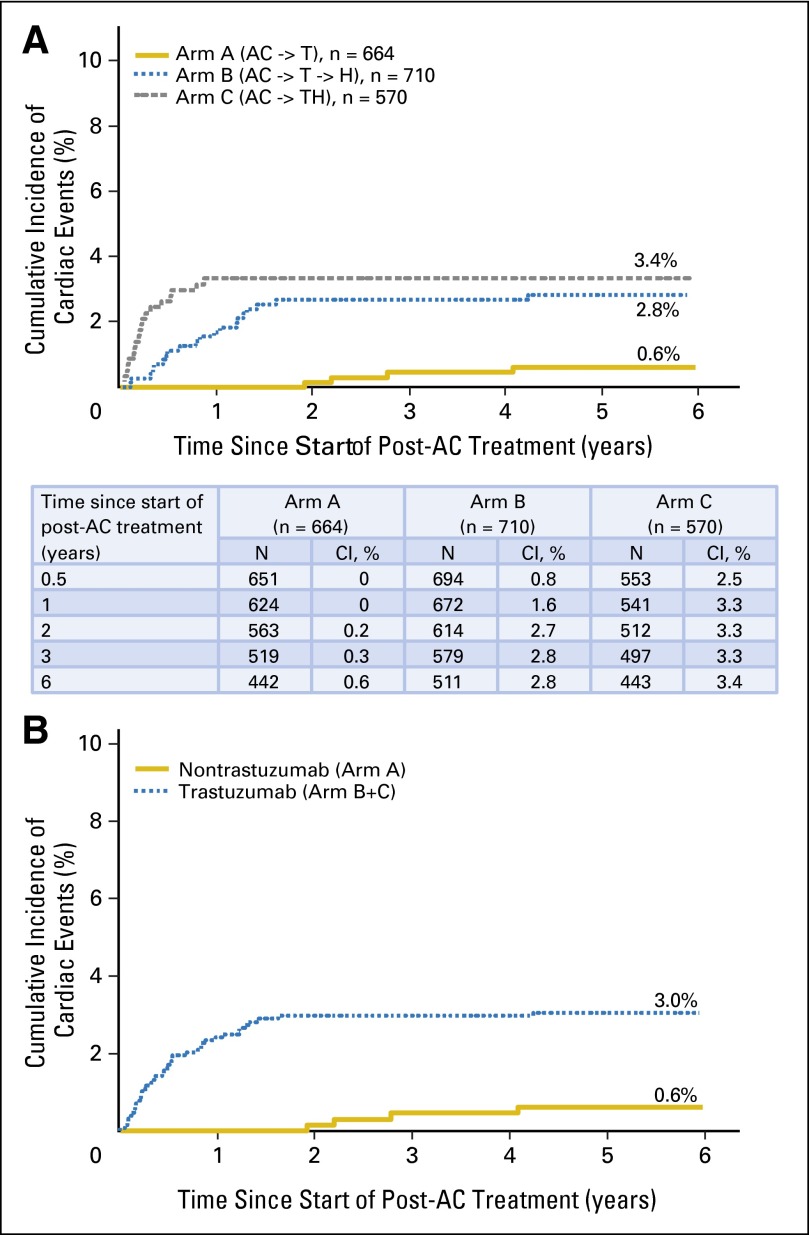
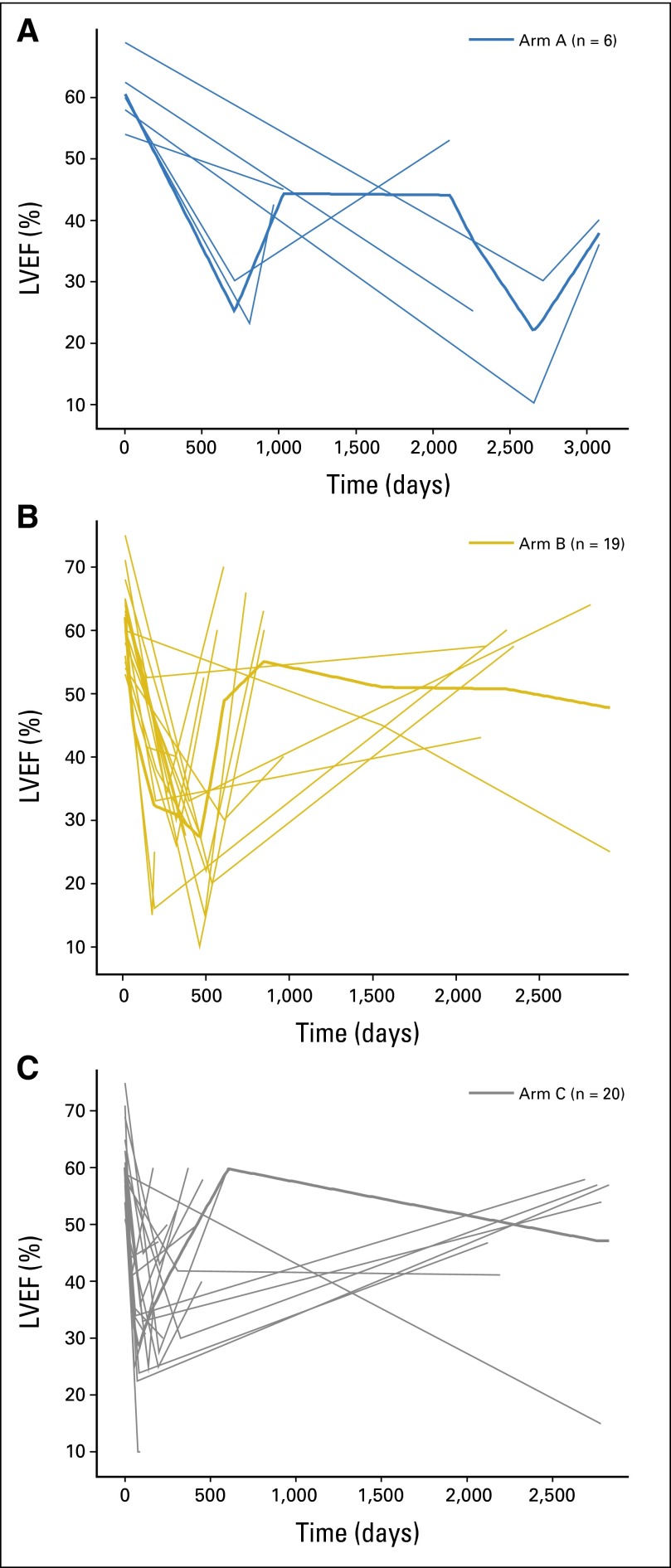
The 6-year cumulative incidence for CEs was 0.6% (95% CI, 0% to 1.2%) in arm A, 2.8% (95% CI, 1.6% to 4.1%) in arm B, and 3.4% (95% CI, 1.9% to 4.8%) in arm C (Fig 3A). The cumulative incidence for CEs in the trastuzumab-containing arms (arms B and C) was 3% (HR for CE in arms B and C, 2.9; P = .0034; Fig 3B). There was a significant increase in risk of CEs in arms B (HR, 2.6; 95% CI, 1.1 to 6.2) and C (HR, 3.4; 95% CI, 1.4 to 8.0) compared with arm A (P = .0259). Treatment arms B and C maintained their increased risk after adjustment for registration risk factors (age, ethnicity, registration LVEF, BMI, radiation therapy, and use of antihypertensive medications), but no other factors were associated with a statistically significant increase in risk. Of the 1,944 patients who began post-AC treatment, 45 patients developed symptomatic CHF (arm A, n = 6; arm B, n = 19; arm C, n = 20). Of these, follow-up data on CHF-related symptoms, use of cardiac medications, and LVEF evaluation by MUGA/ECHO was available for 18 patients at 3 and 6 months and for 16 patients at 12 months post-CHF onset. The use of cardiac medications in patients who developed CHF was not collected beyond 12 months after CHF onset. The majority of patients who developed CHF received cardiac medications, which included diuretics, beta-blockers, and angiotensin-converting enzyme inhibitors. Only five additional diagnoses of CHF occurred beyond our previously reported follow-up time of 3.75 years (three occurrences of 497 patients in arm A and two occurrences of 1,046 patients in arms B and C). Of the 45 patients who developed CHF, LVEF recovered in 28 patients (arm A, n = 4; arm B, n = 12; arm C, n = 12; Fig 4). Twenty-three patients remained symptomatic at the 6-year follow-up (arm A, n = 8; arm B, n = 5; arm C, n = 10). Two CDs occurred in arm A; one, in arm B; and one, in arm C.
Fig 3.
(A) Cumulative incidence of cardiac events by treatment arm. (B) Cumulative incidence of cardiac events for nontrastuzumab arm (arm A) versus trastuzumab arms (arms B and C). AC, doxorubicin plus cyclophosphamide; T, paclitaxel; H, trastuzumab.
Fig 4.
Recovery of cardiac function (left ventricular ejection fraction [LVEF]) in patients with congestive heart failure. (A) No. of patients in arm A = 6; (B) arm B = 19; (C) arm C = 20. Bolded lines represent the average LVEF levels of all patients in each arm through time, and nonbolded lines in each arm represent the LVEF level of an individual patient through time.
Risk Factors for Cardiac Events Among Patients Assigned to Trastuzumab-Containing Arms
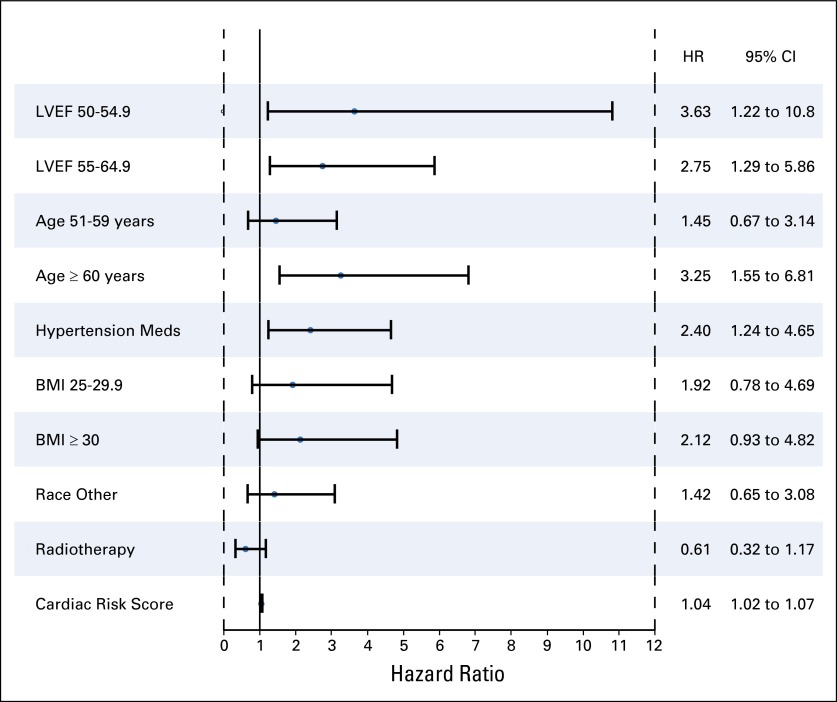
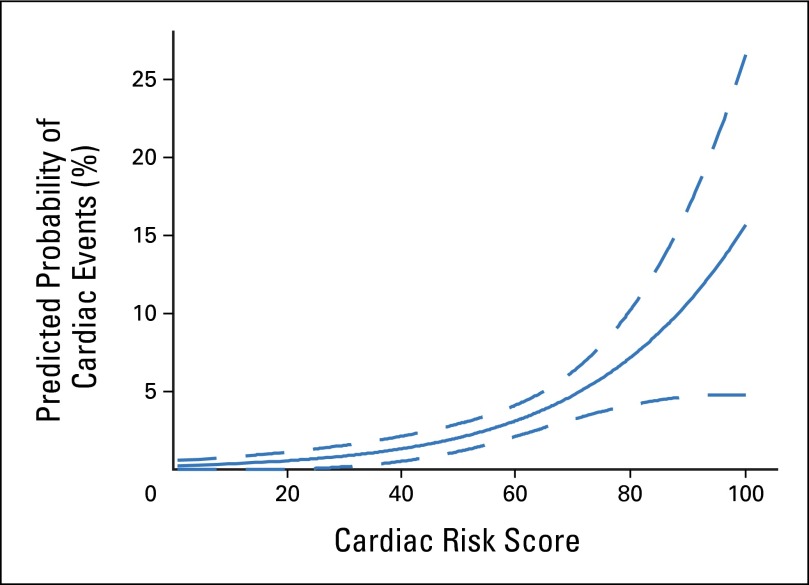
In univariable analysis, risk factors associated with a significant increased risk of CEs with a trastuzumab-containing regimen were age of 60 years or older (HR, 3.2; 95% CI, 1.5 to 6.8; P = .010); registration LVEF between 50% and 54.9% (HR, 3.6; 95% CI, 1.2 to 10.8; P = .020) or 55% and 64.9% (HR, 2.7; 95% CI, 1.3 to 5.9; P = .009), and use of antihypertensive medications (HR, 2.4; 95% CI, 1.2 to 4.6; P = .0148; Fig 5). Radiation therapy (P = .15), BMI (P = .15), and ethnicity (P = .30) were not statistically associated with the development of a CE. The CRS that incorporated age and baseline LVEF and that used the same formula derived by the B-31 investigators was applied to our data set.11 The plot in Figure 6 can be used to determine the predicted probability of a CE on the basis of the CRS value obtained for an individual patient. Similar to the results from B-31, the predicted probability of a CE increases as the CRS increases. Furthermore, CRS was a statistically significant risk factor associated with an increased risk of CEs in the trastuzumab arms (arms B and C) on univariable analysis (P < .001). Multivariable analysis showed a statistically significant association between risk factors of age and baseline LVEF only in a model that did not include CRS (Appendix, online only). When we replaced age and LVEF with CRS, CRS was statistically significant (HR, 1.042; 95% CI, 1.020 to 1.064). The c-statistic for the multivariable model that included CRS did not differ much from the model without CRS (0.6651 v 0.6767).
Fig 5.
Forest plot for univariable risk factors associated with an increased risk of cardiac event in patients who proceeded to post–doxorubicin plus cyclophosphamide therapy. Left ventricular ejection fraction (LVEF) of 65 or greater, age 50 years or younger, body mass index (BMI) of 24.9 or less, white ethnicity, and patients who did not receive radiotherapy were used as reference in each of the groups. HR, hazard ratio.
Fig 6.
Predicted probability of cardiac event (CE) using cardiac risk score (CRS). Plotting the CRS calculated from the formula derived by the investigators of the B-31 trial11 against the predicted probability of cardiac events resulted in similar findings as were reported in B-31. As the CRS increases, the predicted probability of a CE increases as well.
DISCUSSION
Our results demonstrate small but stable rates of cardiac events after adjuvant anthracycline-based chemotherapy plus trastuzumab for patients who have resected, HER2-positive breast cancer. These results are fairly consistent with the cardiac safety analysis of other pivotal adjuvant trastuzumab trials. In the BCIRG-006 (Breast Cancer International Research Group) trial, at a median follow-up of 65 months, the reported incidence of CEs was 0.4% in the docetaxel, carboplatin, and trastuzumab arm, 0.7% in AC followed by docetaxel arm, and 2% in AC followed by docetaxel and trastuzumab arm.4 Of note, in the BCIRG-006 study, greater than 50% of patients were younger than 50 years, and the upper age limit of patients at the time of enrollment was 70 years, which is a potentially relevant factor in the context of our study and those of others that demonstrated that older women have a higher risk of cardiac events. Thus, direct comparisons between N9831 and BCIRG-006 results must be made with extreme caution. Moreover, at a median follow-up of 8 years, severe CHF (New York Heart Association class III or IV) incidence in the Herceptin Adjuvant (HERA) trial was 0% in the observation arm and 0.8% each in the 1-year and 2-year trastuzumab arms.13 The difference in the significant LVEF decrease (ie, asymptomatic or mildly symptomatic decline in LVEF of at least 10% from baseline LVEF and a decline to < 50%) between the 1-year trastuzumab and the observation arm was 3.2%. In the HERA study, patients were randomly assigned after completion of all chemotherapy (adjuvant/neoadjuvant), and baseline LVEF had to be greater than 55% at study entry. Reassuring cardiac safety data were also reported in the long-term analysis of the B-31 trial; the incidence of CEs was 4.0% in the trastuzumab arm and was 1.3% in the nontrastuzumab arm at a median follow-up of 7 years.11 In addition, in 78% of patients (54 of 69 patients) in whom trastuzumab was discontinued because of an asymptomatic decline in cardiac function, the LVEF recovered to at least 50%. An independent retrospective review of 173 patients with CEs from the B-31 and N9831 trials demonstrated a 2% incidence of symptomatic CHF in the trastuzumab-containing arm versus an incidence of 0.45% in the chemotherapy-alone arm.8 Complete or partial recovery was observed in 86.1% of trastuzumab-treated patients who had symptomatic CHF.8
Moja et al14 reported a meta-analysis of eight trastuzumab-based adjuvant or neoadjuvant chemotherapy trials that involved 11,991 patients who had HER2-positive early breast cancer and found an increased risk in patients treated with trastuzumab-based versus non–trastuzumab-based regimens for severe heart failure (2.5 v 0.4%; relative risk, 5.11; P < .001) and for LVEF decline (relative risk, 1.83; P < .001).14 There was no difference in the cardiotoxicity profile in trials with concurrent versus sequential administration of chemotherapy and trastuzumab.
In the recently reported results of the Adjuvant Lapatinib and/or Trastuzumab Treatment Optimization (ALTTO) study, the incidences of significant LVEF decline were 4.5%, 2.4%, and 3.7%, with trastuzumab alone, lapatinib and trastuzumab administered sequentially, or lapatinib and trastuzumab administered in combination, respectively.15 The incidence of CD or New York Heart Association class III or IV CHF was less than 1% in all arms at a median follow-up time of 4.5 years.
Although there is concern about the risk for future cardiac insults from trastuzumab therapy,16 in our study, we observed only two occurrences of CHF more than 3 years after trastuzumab initiation, which suggests that the late onset of cardiac dysfunction with trastuzumab is rare. In addition, LVEF recovered in greater than half of the patients with CHF after trastuzumab was discontinued and heart failure therapy was initiated; this finding was consistent with other studies.11,17,18 One of the limitations of our study is the relatively lower number of patients (33.4%) who consented for 6-year follow-up LVEF evaluation (compared with those who received post-AC therapy); this low number may be due to timing: consent was obtained later in the study period. However, analysis of the cumulative incidence of CEs and related risk factors was performed on the 1,944 patients who proceeded to post-AC therapy. Although the mechanism of trastuzumab-induced cardiac toxicity remains unclear, it has been postulated that it differs from that caused by anthracyclines. Anthracycline-associated ultrastructural changes that show apoptosis and necrosis on cardiac biopsy are not typical of trastuzumab-induced cardiac toxicity, and the toxicity of trastuzumab is usually not dose related.17,18 All patients on this study received anthracyclines before trastuzumab; interestingly, the patients who had LVEF dysfunction at 6 years were the same patients who had LVEF dysfunction during trastuzumab-paclitaxel therapy. In addition, LVEF dysfunction at 6 years was not statistically different among the three treatment arms, so long-term LVEF dysfunction may not be related to trastuzumab but, possibly, to exposure to anthracyclines and/or increasing age.
We also evaluated serum cardiac factors (including troponin-1 and B natuiretic peptide) in our study but did not find clear evidence that they were predictive of clinical cardiac events (Appendix). Of note, the serial blood levels were obtained at baseline and then before the first therapy (instead of immediately or shortly after anthracyclines, taxanes, or trastuzumab), although all patients at 9 and 12 months had steady-state levels of trastuzumab if they were randomly assigned to arms B or C, and those in arm B of the N9831 study already had steady-state levels at the 6-month blood draw. Serum cardiac factors have been of interest for many investigators, but there is no conclusive proof that they can be helpful.
With the SEER–Medicare database, Ezaz et al19 identified older women (age range, 67 to 94 years) with breast cancer who had received adjuvant trastuzumab therapy and developed a risk model to incorporate age, adjuvant chemotherapy, coronary artery disease, atrial fibrillation or flutter, diabetes, hypertension, and renal failure.19 This seven-factor score was used to stratify 1,664 women into low, medium, and high scores that corresponded to a 3-year risk of heart failure of 16.2%, 26%, and 39.5%, respectively. However, use of the SEER data may not be ideal, because the score is derived from administrative rather than clinical data, because information of specific anticancer agents or cumulative doses are not available, because the baseline LVEF data are not available, or because standardized clinical definitions of cardiac events are not used. The cardiac risk score calculated with the patient's age and baseline LVEF was developed by the B-31 investigators11 and was validated in our study; this score serves as a useful tool for clinicians to estimate the risk of trastuzumab-induced cardiac toxicity in a given patient and allows for a more individualized benefit-risk assessment. This individualization, in turn, may enable clinicians to consider an alternative treatment regimen and allow more frequent monitoring for at-risk patients. These patients may also benefit from administration of cardioprotective medications, although this strategy is under investigation.
In summary, the incidence of trastuzumab-related CEs with the N9831 regimen is low and does not appear to be increasing over time, which affirms that doxorubicin at a cumulative dose of 240 mg/m2 administered over four doses plus cyclophosphamide followed by paclitaxel and trastuzumab is a highly appropriate therapy to consider and administer, especially in the context of benefits of therapy to reduce breast cancer recurrence and improve survival. Certain risk factors, such as older age, low pretreatment LVEF, and hypertension, appear to increase the risk of trastuzumab-related CEs according to our data. We could not validate the predictive utility of biomarkers, such as troponin I or BNP, with clinical cardiac events. Correlation of pharmacogenetic markers with the development of cardiac toxicity, serial evaluation of serum markers measured within 24 hours of drug administration, and prophylactic use of cardioprotective medications (eg, angiotensin-converting enzyme inhibitors and beta-blockers) in women deemed at high risk for CHF should be investigated in the setting of large prospective trials.
Supplementary Material
Appendix
Biomarker Section
Blood and plasma for circulating markers (B natriuretic peptide [BNP], troponin T [TnT], troponin L, C-reactive protein, tumor necrosis factor-α, interleukin [IL] -6, and IL-1β) were drawn before first cycle of doxorubicin plus cyclophosphamide (at baseline) and within 1 hour of completion of the first or second dose of doxorubicin plus cyclophosphamide, paclitaxel, and trastuzumab. Sixty-seven patients (median age, 52 years) had baseline and at least one post-treatment samples and serial left ventricular ejection fraction (LVEF) determination. LVEF evaluation by multigated acquisition scan, echocardiography, or both was available in 69%, 24%, and 7% of these patients. None of these patients experienced a protocol-defined CE; however, 21% (14 of 67 patients) met criteria for LVEF decline, with a median LVEF of 50%. Each marker was evaluated at baseline and for any serial elevation from baseline for each patient. Then, levels were correlated with LVEF decline (Appendix Tables A1 to A4).
For C-reactive protein and tumor necrosis factor-α, normal values were seen at baseline and subsequently became abnormal in 45% and 16% of patients, respectively, but there was no correlation with cardiac dysfunction. In addition, IL-6 and IL-1β levels also had no association with cardiac dysfunction, and there was no significant change over time in any of these values. No patient had an elevated TnT (ie, > 0.01 μg/mL) at baseline. Elevation in baseline troponin I (> 0.04 μg/mL) was observed in 21% of patients with subsequent cardiac dysfunction and in 6% of patients without (P = .10). Baseline elevation of BNP (> 40 ng/mL) was also observed in 21% of patients with subsequent cardiac dysfunction and in 8% of those without (P = .18; Appendix Table A3). Six patients (11%) had a normal BNP at baseline with a subsequent doubling to greater than 40 ng/mL, at least. The rate of doubling in BNP to greater than 40 ng/mL was numerically higher in patients who had subsequent cardiac dysfunction than in patients who did not (27% v 7%; P = .09; Appendix Table A4), although this difference did not reach statistical significance In patients with normal TnT and troponin I at baseline (31%), there was a subsequent increase to an abnormal value. However, an elevation in either marker or both markers in the same patient did not correlate with risk of cardiac dysfunction (Appendix Table A4).
Table A1.
Multivariable Analysis of Risk Factors Associated With an Increased Risk of Cardiac Event in Patients Who Proceeded to Post-AC Therapy
| Characteristic | Hazard Ratio | 95% CI |
|---|---|---|
| Age, years | ||
| ≥ 60 | 2.755 | 1.237 to 6.137 |
| 50-59 | 1.350 | 0.615 to 2.964 |
| < 50 | Reference | Reference |
| Current or prior antihypertensive medications | ||
| Yes | 1.821 | 0.890 to 3.725 |
| No | Reference | Reference |
| LVEF at registration, % | ||
| ≥ 65 | Reference | Reference |
| 55-64.9 | 2.908 | 1.361 to 6.210 |
| 50-54.9 | 3.975 | 1.329 to 11.888 |
NOTE. This analysis only includes variables that were found to be significant in univariable analysis. Cardiac risk score (which incorporated age and baseline LVEF) is excluded from this analysis.
Abbreviations: AC, doxorubicin plus cyclophosphamide; LVEF, left ventricular ejection fraction.
Table A2.
Cardiac Marker Data
| Cardiac Marker (cutoff for abnormal) | Baseline Data |
Treatment Regimen |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AC |
T |
T + H |
H |
||||||||||||
| No. of Patients | Median (min, max) | No. Abnormal | No. of Patients | Median (min, max) | No. Abnormal | No. of Patients | Median (min, max) | No. Abnormal | No. of Patients | Median (min, max) | No. Abnormal | No. of Patients | Median (min, max) | No. Abnormal | |
| BNP (> 40 ng/mL) | 65 | 13.7 (< 4.0, 156) | 7 | 68 | 15.6 (< 4.0, 179) | 8 | 5 | 17.2 (12.3, 49.9) | 1 | 73 | 14.1 (< 4.0, 191) | 9 | 61 | 4.7 (< 4.0, 120) | 3 |
| CRP (> 0.3 mg/L) | 69 | 2 (0.2, 25.6) | 26 | 68 | 2.4 (0.3, 40.7) | 31 | 5 | 3.3 (1.9, 4.4) | 3 | 73 | 2 (0.1, 32.8) | 29 | 63 | 2.1 (< 0.1, 40.1) | 27 |
| TnT (> 0.01 μg/mL) | 68 | < 0.01 (< 0.01, 0.01) | 0 | 68 | < 0.01 (< 0.01, 0.01) | 0 | 5 | < 0.01 (< 0.01, 0.01) | 0 | 73 | < 0.01 (< 0.01, 0.06) | 7 | 63 | < 0.01 (< 0.01, 0.02) | 2 |
| TNF-α (> 4.71 pg/mL) | 69 | 1.6 (< 0.05, 14) | 3 | 68 | 1.2 (< 0.5, 3.4) | 0 | 5 | 1.4 (0.56, 2.2) | 0 | 72 | 1.4 (< 0.5, 25) | 2 | 62 | 1.5 (< 0.5, 35) | 8 |
| IL-1β (> 0.54 pg/mL) | 67 | < 0.5 (< 0.5, 1.9) | 5 | 68 | < 0.5 (< 0.5, 3.7) | 1 | 5 | < 0.5 (< 0.05, < 0.05) | 0 | 73 | < 0.5 (< 0.5, 0.9) | 1 | 61 | < 0.5 (< 0.5, 1.9) | 2 |
| TnI (> 0.04 mcg/mL) | 68 | 0.01 (< 0.01, 0.07) | 6 | 68 | 0.01 (< 0.01, 24.1) | 6 | 5 | 0.04 (0.01, 0.08) | 1 | 73 | 0.03 (< 0.01, 8.16) | 17 | 61 | 0.02 (< 0.01, 10) | 3 |
| IL-6 (> 10 pg/mL) | 68 | 2 (0.8, 22.4) | 4 | 69 | 1.9 (0.6, 27.5) | 1 | 5 | 1.3 (0.4, 3.7) | 0 | 73 | 1.8 (< 0.3, 27.6) | 3 | 60 | 3.55 (0.4, 128) | 5 |
Abbreviations: AC, doxorubicin plus cyclophosphamide; BNP, brain natriuretic peptide; CRP, C-reactive protein; IL, interleukin; T, paclitaxel; T + H, paclitaxel plus trastuzumab; Tn, troponin; TNF, tumor necrosis factor.
Table A3.
Decrease in LVEF by Baseline Markers
| Baseline Marker | Decrease in LVEF* |
P | |
|---|---|---|---|
| Yes | No | ||
| BNP > 40 ng/mL | 3/14 (21%) | 4/49 (8%) | .18 |
| TnT > 0.01 mcg/mL | 0/14 | 0/52 | |
| TnI > 0.04 mcg/mL | 3/14 (21%) | 3/52 (6%) | .10 |
| CRP > 3 mg/L | 6/14 (43%) | 20/53 (38%) | .76 |
| TNF-α > 4.71 pg/mL | 0/14 | 3/53 (6%) | 1.00 |
| IL-6 > 10 pg/mL | 1/14 (7%) | 3/52 (6%) | 1.00 |
| IL-1β > 0.54 pg/mL | 0/13 | 0/47 | |
Abbreviations: BNP, brain natriuretic peptide; CRP, C-reactive protein; IL, interleukin; LVEF, left ventricular ejection fraction; Tn, troponin; TNF, tumor necrosis factor.
Decrease in LVEF measure was defined as a decrease of 10% to 15% from baseline to below the lower limit of normal or a decrease in LVEF > 15%.
Table A4.
Decreases in LVEF by Change of Markers From Baseline Levels
| Baseline Marker Change | No. of Patients/Total No. of Patients (%) With Decrease in LVEF* |
P | |
|---|---|---|---|
| Yes | No | ||
| BNP normal at baseline to double postbaseline and > 40 ng/mL | 3/11 (27) | 3/44 (7) | .09 |
| TnT normal at baseline to abnormal postbaseline | 2/14 (14) | 6/51 (12) | 1.00 |
| TnI normal at baseline to abnormal postbaseline | 3/11 (27) | 14/49 (29) | 1.00 |
| TnT and TnI both normal at baseline to both abnormal postbaseline | 1/11 (9) | 5/48 (10) | 1.00 |
| CRP normal at baseline to abnormal postbaseline | 5/8 (63) | 13/32 (41) | .43 |
| TNF-α normal at baseline to abnormal postbaseline | 2/14 (14) | 6/51 (12) | 1.00 |
| IL-6 normal at baseline to abnormal postbaseline | 0/13 | 3/48 (6) | 1.00 |
| IL-1β normal at baseline to abnormal postbaseline | 0/13 | 2/46 (4) | 1.00 |
Abbreviations: BNP, brain natriuretic peptide; CRP, C-reactive protein; IL, interleukin; LVEF, left ventricular ejection fraction; Tn, troponin; TNF, tumor necrosis factor.
Decrease in LVEF measure was defined as a decrease of 10% to 15% from baseline to below the lower limit of normal or a decrease in LVEF > 15%.
Fig A1.
NCCTG N9831 clinical trial schema. Doxorubicin (A) 60 mg/m2; cyclophosphamide (C) 600 mg/m2; paclitaxel 80 mg/m2; trastuzumab 4 mg/kg loading dose followed by 2 mg/kg weekly (qw). Eval, evaluation; LVEF, left ventricular ejection fraction; q3w, every 3 weeks.
Footnotes
Supported by the National Cancer Institute of the National Institutes of Health under Awards No. U10CA180821 and U10CA180882 to the Alliance for Clinical Trials in Oncology and Award No. CA25224 to the legacy North Central Cancer Treatment Group.
Presented in part at the 50th Annual Meeting of the American Society of Clinical Oncology, Chicago, IL, May 30-June 3, 2014.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Clinical trial information: NCT00005970.
AUTHOR CONTRIBUTIONS
Conception and design: Pooja P. Advani, Karla V. Ballman, Edith A. Perez
Provision of study materials or patients: Edith A. Perez
Collection and assembly of data: Pooja P. Advani, Edith A. Perez
Data analysis and interpretation: All authors
Manuscript writing: All authors
Final approval of manuscript: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Long-Term Cardiac Safety Analysis of NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or jco.ascopubs.org/site/ifc.
Pooja P. Advani
No relationship to disclose
Karla V. Ballman
Consulting or Advisory Role: Astellas Pharma
Patents, Royalties, Other Intellectual Property: Patent No. 20090298082/pending 61/982,251
Travis J. Dockter
No relationship to disclose
Gerardo Colon-Otero
Research Funding: Novartis (Inst)
Edith A. Perez
No relationship to disclose
REFERENCES
- 1.Owens MA, Horten BC, Da Silva MM. HER2 amplification ratios by fluorescence in situ hybridization and correlation with immunohistochemistry in a cohort of 6,556 breast cancer tissues. Clin Breast Cancer. 2004;5:63–69. doi: 10.3816/cbc.2004.n.011. [DOI] [PubMed] [Google Scholar]
- 2.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med. 2011;365:1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez EA, Romond EH, Suman VJ, et al. Four-year follow-up of trastuzumab plus adjuvant chemotherapy for operable human epidermal growth factor receptor 2-positive breast cancer: Joint analysis of data from NCCTG N9831 and NSABP B-31. J Clin Oncol. 2011;29:3366–3373. doi: 10.1200/JCO.2011.35.0868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viani GA, Afonso SL, Stefano EJ, et al. Adjuvant trastuzumab in the treatment of HER2-positive early breast cancer: A meta-analysis of published randomized trials. BMC Cancer. 2007;7:153. doi: 10.1186/1471-2407-7-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perez EA, Romond EH, Suman VJ, et al. Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2–positive breast cancer: Planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831. J Clin Oncol. 2014;32:3744–3752. doi: 10.1200/JCO.2014.55.5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Russell SD, Blackwell KL, Lawrence J, et al. Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: A combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol. 2010;28:3416–3421. doi: 10.1200/JCO.2009.23.6950. [DOI] [PubMed] [Google Scholar]
- 9.Chavez-MacGregor M, Zhang N, Buchholz TA, et al. Trastuzumab-related cardiotoxicity among older patients with breast cancer. J Clin Oncol. 2013;31:4222–4228. doi: 10.1200/JCO.2013.48.7884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Perez EA, Suman VJ, Davidson NE, et al. Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol. 2008;26:1231–1238. doi: 10.1200/JCO.2007.13.5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol. 2012;30:3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gray RJ. A class of K sample tests for comparing the cumulative incidence of a competing risk. Ann Statist. 1988;16:1141–1154. [Google Scholar]
- 13.de Azambuja E, Procter MJ, van Veldhuisen DJ, et al. Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin adjuvant trial (BIG 1-01) J Clin Oncol. 2014;32:2159–2165. doi: 10.1200/JCO.2013.53.9288. [DOI] [PubMed] [Google Scholar]
- 14.Moja L, Tagliabue L, Balduzzi S, et al. Trastuzumab-containing regimens for early breast cancer. Cochrane Database Syst Rev. 2012;4:CD006243. doi: 10.1002/14651858.CD006243.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perez EA, Holmes E, De Azambuja E, et al. Disease-free survival (DFS) in the lapatinib alone arm and expanded results of the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) in the adjuvant treatment of HER2-positive early breast cancer (EBC) Ann Oncol. 2014;25(suppl 5):v1–v41. (suppl 5; abstr LBA7) [Google Scholar]
- 16.Telli ML, Hunt SA, Carlson RW, et al. Trastuzumab-related cardiotoxicity: Calling into question the concept of reversibility. J Clin Oncol. 2007;25:3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 17.Ewer MS, Lippman SM. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J Clin Oncol. 2005;23:2900–2902. doi: 10.1200/JCO.2005.05.827. [DOI] [PubMed] [Google Scholar]
- 18.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: Clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 19.Ezaz G, Long JB, Gross CP, et al. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.