Abstract
Freshwater food webs can be partly supported by terrestrial primary production, often deriving from plant litter of surrounding catchment vegetation. Although consisting mainly of poorly bioavailable lignin, with low protein and lipid content, the carbohydrates from fallen tree leaves and shoreline vegetation may be utilized by aquatic consumers. Here we show that during phytoplankton deficiency, zooplankton (Daphnia magna) can benefit from terrestrial particulate organic matter by using terrestrial-origin carbohydrates for energy and sparing essential fatty acids and amino acids for somatic growth and reproduction. Assimilated terrestrial-origin fatty acids from shoreline reed particles exceeded available diet, indicating that Daphnia may convert a part of their dietary carbohydrates to saturated fatty acids. This conversion was not observed with birch leaf diets, which had lower carbohydrate content. Subsequent analysis of 21 boreal and subarctic lakes showed that diet of herbivorous zooplankton is mainly based on high-quality phytoplankton rich in essential polyunsaturated fatty acids. The proportion of low-quality diets (bacteria and terrestrial particulate organic matter) was <28% of the assimilated carbon. Taken collectively, the incorporation of terrestrial carbon into zooplankton was not directly related to the concentration of terrestrial organic matter in experiments or lakes, but rather to the low availability of phytoplankton.
Primary production is a key process in the biosphere that synthesizes organic compounds (monosaccharides, amino acids, and fatty acids) fueling biomass production and function of organisms across the food webs. Both marine and freshwater food webs are rich in essential ω-3 and ω-6 polyunsaturated fatty acids (PUFA; refs 1,2), whereas terrestrial food webs are low in physiologically important eicosapentaenoic acid (EPA, 20:5ω3) and docosahexaenoic acid (DHA, 22:6ω3)3. Lipids and essential fatty acids (FA) synthesized by aquatic primary producers (phytoplankton, littoral algae) not only support aquatic food webs4,5,6, but also many insect, bird and mammalian species living at the interface of aquatic and terrestrial ecosystems, highlighting the importance of aquatic support to terrestrial food webs7,8,9. Aquatic subsidies to terrestrial ecosystems can be important since terrestrial food webs (e.g. arthropods) can become lipid-limited10. During recent decades, increased loading of terrestrial organic matter to freshwater systems has been detected in several boreal and temperate regions11. This may have direct and indirect effects on the availability and quality of diet sources for consumers, such as herbivorous zooplankton and fish in freshwater systems12,13,14. More studies on biochemical composition and assimilation of both terrestrial and aquatic diet sources are needed for understanding costs and benefits for aquatic consumers utilizing terrestrial resources.
Somatic growth and reproduction of consumers is strongly affected by the quality of lipids and proteins, specifically by the concentration and composition of ω-3 and ω-6 PUFA and amino acids2. Additionally, non-essential biomolecules such as carbohydrates are important dietary energy sources for consumers, which can save more valuable biomolecules, such as proteins for reproduction and somatic growth. This phenomenon is called “protein sparing” and is found in a wide range of organisms15,16. However, carbohydrates can only fulfill a consumer’s short-term energy demands, whereas lipids are important as long-term energy storage and consumers with high lipid content, in general, have high energy density. Lipid energy storage is important for some zooplankton taxa (e.g., Eudiaptomus sp., Limnocalanus macrurus, ref. 17) for over winter survival. In addition, the deficit of dietary lipids has shown to decrease somatic growth and reproduction in consumers at different trophic levels in the food chain up to predators (e.g. beetles, seabirds, sea lions; refs 18, 19, 20).
The role of terrestrial organic matter in supporting aquatic food webs (allochthony) is estimated to be significant in brown-water lakes and headwater rivers21,22,23, which receive large inputs of terrestrial organic matter from the catchment24,25. In food web studies based on stable carbon and/or hydrogen isotopes, dietary sources of consumers are often treated as biochemically homogenous groups (autochthonous or allochthonous), which may be difficult to separate due to overlapping isotope values, and rely on many assumptions in calculations for phytoplankton fractionation and ‘environmental water’26,27,28. Moreover, the isotope values of consumers are combinations of different organic carbon molecules (proteins, saccharides and lipids) often originating from distinct dietary sources29.
More than >90% of the allochthonous, terrestrial organic matter in aquatic ecosystems is in dissolved form and consists mainly of poorly bioavailable recalcitrant humic substances30,31. Only a small fraction (<20%) of the dissolved organic carbon (DOC) in freshwater systems consists of biodegradable low-molecular weight fraction, such as organic acids, free amino acids and carbohydrates32,33. These biodegradable molecules may maintain heterotrophic bacterial production and potentially support the higher trophic levels34. Many cladoceran zooplankton taxa are capable of utilizing bacteria as a dietary resource35,36. However, due to lack of essential PUFA and sterols, bacteria are not a nutritionally adequate resource, and cannot solely support somatic growth and reproduction of cladoceran zooplankton37,38.
In addition to bacteria, detrital particulate organic matter from terrestrial ecosystems, e.g., originating from fallen tree leaves or shoreline vegetation, can be directly utilized by primary consumers39. Terrestrial particulate organic matter (t-POM) in lakes is highly degraded and is mostly composed of lignin and cellulose, containing only trace amounts of fatty acids and sterols12,40,41. The predominant class of fatty acids in t-POM is the long chain saturated fatty acids (LC-SAFA) (>60%), whereas the proportion of PUFA (predominately α-linolenic acid, ALA, 18:3ω3) is <1% 41. Herbivorous zooplankton (crustacean Daphnia) can utilize t-POM, but as a sole diet source it yields poor growth and reproduction12,38,42.
In the current study, we combined experimental and field data to assess the importance of t-POM to cladoceran zooplankton. Based on previous experimental and field studies (e.g., Taipale et al. 2008, Galloway et al. 2014) we tested two hypotheses: 1) terrestrial-origin carbohydrates and proteins are assimilated efficiently and support biomass growth of cladoceran zooplankton, and 2) the utilization t-POM and bacteria by zooplankton is related to the concentration of allochthonous organic carbon in the lakes. Firstly, we analyzed biochemical composition of major potential aquatic (phytoplankton) and terrestrial food sources (t-POM of wetland reed and deciduous tree leaves) available for herbivorous zooplankton. Secondly, we tested how different levels of t-POM affect somatic growth of a model consumer organism, the cladoceran crustacean Daphnia magna, using these t-POM types and phytoplankton as diet sources as well as to estimate threshold values for autochthonous essential biomolecules support needed to maintain the optimal growth. We analyzed how the proportions of different biomolecules (carbohydrates, lipids, proteins) in the total body carbon of Daphnia changed with increasing diet allochthony and investigated if Daphnia was able to bioconvert terrestrial-originated carbohydrates to fatty acids. Thirdly, we studied zooplankton dietary assimilation in 16 boreal and in 5 subarctic lakes using fatty acid based mixing model calculations38,43, to identify the importance t-POM subsidy in different lake types.
Results
Quality of phytoplankton and t-POM
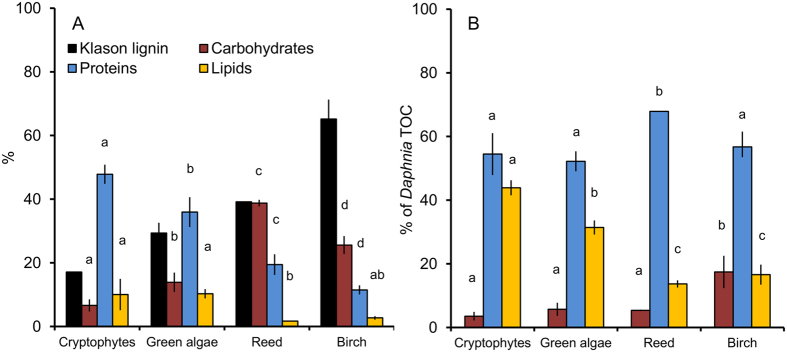
The biochemical composition of phytoplankton and t-POM, originating from tree and reed leaf litter, showed clear qualitative differences (Fig. 1A, Table 1). A high proportion of the leaves consisted of lignin (birch 65 ± 7%, reed 39 ± 0.1% of total organic carbon (TOC). Total carbohydrate content of reed leaves (>75% consisting of glucose) was significantly higher (39 ± 1% of TOC) than that of tree leaves (29 ± 5% of TOC) and phytoplankton (7–10% of TOC). In phytoplankton, the proportions of proteins and lipids of TOC were greater than in tree and reed leaves (Fig. 1A, Table 1).
Figure 1.
(A) Biochemical content (mean ± SD% of total organic carbon, TOC) of cryptophytes (Cryptomonas ozolinii), and green algae (Acutodesmus sp.), particles of dried birch (average of Betula pedula and B. pubescens czerepanovii) and reed (Phragmites australis) leaf litter and (B) corresponding values of Daphnia fed with those diets. Different letters (a–d) denote significant differences in carbohydrate, protein and lipid content between diet sources (A) and Daphnia fed solely with those diets (B).
Table 1. Klason lignin, carbohydrate, protein and lipid contribution of total organic carbon (TOC) in phytoplankton and in leaves of terrestrial trees and reed (na = not available).
| Common name | Functional Group | Strain | Media | Klason lignin | Carbohydrates | Proteins | Lipids |
|---|---|---|---|---|---|---|---|
| Phytoplankton | Mean ± SD | Mean ± SD | Mean ± SD | Mean ± SD | |||
| Green algae | Acutodesmus sp. | University of Basel | MWC64 | 30.2 ± 0.3 | 18.8 ± 2.6 | 40.0 ± 0.0 | 11.0 ± 1.4 |
| Monoraphidium griffithii | NIVA-CHL 8 | MWC64 | na | 11.6 ± 1.9 | 31.9 ± 0.6 | 13.3 ± 7.4 | |
| Average | 30.2 ± 0.3 | 15.2 ± 5.1 | 36.0 ± 5.7 | 12.2 ± 1.6 | |||
| Cryptophytes | Cryptomonas ozolinii | UTEX LB 2782 | AF6 64 | 26.0 | 4.4 ± 1.1 | 50.6 ± 3.0 | 14.1 ± 2.5 |
| Cryptomonas erosa | CPCC 466 | AF6 65 | na | 8.2 ± 0.5 | 48.3 ± 1.9 | 5.9 ± 0.3 | |
| Rhodomonas minuta | CPCC 344 | Z8 66 | na | 7.8 ± 1.3 | na | na | |
| Rhodomonas lacustris | NIVA 8/82 | Z8 66 | na | 8.7 ± 0.1 | 44.2 ± 1.2 | 6.7 ± 2.4 | |
| Average ± SD | 7.3 ± 2.0 | 47.7 ± 3.2 | 8.9 ± 4.5 | ||||
| Diatoms | Diatoma tenuis | CPCC 62 | Chu 10 67 | na | 13.7 ± 2.2 | 38.1 ± 0.4 | 18.2 ± 2.0 |
| Navicula pellicosa | UTEX B 664 | Chu 10 67 | na | 10.9 ± 0.9 | 43.8 ± 5.4 | 9.2 ± 3.3 | |
| Average ± SD | 12.3 ± 2.0 | 41.0 ± 4.0 | 13.7 ± 6.4 | ||||
| Dinoflagellates | Peridinium cintum | SCCAP K-1721 | MWC64 | na | 9.6 ± 0.7 | 33.8 ± 0.6 | 5.5 ± 2.4 |
| Cyanobacteria | Limnothrix planktonica | NIVA-CYA 107 | MWC64 | na | 13.5 ± 9.1 | 46.0 ± 1.1 | 3.6 ± 0.0 |
| Pseudanabaena limnetica | NIVA 276/11 | MWC64 | na | 7.1 ± 1.4 | 57.5 ± 0.9 | 4.7 ± 0.5 | |
| Synechococcus elongatus | MWC64 | na | na | na | 3.4 ± 0.7 | ||
| Average ± SD | 10.3 ± 4.5 | 51.8 ± 8.1 | 4.2 ± 0.7 | ||||
| Chrysophytes | Mallomonas caudata | Lake Horkkajärvi | na | na | 39.6 ± 0.7 | 18.2 ± 2.7 | |
| Leaves of terrestrial trees | |||||||
| Arctic Birch | Betula pubescens subsp. czerepanovii | Kilpisjärvi, Finland | 60.0 ± 2.4 | 23.6 ± 2.6 | 12.4 ± 0.6 | 3.3 ± 0.8 | |
| Silver Birch | Betula pendula | Jyväskylä, Finland | 70.3 ± 0.5 | 27.6 ± 0.9 | 12.6 ± 0.84 | 12.6 ± 0.8 | |
| Red alder | Alnus rubra | Seattle, USA | 39.8 ± 2.0 | 26.1 | 12.4 ± 0.6 | 2.6 ± 0.1 | |
| Black cottonwood | Populus trichocarpa | Seattle, USA | 32.8 ± 2.0 | 33.5 ± 4.9 | na | na | |
| Willow | Salix spp. | Seattle, USA | 46.0± | 27.5 | na | na | |
| Bigleaf maple | Acer macrophyllum | Seattle, USA | 63.8 ± 0.7 | 37.7 ± 12.9 | na | na | |
| Average ± SD | 52.1 ± 14.8 | 29.3 ± 5.2 | 12.5 ± 0.1 | 6.2 ± 5.6 | |||
| Reed | Phragmites australis | Joensuu, Finland | 39.2 ±0.1 | 39.0 ± 1.1 | 16.9 ± 0.0 | 1.5 ± 0.1 | |
The fate of terrestrial carbohydrates, lipids and proteins in zooplankton
Our laboratory experiments showed that herbivorous zooplankton (Daphnia) fed on phytoplankton had significantly higher lipid content (Cryptophyte diet 44 ± 2%, green algal diet 31 ± 3% of TOC) than those fed solely on terrestrial diets (14–17% of TOC) (Fig. 1B). The contribution of carbohydrates to Daphnia (Fig. 1B) was higher in those fed on birch leaf particles (15 ± 3% of TOC) compared with those with the other diets. About half of the organic carbon in Daphnia consisted of proteins, the proportion being highest with reed diet (Fig. 1B).
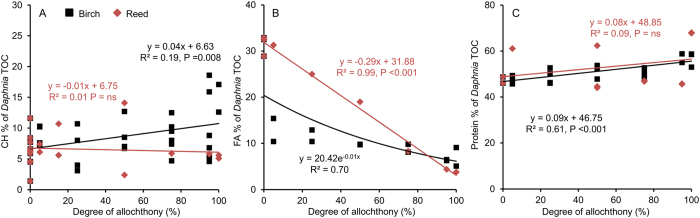
Along with increasing allochthony with the mixed diet of Acutodesmus green alga and t-POM, no trend in carbohydrate content was seen in Daphnia fed with reed leaf particle mixtures (Fig. 2A). In Daphnia fed with birch leaf particle mixtures, the proportion of carbohydrates of TOC increased significantly with allochthony, although the variation was rather high (Fig. 2A). The proportion of fatty acids in Daphnia decreased significantly with the diet allochthony both with birch and reed particle mixed diets (Fig. 2B). Until ca. 90% allochthony, the fatty acid content was higher in those fed with reed mixed particles compared with the birch treatment. The contribution of proteins increased slightly with allochthony gradient with birch mixed particles, while in reed treatment the variation between replicates was too high to produce a significant regression (Fig. 2C).
Figure 2.
(A) Proportion of carbohydrates, (B) fatty acids (FA) and (C) proteins in Daphnia body carbon (% of TOC) grown with diets mixed with Acutodesmus green alga and birch or reed particles. Best fit linear or exponential model equations with R2 and P values are also shown.
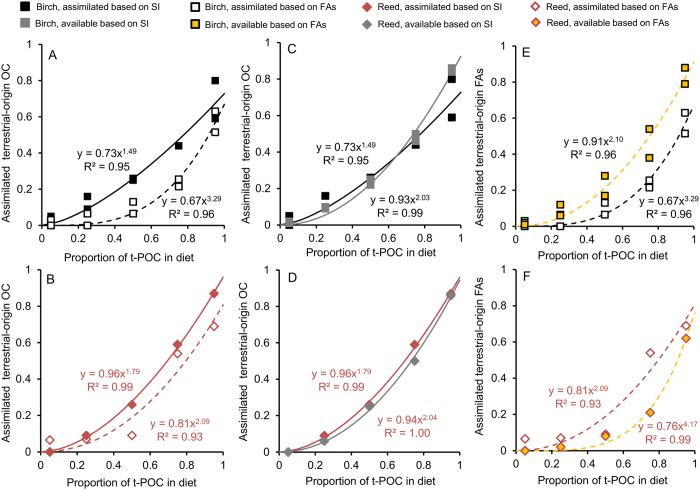
The proportion of assimilated terrestrial organic carbon increased with the proportion of t-POM in the diets. The contribution of assimilated terrestrial organic carbon was greater based on stable isotopes than on fatty acids in both terrestrial diets (Fig. 3A,B), but the difference was greater in the birch experiment. Based on stable isotopes, the assimilated proportion of terrestrial-origin organic carbon was similar than that available in the diet with both t-POM sources (Fig. 3C,D). In the reed experiment, the proportion of assimilated saturated fatty acids in Daphnia exceeded that of available in the diet (Fig. 3F), whereas in the birch experiment, the proportion of assimilated saturated fatty acids was lower than what was available in t-POM (Fig. 3E). Thus, Daphnia fed on reed leaf particles had 6-times higher fatty acid assimilation rate relative to the ones fed on birch leaf particles.
Figure 3.
Assimilation of terrestrial-origin carbon by Daphnia in relation to the proportion of terrestrial particulate organic carbon (t-POC) in (A) mixed birch-algae diet (square) and (B) in mixed reed-algae diet (diamond). The assimilation of terrestrial-origin organic carbon (OC) (filled black and red symbols), based on stable isotope analyses, did not differ to that available in the diets (filled grey symbols; (C,D). The proportion of assimilated terrestrial-origin fatty acids (FAs) by Daphnia (white squares) was lower than that available in mixed birch-algae diet (yellow squares) but higher (white diamonds) than that available in mixed reed-algae diet (yellow diamonds; E,F).
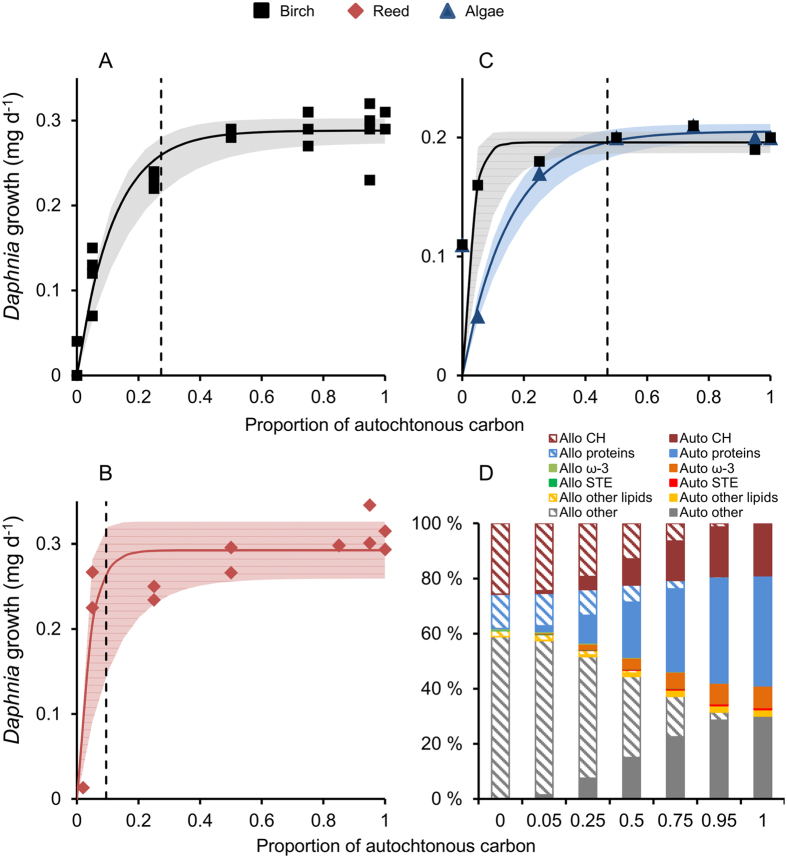
In the 10-day experiment, Daphnia achieved optimal somatic growth rate (90% of maximal somatic growth) when the proportion of autochthonous (Acutodesmus) carbon was ≥27% and ≥9.5% of total dietary carbon in birch-algae and reed-algae diet mixtures, respectively (dashed lines in Fig. 4A,B). In terms of biochemical content, this meant that Daphnia required 108 μg of ω-3 fatty acids mg C−1, 11 μg of sterols mg C−1 and 0.5 mg proteins mg C−1 from Acutodesmus to achieve optimal somatic growth in the birch-algae experiment. The autochthonous supplements needed in reed-algae experiment were much smaller: 37 μg of ω-3 fatty acids mg C−1, 4 μg of sterols mg C−1, and 0.2 mg proteins mg C−1. In the second experiment lasting 21 days, Daphnia were cultured either solely with Acutodesmus concentration gradient from 0.25 to 5 mg C L−1 or with the same concentration gradient of Acutodesmus, but supplemented with birch leaf particles up to concentration 5 mg C L−1 (Fig. 4C). This experiment demonstrated that when the proportion of autochthonous algae in Daphnia diet was less than 47% of total carbon content (equals to < 2.4 mg C L−1), somatic growth of Daphnia benefitted from birch leaf particles (Fig. 4C). At this threshold 7% of ω-3 fatty acids, 23% of sterols, 60% of carbohydrates and 26% of proteins in the diet were of terrestrial origin (Fig. 4D).
Figure 4.
Somatic growth rate of Daphnia in relation to the proportion of autochthonous carbon during a 10-day experiment, fed with (A) mixed birch-algae diet (square), and (B) with mixed reed-algae diet (diamond) in non-limiting food conditions (5 mg C L−1). Dashed lines refer to optimal growth rate threshold (or 90% of maximal somatic growth). (C) Biomass growth rate of Daphnia in relation to the proportion of autochthonous carbon during a 21-day experiment fed with pure green algae (Acutodesmus sp.; triangle) in food gradient from 0.25 to 5 mg C L−1 and with mixed diet of green algae and silver birch leaf particles (total food concentration 5 mg C L−1; square). Daphnia benefitted from additional terrestrial carbon when algal food concentration was < 2.4 mg C L−1. Dashed line refers to threshold point where terrestrial addition did not benefit Daphnia anymore. Shaded areas in A, B and C are the 95% confidence intervals. (D) The proportions of biochemical compounds in Daphnia diet along with decreasing allochthonous (allo) and increasing autochthonous (auto) carbon. Pure terrestrial diet mainly consisted of carbohydrates (CH) and undigestible compounds (others), whereas pure autochthonous diet had high protein and lipid content. (Symbols: ω-3 = ω-3 fatty acids, STE = sterols).
Cladoceran allochthony in lakes
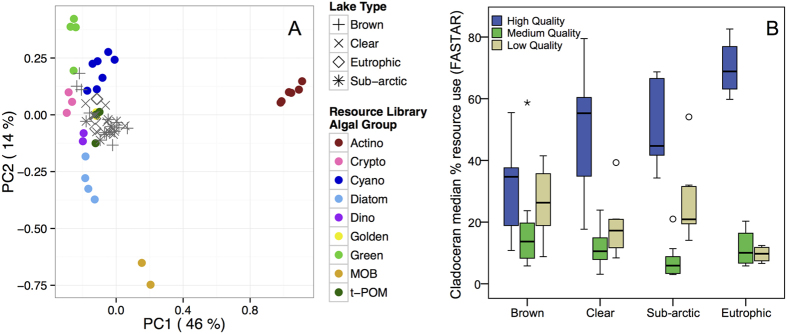
The sampled wild zooplankton from all lakes fell inside of the multivariate resource-polygons of the resource library of the fatty acid profiles of Daphnia fed end members in controlled feeding trial (Fig. 5A), a prerequisite best practice for bio-tracer based mixing model analysis43,44. The contribution of high-quality phytoplankton (rich in EPA; ref. 12) diets in cladocerans differed among lake types (ANOVA, F3,27 = 6.97, p = 0.001), and appeared to be a less important dietary resource for cladocerans in brown-water lakes as compared with the other lake types (Fig. 5B). In clear-water, subarctic and eutrophic lakes 50–70% of cladoceran diets consisted of high-quality phytoplankton, the greatest proportion appearing in the eutrophic lakes. In the utilization of high-quality resources, post-hoc tests found significant differences between brown-water lakes and eutrophic lakes (p = 0.001), but differences were not statistically significant between brown-water and clear or subarctic lakes. The proportion of medium-quality (phytoplankton rich in ALA; 18:3ω3) diets did not differ among lake types (ANOVA, F3,27 = 1.48, p = 0.243), but support from low-quality diets (the lowest content of ω-3 fatty acids, i.e. t-POM and bacteria) did differ among lake types (ANOVA, F3,27 = 3.72, p = 0.023). However, post-hoc tests between lake types for low-quality diets did not differ (Fig. 5B).
Figure 5.
(A) Principal components analysis (PCA) visualizing the multivariate fatty acid resource-library of experimentally fed Daphnia fed known basal end-members and the wild cladocerans collected in the study lakes. Each colored filled circles is the mean fatty acid profile of Daphnia fed a different basal taxon within each of the nine end-member groups [abbreviations: Actino (Actinobacteria); Crypto (Cryptophyceae); Cyano (Cyanophyceae); Diatom (Heterokontophyta); Dino (Dinophyceae); Golden (Synurophyceae); Green (Cryptophyceae); MOB (methane oxidizing bacteria); t-POM (terrestrial particulate organic matter, Plantae)]. Gray symbols are the fatty acid profiles of wild cladocerans collected in each of the 4 lake types. The total variation explained by each PC is displayed on the axes. PC3 (not presented) explained 12% of the total variation. (B) The contribution (Boxplots with median, 25th and 75th percentiles, with whiskers for 5th and 95th percentiles) of diet nutritional quality for Daphnia was divided into high (phytoplankton with EPA), intermediate (phytoplankton with high levels of ALA) and poor (low content of ω-3 fatty acids; Actinobacteria, MOB and t-POM) quality diets, as modeled by FASTAR.
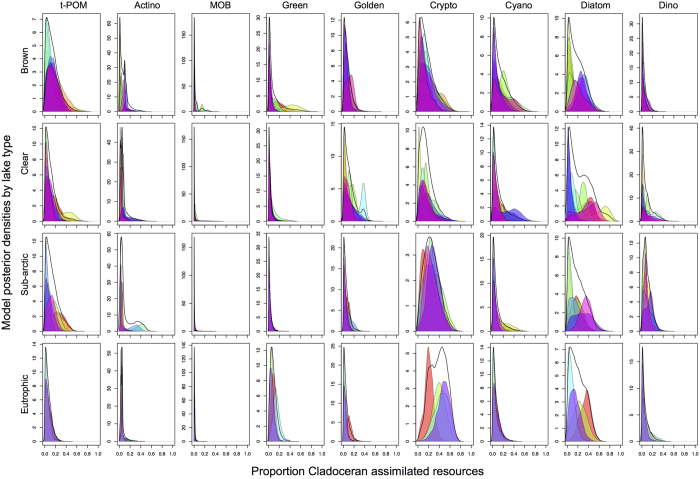
Terrestrial particulate organic matter (t-POM) contributed approximately 11 ± 6% (Mean ± SD of median values), but varied between lakes (Fig. 6, Table 2). Assimilated t-POM content differed among lake types (ANOVA, F3,27 = 5.45, p = 0.005), being higher (15 ± 7% of all) in brown-water, clear-water (10 ± 5% of all), and subarctic lakes (14 ± 3% of all) than in the eutrophic lakes (3 ± 2% of all). Correspondingly, the sum of autochthonous phytoplankton consisted in average 64 ± 24% of cladoceran diets and the proportion differed among the lake types (ANOVA, F3,27 = 4.96, p = 0.007), with eutrophic lakes having the highest (86 ± 5%) phytoplankton assimilation by zooplankton (Fig. 6). Cryptophytes and diatoms were the most important dietary resources in all lake types. Cryptophytes were the major dietary resource for cladocerans in eutrophic (49 ± 16% of all) and subarctic lakes (28 ± 11% of all), whereas diatoms were the major dietary source in clear-water (22 ± 11% of all) and brown-water lakes (15 ± 11% of all). Additionally, green algae contributed 12 ± 14% of all diet sources in brown-water lakes and dinoflagellates contributed 13 ± 15% of all diets in clear-water lakes.
Figure 6. FASTAR mixing model estimates of the proportional assimilation of basal resources by wild cladocera from 9 basal resource groups at the four lake types (rows).
The model was run using all 24 fatty acids in the profiles of Daphnia fed known end-member diets (see Methods; Fig. 4A) in independent feeding trials. Each plot shows the posterior density (y-axis) of results from the mixing model for a given dietary source (x-axis). The FASTAR solution for Daphnia from each individual lake are reported as different colored distributions for each replicate [brown lakes (n = 10); clear lakes (n = 10); subarctic lakes (n = 7); and eutrophic lakes (n = 4)], along with median ‘lake-level’ solution compiled from all replicates within that lake type (non-colored distribution). Resource group names (columns) follow abbreviations defined in the Fig. 5 caption.
Table 2. Brown-water, clear-water, subarctic and eutrophic lakes were sampled between 2006 and 2015.
| Lake | Latitude (°N) | Longitude (°E) | Month and year | Zooplankton | Fraction | DOC (C mg/l) | tot N μg/l | tot P μg/l | Chl α μg/l | Phytop (%) | MOB (%) | t-POM (%) | Actino (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nimetön | 61.220 | 25.190 | May 2006 | Daphnia longispina | PLFA | 22.3 | 828 | 31 | 8.5 | 66.7 | 0.3 | 17.4 | 1.2 |
| Nimetön | 61.220 | 25.190 | May 2006 | Daphnia + Holopedium | PLFA | 22.3 | 828 | 31 | 8.5 | 55.9 | 0.3 | 15.4 | 1.4 |
| Mekkojärvi | 61.230 | 25.140 | May 2006 | Daphnia longispina | PLFA | 26.3 | 419 | 17 | 11.3 | 75.0 | 1.5 | 6.3 | 1.0 |
| Mekkojärvi | 61.230 | 25.140 | Jul 2006 | Daphnia longispina | TFA | 22 | 419 | 17 | 60.7 | 15.1 | 11.5 | 3.1 | |
| Mekkojärvi | 61.230 | 25.140 | Oct 2006 | Daphnia longispina | PLFA | 17.6 | 786 | 27 | 12.9 | 55.6 | 5.3 | 14.0 | 4.0 |
| Horkkajärvi | 61.210 | 25.150 | Aug 2013 | Daphnia | TFA | 31.6 | 766 | 17 | 13.8 | 47.9 | 13.5 | 4.8 | 17.4 |
| Harkkojärvi | 62.960 | 31.040 | Aug 2013 | Daphnia | TFA | 16.6 | 554 | 18.3 | 8.2 | 67.1 | 0.5 | 9.1 | 10.4 |
| Harkkojärvi | 62.960 | 31.040 | Aug 2013 | Daphnia | TFA | 16.6 | 554 | 18.3 | 8.2 | 52.0 | 0.9 | 27.4 | 12.8 |
| Harkkojärvi | 62.960 | 31.040 | Aug 2013 | Daphnia | TFA | 16.6 | 554 | 18.3 | 8.2 | 57.3 | 0.2 | 19.4 | 9.8 |
| Nuorajärvi | 62.680 | 31.120 | Aug 2013 | Daphnia | TFA | 16.1 | 478 | 22 | 6.1 | 55.7 | 0.4 | 20.8 | 20.4 |
| Vähä-Valkjärvi | 61.190 | 25.090 | Aug 2011 | Bosmina | TFA | 6.0 | 581 | 17 | 19.9 | 72.4 | 0.4 | 12.0 | 3.9 |
| Valkea Mustajärvi | 61.220 | 25.120 | Aug 2011 | Daphnia | TFA | 5.1 | 349 | 12 | 16.3 | 50.2 | 0.3 | 17.1 | 2.8 |
| Valkea Mustajärvi | 61.220 | 25.120 | May 2006 | Holopedium, Bosmina | PLFA | 5.1 | 367 | 8 | 3 | 85.8 | 0.2 | 10.1 | 1.3 |
| Syrjänalunen | 61.190 | 25.140 | Aug 2013 | Daphnia | TFA | 2.5 | 271 | 7 | 3.1 | 82.7 | 1.8 | 3.0 | 7.7 |
| Iso Valkjärvi | 61.190 | 25.110 | Aug 2013 | Daphnia | TFA | 4.5 | 402 | 12 | 6.4 | 64.5 | 0.4 | 11.4 | 6.4 |
| Ylinen | 62.600 | 30.220 | Aug 2013 | Daphnia | TFA | 6.9 | 458 | 7 | 2.3 | 72.6 | 0.4 | 15.4 | 5.1 |
| Ylinen | 62.600 | 30.220 | Aug 2013 | Daphnia | TFA | 6.9 | 378 | 3.6 | 2.3 | 48.4 | 0.4 | 9.4 | 29.5 |
| Kuorinka | 62.620 | 29.420 | Aug 2013 | Daphnia | TFA | 2.8 | 190 | 1.6 | 1.3 | 46.9 | 0.3 | 9.6 | 11.1 |
| Kuorinka | 62.620 | 29.420 | Aug 2013 | Daphnia | TFA | 2.8 | 190 | 1.6 | 1.3 | 85.6 | 0.2 | 4.2 | 4.7 |
| Kermajärvi | 62.430 | 28.720 | Aug 2013 | Daphnia | TFA | 8.0 | 417 | 4.6 | 2.3 | 77.1 | 1.5 | 5.0 | 1.9 |
| Oikojärvi | 68.500 | 21.130 | Sept 2011 | Daphnia | TFA | 5.6 | 215 | 7 | 2.6 | 60.3 | 0.3 | 12.7 | 5.5 |
| Oikojärvi | 68.500 | 21.130 | Sept 2011 | Daphnia | TFA | 5.6 | 215 | 7 | 2.6 | 51.4 | 0.3 | 14.7 | 16.1 |
| Ropijärvi | 68.410 | 21.350 | Sept 2011 | Daphnia | TFA | 5.4 | 273 | 10 | 2.9 | 76.0 | 0.2 | 16.2 | 4.5 |
| Kuohkimajärvi | 69.030 | 20.330 | Sept 2012 | Daphnia | TFA | 3.0 | 269 | 18 | 1.3 | 41.8 | 0.3 | 13.9 | 40.0 |
| Kuohkimajärvi | 69.030 | 20.330 | Sept 2012 | Daphnia | TFA | 3.0 | 269 | 18 | 1.3 | 65.3 | 0.2 | 8.6 | 23.2 |
| Siilasjärvi | 69.040 | 20.450 | Sept 2012 | Daphnia | TFA | 1.9 | 159 | 5 | 1.2 | 73.0 | 0.4 | 11.7 | 2.0 |
| Kilpisjärvi | 69.000 | 20.490 | Sept 2012 | Daphnia | TFA | 2.8 | 351 | 9 | 0.9 | 74.4 | 0.3 | 17.1 | 3.0 |
| Lovonjärvi | 61.080 | 25.030 | Jul 2015 | Daphnia, Bosmina | TFA | 13.5 | 785 | 49 | 24.4 | 89.1 | 0.7 | 3.4 | 2.6 |
| Kyynäröinen | 60.870 | 24.170 | Jul 2015 | Cladocerans | TFA | 16.9 | 965 | 77 | 44.9 | 89.9 | 1.3 | 2.6 | 4.4 |
| Kataloistenjärvi | 61.020 | 24.940 | Jul 2015 | Cladocerans | TFA | 8.9 | 620 | 35 | 11.1 | 80.5 | 0.4 | 2.3 | 9.7 |
| Tuusulanjärvi | 60.400 | 25.100 | Jul 2015 | Daphnia | TFA | 9.2 | 940 | 69 | 29.0 | 82.7 | 0.7 | 5.5 | 4.9 |
Some of them were sampled several times or at 2–3 sites. Mean values of dissolved organic carbon (DOC), total nitrogen (tot N), total phosphorus (tot P) and chlorophyll a content (Chl a) are also shown. Cladoceran zooplankton consisted of different species (Daphnia, Holopedium and Bosmina). Total fatty acids (TFA) or phospholipids fatty acids (PLFA) were analyzed from zooplankton. FASTAR results of median contribution (%) of phytoplankton (Phytop), methane oxidizing bacteria (MOB), terrestrial particulate organic matter (t-POM) and Actinobacteria (Actino) are presented here.
The proportion of methane oxidizing bacteria (MOB) in cladoceran diets was generally low (<2%) with the exception of two small brown-water lakes (Mekkojärvi and Horkkajärvi, Table 2). The proportion of Actinobacteria in the cladoceran diet varied within and between lakes, maximum in the subarctic Lake Kuohkimajärvi (32 ± 12%). If all Actinobacteria are assumed to be 100% supported by allochthonous organic carbon, the average zooplankton allochthony would have been 19 ± 10%. The proportions of t-POM and Actinobacteria in the zooplankton diets were not correlated with water colour, DOC, nitrogen, phosphorus or chlorophyll-a concentration of the lakes (r < 0.1, P > 0.05).
Discussion
Our field and laboratory study of herbivorous zooplankton demonstrates that although freshwater ecosystems are strongly affected by their catchment, terrestrial organic matter can only limitedly support zooplankton production and its contribution varies greatly among lakes. We show that the assimilation of terrestrial organic carbon is not directly related to its availability, but rather to the lack of better quality diets. We demonstrated that this is due to the fact that terrestrial plant litter entering lakes contains high amounts of biologically unavailable lignin, and more carbohydrates, but less proteins and lipids (including essential fatty acids and amino acids) per carbon unit than aquatic primary consumers require for their optimal growth and reproduction. In the absence of phytoplankton and under circumstances of forced high allochthony, the carbohydrate content of herbivorous zooplankton increased from ~6 even up to 10% of TOC, reflecting the poor biochemical quality of terrestrial organic matter. We found that when algal food availability is low, t-POM supplements benefit somatic growth of Daphnia, thus, partly supporting our first hypothesis. However, due to the low-lipid and low-protein content, terrestrial organic carbon could support growth only up to a certain threshold, indicating that herbivorous zooplankton have to satisfy the most essential biochemical demands mainly with phytoplankton (e.g., refs 42 and 45).
Leaf litter of common deciduous trees in the North-America and Scandinavia consists mainly of lignin, which is non-digestible for zooplankton, and is a poor source for proteins, ω-3 FA and sterols, shown by our biochemical analyses (this study, refs 12 and 38). Carbohydrates are the most beneficial compounds of t-POM, which can be utilized by aquatic primary consumers. Due to their higher carbohydrate and protein content, the quality of reed leaf particles was a better dietary resource for herbivorous cladocerans than that of tree leaves. In fact, Daphnia fed with mixed reed-algae diet had a higher somatic growth rates than those grown with mixed birch-algae diet. Glucose is the major oligosaccharide in the leaves, suggesting that t-POM can be an optimal short-term energy source for zooplankton. This was observed in our 20-day laboratory experiments, where birch supplementation enhanced Daphnia growth significantly when phytoplankton concentration was low. The results show that Daphnia uses a ‘sparing strategy’ in circumstances of high carbohydrate, but low lipid and protein availability to maximize its somatic growth. Thus, Daphnia is able to use terrestrial carbohydrates for energy and save proteins (amino acids) and lipids (fatty acids) for structural components.
Daphnia fed on tree leaf particles had ~twice higher carbohydrate content than what was found in those fed on reed. This, together with low ω-3:ω-6 –ratio of tree leaves12,38,41, indicated higher nutritional stress in Daphnia using the birch diet than in those using reed or phytoplankton diets. Furthermore, the assimilated proportion of terrestrial-origin fatty acids by Daphnia in the reed experiment was higher than the proportion available in the diet, suggesting that Daphnia is able to convert excess carbohydrates from reed leaves to fatty acids. However, this was not observed in Daphnia with birch leaf diet, for which the lower proportion of carbohydrates in the diet possibly forced Daphnia to use both terrestrial-origin carbohydrates and fatty acids to meet its energy demand. Since the carbon isotope signal comes from all organic compounds, the difference between stable isotopes and fatty acids (two source mixing model) results is due to the utilization of terrestrial-origin proteins and carbohydrates. The difference between the methods was smaller in the reed diet than in the birch diet even though the reed diet initially contained less fatty acids than birch diets. Therefore, Daphnia has likely obtained fatty acids by conversion from carbohydrates in the reed experiment. Our estimates of t-POM utilization by herbivorous zooplankton in 21 lakes based on fatty acid mixing model (FASTAR) results are within the range of zooplankton allochthony estimates for temperate and boreal lakes based on stable carbon (δ13C) and/or hydrogen (δD) isotope analyses46,47,48. In stable isotope mixing-model calculations phytoplankton is generally assumed to be one solid group, because although carbon isotope values can differ greatly between taxa49,50, this is rarely if ever measured. This problem does not occur in fatty-acid based modeling since group-specific fatty acid characteristics of phytoplankton and other resources are included in the resource library of the model. The fatty acid mixing model analyses (FASTAR) show that in all four lake types, the high-quality phytoplankton (cryptophytes and diatoms) form the base of herbivorous zooplankton diet. Use of these high quality resources, which are rich in essential amino acids, fatty acids, and sterols51,52. Also makes somatic growth and reproduction for zooplankton possible during utilization of lower quality diets12,37.
Our second hypothesis, that the utilization t-POM and bacteria by zooplankton is related to the concentration of allochthonous organic carbon in the lakes was not supported. We found that the contribution of t-POM in zooplankton diet varies greatly within and among the lakes (from 2 to 27%). Even if >90% of allochthonous carbon inputs is in dissolved form30,31, suggesting that microbial food chain could be the major link between terrestrial food sources and herbivorous zooplankton22, our fatty acid-based mixing model results from 21 lakes indicate that in most cases the contribution of t-POM exceeded that of Actinobacteria. This is in agreement with our previous results analyzing cladoceran basal resource support in large boreal lakes43. Furthermore, the DOC concentration of lakes did not correlate with the contribution of Actinobacteria in the cladoceran diets. Thus, our results suggest that the major pathway of terrestrial organic carbon to zooplankton is not diverted via heterotrophic DOC-utilizing bacteria53. Strong positive relationships found between biomasses and production of phytoplankton and bacteria, generally observed in lake ecosystems, suggest that phytoplankton-origin DOC is a very important carbon source for heterotrophic bacteria in all kinds of lakes54,55. Moreover, the poor growth efficiency of heterotrophic bacteria utilizing terrestrial DOC56,57 also supports this conclusion. However, the microbial food chain, via bacteria to protozoans, may have some importance in the transfer of terrestrial carbon to higher trophic levels. Here, the quality of organic matter and picoplankton prey available for heterotrophic protozoans affect their quality as diet source to the next trophic level, metazoan plankton58. It should also be noted that each additional step in the food chain cause respiratory losses lowering the transfer efficiency of terrestrial carbon to higher trophic levels44,59.
The high variation in the estimated cladoceran t-POM utilization indicates that when lipid and protein rich autochthonous organic carbon sources are scarce, herbivorous zooplankton can use terrestrial-origin carbohydrates, lipids, and proteins more intensively. This finding is supported by results of 21 lakes, where the assimilation of t-POM was not correlated with DOC concentration, although, in general, higher cladoceran allochthony was detected in the brown-water lakes. For example, in Lake Horkkajärvi, which had the highest DOC concentration of all the sampled lakes, the observed proportion of t-POM assimilated by cladocerans was very low, presumably due to the high densities of better quality food sources (e.g. autotrophic and mixotrophic algae)48,54 during the sampling season. In small stratified lakes methane-oxidizing bacteria (MOB) can significantly contribute to zooplankton diets60,61 as found in the two lakes of this study. The trophic pathway from methane to higher trophic levels via MOB may be more related to anaerobic decomposition of fresh, autochthonous organic matter than to allochthonous sources62.
Our results are in accordance with recent model results based on field experiments in five lakes63, in which zooplankton biomasses and production were low when allochthony exceeded 30%. This was related to the light extinction by brown-coloured terrestrial DOC suppressing phytoplankton primary production and biomass64,65 available for zooplankton grazers. The high level of zooplankton allochthony seems to be a consequence of the absence of better quality dietary sources rather than the result of the high availability of terrestrial organic matter. Zooplankton allochthony was not the highest in the lakes with the most pronounced loadings of terrestrial matter and this was likely explained by the biochemical composition of t-POM and by nutritional requirements of herbivorous zooplankton. The high estimates of assimilated t-POM by cladoceran zooplankton obtained with the fatty acid mixing model could also indicate low nutritional status of zooplankton in the lakes41,43.
In conclusion, allochthony of herbivorous zooplankton varies among different type of boreal lakes, and is mainly defined by the availability of lipid and protein-rich phytoplankton. We show that cladocerans use primarily carbohydrates for energy, but can also exploit some lipids and proteins of terrestrial matter for somatic growth. Leaves of shoreline vegetation (reed) had better dietary quality than those of birch, containing less lignin and more glucose, which Daphnia was able to partially bioconvert to more usable saturated fatty acids. However, this was not observed in birch diet with low carbohydrate content. Overall, we suggest that relatively high proportion of terrestrial organic carbon in cladocerans and in other aquatic herbivores can result from multiple biochemical processes and that the degree to which organic matter produced by terrestrial plants can support freshwater food webs may depend upon the biochemical content of different terrestrial vegetation. However, the high content of lipids, proteins and other essential biomolecules produced by phytoplankton are needed to sustain all types of aquatic food webs.
Methods
Zooplankton and phytoplankton cultures
All experiments were conducted using a clone of Daphnia magna (DK-35-9, hereafter Daphnia), initially grown and maintained on Acutodesmus sp. which was obtained from the Institute of Zoology, University of Basel. We also used Cryptomonas erosa (CPCC 466) as high quality diet control in our experiments. Acutodesmus sp. was cultured using modified WC solution supplemented with biotin and cyanocobalamin (B12)66. Cryptomonas erosa was cultured using AF6 media67. In addition, we cultured some more phytoplankton strains listed in Table 1. Each strain was cultured in a medium specific to that strain (Table 1) and were grown at 20 °C under a 14 h:10 h light:dark cycle with light intensity of 30–70 μmol m−2 s−1. To obtain differences in carbon isotope signals between the diets, Acutodesmus sp. cultures were enriched with 13C, 3% of the NaHCO3 in the MWC media consisted of NaH13CO3 (99%), Cambridge Isotope Laboratories].
Terrestrial carbon source
We used leaf litter particles of common reed [Phragmites australis (Cav.) Trin. ex Steud], silver birch (Betula pendulata) and arctic birch (Betula pubescens subsp. czerepanovii) as terrestrial particulate organic matter (hereafter called t-POM) food resources for zooplankton Daphnia magna. We collected reed leaves from the shore of Lake Pyhäselkä (eastern Finland), and ground it to small particles using a Fritsch Planetary Mono Mill Pulverisette 638. The particles were then diluted into the WC Media directly and incubated for one month in the. Silver birch and arctic birch leaves were ground to fine particles using a Retch ZM 100 GWB ultra centrifugal Mill41. For this experiment ground t-POM was diluted into modified Woods Hole (WC) medium68 and filtered through a 50 μm screen and incubated one month in the dark with continuous shaking at 120 rpm.
Batch experiment
Daphnia neonates (~6 h old) were used for all experiments. Neonates from specific adults were divided equally between treatments to minimize maternal effects69 and distributed individually into glass vials (40 mL of L16 media) with each treatment consisting of 10 replicates. The media was changed and the Daphnia fed every other day with total food concentrations of 1.5, 2 and 5 mg C L−1 for ages 2, 4 and 6 + days, respectively. These food concentrations were above the incipient limiting level for ingestion70. In a 10-day experiment Daphnia was fed with pure (100%) diets of t-POM, and each taxon of phytoplankton (Cryptomonas erosa and Acutodesmus sp.), and also in gradients of diets consisting 95%, 75%, 50%, 25% and 5% of t-POM and mixed with 5%, 25%, 50%, 75%, 95% of intermediate quality phytoplankton (Acutodesmus sp.), respectively, to evaluate how the allochthonous diet impacts on Daphnia lipid, protein and carbohydrate content.
Life table experiment
In the life table experiment lasting 21 days, Daphnia were cultured solely with Acutodesmus in a food concentration gradient, 0.25, 1.25, 2.5, 3.75, 4.75 and 5 mg C L−1, and in parallel cultures with the same concentration gradient of Acutodesmus, but added with birch leaf particles up to concentration 5 mg C L−1. We compared somatic growth of Daphnia in these treatments to find out does birch leave particles enhance growth under low availability of autochthonous carbon. This experiment was carried out in 40 mL vials. At the end of the experiment the size of Daphnia was measured under a microscope, and the individuals were then placed into 1.5 mL Eppendorf® tubes, freeze-dried and stored at −80 °C. Preserved individuals were randomly divided between lipid, fatty acid, carbohydrate and stable isotope analyses. Total biomass growth rate (g) of pooled Daphnia for each treatment were calculated as g = (lnBt21 − lnBt0)/t, where B is biomass (dry weight) at the end (t21) and at the beginning (t0) of the experiment.
Lipid, fatty acid and sterol analyses
Lipids were extracted with chloroform:methanol:water (2:1:0.75) from freeze-dried, homogenized cladocerans (0.1–1 mg), terrestrial matter (3–7 mg) and phytoplankton (1–4 mg) samples. The organic phases were pooled, chloroform evaporated off under nitrogen and lipids were measured gravimetrical difference in smooth wall tin capsules. Fatty acid samples were transmethylated with 1% H2SO4 in methanol and FA methyl esters were run with a gas chromatograph equipped with a mass spectrometer (GC-MS, Shimadzu Ultra) at University of Jyväskylä or at University of Helsinki. Both instruments were equipped with an Agilent® DB-23 column (30 m × 0.25 mm × 0.25 μm), under the following temperature program: 60 °C for 1.5 min, then the temperature was increased at 10 °C min−1 to 100 °C, followed by 2 °C min−1 to 140 °C, and 1 °C min−1 to 180 °C and finally heated at 2 °C min−1 to 210 °C and held for 6 min6. Sterols were silylated and analyzed with a gas chromatograph (Shimadzu) equipped with a mass detector using Phenomenex ® (Torrance, California, USA) ZB-5 Guardian column (30 m × 0.25 mm × 0.25 μm) and previously published temperature program71.
Klason lignin analysis
Klason lignin content of terrestrial particulate matter and phytoplankton was determined by two-step strong acid hydrolysis with sulfuric acid according to National Renewable Energy Laboratory72. In the first stage, about 0.01–0.3 g of sample and 3 ml of 72% H2SO4 was added to tube and the tubes were placed in a water bath at temperature 30 °C. The samples were stirred during the treatment. After the hydrolysis, the acid was diluted to a 4% concentration by adding 84 ml Millipore-grade water. In the second stage the sample tubes were autoclaved for 1 h in pressure 1 bar (at 121 °C). After autoclaving the samples were separated using sinter glasses (ROBU, 42 mm, 30 mL) and vacuum filtrate system. The sinter glasses with the precipitate were dried at 105 °C for 12 h and weighted, which after sinter glasses were burnt at 550 °C for 3 h, cooled and weighted again. Klason-lignin content was calculated as difference of sinter glasses after filtering and dried at 105 °C and burnt at 550 °C.
Carbohydrate analyses
Total carbohydrate content was measured using Dubois et al.73 protocol in which glucose is dehydrated to hydroxymethyl furfural in hot acidic medium. Practically, 0.1–1 mg of freeze-dried Daphnia, terrestrial organic matter or phytoplankton was diluted with 1 mL of deionized (MQ) water and 1 mL of 5% phenol solution and 5 mL of sulphuric acid (96% reagent grade) was added into the vials. These were shaken well and placed in 20 °C water bath for 20 minutes. Carbohydrates were measured with a Shimadzu UV-240 spectrophotometer at 490 nm and quantified against calibration curve with glucose (Sigma-Aldrich, 0.05; 0.1; 0.2; 0.5; 0.7; 1 μg μL−1). Additionally, we measured contribution (area %) of different types of monosaccharaides based on Laboratory Analytical Procedure (LAP) for determination of carbohydrates in algal biomass (N-REL 2013). For the determination of monosaccharides we used HPLC (Shimadzu) and RID detector (RID-20, Shimadzu) using Phenomenex Relex RHM-Monosaccharide H+ (8%) column (size 300 mm × 7.8 mm). Deionized water was used as an eluent with a flow rate of 0.6 mL min−1, the running temperature was constantly at +85 °C and the running time was 30 min per sample. The instrument was calibrated using five standards mixed of l-rhamnose, ribose, fructose, mannose, xylose, glucose, galactose and arabinose (Sigma-Aldrich) diluted to deionized water (containing each of 0.0313, 0.125, 0.25, 0.5, 0.75 and 1 g/l). The column could not separate xylose, rhamnose, galactose and mannose from each other, but they eluted at the same time.
Protein content
Protein content was calculated by conversion elemental nitrogen to protein from equation (N-REL):
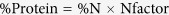 |
where %N is elemental nitrogen content determined by combustion (Carlo-Erba Flash 1112 series Element Analyzer) and Nfactor is the specific conversion factor (nitrogen content of proteins) for phytoplankton, terrestrial matter and zooplankton. Here, we used 4.78 for phytoplankton and t-POM74 and 6.3 for zooplankton75.
Stable isotope analyses
Approximately 0.2–0.6 mg of zooplankton and ≈1.0 mg of phytoplankton and t-POM were weighed in tin cups for δ13C and δ15N analyses, which were carried out on a Carlo-Erba Flash 1112 series Element Analyzer connected to a Thermo Finnigan Delta Plus Advantage IRMS at the University of Jyväskylä, Finland. These samples were compared to the NBS-22 standard using fish muscle as a laboratory-working standard. The precision of the δ13C and the δ15N analyses were 0.2‰ and 0.3‰, respectively, for all samples.
Carbon content of biomolecules
The final results of lipids, proteins, carbohydrates and fatty acids were converted to percent (%) of total organic carbon (TOC) to be able to calculate the contribution of different biochemical groups based on carbon isotope results. This means that stable isotope carbon signal contains only carbon of lipids, proteins, carbohydrates and fatty acids. Therefore, we converted concentrations of the biomolecules to carbon content. The most common biochemical compounds were used to calculate the carbon content of each biochemical group. Lignin carbon content of trees is usually 60–65%, and here we used the average value of 63.9% 76. For carbohydrates, we used the carbon content of glucose which is 40%. Carbon content of fatty acids with 14–22 carbon chain length varies between 75–80%, the proportion being the highest in highly polyunsaturated fatty acids (e.g. DHA). Carbon content of lipids (e.g. triaglycerol, phosphatidylcholine, phosphatidylethanolamine) varies between 69–77%, and we used the average value of 63% for the above mentioned lipids when fatty acid chain length was estimated to be 16.We used 46% as carbon percentage for both proteins and amino acids in this study75.
Fatty acid calculations of batch experiment
We calculated the proportions (mean ± SD) of different FA sources in Daphnia in the mixed diet treatments originating from terrestrial particulate organic carbon (t-POC), bacteria and phytoplankton by comparing the actual Daphnia FA profiles to hypothetical Daphnia FA profiles12. A hypothetical FA profile for a mixed diet was calculated = X × (the percentage of total FAs for a particular FA in the 100% Cryptomonas diet) + (1 − X) × (the percentage of FAs for a particular FA in the 100% bacterial or t-POC diet). We then compared this hypothetical FA profile to the Daphnia FA profile for the t-POC or algal diet and used the Solver function in Microsoft Excel to find the value of X that minimized the Error Sum of Squares between these two profiles. We also used Excel Solver to find the value of X that maximized the fit (r2) between the predicted and observed FA profiles.
Isotope modeling for experiments
The contribution of ingested phytoplankton and t-POM in Daphnia was calculated using δ13C values of the diet in both life table and batch experiments. Mean (±SD) carbon assimilation based on δ13C values, was calculated with IsoError software (version 1.04; ref. 77). In all cases we had only two diet sources and, thus, the uncertainty caused by variability of both sources was taken into account.
Statistical analyses
The differences in the biochemical composition of diet sources or Daphnia fed with different diets (Cryptophytes, Green algae, Reed, Birch) were tested using 1-way ANOVA, or if normality assumptions were not met, using Kruskal-Wallis test. Pairwise comparisons were conducted with least significant difference test. The relationships between proportions of carbohydrates, fatty acids or proteins in Daphnia and the degree of allocthtony were examined using linear (x + b) or nonlinear (y = ae−xb) regression models. Similarly, the relationships between the proportion of terrestrial carbon (t-POC) in the diet and the proportion of assimilated terrestrial-origin carbon by Daphnia (estimated with stable carbon isotope or fatty acid analyses) were examined using linear or nonlinear (y = axb) regression models.
Growth response and dietary thresholds of Daphnia in the experiments were estimated with nonlinear regression analysis. The model was modified from von Bertalanffy growth equation78, describing somatic growth rates (Wg mg DW d−1) in relation to the proportion of autochthonous carbon in the diet:
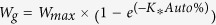 |
where Wmax represents the maximum growth rate, K = von Bertalanffy growth coefficient and Auto% the proportion of autochthonous carbon in the diet. Because the Wmax cannot be used for estimating growth saturation, we used 90% growth rate estimates79.
Cladoceran sample collections
Herbivorous cladoceran (mostly Daphnia) samples were collected from lakes in southern and eastern Finland and subarctic Finnish Lapland between May and September during several years (Table 2). The lakes were classified as brown-water, clear-water, subarctic and eutrophic lakes based on dissolved organic carbon (DOC), total phosphorus (TP) concentration and geographical location; brown-water lakes: DOC > 10 mg C L−1, TP < 35 μg P L−1, 61–62°N; clear-water lakes DOC < 10 mg DOC L−1, TP < 35 μg P L−1, 62°N; subarctic lakes DOC < 10 mg DOC L−1, TP < 20 μg P L−1, 68–69°N and eutrophic lakes DOC < 10 mg DOC L−1, TP > 35 μg P L−1, 60–61°N. Zooplankton samples were collected with vertical or horizontal tows of zooplankton net with mesh size of 25 μm. Total phosphorus (TP), total nitrogen (TN), chlorophyll a and dissolved organic carbon (DOC) concentration was analyzed using validated routine methods of the Finnish Standard Association ( htpp://www.sfs.fi/en/). The pigments were extracted in ethanol80 and measured with a Shimadzu UV-240 spectrophotometer at 665 nm and 750 nm for chlorophyll a.
Fatty acid based modeling of field collected cladocerans
To generate estimates of dietary resource assimilation by zooplankton of different basal resources, we used the Bayesian mixing model FASTAR43,81, which is adapted for analysis of fatty acids from the isotope mixing models MixSIR82 and SIAR83. The model uses a ‘resource library’ file consisting of means ± sd fatty acids of Daphnia fed a diversity of known basal monocultures in controlled laboratory feeding trials43,79. Each distinct end member (points in Fig. 5A) is a unique mean fatty acid profile of Daphnia in a fully replicated feeding trial fed one algal taxon from the nine potential basal resource groups considered here. The available phytoplankton, DOC content, and bacteria observed in these lakes was initially used to determine which end members would need to be experimentally fed to Daphnia in the feeding trials to establish the resource library (e.g. 43, ref. 84). The mixing model aggregates the unique species fatty acid profiles to a single ‘group-level’ source (e.g., cryptophytes) as the mean of the fatty acid profiles for Daphnia fed the different cryptophyte taxa. Of the bacterial groups we included Actinobacteria, which generally represent ~30% of heterotrophic bacteria in boreal lakes85,86, and methane-oxidizing bacteria (MOB), important in small stratified lakes, in the model. The third abundant bacterial group in boreal lakes, Polynucleobacter, was not included in the model because in previous laboratory experiments these bacteria proved to be toxic to Daphnia37.
Uncertainty in the model sources at the group level is accounted for by using the calculated standard deviations of fatty acid values across diets within a given phytoplankton group81. Our analysis makes the general assumption that, at this group scale, we are accounting for all of the important potential prey items for Daphnia in the lakes studied. This is a reasonable assumption because the available phytoplankton, DOC and bacteria have been identified for these lakes, and most importantly, potentially missing individual basal resource groups are expected to group largely according to taxonomy6,87,88. This means that even if a particular individual taxon was not included in the model, the group is adequately characterized with multiple end members that are representative of the group mean ± sd fatty acid values. Moreover, it is evident that the sampled wild zooplankton from all lakes fall inside of the multivariate resource-polygons of the resource library of the fatty acid profiles of Daphnia fed end members in controlled feeding trial (Fig. 5A).
As the resource library natively accounts for trophic modification of fatty acids by the consumer, the model does not assume universal or non-species specific trophic fractionation constants43,58. This means that, in practice, the resource library is the fatty acid profiles of Daphnia fed the known diets (Fig. 5A) rather than just the values the raw phytoplankton FA profiles43,81. For this analysis, FASTAR differed from Galloway et al.43 in that SIAR was used as the underlying model, and the model was run with an expanded nine-source resource library, using all 24 fatty acids to solve likely dietary contribution of these resources to Cladocerans in all 21 study lakes. The posterior distributions (Fig. 6) were estimated using the Gibbs sampling algorithm of Markov Chain Monte Carlo (MCMC) in R ref. 89 implemented as described in Galloway et al.43. The model was run for each individual lake, and in addition to showing the full posterior distributions (Fig. 6) we report the median model solution for each lake, with summaries by lake type computed from the individual lake medians. Resource group median FASTAR model results were summed for the post-hoc summary comparisons of high, medium, and low quality resources by lake type.
The comparisons of zooplankton resource assimilation of the different food quality resources (high: cryptophytes, diatoms, dinoflagellates; medium: green algae and golden algae; and low: t-POM, Actinobacteria, MOB) were made using one-way ANOVA followed by Hochberg’s GT2 posthoc tests (due to unequal sample sizes between treatments) using SPSS v. 19. Principal components analysis89 was used to visualize the multivariate fatty acid resource-library of experimentally fed Daphnia fed known basal end-members and the wild cladocerans collected in the study lakes.
Additional Information
How to cite this article: Taipale, S. J. et al. Terrestrial carbohydrates support freshwater zooplankton during phytoplankton deficiency. Sci. Rep. 6, 30897; doi: 10.1038/srep30897 (2016).
Acknowledgments
This research was supported by Academy of Finland research grant 251665 awarded to S.J.T., 263350 to P.K. and 1140903 to K.K.K.
Footnotes
Author Contributions S.J.T., P.K., U.S. and K.K.K. designed field sampling of zooplankton. S.J.T. designed and carried out laboratory experiments and analyzed biochemical composition of Daphnia and their diets. S.J.T. and U.S. collected zooplankton from the lakes and analyzed their fatty acid composition. A.W.E.G., S.L.A. and S.J.T. performed statistical tests for the laboratory experiments and prepared the figures. A.W.E.G performed mixing model analyses for fatty acid data of field zooplankton. S.J.T. and P.K. initially wrote the manuscript. All authors discussed the results and contributed to writing the manuscript.
References
- Ackman R. G. Characteristics of the fatty acid composition and biochemistry of some fresh-water fish oils and lipids in comparison with marine oils and lipids. J. Com. Biochem. Physiol. 22, 907–922 (1967). [Google Scholar]
- Arts M. T., Brett M. T. & Kainz M. J. Lipids in Aquatic Ecosystems. Springer, New York (2009). [Google Scholar]
- Hixson S. M., Sharma B., Kainz M. J., Wacker A. & Arts M. T. Production, distribution, and abundance of long-chain omega-3 polyunsaturated fatty acids: a fundamental dichotomy between freshwater and terrestrial ecosystems. Environ. Rev. 23, 414–424 (2015). [Google Scholar]
- Ahlgren G., Gustafsson I.-B. & Boberg M. Fatty acid content and chemical composition of freshwater microalgae. J. Phycol. 28, 37‒50 (1992). [Google Scholar]
- Galloway A. W. E., Britton-Simmons K. H., Duggins D. O., Gabrielson. P. W. & Brett M. T. Fatty acid signatures differentiate marine macrophytes at ordinal and family ranks. J. Phycol. 48, 956–965 (2012). [DOI] [PubMed] [Google Scholar]
- Taipale S. J. et al. Fatty acid composition as biomarkers of freshwater microalgae: analysis of 37 strains of microalgae in 22 genera and in 7 classes. Aquat. Microb. Ecol. 71, 165–178 (2013). [Google Scholar]
- Polis G. A., Anderson W. B. & Holt R. D. Toward an integration of landscape and food web ecology: the dynamics of spatially subsidized food webs. Annu. Rev. Ecol. Syst. 28, 289–316 (1997). [Google Scholar]
- Bartels P. et al. Reciprocal subsidies between freshwater and terrestrial ecosystem structure consumer resource dynamics. Ecology 93, 1173–1182 (2012). [DOI] [PubMed] [Google Scholar]
- Soininen J., Bartels P., Heino J., Luoto M. & Hillebrand H. Toward more integrated ecosystem research in aquatic and terrestrial environments. BioScience 65, 174–182 (2015). [Google Scholar]
- Wilder S. M., Norris M., Raymond W. L., Raubenheimer D. & Simpson S. J. Arthropod food webs become increasingly lipid-limited at higher trophic levels. Ecol. Lett. 16, 895–902 (2013). [DOI] [PubMed] [Google Scholar]
- Monteith D. T. et al. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 22, 537–540 (2007). [DOI] [PubMed] [Google Scholar]
- Brett M. T., Kainz M. J., Taipale S. J. & Seshan H. Phytoplankton, not allochthonous carbon sustains herbivorous zooplankton production. Proc. Natl. Acad. Sci. USA 106, 21197–21201 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. T., Solomon C. T., Weidel B. C. & Jones S. E. Terrestrial carbon is a resource, but not a subsidy, for lake zooplankton. Ecology 95, 1236–1242 (2014). [DOI] [PubMed] [Google Scholar]
- Solomon C. T. et al. Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. Ecosystems 18, 376–389 (2015). [Google Scholar]
- Kuijper L. D. J., Anderson T. R. & Kooijman S. A. L. M. C and N gross growth efficiencies of copepod egg production studied using a dynamic energy budget model. J. Plankton Res. 26, 213–226 (2004). [Google Scholar]
- Arnould J. P. Y., Green J. A. & Rawlins D. R. Fasting metabolism in Antarctic fur seal (Arctocephalus 23azelle) pups. Comp. Biochem. Physiol. A 129, 829–841 (2001). [DOI] [PubMed] [Google Scholar]
- Hiltunen M., Strandberg U., Taipale S. J. & Kankaala P. Taxonomic identity and phytoplankton diet affect fatty acid composition of zooplankton in large lakes with differing dissolved organic carbon concentration. Limnol. Oceanogr. 60, 303–317 (2015). [Google Scholar]
- Jensen K. et al. Optimal foraging for specific nutrients in predatory beetles. Proc. Biol. Sci. 7, 2212–2218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M. D., Piatt J. F. & Roby D. D. Testing the junk-food hypothesis on marine birds: effects of prey type on growth and development. J. Waterbird Soc. 29, 407–524 (2006). [Google Scholar]
- Trites A. W. & Donnelly C. P. The decline of Stellar sea lions Eumetopias jubatus in Alaska: a review of the nutritional stress hypothesis. Mamm. Rev. 33, 3–28 (2003). [Google Scholar]
- Carpenter S. R. et al. Ecosystem subsidies: terrestrial support of aquatic food webs from 13C addition to contrasting lakes. Ecology 86, 2737–2750 (2005). [Google Scholar]
- Karlsson J. et al. Terrestrial organic matter support of lake food webs: Evidence from lake metabolism and stable hydrogen isotopes of consumers. Limnol Oceanogr 57, 1042–1048 (2012). [Google Scholar]
- Jardine T. D. et al. Reconciling the role of organic matter pathways in aquatic food webs by measuring multiple tracers in individuals. Ecology 96, 3257–3269 (2015). [DOI] [PubMed] [Google Scholar]
- Xenopoulos M. A. et al. Regional comparisons of watershed determinants of dissolved organic carbon from the Upper Great Lakes region and selected regions globally. Limnol. Oceanogr. 48, 2321–2334 (2003). [Google Scholar]
- Mattsson T. et al. Export of dissolved organic matter in relation to land use along a European climatic gradient. Sci. Tot. Environ. 407, 1967–1976 (2009). [DOI] [PubMed] [Google Scholar]
- Marty J. & Planas D. Comparison of methods to determine algal δ13C in freshwater. Limnol. Oceanogr. Methods 6, 51–53 (2008). [Google Scholar]
- Solomon C. T. et al. Terrestrial, benthic, and pelagic resource use in lakes: results from a three-isotope Bayesian mixing model. Ecology 92, 1115–1125 (2011). [DOI] [PubMed] [Google Scholar]
- Hondula K. L., Pace M. L., Cole J. J. & Batt R. D. Hydrogen isotope discrimination in aquatic primary producers: Implications for aquatic food web studies. Aquat. Sci. 76, 217–229 (2014). [Google Scholar]
- Hayes J. M. Fractionation of carbon and hydrogen isotopes in biosynthetic processes. In: Stable isotope geochemistry. Reviews in mineralogy and geochemistry. (eds Valley J. W. & Cole D. R. ), 225–278 (Mineralogical Society of America, Washington, DC, 2001). [Google Scholar]
- Münster U. Concentrations and fluxes of organic carbon substrates in the aquatic environment. Antonie Van Leeuwenhoek 63, 243–274 (1993). [DOI] [PubMed] [Google Scholar]
- Koehler B., von Wachenfeldt E., Kothawala D. & Tranvik L. J. Reactivity continuum of dissolved organic carbon decomposition in lake water. J. Geophys. Res.—Biogeo. 117, G01024 (2012). [Google Scholar]
- Søndergaard M. & Middelboe M. A cross-system analysis of labile dissolved organic carbon, Mar. Ecol. Prog. Ser. 118, 283–294 (1995). [Google Scholar]
- Hulatt C. J. et al. Bioavailability and radiocarbon age of fluvial dissolved organic matter (DOM) from a northern peatland-dominated catchment: effect of land-use change. Aquat. Sci. 76, 393–404 (2014). [Google Scholar]
- Jansson M., Persson L., De Roos A. M., Jones R. I. & Tranvik L. J. Terrestrial carbon and intraspecific size variation shape lake ecosystems. Trends Ecol. Evol. 22, 316–322 (2007). [DOI] [PubMed] [Google Scholar]
- Brendelberger H. Filter mesh size of cladocerans predicts retention efficiency for bacteria. Limnol. Oceanogr 36, 884‒894 (1991). [Google Scholar]
- Jurgens K. Impact of Daphnia on planktonic microbial food webs - A review. Mar. Microb. Food Webs 8, 295‒324 (1994). [Google Scholar]
- Taipale S. J., Brett M. T., Pulkkinen K. & Kainz M. J. The influence of bacteria dominated diets on Daphnia magna somatic growth, reproduction and lipid composition. Fems Microbiol. Ecol. 82, 50‒62 (2012). [DOI] [PubMed] [Google Scholar]
- Taipale S. J. et al. Differing Daphnia magna assimilation efficiencies for terrestrial, bacterial and algal carbon and fatty acids. Ecology 95, 563‒576 (2014). [DOI] [PubMed] [Google Scholar]
- Scharnweber K. J. et al. Whole-lake experiments reveal the fate of terrestrial particulate organic carbon in benthic food webs of shallow lakes. Ecology 95, 1496‒1505 (2014). [DOI] [PubMed] [Google Scholar]
- Taylor B. R., Parkinson D. & Parsons W. F. J. Nitrogen and lignin content as predictors of litter decay rates. A microcosm test. Ecology 70, 97–104 (1989). [Google Scholar]
- Taipale S. J., Kainz M. J. & Brett M. T. A low ω-3:ω-6 ratio in Daphnia indicates terrestrial resource utilization and poor nutritional condition. J. Plankton Res 37, 596–610 (2015). [Google Scholar]
- Wenzel A., Bergstrom A. K., Jansson M. & Vrede T. Poor direct exploitation of terrestrial particulate organic material from peat layers by Daphnia galeata. Can. J. Fish Aquat. Sci. 69, 1870–1880 (2012). [Google Scholar]
- Galloway A. W. E. et al. Diet specific biomarkers show that high-quality phytoplankton fuels herbivorous zooplankton in large boreal lakes. Freshw. Biol. 59, 1902–1915 (2014). [Google Scholar]
- Kankaala P. et al. Impacts of added dissolved organic carbon on boreal freshwater pelagic metabolism and food webs in mesocosm experiments. Fundam. Appl. Limnol. 177, 161–176 (2010). [Google Scholar]
- McMeans B. C., Koussoroplis A.-M., Arts M. T. & Kainz M. J. Terrestrial dissolved organic matter supports growth and reproduction of Daphnia magna when algae are limiting. J. Plankton Res. 37, 1201–1209 (2015). [Google Scholar]
- Cole J. J. et al. Strong evidence for terrestrial support of zooplankton in small lakes based on stable isotopes of carbon, nitrogen, and hydrogen. Proc. Natl. Acad. Sci. USA 108, 1975–1980 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rautio M., Mariash H. & Forsström L. Seasonal shifts between autochthonous and allochthonous carbon contributions to zooplankton diets in a subarctic lake. Limnol. Oceanogr. 56, 1513–1524 (2011). [Google Scholar]
- Mohamed M. N. & Taylor W. D. Relative contribution of autochthonous and allochthonous carbon to limnetic zooplankton: A new cross-system approach. Fund. Appl. Limnol. 175, 113–124 (2009). [Google Scholar]
- Vuorio K., Meili M. & Sarvala J. Taxon-specific variation in the stable isotopic signatures (δ13C and δ15N) of lake phytoplankton. Freshwater Biol. 51, 807–822 (2006). [Google Scholar]
- Taipale S. J. et al. Lake zooplankton δ13C values are strongly correlated with the δ13C values of distinct phytoplankton taxa. Ecosphere, in press (2016).
- Brett M. T. & Müller-Navarra D. C. The role of highly unsaturated fatty acids in aquatic food web processes. Freshwater Biol. 38, 483–499 (1997). [Google Scholar]
- Martin-Creuzburg D. & Von Elert E. “Ecological significance of sterols in aquatic food webs”, In Lipids in Aquatic Ecosystems, (eds Arts M. T., Brett M. T. & Kainz M. J. ), 25–42 (Springer, New York, 2009). [Google Scholar]
- Cole J. J. et al. Differential support of lake food webs by three types of terrestrial organic carbon. Ecol. Lett. 9, 558–568 (2006). [DOI] [PubMed] [Google Scholar]
- Simon M., Cho B. C. & Azam F. significance of bacterial biomass in lakes and the ocean: comparisons to phytoplankton biomass and biogeochemical implications. Mar. Ecol. Prog. Ser. 86, 103–110 (1992). [Google Scholar]
- Kankaala P., Lopez Bellido J., Ojala A., Tulonen T. & Jones R. I. Variable production by different pelagic energy mobilizers in boreal lakes. Ecosystems 16, 1152–1164 (2013). [Google Scholar]
- del Giorgio P. A. & Cole J. J. Bacterial growth efficiency in natural aquatic systems. Annu. Rev. Ecol. Syst. 29, 503–541 (1998). [Google Scholar]
- Berggren M., Laudon H. & Jansson M. Aging of allochthonous organic carbon regulates bacterial production in unproductive boreal lakes. Limnol. Oceanogr. 54, 133–1342 (2009). [Google Scholar]
- Véra A., Desvilettes C., Bec A. & Bourdier G. Fatty acid composition of freshwater heterotrophic flagellates: an experimental study. Aquat. Microb. Ecol. 25, 271–279 (2001). [Google Scholar]
- Blomquist P., Jansson M., Drakare S., Bergström A.-K. & Brydsten L.. Effects of additions of DOC on pelagic biota in a clearwater system: Results from a whole lake experiment in northern Sweden. Microb. Ecol. 42, 383–394 (2001). [DOI] [PubMed] [Google Scholar]
- Taipale S. J., Kankaala P., Tiirola M. & Jones R. I. Whole-lake dissolved inorganic 13C addition reveals seasonal shifts in zooplankton diet. Ecology 89, 463–474 (2008). [DOI] [PubMed] [Google Scholar]
- Schilder J. et al. The stable carbon isotope composition of Daphnia ephippia in small temperate lakes reflects in-methane availability. Limnol. Oceanogr. 60, 1064–1075 (2015). [Google Scholar]
- Bastviken D., Cole J. J., Pace M. L. & Van de Bogart M. C. Fates of methane from different lake habitats: Connecting whole-lake budgets and CH4 emissions. J. Geophys. Res. 113, G02024 (2008). [Google Scholar]
- Carpenter S. R., Cole J. J., Pace M. L. & Wilkinson G. M. Response of plankton to nutrients, planktivory and terrestrial organic matter: a model analysis of whole-lake experiments. Ecol. Lett. 19, 230–239 (2016). [DOI] [PubMed] [Google Scholar]
- Jones R. I. The influence of humic substances on lacustrine planktonic food-chains. Hydrobiologia 229, 73‒91 (1992). [Google Scholar]
- Thrane J. E., Hessen D. O. & Andersen T. The absorption of light in lakes: negative impact of dissolved organic carbon on primary productivity. Ecosystems 17, 1040‒1052 (2014). [Google Scholar]
- Guillard R. R. L. & Lorenzen C. J. Yellow-green algae with chlorophyllide. J. Phycol. 8, 10–14 (1972). [Google Scholar]
- Watanabe M. M., Kawachi M., Hiroki M. & Kasai F. (eds.) NIES Collection List of Strains: Microalgae and Protozoa. 6th edition. National Institute for Environmental Studies, Environment: Agency, Japan, , pp 167 (2000). [Google Scholar]
- Guillard R. R. L. Culture of phytoplankton for feeding marine invertebrates. In: Culture of Marine Invertebrate Animals. (eds Smith W. L. & Chantey M. H. ), 29–60 (Plenum Publishers, 1975). [Google Scholar]
- Brett M. T. Resource quality effects on Daphnia longispina offspring fitness. J. Plankton Res. 15, 403–412 (1993). [Google Scholar]
- Lampert W. Feeding and nutrition in Daphnia. In Daphnia. (eds Peters R. H. & de Bernardi R. ), 143–192 (Memorie dell’Istituto Italiana di Idrobiologia, 1987). [Google Scholar]
- Taipale S. J., Hiltunen M., Vuorio K. & Peltomaa E. Suitability of phytosterols alongside fatty acids as chemotaxonomic biomarkers for phytoplankton. Frontiers in Plant Science 7, 212 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluiter A. et al. Determination of structural carbohydrates and lignin in biomass. NREL Laboratory Analytical Procedure NREL/TP-510-42618. Golden, CO. National Renewable Energy Laboratory (2008).
- Dubois M., Gilles K. A., Hamilton J. K., Rebers P. A. & Smith F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 28, 350–356 (1956). [Google Scholar]
- Lourenco S. O. et al. Distribution of intracellular nitrogen in marine microalgae: Calculation of new nitrogen-to-protein conversion factors. Eur. J. Phycol. 39, 17–32 (2004). [Google Scholar]
- Postel L., Fock H. & Hagen W. Biomass and Abundance. In: ICES zooplankton methodology manual. (eds Harris R. P. et al. .), 83–192 (Academic Press, London, 2000). [Google Scholar]
- Calvo-Flores F. G., Dobado J. A., García J. I. & Martin-Martinez F. J. Lignin and Lignans as Renewable Raw Materials: Chemistry, Technology and Applications. (John Wiley & Sons Ltd, 2015) [Google Scholar]
- Phillips D. L. & Gregg J. W. Uncertainty in source partitioning using stable isotopes. Oecologia 127, 171–179 (2001). [DOI] [PubMed] [Google Scholar]
- von Bertalanffy L. A quantitative theory of organic growth. Hum. Biol. 10, 181–213 (1938). [Google Scholar]
- Sperfeld E. & Wacker A. Temperature- and cholesterol-induced changes in eicosapentaenoic acid limitation of Daphnia magna determined by a promising method to estimate growth saturation thresholds. Limnol. Oceanogr. 56, 1273–1284 (2011). [Google Scholar]
- Arvola L. Spectrophotometric determination of chlorophyll a and phaeopigments in ethanol extractions. Ann. Bot. Fenn. 18, 221–227 (1981). [Google Scholar]
- Galloway A. W. E. et al. A fatty acid based Bayesian approach for inferring diet in aquatic consumers. PloS ONE 10, e0129723 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. W. & Semmens B. X. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol. Lett. 11, 470–480 (2008). [DOI] [PubMed] [Google Scholar]
- Parnell A. C., Inger R., Bearhop S. & Jackson A. L. Source partitioning using stable isotopes: coping with too much variation. PloS ONE 5, e9672 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strandberg U. et al. Inferring heterogeneous phytoplankton composition with a fatty acid mixing model. Ecosphere 6, doi: 10.1890/ES14-00382.1 (2015). [DOI] [Google Scholar]
- Zwart B. et al. Typical freshwater bacteria: an analysis of available 16S rRNA gene sequencing from plankton of lakes and rivers. Aquat. Microb. Ecol. 28, 141–155. [Google Scholar]
- Taipale S. J., Jones R. I. & Tiirola M. Vertical diversity of bacteria in an oxygen-stratified humic lake, evaluated using DNA and phospholipid analyses. Aquat. Microb. Ecol. 55, 1–16 (2009). [Google Scholar]
- Galloway A. W. E. & Winder M. Partitioning the relative importance of phylogeny and environmental conditions on phytoplankton fatty acids. PLoS One 10, e0130053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard J., John M. St., Kattner G., Müller-Navarra D. C. & Hagen W. Fatty acid trophic markers in the pelagic marine food environment. Adv. Mar. Biol. 46, 226–340 (2003). [DOI] [PubMed] [Google Scholar]
- Development Core Team. R: A language and environment for statistical computing. R Foundataion for Statistical Computing, Vienna, Austria (2016).