Abstract
The Endoplasmic Reticulum (ER) is most notable for its central roles in calcium ion storage, lipid biosynthesis, and protein sorting and processing. By virtue of its extensive membrane contact sites that connect the ER to most other organelles and to the plasma membrane, the ER can also regulate diverse cellular processes including inflammatory and insulin signaling, nutrient metabolism, and cell proliferation and death via a signaling pathway called the unfolded protein response (UPR). Chronic UPR activation has been observed in liver and/or adipose tissue of dietary and genetic murine models of obesity, and in human obesity and non-alcoholic fatty liver disease (NAFLD). Activation of the UPR in obesity and obesity-related disorders likely has two origins. One linked to classic ER stress involving the ER lumen and one linked to alterations to the ER membrane environment. This review discusses both of these origins and also considers the role of post-translational protein modifications, such as acetylation and palmitoylation, and ER-mitochondrial interactions to obesity-mediated impairments in the ER and activation of the UPR.
Keywords: Unfolded Protein Response, Non-alcoholic fatty liver disease, Adipose Tissue, Liver, Lipid Composition, Calcium
Introduction
The Endoplasmic Reticulum (ER) is most notable for its central roles in calcium ion storage, lipid biosynthesis, and protein sorting and processing. By virtue of its extensive membrane contact sites that connect the ER to most other organelles and to the plasma membrane, the ER can also regulate diverse cellular processes including inflammatory and insulin signaling, nutrient metabolism, and cell proliferation and death via a signaling pathway called the unfolded protein response (UPR). The UPR has traditionally been regarded as a homeostatic mechanism that preserves ER function in response to imbalances between the protein load delivered to the ER and the ability of the ER to process this load. This adaptive response is especially vital to secretory cells that maintain high levels of protein synthesis, such as hepatocytes and insulin-producing β-cells. Persistent activation of the UPR, on the other hand, is associated with the pathogenesis of a number of metabolic diseases [1, 2]. Chronic UPR activation has been observed in liver and/or adipose tissue of dietary and genetic murine models of obesity, and in human obesity and non-alcoholic fatty liver disease (NAFLD) [3–9]. Activation of the UPR typically occurs in response to the accumulation of unfolded proteins in the ER lumen, termed ER stress, however recent studies have demonstrated that ER membrane events independent of the ER lumen can trigger the UPR. In this review we will present an expanded view of the ER, one that considers the role of the ER membrane and ER-mitochondrial interactions in UPR activation and their relevance to the relationship between the ER and chronic, metabolic diseases.
Endoplasmic Reticulum and the Unfolded Protein Response
The smooth ER produces the majority of membrane lipids and their intermediates including glycerophospholipids, ceramides, and cholesterol [10, 11]. Ceramide traffics to the Golgi for conversion to complex sphingolipids and much of the cholesterol is transported to other cellular compartments, leaving relatively little in the ER membrane [10, 12]. Therefore, the ER membrane is comprised of very low concentrations of cholesterol and complex sphingolipids [10]. The presence of such a specialized membrane lipid environment may be particularly relevant to chronic, metabolic diseases such as obesity and NAFLD, that are characterized by ectopic lipid accumulation and elevated circulating lipids.
The rough ER acts as the entry point to the protein secretory pathway. Proteins destined for secretion from the cell or for insertion into the plasma membrane first translocate to the rough ER en route to their final destinations. The ER lumen provides a specialized environment for protein folding and maturation, that includes high concentrations of calcium and a unique complement of molecular chaperones and folding enzymes [13]. A quality control system known as ER-associated degradation (ERAD) recognizes and removes nonnative proteins for degradation via the cytosolic ubiquitin-proteasome system [14, 15]. In this context, a central function of the UPR is to monitor and respond to the accumulation of improperly folded proteins in the ER lumen [16].
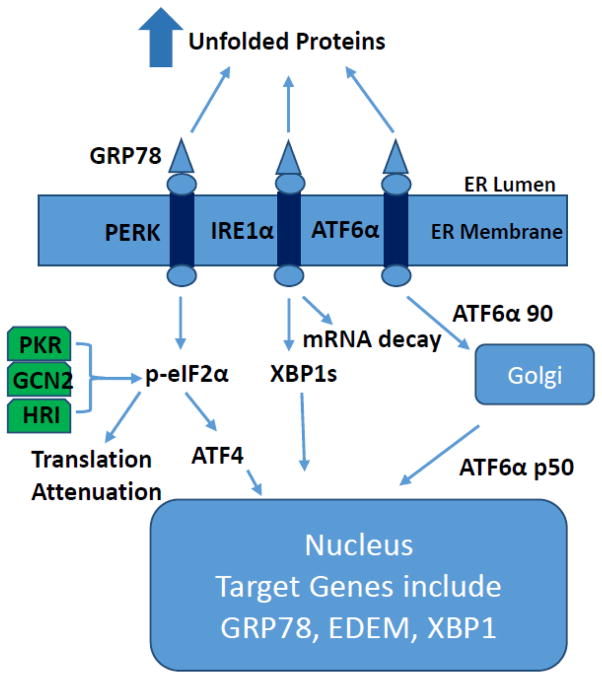
The UPR is now a well-characterized signaling pathway. In mammalian cells, UPR signaling is initiated by three ER-localized proteins (Fig. 1): double-stranded RNA-dependent protein kinase-like ER kinase (PERK), inositol-requiring 1α (IRE1α), and activating transcription factor-6α (ATF6α) [17]. It is currently thought that in the un-stressed state IRE1α, PERK, and ATF6α are maintained in an inactive conformation via their association with glucose-regulated protein 78/immunoglobulin-heavy chain-binding protein (GRP78). Accumulation of unfolded proteins titrates GRP78 from the proximal UPR sensors permitting dimerization/activation of these proteins and GRP78 migration into the ER lumen where it facilitates chaperone-mediated protein folding [18]. PERK activation leads to phosphorylation of the α-subunit of the translation initiation factor eIF2 (p-eIF2α) and subsequent attenuation of translation initiation, as well as selective translation of activating transcription factor-4 (ATF4) [19, 20]. Activation of IRE1α leads to the splicing of X-box-binding protein-1 (XBP1s) mRNA and subsequent transcription of molecular chaperones (e.g. GRP78) and genes involved in ERAD (e.g. ER degradation-enhancing α-like protein (EDEM)) [20]. IRE1α also appears to mediate rapid degradation of specific mRNAs, presumably in an effort to reduce production of proteins that require folding in the ER lumen [21, 22]. In addition, it appears that mRNAs encoding membrane and secreted proteins can be rapidly released from the ER upon induction of the UPR [23]. Activation of ATF6α leads to its release from the ER membrane, processing in the Golgi, and entry into the nucleus. Transcriptional targets of ATF6α include protein chaperones and XBP1 [24]. If these adaptive responses fail to resolve ER stress, UPR signaling can initiate regulated cell death.
Figure 1.
Overview of the mammalian unfolded protein response. Accumulation of unfolded proteins in the ER lumen results in activation of PERK, IRE1α, and ATF6α, and subsequent translational attenuation and activation of gene transcription.
Several studies and reviews have documented the interaction of the UPR with inflammatory signaling pathways, insulin signaling, glucose and lipid metabolism, cell proliferation, autophagy, and apoptosis [2, 3, 25–31]. The ability of the UPR to interact with such a diverse set of signaling and metabolic pathways suggests that ER protein quality control and UPR activation may play an important role in chronic, metabolic diseases characterized by inflammation, insulin resistance, and impairments in nutrient metabolism [2, 30, 32]. Often overlooked in discussions involving the UPR is the fact that PERK is only one of four protein kinases that can phosphorylate eIF2α; the other three being double-stranded RNA-activated protein kinase (PKR) which is activated in response to viral infection, general control non-derepressible 2 kinase (GCN2) which is activated in response to amino acid deprivation, and heme-regulated inhibitor kinase (HRI) which is primarily expressed in reticulocytes and appears to coordinate globin polypeptide synthesis with heme availability (Fig. 1) [25].
Activation of PKR has been observed in the liver and adipose tissue taken from dietary and genetic murine models of obesity, and the absence of PKR results in amelioration of many of the metabolic impairments observed in these models [33]. More recent studies have identified TAR RNA-binding protein (TRBP) and endogenous RNAs, small nucleolar RNAs (snoRNAs) that interact with and activate PKR in response to metabolic stress [34, 35]. Other research that has linked snoRNAs to lipotoxicity support the notion that both snoRNA- and TRBP-mediated regulation of PKR likely play important roles in metabolic diseases characterized by UPR activation [36, 37].
GCN2, as a sensor of amino acid deficiency, plays a key role in adapting amino acid metabolism to nutrient deprivation [38]. Amino acid deprivation also has profound effects on lipid metabolism, including reduced expression of lipogenic genes in the liver and mobilization of adipose tissue lipid stores [38]. However, GCN2 deficient mice developed hepatic steatosis and were characterized by reduced adipose tissue lipid mobilization in response to amino acid deprivation [38]. These data suggest that GCN2 can also regulate lipid metabolism during amino acid deficiency. Whether GCN2 plays a role in metabolic diseases defined by nutrient excess is presently unknown, however given its ability to regulate lipid metabolism in liver and adipose tissue, it is also a protein that must be considered when evaluating the role of the UPR in obesity and obesity-related disorders.
ER stress and activation of the UPR in obesity and obesity-related disorders
UPR activation is typically discussed in the context of ER stress or the accumulation of mis- or un-folded proteins in the ER lumen [39]. The primary evidence that obesity-mediated UPR activation involves ER stress has come from studies employing chemical chaperones [40]. Chemical chaperones, such as glycerol, trimethylamine-N-oxide, methyl-β-cyclodextrin, and 4-phenyl butyric acid (PBA), represent a group of low molecular weight compounds that can stabilize protein conformation, improve ER folding capacity, and facilitate the appropriate trafficking of mutant proteins [41–44]. Endogenous bile acid derivatives, such as ursodeoxycholic acid and taurine-conjugated ursodeoxycholic acid (TUDCA), can also modify ER function [45]. A number of misfolded proteins have been rescued by chemical or pharmacological chaperone intervention [46]. In addition, studies have demonstrated that chemical chaperones can reduce UPR activation in model systems of lysosomal storage disease, hereditary hemochromatosis, and cholangiocarcinoma [47–49].
Oral administration of PBA or TUDCA to ob/ob mice reduced UPR activation in liver and adipose tissue and improved insulin action [50]. Elevated free fatty acids and increased free fatty acid delivery to tissues is a common feature of obesity. Increased free fatty acid delivery in vivo results in the activation of the UPR in both adipose tissue and liver [51, 52]. Pretreatment of McA (Morris hepatoma 7777) liver cells with PBA or co-treatment of primary hepatocytes with PBA or TUDCA reduced UPR activation in response to increased free fatty acid delivery [53, 54]. These data are consistent with the notion that activation of the UPR in obesity involves ER stress; that is, the accumulation of unfolded proteins. However, it is important to note that TUDCA has been reported to also influence the release of calcium from the ER and the regulation of both protein kinase C and mitogen-activated kinase p42/44 [45, 48]. Thus, while chemical chaperones appear to alleviate obesity-mediated activation of the UPR, the mechanism of action through which this is accomplished remains unclear.
Endogenous chaperones, such as GRP78, when overexpressed in the liver of ob/ob mice reduced hepatic steatosis and markers of UPR activation, and improved insulin action [55]. GRP78 heterozygosity resulted in a compensatory UPR response that included upregulation of proteins involved in ERAD and other ER chaperone proteins, such as GRP94, calnexin and calreticulin in white adipose tissue [56]. In total, these data suggest that ER protein folding is impaired in the liver and white adipose tissue of obese mice.
An increase in protein synthesis represents one mechanism that could result in obesity-mediated ER stress. However, basal rates of protein synthesis were not increased in skeletal muscle of high-fat diet-fed rats [57]. In addition, ER-associated polysome profiling from liver tissue of lean, wildtype, and ob/ob mice at 2, 3, and 6 months of age suggested that the ER from livers of obese mice exhibited reduced protein synthesis [58]. In contrast, islet cells isolated from mice fed a high-fat diet for 7 days were characterized by increased polyribosome-associated RNA and activation of mammalian Target of Rapamycin (mTOR) [59]. Increased activation of the mTOR pathway was also observed in liver and skeletal muscle of obese rats, although it is not clear whether this increase was linked to protein synthesis or as a feature of the ensuing insulin resistance [60]. In total, these data suggest that an increase in the protein load to the ER does not contribute to the activation of the UPR in the liver of obese rodents, although more data are needed on the early stages of obesity development and UPR activation.
A reduction in folding capacity is a second mechanism that can lead to ER stress. The concentration of calcium within the ER lumen is an order of magnitude higher than the cytosol and several ER luminal chaperones require calcium to assist in protein folding [61, 62]. The sarco-/endoplasmic reticulum calcium ATPase (SERCA) is an ER membrane-bound protein that serves to pump calcium back in to the ER lumen, thus it plays an important role in both overall cellular calcium homeostasis and maintenance of ER lumen calcium stores [63]. Two studies have observed a reduction of SERCA2b (activity or protein level) in the liver of obese mice [64, 65]. Overexpression of SERCA2b in obese mice reduced activation of the UPR and improved glucose homeostasis in both studies. These data suggest that obesity-mediated changes in SERCA2b, and potentially ER calcium stores, may lead to a reduced capacity for chaperone-mediated protein folding, ER stress, and activation of the UPR.
A reduction in the degradation of mis- or un-folded proteins in the ER lumen is a third mechanism that can lead to ER stress. The ubiquitin-proteasome system (UPS) is required for the degradation of terminally misfolded ER proteins [14, 66]. Proteasome activity was reduced and polyubiquinated proteins increased in the liver of C57BL/6 mice fed a high-fat diet for 28 wks, 20 wk old db/db mice and 20 wk old ob/ob mice [67]. In this study, mice with impaired proteasome function (proteasome activator-28 null mice) were characterized by hepatic steatosis, hepatic insulin resistance and activation of the UPR, all of which were partially rescued by treatment with the chemical chaperone, PBA. These data suggest that obesity-induced activation of the UPR and insulin resistance in the liver may be mediated by proteasome dysfunction and further support the notion that ER stress triggers UPR activation in obesity.
Fatty acids and UPR activation
Increased free fatty acids, and in particular saturated fatty acids, have been linked to UPR activation in a number of tissues/organs and cell types [51, 52, 68, 69]. Palmitate is the most prevalent circulating saturated fatty acid and, along with the total free fatty acid pool, is elevated in human with obesity and NAFLD. The covalent attachment of palmitate to substrate proteins, palmitoylation, is recognized as an important post-translational modification that occurs in the ER. Palmitoylation is reversible, thereby allowing it to dynamically regulate a range of protein functions including trafficking, localization, stability, aggregation, and interaction with effectors [70–75]. Obesity appears to influence the palmitoylated proteome in adipose tissue. For example, palmitoylation of both GLUT4 and insulin responsive amino peptidase was increased in mice fed a high-fat diet for 8 wks [76]. Calnexin and calreticulin comprise an ER chaperone system that functions in the folding and quality control of newly synthesized glycoproteins [77]. A recent study demonstrated that palmitoylation of calnexin redirected this protein away from its quality control function to an interaction with SERCA2b on the mitochondria-associated ER membrane, where the ER and mitochondria juxtapose [78]. These data suggest that palmitoylation can modulate ER proteostasis and ER-mitochondrial calcium crosstalk. Although it is not clear whether obesity increases palmitoylation of calnexin, this modification may have implications for obesity-associated changes in SERCA2b (described above) and hence obesity-associated ER stress and UPR activation.
The ER membrane and activation of the UPR
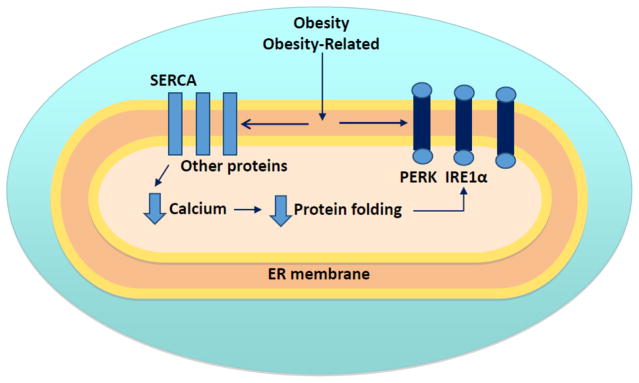
A growing body of evidence indicates that changes in the composition of the ER membrane may mediate UPR activation. The plasma membrane composition is estimated to contain 60–80% of total cellular cholesterol, whereas less than 1% resides in the ER membrane [79, 80]. Therefore, even small changes in the amount of cholesterol in the ER membrane translates to large alterations in overall composition. The cholesterol-loaded macrophage is a characteristic feature of advanced atherosclerotic lesions [81]. Experimental enrichment of the macrophage ER membrane with free cholesterol or 14:0–18:0 phosphatidylcholine (PC) inhibited both the ATPase activity and calcium sequestration function of SERCA2b in macrophages [82]. These data suggest that changes in the cholesterol content of the ER membrane can influence the function of SERCA2b, and ER membrane-bound protein, in macrophages. In a similar vein, changes in the phospholipid profile of the ER membrane have recently been linked to changes in SERCA2b in the liver. Obesity-associated reductions in hepatic SERCA2b activity were linked to an increase in the PC to phosphatidylethanolamine (PE) ratio in the ER [65]. Experimental knockdown of the protein involved in the conversion of PC to PE, phosphatidylethanolamine N-methyltransferase, reduced the PC to PE ratio, significantly improved the calcium transport activity of the ER, and reduced UPR activation and insulin resistance in ob/ob mice [65]. The ratio of monounsaturated to saturated fatty acids has been shown to play a role in both the activation of the UPR in the liver and liver damage in rodent models of NAFLD [4, 83]. These data suggest that changes to ER membrane lipid composition may mediate obesity-associated UPR activation in the liver via mechanisms that involve changes in the regulation of ER calcium (Fig. 2).
Figure 2.
Links between membrane composition and UPR activation. Changes in the lipid composition of the ER membrane can a) lead to impairments in SERCA, reduced luminal calcium stores and a reduction in the folding capacity of the ER lumen, and, b) direct activation of PERK, IRE1α, and possibly ATF6α.
Several recent studies have demonstrated that changes in membrane lipids can also activate the UPR in the absence of ER stress [84–88]. In one of these studies, an ER-targeted IRE1α or PERK mutant lacking the luminal stress-sensing domain was expressed in mutant cells that lacked either endogenous IRE1α or PERK protein or activity [85]. IRE1α-mediated XBP1 splicing or PERK phosphorylation was not observed when cells were treated with chemical agents such as tunicamycin, which inhibits protein glycosylation, thapsigargin, which inhibits SERCA or the reducing agent dithiothreital. In contrast, changes in membrane lipid saturation, induced by inhibition of steroyl-CoA desaturase 1 or the provision of palmitate, resulted in IRE1α-mediated XBP1 splicing or PERK phosphorylation. These results suggest that changes in membrane lipid composition can activate IRE1α and PERK directly and independently of ER stress. The authors postulated that “direct modulation of IRE1α and PERK activity by the lipid environment and luminal stress-induced activation are two parallel pathways that likely intertwine”. It has also been suggested that remodeling of the protein homeostasis network is a “signature response against lipid disequilibrium, particularly during loss of PC/PE homeostasis” [89]. In this context, the UPR appears to help support protein biogenesis, ER-associated degradation and membrane integrity [89]. In total, these studies suggest that the ER membrane may mediate UPR activation via effects on protein folding (mediated through SERCA2b) and direct effects on the membrane-bound ER sensors, IRE1α and PERK (Fig. 2). Such a model provides a plausible explanation for the presence of chronic activation of the UPR in chronic metabolic diseases.
ER-mitochondrial interactions and the UPR
The ER interacts and communicates with a number of cellular structures including the nucleus, plasma membrane, endosomes, and mitochondria [90, 91]. The physical coupling of the ER and mitochondria, often referred to as the mitochondria-associated ER membrane (MAM), facilitates the exchange of metabolites that have direct bearing on stress-responsive pathways, including the UPR [92]. The ER depends on mitochondrial-derived ATP to support protein folding so any deterioration of mitochondrial ATP supply, such as impaired ATP production or transport to the ER, could promote ER stress and UPR activation. In turn, mitochondria depend on ER-derived calcium to support enzymes within the Krebs cycle. Indeed, recent studies have demonstrated close links between the ER and mitochondrial bioenergetics [93–95]. Lipid exchange between these two organelles also occurs through the MAM. Phosphatidylserine (PS), synthesized in the ER, is transported to and decarboxylated in mitochondria to PE [96]. PE then returns to the ER for further conversion to PC. This means that ER-mitochondrial coupling may, at least in part, influence the PC/PE ratio of the ER membrane, and thereby SERCA2B activity [97].
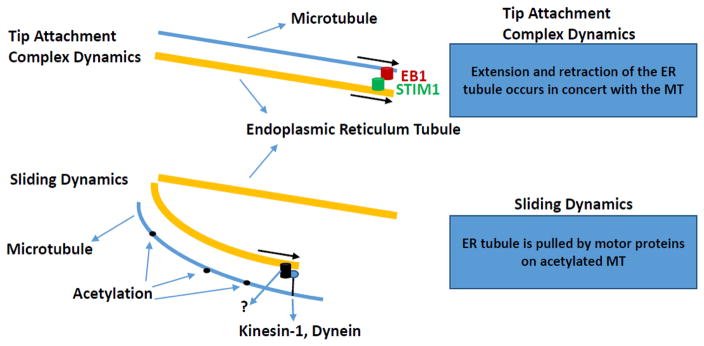
ER-mitochondrial coupling can be maintained despite the fact that these two organelles are constantly moving in the cell [91, 98]. Thus, the regulation of ER-mitochondrial coupling in the context of the dynamic nature of these two organelles may be relevant to obesity-mediated changes in ER and mitochondrial function. ER movement occurs along microtubules via ER sliding (where the tip of the ER tubule binds to the shaft of a microtubule and slides along it) and tip attachment complex dynamics (ER grows or retracts in concert with a partner microtubule, (Fig. 3)) [98]. ER sliding, as well as ER-mitochondria coupling appear to preferentially occur on acetylated microtubules [98]. Nutrient starvation generally results in a reduction in acetylated proteins, however this is not the case with tubulin [99]. Acetylation of α-tubulin via the tubulin acetyltransferase, MEC-17, marks very stable microtubule structures [100]. This protein appears to be characterized by an enzymatic rate that is an order of magnitude slower than the lifetime of dynamic microtubules [101]. The role of tubulin acetyltransferase in the regulation of ER-mitochondrial interactions is presently unknown. Sirtuin 2 (SIRT2) and histone deacetylase II enzyme (HDAC6) have been identified as tubulin deacetylases [102–105]. Recent evidence has suggested that the NAD-dependent SIRT2 plays an important role in the regulation of programmed necrosis [106]. HDAC6 has been linked to the maintenance of mitochondrial fusion under hypoxic stress conditions and overexpression of HDAC6 can enhance resistance to virus infection in mice [107, 108]. It is presently unclear whether phenotypes resulting from changes to HDAC6 or SIRT2 involve ER-mitochondrial interactions or whether the regulation of microtubule acetylation is an important component of ER and mitochondrial dysfunction in obesity and obesity-related disorders.
Figure 3.
Tip attachment complex and sliding dynamics. Tip attachment complex dynamics involve simultaneous movement of ER tubules and microtubules from the plus-end of the microtubule. EB1 is end binding protein-1; STIM1 is stromal interaction molecule-1; ER is endoplasmic reticulum; MT is microtubule. Figure adapted from Friedman and Voeltz with permission [111].
Recent studies have demonstrated that the physical interaction between the ER and mitochondria plays a role in obesity-related hepatic insulin resistance, and impairments in ER and mitochondrial function [109, 110]. In one of these studies, interactions between the voltage-dependent anion channel and inositol 1,4,5-triphosphate receptor (IP3R1), and glucose-regulated protein 75 and IP3R1 were monitored, by in situ proximity ligation assay, as surrogate markers for ER-mitochondrial interactions [110]. Livers from both ob/ob mice and mice fed a high-fat diet for 16 wks were characterized by a reduction of ER-mitochondrial interactions. Overexpression of cyclophilin D in hepatocytes from ob/ob and high-fat diet fed mice increased ER-mitochondrial interactions and improved hepatic insulin action. In the second study, livers from 8–12 wk old ob/ob mice and mice fed a high-fat diet for 16 wks were characterized by an increase in ER-mitochondrial interactions as measured by transmission electron microscopy [109]. Adenovirus-mediated gene transfer of a synthetic linker in the liver of mice fed a high-fat diet for 4 wks resulted in increased ER-mitochondrial interactions and increased hepatic lipid accumulation, lower mitochondrial oxidative function and impaired insulin-mediated phosphorylation of insulin signaling proteins. In total, these studies suggest that ER-mitochondrial interactions play an important role in obesity-mediated impairments in insulin action, steatosis, and mitochondrial function in the liver. More research is required to determine whether the differences between the studies are due to experimental methodologies or to the dynamic nature of ER-mitochondrial interactions.
Summary
Activation of the UPR in obesity and obesity-related disorders likely has two origins. One linked to classic ER stress involving the ER lumen and one linked to alterations to the ER membrane environment (Fig. 2). These two origins are not mutually exclusive. For example, changes to the ER membrane environment can impact ER membrane proteins such as SERCA which in turn can influence protein folding within the ER lumen. However, changes to the ER membrane environment can also influence the activation state of IRE1 and PERK directly and independently of the accumulation of unfolded proteins in the ER lumen. Post-translational protein modifications, such as acetylation and palmitoylation, may also play a role in obesity-mediated disturbances in ER function and UPR activation. Organelle interactions, particularly those involving the ER and mitochondria, may be important to our understanding of obesity-mediated impairments in both the ER and mitochondria.
Acknowledgments
MJP is supported by NIH grant DK072017 and the Lillian Fountain Smith Endowment.
PYK is supported by the Louisiana Biomedical Research Network (NIGMS 8P20GM103424) and the American Society for Cell Biology
Abbreviations
- ATF4
activating transcription factor-4
- ATF6α
activating transcription factor-6α
- EB1
end binding protein-1
- EDEM
ER degradation-enhancing α-like protein
- ER
endoplasmic reticulum
- ERAD
ER-associated degradation
- GCN2
general control non-derepressible 2 kinase
- GRP78
glucose-regulated protein 78/immunoglobulin-heavy-chain-binding protein
- HRI
heme-regulated inhibitor kinase
- IP3R1
inositol 1,4,5-triphosphate receptor
- IRE1α
inositol-requiring 1α
- MAM
mitochondria-associated ER membrane
- McA
Morris hepatoma 7777
- MT
microtubule
- mTOR
mammalian Target of Rapamycin
- PBA
4-phenyl butyric acid
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- p-eIF2α
phosphorylation of the α-subunit of eukaryotic initiation factor-2
- PERK
double-stranded RNA-dependent protein kinase-like ER kinase
- PKR
double-stranded RNA-activated protein kinase
- PS
phosphatidylserine
- SERCA
sarco-endoplasmic reticulum calcium ATPase
- snoRNAs
small nucleolar RNAs
- STIM1
stromal interaction molecule-1
- TRBP
TAR RNA-binding protein
- TUDCA
taurine-conjugated ursodeoxycholic acid
- UPR
unfolded protein response
- UPS
ubiquitin-proteasome system
- XBP1s
spliced X-box binding protein-1
Footnotes
Author Contributions
MJP, PYK, ALE and CMS wrote portions of the manuscript and edited the manuscript. CLG edited the manuscript.
Conflict of Interest
None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lee J, Ozcan U. Unfolded protein response signaling and metabolic diseases. J Biol Chem. 2014;289:1203–1211. doi: 10.1074/jbc.R113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ozcan U, Cao Q, Yilmaz E, Lee AH, Iwakoshi NN, Ozdelen E, Tuncman G, Gorgun C, Glimcher LH, Hotamisligil GS. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 4.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 5.Boden G, Duan X, Homko C, Molina EJ, Song W, Perez O, Cheung P, Merali S. Increase in endoplasmic reticulum stress-related proteins and genes in adipose tissue of obese, insulin-resistant individuals. Diabetes. 2008;57:2438–2444. doi: 10.2337/db08-0604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gregor MF, Yang L, Fabbrini E, Mohammed BS, Eagon JC, Hotamisligil GS, Klein S. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58:693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 8.Sharma NK, Das SK, Mondal AK, Hackney OG, Chu WS, Kern PA, Rasouli N, Spencer HJ, Yao-Borengasser A, Elbein SC. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. J Clin Endocrinol Metab. 2008;93:4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gawrieh S, Baye TM, Carless M, Wallace J, Komorowski R, Kleiner DE, Andris D, Makladi B, Cole R, Charlton M, et al. Hepatic gene networks in morbidly obese patients with nonalcoholic fatty liver disease. Obes Surg. 2010;20:1698–1709. doi: 10.1007/s11695-010-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fagone P, Jackowski S. Membrane phospholipid synthesis and endoplasmic reticulum function. J Lipid Res. 2009;50(Suppl):S311–316. doi: 10.1194/jlr.R800049-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soccio RE, Breslow JL. Intracellular cholesterol transport. Arterioscler Thromb Vasc Biol. 2004;24:1150–1160. doi: 10.1161/01.ATV.0000131264.66417.d5. [DOI] [PubMed] [Google Scholar]
- 13.Schroder M, Kaufman RJ. ER stress and the unfolded protein response. Mutat Res. 2005;569:29–63. doi: 10.1016/j.mrfmmm.2004.06.056. [DOI] [PubMed] [Google Scholar]
- 14.Demartino GN, Gillette TG. Proteasomes: machines for all reasons. Cell. 2007;129:659–662. doi: 10.1016/j.cell.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Hershko A, Ciechanover A. The ubiquitin system. Annu Rev Biochem. 1998;67:425–479. doi: 10.1146/annurev.biochem.67.1.425. [DOI] [PubMed] [Google Scholar]
- 16.Rutkowski DT, Hegde RS. Regulation of basal cellular physiology by the homeostatic unfolded protein response. J Cell Biol. 2010;189:783–794. doi: 10.1083/jcb.201003138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rutkowski DT, Kaufman RJ. A trip to the ER: coping with stress. Trends Cell Biol. 2004;14:20–28. doi: 10.1016/j.tcb.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Pfaffenbach KT, Lee AS. The critical role of GRP78 in physiologic and pathologic stress. Curr Opin Cell Biol. 2011;23:150–156. doi: 10.1016/j.ceb.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaufman RJ. Orchestrating the unfolded protein response in health and disease. J Clin Invest. 2002;110:1389–1398. doi: 10.1172/JCI16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schroder M, Kaufman RJ. The mammalian unfolded protein response. Annu Rev Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 21.Hollien J, Weissman JS. Decay of endoplasmic reticulum-localized mRNAs during the unfolded protein response. Science. 2006;313:104–107. doi: 10.1126/science.1129631. [DOI] [PubMed] [Google Scholar]
- 22.Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol. 2009;186:323–331. doi: 10.1083/jcb.200903014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Reid DW, Chen Q, Tay AS, Shenolikar S, Nicchitta CV. The unfolded protein response triggers selective mRNA release from the endoplasmic reticulum. Cell. 2014;158:1362–1374. doi: 10.1016/j.cell.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu J, Kaufman RJ. From acute ER stress to physiological roles of the Unfolded Protein Response. Cell Death Differ. 2006 doi: 10.1038/sj.cdd.4401840. [DOI] [PubMed] [Google Scholar]
- 25.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 26.Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell. 2001;7:1165–1176. doi: 10.1016/s1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 27.Lee AH, Scapa EF, Cohen DE, Glimcher LH. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sriburi R, Jackowski S, Mori K, Brewer JW. XBP1: a link between the unfolded protein response, lipid biosynthesis, and biogenesis of the endoplasmic reticulum. J Cell Biol. 2004;167:35–41. doi: 10.1083/jcb.200406136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gentile CL, Frye M, Pagliassotti MJ. Endoplasmic reticulum stress and the unfolded protein response in nonalcoholic fatty liver disease. Antioxid Redox Signal. 2011;15:505–521. doi: 10.1089/ars.2010.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pagliassotti MJ. Endoplasmic reticulum stress in nonalcoholic fatty liver disease. Annu Rev Nutr. 2012;32:17–33. doi: 10.1146/annurev-nutr-071811-150644. [DOI] [PubMed] [Google Scholar]
- 31.Hetz C. The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol. 2012;13:89–102. doi: 10.1038/nrm3270. [DOI] [PubMed] [Google Scholar]
- 32.Zhang K, Kaufman RJ. The unfolded protein response: a stress signaling pathway critical for health and disease. Neurology. 2006;66:S102–109. doi: 10.1212/01.wnl.0000192306.98198.ec. [DOI] [PubMed] [Google Scholar]
- 33.Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell. 2010;140:338–348. doi: 10.1016/j.cell.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Youssef OA, Safran SA, Nakamura T, Nix DA, Hotamisligil GS, Bass BL. Potential role for snoRNAs in PKR activation during metabolic stress. Proc Natl Acad Sci U S A. 2015;112:5023–5028. doi: 10.1073/pnas.1424044112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakamura T, Kunz RC, Zhang C, Kimura T, Yuan CL, Baccaro B, Namiki Y, Gygi SP, Hotamisligil GS. A critical role for PKR complexes with TRBP in Immunometabolic regulation and eIF2alpha phosphorylation in obesity. Cell Rep. 2015;11:295–307. doi: 10.1016/j.celrep.2015.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michel CI, Holley CL, Scruggs BS, Sidhu R, Brookheart RT, Listenberger LL, Behlke MA, Ory DS, Schaffer JE. Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metab. 2011;14:33–44. doi: 10.1016/j.cmet.2011.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scruggs BS, Michel CI, Ory DS, Schaffer JE. SmD3 regulates intronic noncoding RNA biogenesis. Mol Cell Biol. 2012;32:4092–4103. doi: 10.1128/MCB.00022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo F, Cavener DR. The GCN2 eIF2alpha kinase regulates fatty-acid homeostasis in the liver during deprivation of an essential amino acid. Cell Metab. 2007;5:103–114. doi: 10.1016/j.cmet.2007.01.001. [DOI] [PubMed] [Google Scholar]
- 39.Kaufman RJ. Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev. 1999;13:1211–1233. doi: 10.1101/gad.13.10.1211. [DOI] [PubMed] [Google Scholar]
- 40.Gentile CL, Pagliassotti MJ. The endoplasmic reticulum as a potential therapeutic target in nonalcoholic fatty liver disease. Curr Opin Investig Drugs. 2008;9:1084–1088. [PMC free article] [PubMed] [Google Scholar]
- 41.Welch WJ, Brown CR. Influence of molecular and chemical chaperones on protein folding. Cell Stress Chaperones. 1996;1:109–115. doi: 10.1379/1466-1268(1996)001<0109:iomacc>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolter T, Wendeler M. Chemical chaperones--a new concept in drug research. Chembiochem. 2003;4:260–264. doi: 10.1002/cbic.200390045. [DOI] [PubMed] [Google Scholar]
- 43.Brown CR, Hong-Brown LQ, Biwersi J, Verkman AS, Welch WJ. Chemical chaperones correct the mutant phenotype of the delta F508 cystic fibrosis transmembrane conductance regulator protein. Cell Stress Chaperones. 1996;1:117–125. doi: 10.1379/1466-1268(1996)001<0117:ccctmp>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci U S A. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xie Q, Khaoustov VI, Chung CC, Sohn J, Krishnan B, Lewis DE, Yoffe B. Effect of tauroursodeoxycholic acid on endoplasmic reticulum stress-induced caspase-12 activation. Hepatology. 2002;36:592–601. doi: 10.1053/jhep.2002.35441. [DOI] [PubMed] [Google Scholar]
- 46.Loo TW, Clarke DM. Chemical and pharmacological chaperones as new therapeutic agents. Expert Rev Mol Med. 2007;9:1–18. doi: 10.1017/S1462399407000361. [DOI] [PubMed] [Google Scholar]
- 47.Wei H, Kim SJ, Zhang Z, Tsai PC, Wisniewski KE, Mukherjee AB. ER and oxidative stresses are common mediators of apoptosis in both neurodegenerative and non-neurodegenerative lysosomal storage disorders and are alleviated by chemical chaperones. Hum Mol Genet. 2008;17:469–477. doi: 10.1093/hmg/ddm324. [DOI] [PubMed] [Google Scholar]
- 48.Alpini G, Kanno N, Phinizy JL, Glaser S, Francis H, Taffetani S, LeSage G. Tauroursodeoxycholate inhibits human cholangiocarcinoma growth via Ca2+-, PKC-, and MAPK-dependent pathways. Am J Physiol Gastrointest Liver Physiol. 2004;286:G973–982. doi: 10.1152/ajpgi.00270.2003. [DOI] [PubMed] [Google Scholar]
- 49.de Almeida SF, Picarote G, Fleming JV, Carmo-Fonseca M, Azevedo JE, de Sousa M. Chemical chaperones reduce endoplasmic reticulum stress and prevent mutant HFE aggregate formation. J Biol Chem. 2007;282:27905–27912. doi: 10.1074/jbc.M702672200. [DOI] [PubMed] [Google Scholar]
- 50.Ozcan U, Yilmaz E, Ozcan L, Furuhashi M, Vaillancourt E, Smith RO, Gorgun CZ, Hotamisligil GS. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Boden G, Song W, Duan X, Cheung P, Kresge K, Barrero C, Merali S. Infusion of glucose and lipids at physiological rates causes acute endoplasmic reticulum stress in rat liver. Obesity (Silver Spring) 2011;19:1366–1373. doi: 10.1038/oby.2011.71. [DOI] [PubMed] [Google Scholar]
- 52.Nivala AM, Reese L, Frye M, Gentile CL, Pagliassotti MJ. Fatty acid-mediated endoplasmic reticulum stress in vivo: differential response to the infusion of Soybean and Lard Oil in rats. Metabolism. 2013;62:753–760. doi: 10.1016/j.metabol.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfaffenbach KT, Gentile CL, Nivala AM, Wang D, Wei Y, Pagliassotti MJ. Linking endoplasmic reticulum stress to cell death in hepatocytes: roles of C/EBP homologous protein and chemical chaperones in palmitate-mediated cell death. Am J Physiol Endocrinol Metab. 2010;298:E1027–1035. doi: 10.1152/ajpendo.00642.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ota T, Gayet C, Ginsberg HN. Inhibition of apolipoprotein B100 secretion by lipid-induced hepatic endoplasmic reticulum stress in rodents. J Clin Invest. 2008;118:316–332. doi: 10.1172/JCI32752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kammoun HL, Chabanon H, Hainault I, Luquet S, Magnan C, Koike T, Ferre P, Foufelle F. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. J Clin Invest. 2009;119:1201–1215. doi: 10.1172/JCI37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ye R, Jung DY, Jun JY, Li J, Luo S, Ko HJ, Kim JK, Lee AS. Grp78 heterozygosity promotes adaptive unfolded protein response and attenuates diet-induced obesity and insulin resistance. Diabetes. 2010;59:6–16. doi: 10.2337/db09-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Anderson SR, Gilge DA, Steiber AL, Previs SF. Diet-induced obesity alters protein synthesis: tissue-specific effects in fasted versus fed mice. Metabolism. 2008;57:347–354. doi: 10.1016/j.metabol.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fu S, Fan J, Blanco J, Gimenez-Cassina A, Danial NN, Watkins SM, Hotamisligil GS. Polysome profiling in liver identifies dynamic regulation of endoplasmic reticulum translatome by obesity and fasting. PLoS Genet. 2012;8:e1002902. doi: 10.1371/journal.pgen.1002902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hatanaka M, Maier B, Sims EK, Templin AT, Kulkarni RN, Evans-Molina C, Mirmira RG. Palmitate induces mRNA translation and increases ER protein load in islet beta-cells via activation of the mammalian target of rapamycin pathway. Diabetes. 2014;63:3404–3415. doi: 10.2337/db14-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khamzina L, Veilleux A, Bergeron S, Marette A. Increased activation of the mammalian target of rapamycin pathway in liver and skeletal muscle of obese rats: possible involvement in obesity-linked insulin resistance. Endocrinology. 2005;146:1473–1481. doi: 10.1210/en.2004-0921. [DOI] [PubMed] [Google Scholar]
- 61.Burdakov D, Petersen OH, Verkhratsky A. Intraluminal calcium as a primary regulator of endoplasmic reticulum function. Cell Calcium. 2005;38:303–310. doi: 10.1016/j.ceca.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 62.Gorlach A, Klappa P, Kietzmann T. The endoplasmic reticulum: folding, calcium homeostasis, signaling, and redox control. Antioxid Redox Signal. 2006;8:1391–1418. doi: 10.1089/ars.2006.8.1391. [DOI] [PubMed] [Google Scholar]
- 63.Zarain-Herzberg A, Alvarez-Fernandez G. Sarco(endo)plasmic reticulum Ca2+-ATPase-2 gene: structure and transcriptional regulation of the human gene. Scientific World Journal. 2002;2:1469–1483. doi: 10.1100/tsw.2002.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Park SW, Zhou Y, Lee J, Lee J, Ozcan U. Sarco(endo)plasmic reticulum Ca2+-ATPase 2b is a major regulator of endoplasmic reticulum stress and glucose homeostasis in obesity. Proc Natl Acad Sci U S A. 2010;107:19320–19325. doi: 10.1073/pnas.1012044107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, Lin X, Watkins SM, Ivanov AR, Hotamisligil GS. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. 2011;473:528–531. doi: 10.1038/nature09968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hartley T, Brumell J, Volchuk A. Emerging roles for the ubiquitin-proteasome system and autophagy in pancreatic beta-cells. Am J Physiol Endocrinol Metab. 2009;296:E1–10. doi: 10.1152/ajpendo.90538.2008. [DOI] [PubMed] [Google Scholar]
- 67.Otoda T, Takamura T, Misu H, Ota T, Murata S, Hayashi H, Takayama H, Kikuchi A, Kanamori T, Shima KR, et al. Proteasome dysfunction mediates obesity-induced endoplasmic reticulum stress and insulin resistance in the liver. Diabetes. 2013;62:811–824. doi: 10.2337/db11-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moffitt JH, Fielding BA, Evershed R, Berstan R, Currie JM, Clark A. Adverse physicochemical properties of tripalmitin in beta cells lead to morphological changes and lipotoxicity in vitro. Diabetologia. 2005;48:1819–1829. doi: 10.1007/s00125-005-1861-9. [DOI] [PubMed] [Google Scholar]
- 69.Borradaile NM, Han X, Harp JD, Gale SE, Ory DS, Schaffer JE. Disruption of endoplasmic reticulum structure and integrity in lipotoxic cell death. J Lipid Res. 2006;47:2726–2737. doi: 10.1194/jlr.M600299-JLR200. [DOI] [PubMed] [Google Scholar]
- 70.Abrami L, Kunz B, Iacovache I, van der Goot FG. Palmitoylation and ubiquitination regulate exit of the Wnt signaling protein LRP6 from the endoplasmic reticulum. Proc Natl Acad Sci U S A. 2008;105:5384–5389. doi: 10.1073/pnas.0710389105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lynes EM, Bui M, Yap MC, Benson MD, Schneider B, Ellgaard L, Berthiaume LG, Simmen T. Palmitoylated TMX and calnexin target to the mitochondria-associated membrane. EMBO J. 2012;31:457–470. doi: 10.1038/emboj.2011.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thorne RF, Ralston KJ, de Bock CE, Mhaidat NM, Zhang XD, Boyd AW, Burns GF. Palmitoylation of CD36/FAT regulates the rate of its post-transcriptional processing in the endoplasmic reticulum. Biochim Biophys Acta. 2010;1803:1298–1307. doi: 10.1016/j.bbamcr.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 73.Baldwin AC, Green CD, Olson LK, Moxley MA, Corbett JA. A role for aberrant protein palmitoylation in FFA-induced ER stress and beta-cell death. Am J Physiol Endocrinol Metab. 2012;302:E1390–1398. doi: 10.1152/ajpendo.00519.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Linder ME, Deschenes RJ. Palmitoylation: policing protein stability and traffic. Nat Rev Mol Cell Biol. 2007;8:74–84. doi: 10.1038/nrm2084. [DOI] [PubMed] [Google Scholar]
- 75.Greaves J, Chamberlain LH. Palmitoylation-dependent protein sorting. J Cell Biol. 2007;176:249–254. doi: 10.1083/jcb.200610151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ren W, Jhala US, Du K. Proteomic analysis of protein palmitoylation in adipocytes. Adipocyte. 2013;2:17–28. doi: 10.4161/adip.22117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Williams DB. Beyond lectins: the calnexin/calreticulin chaperone system of the endoplasmic reticulum. J Cell Sci. 2006;119:615–623. doi: 10.1242/jcs.02856. [DOI] [PubMed] [Google Scholar]
- 78.Lynes EM, Raturi A, Shenkman M, Ortiz Sandoval C, Yap MC, Wu J, Janowicz A, Myhill N, Benson MD, Campbell RE, et al. Palmitoylation is the switch that assigns calnexin to quality control or ER Ca2+ signaling. J Cell Sci. 2013;126:3893–3903. doi: 10.1242/jcs.125856. [DOI] [PubMed] [Google Scholar]
- 79.Liscum L, Munn NJ. Intracellular cholesterol transport. Biochim Biophys Acta. 1999;1438:19–37. doi: 10.1016/s1388-1981(99)00043-8. [DOI] [PubMed] [Google Scholar]
- 80.Lange Y, Ye J, Rigney M, Steck TL. Regulation of endoplasmic reticulum cholesterol by plasma membrane cholesterol. J Lipid Res. 1999;40:2264–2270. [PubMed] [Google Scholar]
- 81.Ross R. Cell biology of atherosclerosis. Annu Rev Physiol. 1995;57:791–804. doi: 10.1146/annurev.ph.57.030195.004043. [DOI] [PubMed] [Google Scholar]
- 82.Li Y, Ge M, Ciani L, Kuriakose G, Westover EJ, Dura M, Covey DF, Freed JH, Maxfield FR, Lytton J, Tabas I. Enrichment of endoplasmic reticulum with cholesterol inhibits sarcoplasmic-endoplasmic reticulum calcium ATPase-2b activity in parallel with increased order of membrane lipids: implications for depletion of endoplasmic reticulum calcium stores and apoptosis in cholesterol-loaded macrophages. J Biol Chem. 2004;279:37030–37039. doi: 10.1074/jbc.M405195200. [DOI] [PubMed] [Google Scholar]
- 83.Li ZZ, Berk M, McIntyre TM, Feldstein AE. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Promlek T, Ishiwata-Kimata Y, Shido M, Sakuramoto M, Kohno K, Kimata Y. Membrane aberrancy and unfolded proteins activate the endoplasmic reticulum stress sensor Ire1 in different ways. Mol Biol Cell. 2011;22:3520–3532. doi: 10.1091/mbc.E11-04-0295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Volmer R, van der Ploeg K, Ron D. Membrane lipid saturation activates endoplasmic reticulum unfolded protein response transducers through their transmembrane domains. Proc Natl Acad Sci U S A. 2013;110:4628–4633. doi: 10.1073/pnas.1217611110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hou NS, Gutschmidt A, Choi DY, Pather K, Shi X, Watts JL, Hoppe T, Taubert S. Activation of the endoplasmic reticulum unfolded protein response by lipid disequilibrium without disturbed proteostasis in vivo. Proc Natl Acad Sci U S A. 2014;111:E2271–2280. doi: 10.1073/pnas.1318262111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ariyama H, Kono N, Matsuda S, Inoue T, Arai H. Decrease in membrane phospholipid unsaturation induces unfolded protein response. J Biol Chem. 2010;285:22027–22035. doi: 10.1074/jbc.M110.126870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kitai Y, Ariyama H, Kono N, Oikawa D, Iwawaki T, Arai H. Membrane lipid saturation activates IRE1alpha without inducing clustering. Genes Cells. 2013;18:798–809. doi: 10.1111/gtc.12074. [DOI] [PubMed] [Google Scholar]
- 89.Thibault G, Shui G, Kim W, McAlister GC, Ismail N, Gygi SP, Wenk MR, Ng DT. The membrane stress response buffers lethal effects of lipid disequilibrium by reprogramming the protein homeostasis network. Mol Cell. 2012;48:16–27. doi: 10.1016/j.molcel.2012.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lebiedzinska M, Szabadkai G, Jones AW, Duszynski J, Wieckowski MR. Interactions between the endoplasmic reticulum, mitochondria, plasma membrane and other subcellular organelles. Int J Biochem Cell Biol. 2009;41:1805–1816. doi: 10.1016/j.biocel.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 91.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Rainbolt TK, Saunders JM, Wiseman RL. Stress-responsive regulation of mitochondria through the ER unfolded protein response. Trends Endocrinol Metab. 2014;25:528–537. doi: 10.1016/j.tem.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 93.Bravo R, Vicencio JM, Parra V, Troncoso R, Munoz JP, Bui M, Quiroga C, Rodriguez AE, Verdejo HE, Ferreira J, et al. Increased ER-mitochondrial coupling promotes mitochondrial respiration and bioenergetics during early phases of ER stress. J Cell Sci. 2011;124:2143–2152. doi: 10.1242/jcs.080762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lim JH, Lee HJ, Ho Jung M, Song J. Coupling mitochondrial dysfunction to endoplasmic reticulum stress response: a molecular mechanism leading to hepatic insulin resistance. Cell Signal. 2009;21:169–177. doi: 10.1016/j.cellsig.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 95.Brdiczka DG, Zorov DB, Sheu SS. Mitochondrial contact sites: their role in energy metabolism and apoptosis. Biochim Biophys Acta. 2006;1762:148–163. doi: 10.1016/j.bbadis.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 96.Vance JE. Phospholipid synthesis and transport in mammalian cells. Traffic. 2015;16:1–18. doi: 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 97.Vance JE. MAM (mitochondria-associated membranes) in mammalian cells: lipids and beyond. Biochim Biophys Acta. 2014;1841:595–609. doi: 10.1016/j.bbalip.2013.11.014. [DOI] [PubMed] [Google Scholar]
- 98.Friedman JR, Webster BM, Mastronarde DN, Verhey KJ, Voeltz GK. ER sliding dynamics and ER-mitochondrial contacts occur on acetylated microtubules. J Cell Biol. 2010;190:363–375. doi: 10.1083/jcb.200911024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marino G, Pietrocola F, Eisenberg T, Kong Y, Malik SA, Andryushkova A, Schroeder S, Pendl T, Harger A, Niso-Santano M, et al. Regulation of autophagy by cytosolic acetyl-coenzyme A. Mol Cell. 2014;53:710–725. doi: 10.1016/j.molcel.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 100.Davenport AM, Collins LN, Chiu H, Minor PJ, Sternberg PW, Hoelz A. Structural and functional characterization of the alpha-tubulin acetyltransferase MEC-17. J Mol Biol. 2014;426:2605–2616. doi: 10.1016/j.jmb.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Szyk A, Deaconescu AM, Spector J, Goodman B, Valenstein ML, Ziolkowska NE, Kormendi V, Grigorieff N, Roll-Mecak A. Molecular basis for age-dependent microtubule acetylation by tubulin acetyltransferase. Cell. 2014;157:1405–1415. doi: 10.1016/j.cell.2014.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bertos NR, Gilquin B, Chan GK, Yen TJ, Khochbin S, Yang XJ. Role of the tetradecapeptide repeat domain of human histone deacetylase 6 in cytoplasmic retention. J Biol Chem. 2004;279:48246–48254. doi: 10.1074/jbc.M408583200. [DOI] [PubMed] [Google Scholar]
- 103.North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 2003;11:437–444. doi: 10.1016/s1097-2765(03)00038-8. [DOI] [PubMed] [Google Scholar]
- 104.Perdiz D, Mackeh R, Pous C, Baillet A. The ins and outs of tubulin acetylation: more than just a post-translational modification? Cell Signal. 2011;23:763–771. doi: 10.1016/j.cellsig.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Li N, Caron C, Matthias G, Hess D, Khochbin S, Matthias P. HDAC-6 interacts with and deacetylates tubulin and microtubules in vivo. EMBO J. 2003;22:1168–1179. doi: 10.1093/emboj/cdg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Narayan N, Lee IH, Borenstein R, Sun J, Wong R, Tong G, Fergusson MM, Liu J, Rovira II, Cheng HL, et al. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature. 2012;492:199–204. doi: 10.1038/nature11700. [DOI] [PubMed] [Google Scholar]
- 107.Kim HJ, Nagano Y, Choi SJ, Park SY, Kim H, Yao TP, Lee JY. HDAC6 maintains mitochondrial connectivity under hypoxic stress by suppressing MARCH5/MITOL dependent MFN2 degradation. Biochem Biophys Res Commun. 2015;464:1235–1240. doi: 10.1016/j.bbrc.2015.07.111. [DOI] [PubMed] [Google Scholar]
- 108.Wang D, Meng Q, Huo L, Yang M, Wang L, Chen X, Wang J, Li Z, Ye X, Liu N, et al. Overexpression of Hdac6 enhances resistance to virus infection in embryonic stem cells and in mice. Protein Cell. 2015;6:152–156. doi: 10.1007/s13238-014-0120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Arruda AP, Pers BM, Parlakgul G, Guney E, Inouye K, Hotamisligil GS. Chronic enrichment of hepatic endoplasmic reticulum-mitochondria contact leads to mitochondrial dysfunction in obesity. Nat Med. 2014;20:1427–1435. doi: 10.1038/nm.3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tubbs E, Theurey P, Vial G, Bendridi N, Bravard A, Chauvin MA, Ji-Cao J, Zoulim F, Bartosch B, Ovize M, et al. Mitochondria-associated endoplasmic reticulum membrane (MAM) integrity is required for insulin signaling and is implicated in hepatic insulin resistance. Diabetes. 2014;63:3279–3294. doi: 10.2337/db13-1751. [DOI] [PubMed] [Google Scholar]
- 111.Friedman JR, Voeltz GK. The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol. 2011;21:709–717. doi: 10.1016/j.tcb.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]