Abstract
Chronic non-healing skin wounds often contain bacterial biofilms that prevent normal wound healing and closure and present challenges to the use of conventional wound dressings. We investigated inhibition of Pseudomonas aeruginosa biofilm formation, a common pathogen of chronic skin wounds, on a commercially available biological wound dressing. Building upon prior reports, we examined whether the amino acid tryptophan would inhibit P. aeruginosa biofilm formation on the 3-dimensional surface of the biological dressing. Bacterial biomass and biofilm polysaccharides were quantified using crystal violet staining or an enzyme linked lectin, respectively. Bacterial cells and biofilm matrix adherent to the wound dressing were visualized through scanning electron microscopy. D-/L-tryptophan inhibited P. aeruginosa biofilm formation on the wound dressing in a dose dependent manner and was not directly cytotoxic to immortalized human keratinocytes although there was some reduction in cellular metabolism or enzymatic activity. More importantly, D-/L-tryptophan did not impair wound healing in a splinted skin wound murine model. Furthermore, wound closure was improved when D-/L-tryptophan treated wound dressing with P. aeruginosa biofilms were compared with untreated dressings. These findings indicate that tryptophan may prove useful for integration into wound dressings to inhibit biofilm formation and promote wound healing.
Keywords: Biofilm, Wound Dressing, Tryptophan
Introduction
Bacterial biofilms account for nearly 80% of chronic infections, according to the CDC (1). A 2008 study revealed that 60% of chronic skin wounds (i.e. pressure, venous leg, and diabetic foot ulcers) contained bacterial biofilms (2). Biofilms significantly delay normal healing by serving as a reservoir of bacterial cells that resist both antibiotic treatment and clearance by the immune system (3–7). This bacterial burden can trigger a chronic inflammatory state and thus disrupt the normal healing process. The ‘gold standard’ for chronic skin wound treatment is to debride the wound to remove necrotic tissue and microorganisms in order to create a clean wound bed (8). However, bacterial biofilm remaining in the wound may resist the debridement process and serve as a resistant reservoir of bacterial cells leading to additional delays in healing. Novel treatments that disperse or prevent biofilm formation are required to improve the care of chronic non-healing skin wounds (2).
Wound dressings can provide additional surface area for bacterial cell attachment and biofilm formation within chronic wounds (9–11). Various reports have documented the presence of bacterial biofilms on wound dressings and a variety of medical implants, including surgical sutures and wound dressings using scanning electron microscopy (SEM) (12–14). Contamination of wound dressings often necessitates their removal and leads to additional wound debridement, increasing patient discomfort and further delaying the healing process (15–18). There is a pressing clinical need for innovative approaches to prevent adherence of bacterial cells and formation of biofilms on the surfaces of implants, sutures, and wound dressings. Strategies currently employed include chemical modification of the dressing to limit bacterial attachment and controlled release of antibiotics to eliminate planktonic bacterial cells (19–21). An alternative and complimentary strategy is to target the biofilm directly. Incorporating compounds that inhibit and disperse biofilms in the wound dressing itself may help prevent biofilm growth on the dressing and wound bed and therefore help improve healing.
Biofilm inhibitors described in the literature include antimicrobial peptides, metal chelators, quorum sensing inhibitors, and amino acids (22–27). Inhibition of bacterial biofilm growth with D-amino acids was first reported for the gram-positive species Bacillus subtilis and Staphylococcus aureus (28, 29). Inhibition was initially attributed to disruption of the peptidoglycan cell wall, and destabilization of the protein – matrix interaction, but later was credited to disruption of protein synthesis in B. subtilis (30). The amino acid tryptophan has been reported to inhibit biofilm formation by the gram-negative pathogens Escherichia coli (31) and Pseudomonas aeruginosa (32). Our laboratory demonstrated that D- and L-isoforms of tryptophan both inhibited P. aeruginosa biofilm formation and dispersed existing P. aeruginosa biofilms within 24 hours of treatment. Although the mechanism responsible for biofilm inhibition and dispersal by tryptophan remains uncertain, it may involve increased bacterial motility or altered quorum sensing (33–36). An added advantage of using tryptophan as a biofilm inhibitor in chronic wounds is the recently described beneficial effect it has on wound healing and closure (37–39).
One limitation of many in vitro biofilm studies is reliance on a relatively simple 2-dimensional abiotic surface, such as polystyrene microtiter plates, that does not reflect the complexity of biofilms in the wound environment. To investigate inhibition of biofilm formation on complex surfaces, such as within a chronic skin wound, we established a model for P. aeruginosa biofilm formation on a commercially available biological wound dressing (Biobrane). Biobrane was chosen for its complex 3-dimensional geometry and synthetic/biological heterogeneity (40). Using this model system we show that tryptophan dose dependently inhibits biofilm formation on a biological wound dressing. In addition, we demonstrate the absence of in vitro cytotoxicity of tryptophan using two different immortalized human keratinocyte cell lines and observed no deleterious effects when tryptophan was applied topically to experimental full thickness mouse skin wounds. We also demonstrated the potential in vivo benefit of using tryptophan to inhibit P. aeruginosa biofilm formation on the wound dressings using the same full thickness murine skin wound model. These studies provide evidence for the continued exploration and development of tryptophan as an anti-biofilm agent for treatment of chronic skin wounds.
Materials and Methods
Bacterial Strains and Materials
Pseudomonas aeruginosa American Type Culture Collection (ATCC) strain 27853 was used in all experiments. Bacto™ Tryptic Soy Broth (TSB) (Becton, Dickinson, and Company, Sparks, MD) and M63 minimal media (2.0g (NH4)SO4, 13.6g KH2PO4, 0.5mg FeSO4•7H2O, 10ml 20% glycerol, and 1ml 1M MgSO4 in 1.0L of diH2O, pH~7.0) were used for overnight bacterial growth and biofilm experiments, respectively. Saturated solutions of 50 mM D- and L-isoforms of tryptophan (Sigma-Aldrich, St. Louis; Acros Organics, New Jersey) were prepared in 1% Phosphate Buffered Saline (PBS) and filter sterilized using a 0.22μm syringe filter. The wound dressing, Biobrane, was purchased from UDL Laboratories Inc. (Rockford, IL). An 8 mm biopsy punch was used to cut the dressings into discs, which were aseptically placed into separate wells of 48 well microtiter plates for biofilm inhibition and dispersal experiments.
Quantification of Biofilm Formation and Dispersal
P. aeruginosa was incubated overnight (~24h) at 37°C under rotation until a concentration of approximately 109 CFU/ml was obtained. The overnight culture of P. aeruginosa was inoculated into the M63 minimal media at a 1:2500 dilution with or without and equimolar ratio of D- and L-tryptophan (0.5 – 10mM) prior to addition to the wound dressings. For dispersal experiments, 48 hour old biofilms were formed on the dressings in the M63 minimal media without tryptophan at 30°C under static conditions. After 48 hours of growth, planktonic bacterial cells were removed by rinsing the dressings 3 times with sterile 1X PBS. Fresh M63 minimal media containing Tryptophan (0 – 10mM) was then added to the dressings for 24 hours at 30°C prior to biofilm quantification by crystal violet or enumeration of colony forming units (CFU).
To quantify biofilm mass, the dressings were washed 4 times with deionized H2O, air dried, stained with 0.35% filtered crystal violet (EMD Chemicals, Gibbstown, NJ) for 15 minutes, and washed 5 times with tap water as previously reported (32, 41). Bound crystal violet was solubilized with 30% Acetic Acid and solution absorbance measured at 595nm using a microplate reader (Beckman Coulter 880DX). The amount of bound crystal violet was proportional to the bacterial biomass on the surface of the wound dressings and expressed as the mean + the standard error of the mean (SEM) absorbance at 595nm. In some experiments wound dressings were stained with a phosphatase-linked lectin instead of crystal violet. To do so, the wound dressings were washed 3 times with sterile normal saline (0.9% w/v) prior to incubation with alkaline phosphatase conjugated Hippeastrum hybrid lectin (Amaryllis) (E-Y Laboratories, San Mateo, CA) for 18 hours at 4°C. This lectin was chosen because it has been shown to bind extracellular polysaccharide produced in P. aeruginosa biofilms (42). Excess lectin was removed from the wound dressings by washing 3 times with sterile normal saline. The amount of lectin bound to the biofilm was quantified via colorimetric decay of P-nitrophenylphosphate (colorless) to P-nitrophenol (yellow color) by the conjugated phosphatase at a solution absorbance of 405nm. To quantify CFU, the dressings were rinsed 4 times with 1X PBS and placed in sterile Eppendorf tubes containing 1.0ml of sterile 1X PBS and Zirconium Oxide beads. The samples were homogenized with a Bullet Blender® (Next Advance, Averill Park NY), diluted and plated on trypticase soy agar with 5% sheep’s blood, and incubated at 37°C for 18 – 20 hours. Colonies were counted and the values expressed as the mean + SEM log10 (CFU/dressing). Each experimental group was performed in triplicate and repeated for three independent experiments.
Scanning Electron Microscopy
Wound dressings were bathed with P. aeruginosa suspended in M63 minimal media (~5 × 105 CFU/ml) with or without 10.0mM D-/L-tryptophan for up to 72 hours at 30°C. At 24, 48, and 72 hours the dressings were washed with 1X PBS and fixed with 4% (v/v) paraformaldehyde (Electron Microscopy Sciences) for 20 minutes at room temperature. Following fixation the samples were washed with PBS and stored in 2.5% (v/v) gluteraldhyde /1% (v/v) tannic acid at 4°C until stained with 0.5% (v/v) Osmium Tetroxide for 30 minutes. The dressings underwent a series ethanol dehydration and critical point drying with CO2. Samples were mounted onto scanning electron microscope stubs and sputter coated with platin/carbon. Images were acquired at a magnification of 5000× and a working distance of ~9mm using a LEO 1530 scanning electron microscope.
In vitro cytotoxicity
To measure acute cytotoxicity, D-tryptophan was dissolved in Dulbecco’s phosphate buffered saline (DPBS, with Ca2+ and Mg2+, pH 7.4, Thermo Scientific, Logan, UT) at a concentration of 10 mM and serially diluted in DPBS to final concentrations of 1.0 nM – 10.0 mM. Prior to plating cells, 96-well tissue culture plates were coated with 40 μl of fibronectin-collagen mix (FNC, AthenaES, Baltimore, MD) for 15 minutes. The immortalized human keratinocyte cell line, HaCaTs were seeded in the FNC coated 96-well microtiter plates at ~5 × 105 cells/well in 200 μl of DMEM/Endothelial Cell Basal Medium EBM-2 (Lonza, Walkersville, MD) and allowed to adhere overnight. Control conditions included cells treated with 200 μl of normal cell culture medium (positive control), DPBS (vehicle control), or 3% saponin solution (negative control) in DPBS. After a 1 hour incubation period at 37°C in 5% CO2, cells were rinsed 3 times with DPBS. Each well then was incubated for 30 to 60 minutes with 200 μl of calcein-AM (Invitrogen, Eugene, Oregon) solution (1 μM in DPBS). Fluorescence (Ex: 485nm; Em: 528nm) was measured using a Synergy 4 Hybrid Microplate Reader with Gen5 software (Biotek, Winooski, VT). Tryptophan treated wells were expressed as the mean ± SEM percentage of the DPBS control wells and the assay was repeated in triplicate.
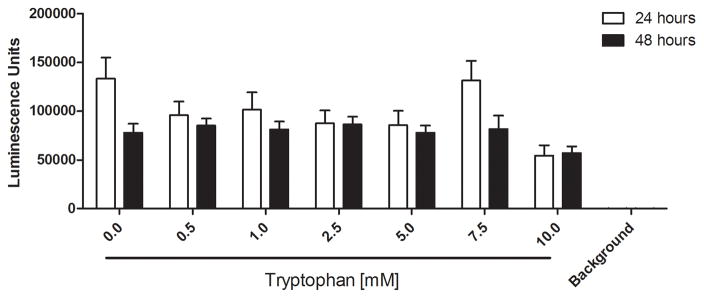
Skin hTERT immortalized normal keratinocytes (STINKs) derived from neonatal human foreskin were kindly provided by Dr. Vladimir S. Speigelman (Department of Dermatology, UW-Madison) and used for 24 and 48 hour cytotoxicity studies. The STINKs were grown in Eagle’s minimum essential medium (EMEM) with nonessential amino acids and L-glutamine without Calcium (Lonza) supplemented with Epidermal Growth Factor (EGF) at 5ng/ml, Bovine Pancreas Insulin at 5μg/ml, Transferrin at 10ng/ml, Ethanolamine at 10μM, Phospho-ethanolamine at 10μM, Calcium Chloride at 20μM (Sigma), 7% Fetal Bovine Serum (Atlanta Biologicals), and 1% Penicillin-streptomycin-amphotericin B (Corning) at 37°C with 5% CO2 (43). Cells were split using 0.25% Trypsin without EDTA (Corning). To measure cell viability and cytotoxicity the RealTime-Glo™ MT Cell Viability Assay (Promega) was multiplexed with CellTox™ Green Cytotoxicity Assay (Promega) and were used according to the manufacturer instructions. Briefly, opaque (white) 96 well plates (NUNC) were seeded with 10,000 STINKs cells/well and allowed to adhere overnight. The media was aspirated and replaced with complete culture medium containing the RealTime-Glo reagents and tryptophan (0 – 10mM). The RealTime-Glo™ MT Cell Viability Assay contains a substrate that is reduced within metabolically active cells to the substrate for the NanoLuc® luciferase and the resulting luminescence is measured. The CellTox™ Green Cytotoxicity Assay contains a cyanine dye that is excluded from viable cells but stains DNA in lysed or membrane permeable cells. Luminescence, the indicator of cellular metabolism and viability, was measured at 24 and 48 hours post tryptophan treatment and expressed as the mean ± SEM Luminescence Units. Thirty minutes before adding the CellTox™ Green Reagent, lysis buffer was added to a set of control wells to determine the maximum fluorescence of dead cells. Once the Cell-Tox Green Reagent was added to the wells, the plates were incubated for 15 minutes at room temperature. Fluorescence, the indicator of cell lysis or death, was measured at EX:485nm/EM:535nm and expressed as the mean ± SEM Relative Fluorescence Units (RFU). The multiplexed assay was repeated four times in triplicate.
Animal Model
To assess the impact of tryptophan on wound healing we utilized an experimental murine skin wound model, previously established in our laboratory (44, 45). All experimental protocols were approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Wisconsin-Madison. Eight to twelve week old BALB/c mice were housed individually in a temperature-controlled facility with a standard light/dark cycle. Environmental enrichment, food, and water ad libitum were provided to all mice. Mice were anaesthetized with isoflurane (Piramal Healthcare, Bethlehem, PA) using an induction chamber. Buprenorphine (0.1 mg/kg) was administered for pain control. The cranial thoracodorsal region was shaved and aseptically prepared for surgery. Silicon O-rings (McMaster-Carr®) were secured with tissue glue (Tissumend II) to the skin 4 mm caudal to the base of the ears on each side of the dorsal midline and were further attached to the skin by six 5-0 interrupted nylon sutures. Two symmetrical wounds within each O-ring were made using a 6mm biopsy punch. Body weights of mice were recorded on post-operative day 1, and every 2–3 days until the end of the study. Upon completion of each study, mice were euthanized by intra-peritoneal injection of Beuthanasia®-D (Schering-Plough) solution (0.5 ml/mouse) after induction of anesthesia with isoflurane.
In vivo wound impairment study
To measure the effects of tryptophan on wound healing, twenty BALB/c mice were randomly assigned to one of 4 treatment groups using a permuted block randomization of block size 4. The treatments consisted of two 8mm diameter circular Kendall Telfa Pads (Tyco Healthcare Group, Mansfield, MA), cut with an 8mm biopsy punch, stacked on top of each other and saturated with 60μl of either A) 1% PBS, B) 50mM D-Tryptophan, C) 50mM L-Tryptophan, or D) 25mM D-/25mM L-tryptophan (46). Every wound was also covered by Tegaderm™ Film (3M Healthcare, St. Paul, MN). Both wounds on a single mouse received the same treatment. The 50 mM concentration was chosen because it is the maximum tryptophan concentration achievable in aqueous media. The surgical team was blinded to the group allocation. Treatments were applied on the day of the surgery and reapplied on days 3 and 6 post surgery. Mice recovered from anesthesia on a warming pad. Each wound was imaged using a Nikon D300 digital camera with an attached Micro-Nikkor lens (105mm f/2.8G) on days 0, 3, 6, and 9 and wound areas calculated. Wound areas from the sequential images were traced and measured by 3 independent technicians blinded to the treatment groups using NIH Image J. The average calculated wound size was normalized to its own baseline (day 0) and wound area remaining on days 3, 6, and 9 was determined.
Effect of inhibiting biofilms with tryptophan in vivo
To determine if inhibition of P. aeruginosa biofilm formation by tryptophan provided an in vivo benefit, the wound dressings were incubated in suspensions of P. aeruginosa in M63 minimal media with and without 10mM D-/L-tryptophan at 30°C for 48 hours. After 48 hours of incubation the dressings were rinsed three times with 1X PBS to remove planktonic bacterial cells and applied to 6mm diameter skin wounds in mice using the same method as previously described. Immediately prior to dressing application, 18 mice were randomly assigned to one of three treatment groups using a permuted block randomization of block size 3. The treatment groups included: 1) dressings without bacterial cells (Control), 2) dressing with intact P. aeruginosa biofilm, and 3) dressing with tryptophan inhibited biofilm. Ten millimeter diameter Kendall Telfa Pads circular cutouts were placed on top of the wound dressing to ensure sustained contact of the dressing with the wound. The wounds were additionally covered with Tegaderm™ Film. Both wounds on each mouse received the same treatment. The surgical team was blinded to the group allocation. On days 3, 6, and 9 post-wounding, 2 mice from each group were euthanized and the wounds imaged to determine the percentage of original wound remaining as previously described, biopsies of the wounds and dressings were taken for quantitative bacteriology. The wound areas and bacterial counts are the expressed as the mean ± SEM of two independent experiments.
Statistical Analysis
Statistical analysis was performed using one-way ANOVA followed by Tukey’s post-test using GraphPad Prism 5.02 software (GraphPad Software, La Jolla, CA). Significance was set at p<0.05.
Results
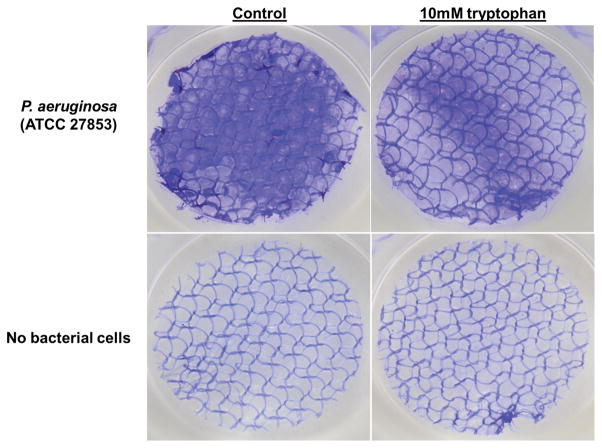
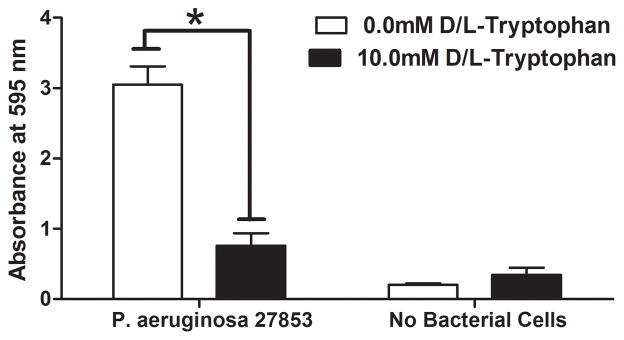
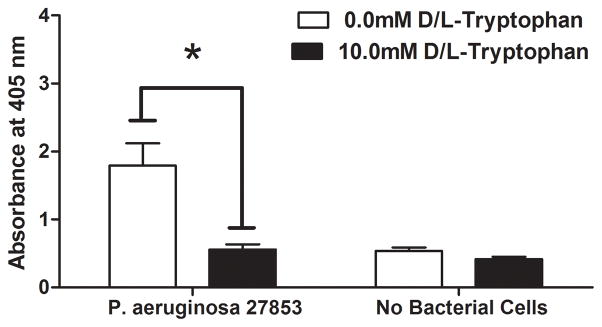
P. aeruginosa readily formed biofilms on the biological wound dressing within 48 hours of incubation in vitro at 30°C. Figure 1A shows representative macroscopic images of wound dressings stained with crystal violet. At 48 hours biofilm growth was readily apparent on the control samples (top left panel of 1A) as compared to an uninoculated control dressing (bottom left panel of 1A). An equimolar ratio of D- and L-tryptophan at a total concentration of 10.0mM significantly inhibited biofilm formation on the wound dressing (top right panel of 1A). Tryptophan alone did not significantly affect background staining of the dressing incubated in bacteriologic broth without bacteria (bottom right panel of 1A). Two independent methods were used to quantify the amount of biofilm formed on the dressing. Figure 1B shows significantly more crystal violet stain solubilized from the control wound dressings than those incubated with 10mM D-L-tryptophan (p<0.05). A phosphatase-linked lectin specific for the polysaccharides present in P. aeruginosa biofilms confirmed the presence of biofilm matrix on the dressings. Figure 1C shows significantly increased amounts of bound lectin on the control dressings compared to those incubated with tryptophan (p<0.05). Both assays produced similar results with biomass (crystal violet) correlating with polysaccharide (lectin) and a relatively low background signal of the dressing itself in both assays.
Figure 1. Tryptophan inhibits P. aeruginosa biofilm formation on a biological wound dressing.
A) Representative samples of eight millimeter diameter sections of the wound dressing incubated for 48 hours at 30°C with P. aeruginosa (ATCC 27853) suspended in M63 minimal media, with or without 10mM D-/L-tryptophan. Crystal violet stained the biofilm on the dressing; samples incubated without bacterial cells were included as controls for background staining of the dressing. B) Solubilized crystal violet bound to the biofilm was quantified at a solution absorbance of 595nm. Tryptophan significantly inhibited biofilm growth on the wound dressing (*, p<0.001). C) A phosphatase linked lectin (HHA) specific for the polysaccharides of P. aeruginosa biofilms stained the biofilm matrix on the dressing. Biofilms were quantified by enzymatic cleavage of P-nitrophenylphosphate to P-nitrophenol at an absorbance of 405nm. Tryptophan significantly inhibited biofilm growth on the wound dressing (*, p=0.0032). Data is presented as the mean ± SEM of three independent experiments performed in triplicate.
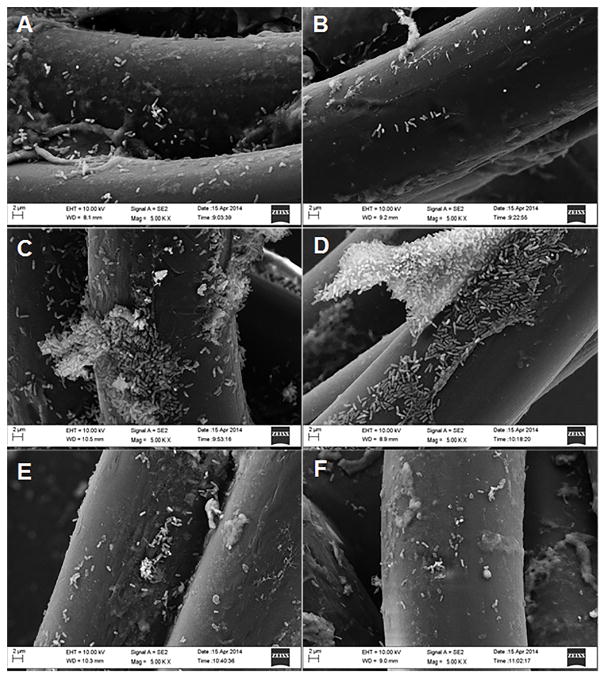
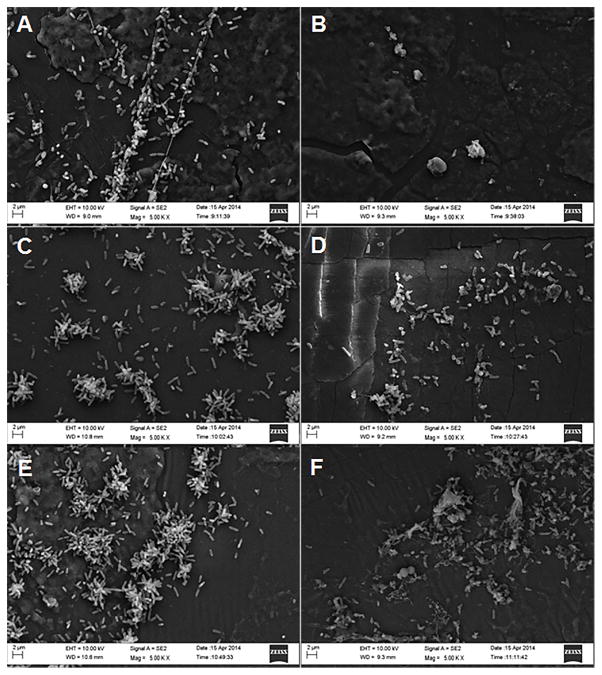
We used scanning electron microscopy to visualize both bacterial cells and biofilm matrix adherent to the collagen coated nylon fibers and silicon backing of the dressing. Representative images of the dressings incubated in P. aeruginosa with and without tryptophan are shown in Figures 2 and 3. Figure 2 shows P. aeruginosa attached to the collagen-coated nylon fibers on control (Figures 2A, 2C, and 2E) and tryptophan-treated (Figures 2B, 2D, and 2F) dressings. Figure 3 shows P. aeruginosa attached to the silicon backing of the dressing in control (Figures 3A, 3C, and 3E) and tryptophan-treated (Figures 3B, 3D, and 3F) dressings. At 24 hours of incubation, bacterial cells are adherent to both the fibers and silicon backing of the dressing (Figures 2A/B and 3A/B). After 48 hours, bacterial aggregates or microcolonies were observed on the dressing (Figures 2C/D and 3C/D). Bacterial cells incubated with the dressing in the presence of tryptophan did not exhibit the same well-defined microcolony structure as the controls (Figures 3C vs 3D). At 72 hours, only single bacterial cells were observed on the fibers of the dressing, although microcolonies were still present on the silicon backing (Figures 2E/F and 3E/F).
Figure 2. Scanning electron microscopy of P. aeruginosa biofilms on collagen coated nylon fibers of a biological wound dressing.
Representative scanning electron microscopy images (5000X) of control P. aeruginosa biofilms (A, C, and E), and 10mM D-/L-tryptophan inhibited biofilms (B, D, and F) grown on the wound dressing for 24 h (A and B), 48 h (C and D), and 72 h (E and F). Images were taken with a LEO 1530 scanning electron microscope.
Figure 3. Scanning electron microscopy images of P. aeruginosa biofilms on the silicon backing of the wound dressing.
Representative scanning electron microscopic images (5000X) of control P. aeruginosa biofilms (A, C, and E) and 10.0mM D-/L-tryptophan treated biofilms (B, D, and F) grown on the wound dressing for 24 h (A and B), 48 h (C and D), and 72 h (E and F). Images were taken with a LEO 1530 scanning electron microscope.
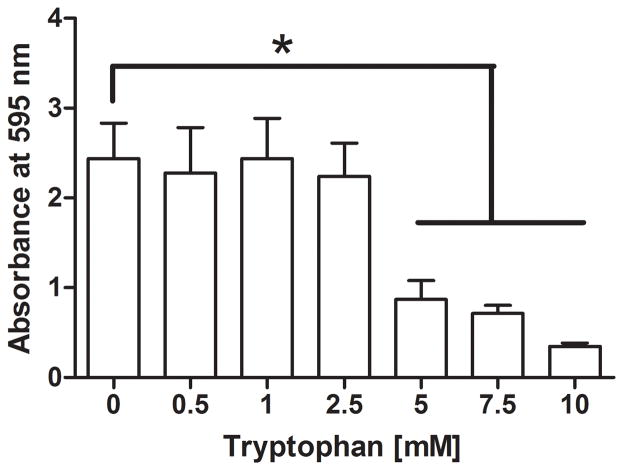
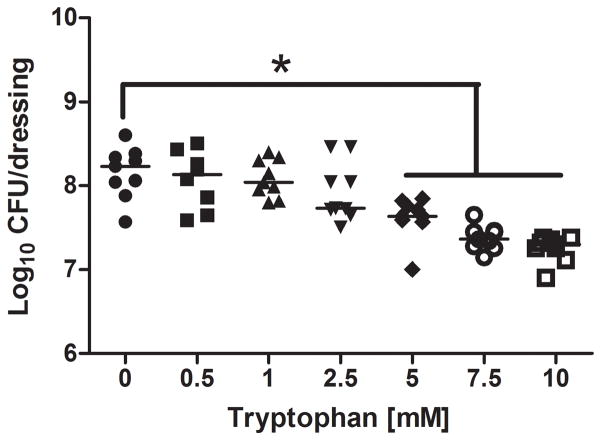
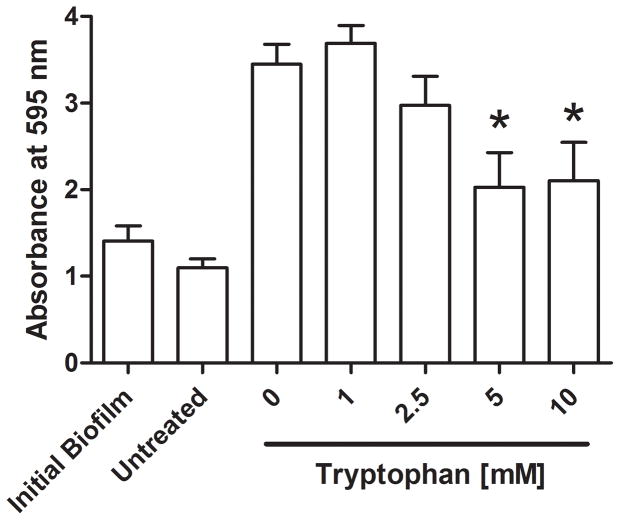
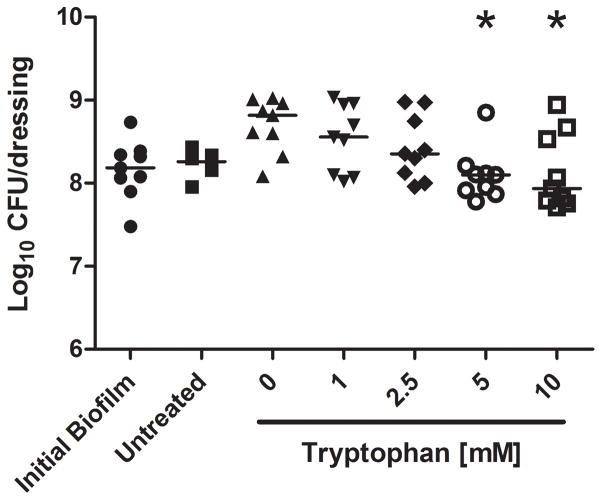
We further characterized the dose response of tryptophan on P. aeruginosa numbers and biofilm biomass. Figure 4 shows significant inhibition by D-/L-tryptophan concentrations above 5mM of biofilm formation as assessed by crystal violet staining and CFU counts, after 48 hours of incubation in M63 minimal media (p<0.05). Figure 5 shows that tryptophan did not significantly disperse 48 hour old P. aeruginosa biofilms from the wound dressing, although 5 and 10mM tryptophan concentrations limited additional biofilm growth as determined by both crystal violet staining and CFU counts (p<0.05).
Figure 4. Dose-dependent inhibition of P. aeruginosa biofilms on a biological wound dressing by tryptophan.
The bioloigcal wound dressing was bathed in P. aeruginosa suspended in M63 minimal media supplemented with tryptophan (0 – 10 mM) for 48 hours at 30°C. A) Tryptophan significantly inhibited biofilm formation on the dressing at concentrations above 5mM as determined by crystal violet staining (*, p<0.05). B) Tryptophan significantly decreased bacterial colonization of the dressing at concentrations above 5mM (*, p<0.05).
Figure 5. Tryptophan treatment of established P. aeruginosa biofilms.
P. aeruginosa biofilms were grown for 48 hours at 30°C on the biological wound dressing prior to treatment with tryptophan (0 – 10 mM). The treatment lasted for an additional 24 hours at 30°C. A) Crystal violet staining revealed that tryptophan significantly inhibited additional biofilm growth (*, p<0.05) and that concentrations above 5mM were not significantly higher than the initial biofilm. B) Quantification of bacterial cells attached to the dressing showed that tryptophan significantly reduced additional bacterial colonization compared to the fresh media alone (*, p<0.05), concentrations of tryptophan above 1mM were not significantly different than the initial bacterial load on the dressing.
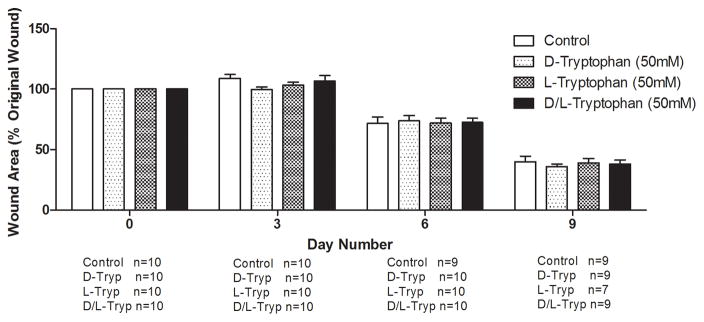
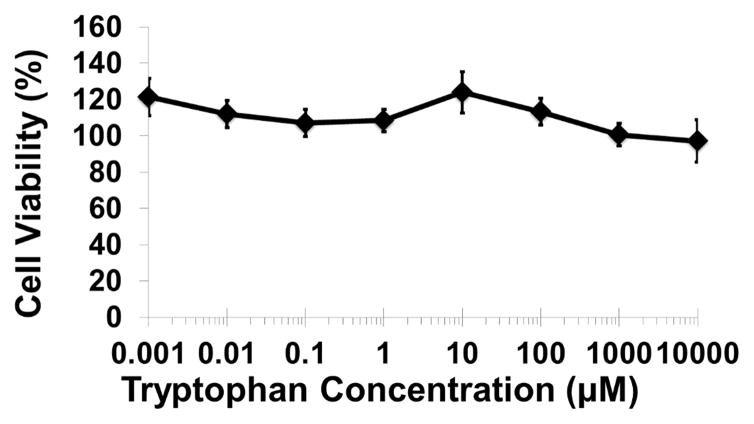
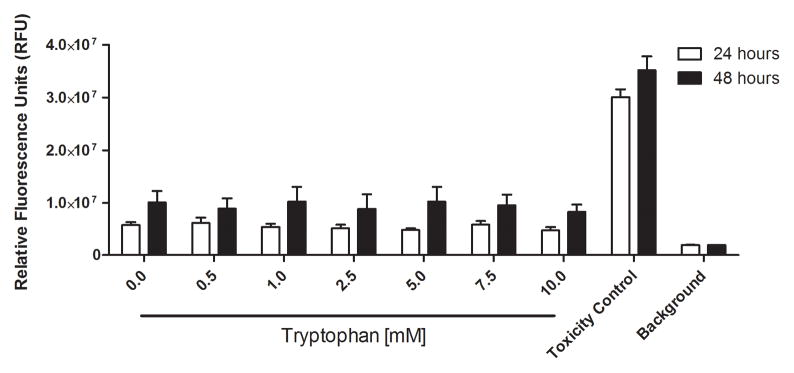
We examined whether the millimolar tryptophan concentrations effective for inhibiting P. aeruginosa biofilms would hinder wound healing or cause cytotoxic effects on mammalian cells in vitro. Figure 6A shows that no significant differences in wound area remaining were observed among four treatment groups (PBS, 50mM D-tryptophan, 50mM L-tryptophan, and 50mM D-/L-tryptophan) over 9 days in a splinted mouse skin wound model. Additionally, no significant side-effects (e.g. weight loss, pain, or irritation) were observed in any mice with any of the tryptophan treatments. To fully characterize any potential toxicity of tryptophan, we used two immortalized human keratinocyte cell lines to examine acute and long term toxicity in vitro. Figure 6B shows that acute exposure of the immortalized human keratinocyte HaCaT cell line to D-tryptophan (1nm – 10mM) for 1 hour resulted in no change in cellular metabolism or viability. We used immortalized keratinocytes from human foreskin (STINKs) to characterize longer term toxicity of tryptophan. Figure 6C shows that for longer incubations (24 and 48 hours) D-/L-tryptophan was not cytotoxic to the keratinocytes as seen by the lack of fluorescence from DNA staining, but Figure 6D indicates that tryptophan did alter the metabolic activity of the STINKs cells.
Figure 6. Tryptophan is not cytotoxic in murine skin wounds or for immortalized human keratinocytes.
A) Twenty BALB/c mice were randomized to four treatment groups. Two splinted full thickness wounds were made on the backs of each mouse, and were treated with two 8mm diameter discs of Telfa® pads soaked with 60μl of either PBS (Control), 50mM D-tryptophan, 50mM L-tryptophan, or a 50:50 combination of D- and L-tryptophan (50mM total tryptophan concentration). Treatments were applied on day 0 and reapplied on days 3 and 6. Images of the wounds were taken on days 0 (baseline), 3, 6, and 9 with a Nikon D300 camera. Image J was used to calculate the wound areas, which were normalized to the baseline (day 0) values. No significant differences in remaining wound area were measured between any treatment and the control, a p-value of below 0.05 was considered significant. B) A one hour exposure of the immortalized HaCaT cell line to D-tryptophan at 37°C exhibited no direct cytotoxicity. Cell viability was measured using calcein-AM and measuring resulting fluorescence at EM:485nm/EX:528nm. C) No loss of cellular membrane integrity was observed in immortalized human keratinocytes (STINKs) with D-/L-tryptophan concentrations up to 10mM over 24 and 48 hour incubations at 37°C using the CellTox™ Green Cytotoxicity Assay. D) Altered or reduced the cellular metabolism of the STINKs cell line was observed over 24 and 48 hour incubations with increasing D-/L-tryptophan concentrations as assessed by the RealTime-Glo MT Cell Viability Assay.
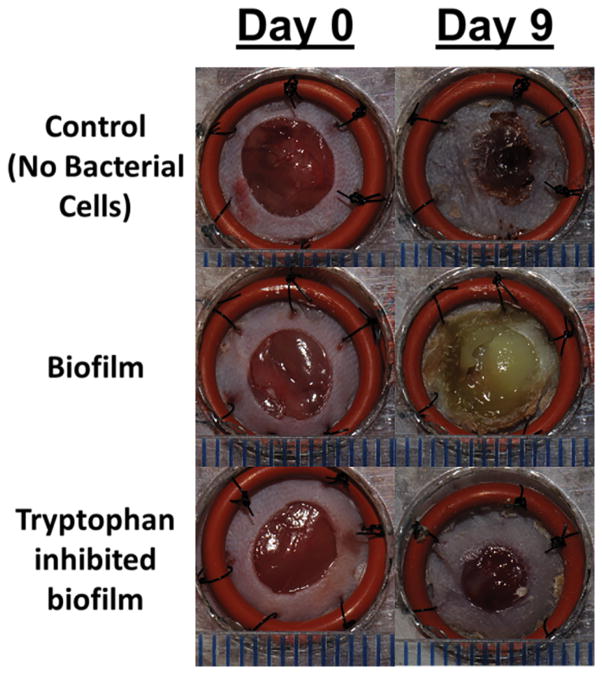
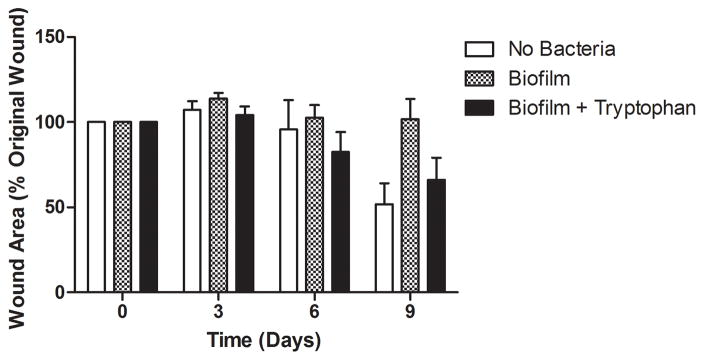
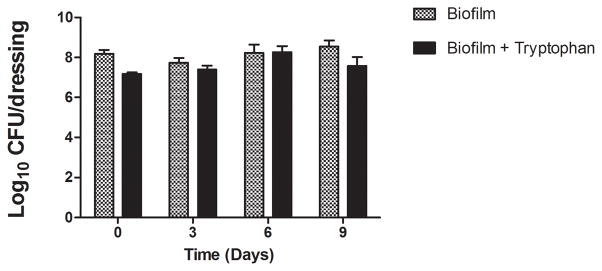
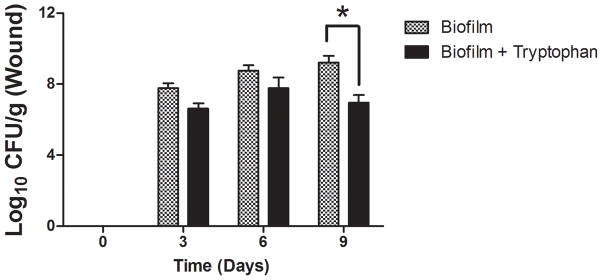
To determine if tryptophan inhibition of P. aeruginosa biofilm formation may provide an in vivo benefit, we applied contaminated wound dressings to experimental mouse skin wounds. Representative images of the wounds on days 0 and 9 are shown in Figure 7A. Figure 7B shows that control dressings with P. aeruginosa biofilms exhibited no wound closure at any time over the 9 days of the study, whereas the sterile dressings showed approximately 50% healing and the tryptophan inhibited biofilm dressings showed approximately 35% healing on day 9. Figure 7C shows that there was slightly fewer bacterial cells on the dressing treated with 10mM D-/L-tryptophan compared to the untreated group (p<0.05) on the day of application, although after 6 days, bacterial counts on the dressings in both the tryptophan-treated and control groups reached comparable levels. Figure 7D shows the bacterial numbers isolated from the wound tissue itself. Significantly fewer bacterial cells were recovered from the wound tissue with the tryptophan-treated biofilms on day 9 compared to the control biofilms.
Figure 7. P. aeruginosa biofilms on wound dressings delay wound healing.
P. aeruginosa biofilms with or without 10mM D-/L-tryptophan were established on wound dressings for 48 hours at 30°C. The contaminated dressings were applied to mouse skin wounds for up to 9 days. On days 3, 6, and 9, two mice from each group were sacrificed for imaging of the wounds, bacterial quantification of the dressing and wound bed. A) Representative images of the wounds in the three groups on days 0 and 9. B) Wound areas are expressed as percentages of the day 0 baseline (100%). By day 9 the control wounds without P. aeruginosa were ~50% closed, the biofilms grown in the presence of tryptophan were ~35% closed, and the biofilms grown without tryptophan remained fully open. C) Bacterial loads on the dressing (log10 CFU/dressing) did not change significantly over the 9 days on the mouse skin wounds. D) Tryptophan slightly reduced the bacterial loads on the wound bed (log10 CFU/g) with significantly lower counts detectible on day 9.
Discussion
Wound dressings and surgical sutures often become contaminated with bacterial cells that can form biofilms on the surface of the wound dressing or implant (9–13). These biofilms can serve as a nidus of infection leading to exacerbation of the wound and delayed healing. While their development and persistence is multifactorial, it is clear that chronic non-healing skin wounds are often infected with pathogenic biofilms that impact the healing process (9, 47). The overall focus of our research team is to develop novel biomaterial-based dressings, which can be applied to chronic non-healing skin wounds, to prevent formation of or eliminate bacterial biofilms on the skin wound bed and aid healing. In the current study we documented that the essential amino acid tryptophan inhibits P. aeruginosa biofilms on a clinically relevant biological wound dressing. We further characterized any potential cytotoxicity of tryptophan both in vitro and in vivo at biofilm inhibitory concentrations, and showed this biofilm inhibition potentially may benefit wound healing.
We previously reported that an equimolar ratio of D- and L-tryptophan inhibited biofilm formation by P. aeruginosa on polystyrene microtiter plates (32). Although plastic microtiter plates are a standard model for studying bacterial biofilm formation, they do not replicate the complex surfaces biofilms grow on in skin wounds or the dressings used to treat such wounds. We developed a model system for studying biofilm inhibition on a complex 3-dimensional substrate by using the biological wound dressing Biobrane. This wound dressing, which is commonly used to treat skin wounds such as burns, is composed of porcine collagen-coated nylon fibers embedded in a sheet of silicon. The dressing is designed to promote re-epithelialization of the wound surface via a scaffolding effect of the collagen-coated fibers (40). Contamination of the dressing with bacterial cells often leads to its failure and requires replacement (15–18). We chose to use P. aeruginosa because it is commonly isolated from skin wound infections and the complex 3-dimensional geometry and biological heterogeneity of the biological dressing is a surface that P. aeruginosa may encounter within wounds. We believe this approach provides a realistic surface for modeling biofilm formation and inhibition on materials used to treat chronic skin wounds.
Macroscopically, P. aeruginosa (ATCC 27853) formed robust amounts of biofilm on the surface of the wound dressing as quantified with crystal violet and enzyme linked lectin staining. Scanning electron microscopy revealed that the bacterial cells attached to both the nylon fibers and the silicon backing of the dressing. It also showed the typical ‘life-cycle’ of P. aeruginosa biofilms in terms of attachment, microcolony formation, and potentially dispersal. Overall, we observed more bacterial cells attached to the silicon backing than the nylon fibers, although more work is required to determine if P. aeruginosa preferentially attaches to silicon versus the collagen-coated fibers. Incubation of wound dressings with 5mM or greater concentrations of D-/L-tryptophan significantly arrested biofilm formation. Microscopy and CFU data suggested that tryptophan may reduce attachment of P. aeruginosa cells to the dressing surfaces (both the nylon fibers and silicon backing), but further quantitative studies are necessary to confirm this observation. Scanning electron microscopy also revealed that tryptophan may inhibit or delay microcolony formation on the silicon backing of the dressing. Our previous work showed that tryptophan increased the swimming motility of P. aeruginosa, indicating that increased flagellar activity may result in decreased biofilm formation (32). Based on the present and previous experimental data, perhaps tryptophan limits bacterial attachment or disrupts microcolony formation through altered bacterial motility. Another potential mechanism for the observed effects of tryptophan may be altered quorum sensing, which could alter bacterial attachment and biofilm formation. Tryptophan feeds into the Pseudomonas quinolone signal (PQS) quorum sensing pathway and may result in altered production of biofilm matrix material through modulation of gene expression (33, 48). Additional studies are in progress to address the mechanism of action through which tryptophan inhibits biofilm formation by P. aeruginosa.
The millimolar concentration required for tryptophan to inhibit P. aeruginosa biofilms raised concerns regarding potentially cytotoxic effects against mammalian cells if tryptophan were to be incorporated into topical skin wound treatments. To address this concern, we performed both in vitro cell viability and in vivo wound healing studies. Acute exposure of HaCaT cells, an immortalized human keratinocyte cell line, to 10.0mM D-tryptophan for 1 hour did not reduce cell viability compared to controls, indicating that tryptophan was not directly cytotoxic to mammalian cells. We used an additional immortalized keratinocyte cell line from neonatal human foreskin (STINKs) for longer term in vitro cell viability tests. Tryptophan did not cause cell lysis or death but did alter or reduce the cellular metabolism of the STINKs cells, potentially through effects on the aryl hydrocarbon receptor (39).
For in vivo experiments, we utilized a blinded, randomized, placebo-controlled trial to compare the effects of tryptophan on wound healing in a splinted mouse skin wound model. We chose this experimental design to eliminate potential bias of animal selection and treatment bias by the surgical team in the study. We asked two separate questions regarding the effect of tryptophan in vivo. The first was if tryptophan would impair wound healing or cause any systemic side effects, thus limiting its applicability as an antibiofilm agent for chronic non-healing skin wounds. We chose a much higher concentration of tryptophan, 50mM, to best evaluate safety of the topical application of tryptophan and determine if there were negative outcomes to its application. This concentration would also provide a margin of safety as it is greater than the concentration required to inhibit P. aeruginosa biofilm formation. No significant differences in wound healing during a 9 day period were observed between control wounds and those treated with tryptophan. In addition, no other systemic side-effects (e.g. weight loss, discomfort, etc.) were observed in any of the study animals, thereby establishing the potential safety of using tryptophan at millimolar concentrations for biofilm treatments in chronic skin wounds. The second question we asked was whether inhibiting P. aeruginosa biofilms with tryptophan provides any benefit in vivo. We bathed wound dressings for 48 hours in P. aeruginosa with or without tryptophan and applied the contaminated dressings to murine skin wounds and assessed wound closure and bacterial counts on the dressing and wound tissue over 9 days. Most interestingly, the wounds that received the control biofilms remained 100% open over the 9 days of the experiment. The wounds, which received dressings on which biofilm formation was inhibited by exposure to tryptophan, remained approximately 65% open over the 9 days. Comparatively, the wounds that received sterile dressings were approximately 50% open after 9 days. We are currently pursuing application of biofilms on wound dressings as a potential model for developing a chronic non-healing skin wound in animal studies.
Other recent evidence supports using tryptophan as a novel wound healing agent. Tryptophan is metabolized in mammalian cells by the enzyme Indoleamine 2,3-dioxygenase (IDO) 1, which converts it into N-formylkynurenine. Mice lacking this enzyme had faster rates of healing than their wild type counterparts, coupled with increased mRNA levels of Interleukin-6 (IL-6), Tumor Necrosis Factor-α (TNF-α), and Matrix Metallopeptidase 9 (MMP9) at wound sites (37). Furthermore, it was shown in the same set of studies that the wild type mice treated with tryptophan had faster rates of healing than control wild type mice, as well as increased IL-6, TNF-α, and MMP9 expression in the wound tissue (37). The effect of tryptophan on wound healing was also observed in chronically stressed mice which exhibited a reduction in Collagen III formation, increased Collagen I deposition, and increased re-epithelialization of the wound (38). The beneficial aspects of tryptophan in wound healing also led to a human clinical trial of application of 50mM L-tryptophan to chronic leg ulcers (39). The tryptophan treated groups had a faster rate of wound healing, which was speculated to be modulated through activation of the aryl hydrocarbon receptor (39). Additionally, the patient group treated with tryptophan reported decreased amounts of pain compared to the control group. Furthermore, levels of systemic tryptophan are reported to be decreased in patients with chronic wounds; while the tryptophan metabolism enzymes IDO and Kynureminase (converts 3-hydroxy-L-kynurenine into 3-hydroxyanthlanilic acid) are reported to be up-regulated at sites of atopic dermatitis and psoriasis (49, 50).
A limitation of our studies is the lack of a defined system or delivery vehicle for releasing tryptophan in a controlled manner. Tryptophan was delivered as a bulk reagent in the in vitro experiments and Telfa® pads were saturated with tryptophan for the in vivo wound healing experiment. Saturated dressings were chosen for their relative ease of manipulation, but do not represent an ideal vehicle for delivery or release of tryptophan to the wound surface. In a separate study we utilized a hyaluronic acid gel for application of tryptophan to the mouse skin wounds and did not observe any detrimental side-effects or impairment of wound healing (data not shown). Ongoing studies are aimed at incorporation of tryptophan into novel polyvinyl alcohol - polyelectrolyte multilayer (PVA-PEMs) microfilms that can release tryptophan in a controlled and sustained manner. Because these microfilms can be directly applied to the skin wound bed to release both antimicrobials and antibiofilm agents into the wound microenvironment, it is hoped they may enhance the overall bio-activity of tryptophan. By releasing tryptophan from the PEMs directly into the local wound microenvironment it may be possible to overcome the millimolar concentration of tryptophan required when provided in bulk solution in vitro. We previously observed similar effects of increased bioactivity with silver loaded PEMs (20, 44, 45). We believe it is possible to modulate release of tryptophan from these PVA-PEMs to allow sustained release over time so that its beneficial effects may be extended beyond the time of application.
In summary, this study demonstrates that the amino acid tryptophan dose dependently inhibits P. aeruginosa biofilm formation on the complex three-dimensional surface of a biological wound dressing currently used for treatment of skin wounds. This system serves as a model for studying biofilm inhibition on a relevant substrate often encountered by the pathogen P. aeruginosa in the wound environment. We also show that tryptophan was not directly cytotoxic to immortalized human keratinocytes in vitro. Additionally, application of tryptophan to murine skin wounds did not inhibit wound closure nor show any toxic side-effects. Recent reports in the literature provide additional evidence that tryptophan is a promising candidate for improving wound healing. The ability of tryptophan to inhibit bacterial biofilm formation, coupled with its lack of toxicity make it a good candidate for development as an antibiofilm treatment for chronic non-healing skin wounds.
Acknowledgments
Assistance with scanning electron microscopy was provided by Joseph Heintz and Dr. Ralph Albrecht, PhD and was performed at the Biological and Biomaterials Preparation, Imaging, and Characterization Facility at UW-Madison. The STINKs cells were kindly provided by Dr. Vladimir S. Speigelman, PhD (Department of Dermatology, UW-Madison). Assistance with tryptophan cytotoxicity assay was additionally provided by Dr. Vijay Raghunathan, PhD. Assistance with the animal studies was kindly provided by Dr. Pablo Mendoza Araya, DVM, Emma T. Watson, Heather L. McAnulty, Madeline Abbott, and Michael Kierski. This project was supported in part by the Dr. E. Michael and Winona Foster Wisconsin Distinguished Fellowship (KSB) and NIH Grants RC2 AR058971-01 and R21 EB014594, and through the TL1 pre-doctoral fellowship (KSB) supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Additionally, NLA, MJS, CJM, JFM, and CJC possess financial interests in Wound Engineering LLC and/or Imbed Biosciences Inc., for-profit companies that have filed patent applications and/or are commercializing aspects of the work reported in this publication.
References
- 1.Akers KS, Mende K, Cheatle KA, Zera WC, Yu X, Beckius ML, et al. Biofilms and persistent wound infections in United States military trauma patients: a case-control analysis. BMC infectious diseases. 2014;14:190. doi: 10.1186/1471-2334-14-190. Epub 2014/04/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James GA, Swogger E, Wolcott R, Pulcini E, Secor P, Sestrich J, et al. Biofilms in chronic wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2008;16(1):37–44. doi: 10.1111/j.1524-475X.2007.00321.x. Epub 2007/12/19. [DOI] [PubMed] [Google Scholar]
- 3.Flemming H-C, Wingender J. The biofilm matrix. Nature reviews Microbiology. 2010;8:623–33. doi: 10.1038/nrmicro2415. [DOI] [PubMed] [Google Scholar]
- 4.López D, Vlamakis H, Kolter R. Biofilms. Cold Spring Harbor perspectives in biology. 2010;2:a000398. doi: 10.1101/cshperspect.a000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ma L, Jackson KD, Landry RM, Parsek MR, Wozniak DJ. Analysis of Pseudomonas aeruginosa conditional psl variants reveals roles for the psl polysaccharide in adhesion and maintaining biofilm structure postattachment. Journal of bacteriology. 2006;188:8213–21. doi: 10.1128/JB.01202-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nithya C, Begum MF, Pandian SK. Marine bacterial isolates inhibit biofilm formation and disrupt mature biofilms of Pseudomonas aeruginosa PAO1. Applied Microbiology and Biotechnology. 2010;88:341–58. doi: 10.1007/s00253-010-2777-y. [DOI] [PubMed] [Google Scholar]
- 7.Sambanthamoorthy K, Gokhale AA, Lao W, Parashar V, Neiditch MB, Semmelhack MF, et al. Identification of a novel benzimidazole that inhibits bacterial biofilm formation in a broad-spectrum manner. Antimicrobial agents and chemotherapy. 2011;55:4369–78. doi: 10.1128/AAC.00583-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nusbaum AG, Gil J, Rippy MK, Warne B, Valdes J, Claro A, et al. Effective method to remove wound bacteria: comparison of various debridement modalities in an in vivo porcine model. J Surg Res. 2012;176(2):701–7. doi: 10.1016/j.jss.2011.11.1040. Epub 2012/03/24. [DOI] [PubMed] [Google Scholar]
- 9.Kathju S, Nistico L, Hall-Stoodley L, Post JC, Ehrlich GD, Stoodley P. Chronic surgical site infection due to suture-associated polymicrobial biofilm. Surgical infections. 2009;10(5):457–61. doi: 10.1089/sur.2008.062. Epub 2009/10/09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Konig L, Klopfleisch R, Kershaw O, Gruber AD. Prevalence of Biofilms on Surgical Suture Segments in Wounds of Dogs, Cats, and Horses. Veterinary pathology. 2014 doi: 10.1177/0300985814535609. Epub 2014/07/06. [DOI] [PubMed] [Google Scholar]
- 11.Masini BD, Stinner DJ, Waterman SM, Wenke JC. Bacterial adherence to suture materials. Journal of surgical education. 2011;68(2):101–4. doi: 10.1016/j.jsurg.2010.09.015. Epub 2011/02/23. [DOI] [PubMed] [Google Scholar]
- 12.Edmiston CE, Jr, Krepel CJ, Marks RM, Rossi PJ, Sanger J, Goldblatt M, et al. Microbiology of explanted suture segments from infected and noninfected surgical patients. J Clin Microbiol. 2013;51(2):417–21. doi: 10.1128/jcm.02442-12. Epub 2012/11/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry-Stanley MJ, Hess DJ, Barnes AM, Dunny GM, Wells CL. Bacterial contamination of surgical suture resembles a biofilm. Surgical infections. 2010;11(5):433–9. doi: 10.1089/sur.2010.006. Epub 2010/08/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lipp C, Kirker K, Agostinho A, James G, Stewart P. Testing wound dressings using an in vitro wound model. Journal of wound care. 2010;19(6):220–6. doi: 10.12968/jowc.2010.19.6.48468. Epub 2010/06/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schierle CF, De la Garza M, Mustoe TA, Galiano RD. Staphylococcal biofilms impair wound healing by delaying reepithelialization in a murine cutaneous wound model. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2009;17(3):354–9. doi: 10.1111/j.1524-475X.2009.00489.x. Epub 2009/08/08. [DOI] [PubMed] [Google Scholar]
- 16.Roche ED, Renick PJ, Tetens SP, Ramsay SJ, Daniels EQ, Carson DL. Increasing the presence of biofilm and healing delay in a porcine model of MRSA-infected wounds. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20(4):537–43. doi: 10.1111/j.1524-475X.2012.00808.x. Epub 2012/06/08. [DOI] [PubMed] [Google Scholar]
- 17.Seth AK, Geringer MR, Gurjala AN, Hong SJ, Galiano RD, Leung KP, et al. Treatment of Pseudomonas aeruginosa biofilm-infected wounds with clinical wound care strategies: a quantitative study using an in vivo rabbit ear model. Plastic and reconstructive surgery. 2012;129(2):262e–74e. doi: 10.1097/PRS.0b013e31823aeb3b. Epub 2012/01/31. [DOI] [PubMed] [Google Scholar]
- 18.Zhao G, Usui ML, Underwood RA, Singh PK, James GA, Stewart PS, et al. Time course study of delayed wound healing in a biofilm-challenged diabetic mouse model. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2012;20:342–52. doi: 10.1111/j.1524-475X.2012.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Agarwal A, Nelson TB, Kierski PR, Schurr MJ, Murphy CJ, Czuprynski CJ, et al. Polymeric multilayers that localize the release of chlorhexidine from biologic wound dressings. Biomaterials. 2012;33(28):6783–92. doi: 10.1016/j.biomaterials.2012.05.068. Epub 2012/07/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Agarwal A, Weis TL, Schurr MJ, Faith NG, Czuprynski CJ, McAnulty JF, et al. Surfaces modified with nanometer-thick silver-impregnated polymeric films that kill bacteria but support growth of mammalian cells. Biomaterials. 2010;31(4):680–90. doi: 10.1016/j.biomaterials.2009.09.092. Epub 2009/10/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Said SS, Aloufy AK, El-Halfawy OM, Boraei NA, El-Khordagui LK. Antimicrobial PLGA ultrafine fibers: interaction with wound bacteria. European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik eV. 2011;79(1):108–18. doi: 10.1016/j.ejpb.2011.03.002. Epub 2011/03/15. [DOI] [PubMed] [Google Scholar]
- 22.Favre-Bonté S, Köhler T, Van Delden C. Biofilm formation by Pseudomonas aeruginosa: role of the C4-HSL cell-to-cell signal and inhibition by azithromycin. The Journal of antimicrobial chemotherapy. 2003;52:598–604. doi: 10.1093/jac/dkg397. [DOI] [PubMed] [Google Scholar]
- 23.Bala A, Kumar R, Harjai K. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. Journal of medical microbiology. 2011;60:300–6. doi: 10.1099/jmm.0.025387-0. [DOI] [PubMed] [Google Scholar]
- 24.Singh PK, Parsek MR, Greenberg EP, Welsh MJ. A component of innate immunity prevents bacterial biofilm development. Nature. 2002;417:552–5. doi: 10.1038/417552a. [DOI] [PubMed] [Google Scholar]
- 25.Mulcahy H, Lewenza S. Magnesium Limitation Is an Environmental Trigger of the Pseudomonas aeruginosa Biofilm Lifestyle. PLoS ONE. 2011;6:e23307. doi: 10.1371/journal.pone.0023307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sanchez CJ, Jr, Prieto EM, Krueger CA, Zienkiewicz KJ, Romano DR, Ward CL, et al. Effects of local delivery of D-amino acids from biofilm-dispersive scaffolds on infection in contaminated rat segmental defects. Biomaterials. 2013;34(30):7533–43. doi: 10.1016/j.biomaterials.2013.06.026. Epub 2013/07/09. [DOI] [PubMed] [Google Scholar]
- 27.Sanchez CJ, Jr, Akers KS, Romano DR, Woodbury RL, Hardy SK, Murray CK, et al. d-Amino Acids Enhance the Activity of Antimicrobials against Biofilms of Clinical Wound Isolates of Staphylococcus aureus and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2014;58(8):4353–61. doi: 10.1128/aac.02468-14. Epub 2014/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kolodkin-Gal I, Romero D, Cao S, Clardy J, Kolter R, Losick R. D-amino acids trigger biofilm disassembly. Science (New York, NY) 2010;328:627–9. doi: 10.1126/science.1188628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hochbaum AI, Kolodkin-Gal I, Foulston L, Kolter R, Aizenberg J, Losick R. Inhibitory effects of D-amino acids on Staphylococcus aureus biofilm development. Journal of bacteriology. 2011;193:5616–22. doi: 10.1128/JB.05534-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Leiman SA, May JM, Lebar MD, Kahne D, Kolter R, Losick R. D-amino acids indirectly inhibit biofilm formation in Bacillus subtilis by interfering with protein synthesis. J Bacteriol. 2013;195(23):5391–5. doi: 10.1128/jb.00975-13. Epub 2013/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimazaki J, Furukawa S, Ogihara H, Morinaga Y. l-Tryptophan prevents Escherichia coli biofilm formation and triggers biofilm degradation. Biochemical and biophysical research communications. 2012;419:715–8. doi: 10.1016/j.bbrc.2012.02.085. [DOI] [PubMed] [Google Scholar]
- 32.Brandenburg KS, Rodriguez KJ, McAnulty JF, Murphy CJ, Abbott NL, Schurr MJ, et al. Tryptophan inhibits biofilm formation by Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2013;57(4):1921–5. doi: 10.1128/aac.00007-13. Epub 2013/01/16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shen DK, Filopon D, Chaker H, Boullanger S, Derouazi M, Polack B, et al. High-cell-density regulation of the Pseudomonas aeruginosa type III secretion system: implications for tryptophan catabolites. Microbiology. 2008;154(Pt 8):2195–208. doi: 10.1099/mic.0.2007/013680-0. Epub 2008/08/01. [DOI] [PubMed] [Google Scholar]
- 34.Farrow JM, 3rd, Pesci EC. Two distinct pathways supply anthranilate as a precursor of the Pseudomonas quinolone signal. J Bacteriol. 2007;189(9):3425–33. doi: 10.1128/jb.00209-07. Epub 2007/03/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palmer GC, Jorth PA, Whiteley M. The role of two Pseudomonas aeruginosa anthranilate synthases in tryptophan and quorum signal production. Microbiology. 2013;159(Pt 5):959–69. doi: 10.1099/mic.0.063065-0. Epub 2013/03/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rutherford ST, Bassler BL. Bacterial quorum sensing: its role in virulence and possibilities for its control. Cold Spring Harbor perspectives in medicine. 2012;2(11) doi: 10.1101/cshperspect.a012427. Epub 2012/11/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ito H, Ando T, Ogiso H, Arioka Y, Saito K, Seishima M. Inhibition of indoleamine 2,3-dioxygenase activity accelerates skin wound healing. Biomaterials. 2015;53:221–8. doi: 10.1016/j.biomaterials.2015.02.098. Epub 2015/04/22. [DOI] [PubMed] [Google Scholar]
- 38.Bandeira LG, Bortolot BS, Cecatto MJ, Monte-Alto-Costa A, Romana-Souza B. Exogenous Tryptophan Promotes Cutaneous Wound Healing of Chronically Stressed Mice through Inhibition of TNF-alpha and IDO Activation. PLoS ONE. 2015;10(6):e0128439. doi: 10.1371/journal.pone.0128439. Epub 2015/06/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barouti N, Mainetti C, Fontao L, Sorg O. L-Tryptophan as a Novel Potential Pharmacological Treatment for Wound Healing via Aryl Hydrocarbon Receptor Activation. Dermatology (Basel, Switzerland) 2015;230(4):332–9. doi: 10.1159/000371876. Epub 2015/03/15. [DOI] [PubMed] [Google Scholar]
- 40.Greenwood JE, Clausen J, Kavanagh S. Experience with biobrane: uses and caveats for success. Eplasty. 2009;9:e25. [PMC free article] [PubMed] [Google Scholar]
- 41.O’Toole GA, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Molecular Microbiology. 1998;28:449–61. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 42.Ma L, Lu H, Sprinkle A, Parsek MR, Wozniak DJ. Pseudomonas aeruginosa Psl is a galactose- and mannose-rich exopolysaccharide. Journal of bacteriology. 2007;189:8353–6. doi: 10.1128/JB.00620-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bhatia N, Demmer TA, Spiegelman VS. Inhibition of beta-TrCP function potentiates UVB-induced apoptosis in hTERT-immortalized normal human keratinocytes. Photochemistry and photobiology. 2008;84(2):376–81. doi: 10.1111/j.1751-1097.2007.00272.x. Epub 2008/01/23. [DOI] [PubMed] [Google Scholar]
- 44.Guthrie KM, Agarwal A, Tackes DS, Johnson KW, Abbott NL, Murphy CJ, et al. Antibacterial efficacy of silver-impregnated polyelectrolyte multilayers immobilized on a biological dressing in a murine wound infection model. Annals of surgery. 2012;256(2):371–7. doi: 10.1097/SLA.0b013e318256ff99. Epub 2012/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herron M, Agarwal A, Kierski PR, Calderon DF, Teixeira LB, Schurr MJ, et al. Reduction in Wound Bioburden using a Silver-Loaded Dissolvable Microfilm Construct. Advanced healthcare materials. 2014 doi: 10.1002/adhm.201300537. Epub 2014/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sterling JP, Heimbach DM, Gibran NS. Management of the Burn Wound. In: Souba WW, Fink MP, editors. ACS Surgery: Principles and Practice. 6. University of Chicago; 2010. [Google Scholar]
- 47.Kathju S, Nistico L, Tower I, Lasko LA, Stoodley P. Bacterial biofilms on implanted suture material are a cause of surgical site infection. Surgical infections. 2014;15(5):592–600. doi: 10.1089/sur.2013.016. Epub 2014/05/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Calfee MW, Coleman JP, Pesci EC. Interference with Pseudomonas quinolone signal synthesis inhibits virulence factor expression by Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 2001;98(20):11633–7. doi: 10.1073/pnas.201328498. Epub 2001/09/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dawson B, Favaloro EJ. High rate of deficiency in the amino acids tryptophan and histidine in people with wounds: implication for nutrient targeting in wound management--a pilot study. Advances in skin & wound care. 2009;22(2):79–82. doi: 10.1097/01.asw.0000345280.20779.17. Epub 2009/01/22. [DOI] [PubMed] [Google Scholar]
- 50.Ito M, Ogawa K, Takeuchi K, Nakada A, Heishi M, Suto H, et al. Gene expression of enzymes for tryptophan degradation pathway is upregulated in the skin lesions of patients with atopic dermatitis or psoriasis. Journal of dermatological science. 2004;36(3):157–64. doi: 10.1016/j.jdermsci.2004.08.012. Epub 2004/11/16. [DOI] [PubMed] [Google Scholar]