Abstract
AIM
To systematically review the medical literature in order to evaluate the safety and efficacy of gastric endoscopic submucosal dissection (ESD).
METHODS
We performed a comprehensive literature search of MEDLINE, Ovid, CINAHL, and Cochrane for studies reporting on the clinical efficacy and safety profile of gastric ESD.
RESULTS
Twenty-nine thousand five hundred and six tumors in 27155 patients (31% female) who underwent gastric ESD between 1999 and 2014 were included in this study. R0 resection rate was 90% (95%CI: 87%-92%) with significant between-study heterogeneity (P < 0.001) which was partly explained by difference in region (P = 0.02) and sample size (P = 0.04). Endoscopic en bloc and curative resection rates were 94% (95%CI: 93%-96%) and 86% (95%CI: 83%-89%) respectively. The rate of immediate and delayed perforation rates were 2.7% (95%CI: 2.1%-3.3%) and 0.39% (95%CI: 0.06%-2.4%) respectively while rates of immediate and delayed major bleeding were 2.9% (95%CI: 1.3-6.6) and 3.6% (95%CI: 3.1%-4.3%). After an average follow-up of about 30 mo post-operative, the rate of tumor recurrence was 0.02% (95%CI: 0.001-1.4) among those with R0 resection and 7.7% (95%CI: 3.6%-16%) among those without R0 resection. Overall, irrespective of the resection status, recurrence rate was 0.75% (95%CI: 0.42%-1.3%).
CONCLUSION
Our meta-analysis, the largest and most comprehensive assessment of gastric ESD till date, showed that gastric ESD is safe and effective for gastric tumors and warrants consideration as first line therapy when an expert operator is available.
Keywords: Endoscopic submucosal dissection, Gastric neoplasms, Meta-analysis
Core tip: Our meta-analysis, the largest and most comprehensive assessment of gastric endoscopic submucosal dissection (ESD) to date, showed that gastric ESD is safe and effective for gastric tumors when an expert operator is available. The most compelling evidence is from Asian countries and we recommend the consideration of the procedure as first line therapy in Western countries.
INTRODUCTION
Advances in diagnostic techniques and an improved understanding of gastric tumors has led to a deepening interest in new management techniques aimed to improve outcomes with minimal complications. In the past, open gastrectomy was the standard of care for gastric tumor but open surgery is typically associated with increased morbidity and mortality rates. Laparoscopy-assisted gastrectomy has also been explored as another option but despite being less invasive, there are known issues with accurately locating the lesion and resection of unnecessary quantities of normal tissue. Endoscopic submucosal dissection (ESD) is an alternative and advance way of managing early-stage lesions in the gastrointestinal tract. It allows for complete resection of early-state lesions with the aim of providing tissue for accurate histological diagnosis as well as preventing the reoccurrence of tumors. While somewhat similar to endoscopic mucosal resection (EMR), ESD is as feasible but more effective[1]. As a minimally invasive management technique developed in Japan in the mid-1990s, ESD has gradually become very widely used in Asia and some part of Europe and America. There is an increasing need to synthesize all the literature currently available to evaluate ESD thoroughly for efficacy and safety profile. We therefore conducted a systematic review and meta-analysis of studies reporting on safety and efficacy of gastric ESD, and evaluated for potential sources of heterogeneity with the aim of elucidating factors affecting these outcomes while utilizing this technique.
MATERIALS AND METHODS
We performed meta-analysis of proportion similar to what has been done in prior studies[2-9]. We followed the recommendations of the meta-analysis of observational studies in epidemiology during all stages of the design, implementation, and reporting of this meta-analysis[10].
Search strategy
We performed a comprehensive literature search of MEDLINE, Ovid, CINAHL, and Cochrane for studies published up to October 2014. Our search query for MEDLINE was (“endoscopic submucosal dissection”[tiab] OR “endoscopic submucosal resection”[tiab] OR “submucosal dissection”[tiab] OR “ESD”[tiab]) AND (“stomach”[Mesh] OR gastr*[tiab] OR “foregut”[tiab]). Similar search terms were adapted for the other databases (Table 1).
Table 1.
Search query
| Medline | (“endoscopic submucosal dissection”[tiab] OR “endoscopic submucosal resection”[tiab] OR “submucosal dissection”[tiab] OR “ESD”[tiab]) AND (“stomach”[Mesh] OR gastr*[tiab] OR “foregut”[tiab]) |
| Ovid | (endoscopic submucosal dissection OR endoscopic submucosal resection OR submucosal dissection OR endoscopic dissection OR ESD) AND (stomach OR gastr* OR foregut) |
| CINAHL | (endoscopic submucosal dissection OR endoscopic submucosal resection OR submucosal dissection OR endoscopic dissection OR ESD) AND (stomach OR gastr* OR foregut) |
| Cochrane | (endoscopic submucosal dissection OR endoscopic submucosal resection OR submucosal dissection OR endoscopic dissection OR ESD) AND (stomach OR gastr* OR foregut) |
Study selection
One investigator screened all titles and abstracts for relevance to our study. Two investigators reviewed full text of these articles and applied our pre-defined inclusion/exclusion criteria independently and in duplicate (Figure 1). Hand searching of reference list of the articles was also done in order to retrieve other articles that might have been missed by our search strategy. We included all full-text publications reporting clinical outcome(s) after gastric ESD. Our exclusion criteria were: Animal studies; case reports; commentaries or general reviews; or overlapping publications from the same center. However, review papers and overlapping publications from the same center were included in the initial screening for further assessment of the full-text and reference list after which, for the overlapping publications, only the most updated and comprehensive publication was retained. For the multicenter studies, we excluded all individual studies from the contributing centers if their sample size is comparable or less than that contributed to the multicenter study. Otherwise, we excluded the multicenter study if there are more updated studies from individual centers that provided more information. Articles in foreign language were translated via Google translator.
Figure 1.
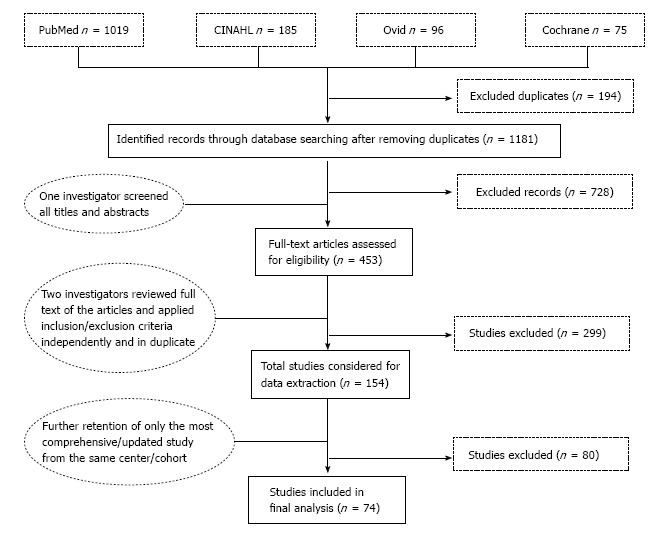
Screening and selection process.
Data extraction
Data from each study were extracted using a standardized data extraction sheet. These included publication information such as author name, year of publication; characteristics of study cohort such as country, name of medical center, study design, number of patients, year of data collection, demographics, setting (single or multi center); characteristics of tumor such as anatomical location, number of tumors, average tumor size, macroscopic or microscopic detail; ESD procedural details such as duration of the procedure and number of failed procedure; and number of patients with clinical success and adverse outcomes.
Endpoints
We assessed both measures of efficacy and adverse outcomes associated with gastric ESD. Our primary measure of efficacy was complete (R0) resection defined as en bloc (i.e., one-piece) resection with histologically confirmed tumor-free lateral and vertical margins. In addition, we evaluated endoscopic en bloc (i.e., one-piece resection without histological confirmation) and curative resection rate as secondary endpoints. Curative resection was defined as resections with both tumor-free lateral and vertical resection margins, minimal submucosal invasion (< 500 μm from the muscularis mucosa), and with no lymphovascular invasion or poorly differentiated component. Adverse outcomes include viscus perforation, major bleeding requiring intervention, and tumor recurrence. Immediate adverse events refers to those occurring within 24 h of the procedure while delayed refers to those occurring after 24 of the procedure. For all endpoints, the rates were evaluated as percentage of number of tumors operated.
Statistical analysis
Proportions from each study were pooled together using logistic-normal random effect model. Study-specific confidence intervals were based on the exact method while confidence intervals for the pooled estimates were based on the Wald method with logit transformation and back transformation. Heterogeneity between studies were assessed via visual inspection of the forest plot and χ2 statistic of the likelihood ratio test comparing the random effect model with its corresponding fixed effect model; Evaluation for potential sources of heterogeneity such as study design, setting, year of data collection (evaluated based on the last year of data collection), region (Asia vs Western world), average age, sex distribution, number of tumors, epithelial vs subepithelial tumor, average tumor size, and duration of the procedure were assessed via meta-regression. Evaluation for publication bias was assessed via visual inspection of the funnel plot and Egger’s test. Potential impact of the bias was evaluated with a cumulative meta-analysis after sorting studies in decreasing order of precision (roughly corresponding to largest to smallest study)[11].
In a subgroup analysis, we evaluated same endpoints in studies reporting outcomes exclusively among patients with cancers, i.e., we excluded studies reporting benign tumors or mixed population of benign and malignant tumors.
Analyses were performed using STATA (Version 13; StataCorp, College Station, TX), 2-tailed α = 0.05.
RESULTS
Of the 1181 citations retrieved through database searching, 728 were excluded because they reported no clinical outcome after ESD procedure in human (Figure 1). Four hundred and fifty-three studies underwent full text review using our pre-defined inclusion and exclusion criteria, after which 74 studies published between 2003 and 2014 were retained for data synthesis.
A total of 29506 tumors in 27155 patients (31% female) with average age 67 years (range: 18-95 years) underwent gastric ESD between 1999 and 2014 (Table 2). The majority of these procedures were performed in the Asian countries of Japan and South Korea with very few experiences in the Western world (Figure 2). Average tumor size was 18 mm (range: 1-150 mm), and the procedures were completed in an average time of 73 min (range: 4-750 min).
Table 2.
Characteristics of studies included in the meta-analysis of gastric endoscopic submucosal dissection
| Ref. | Data period, yr | Country | Patients, n | Age, mean (range), yr | Female, % | Tumor, n | Tumor size, mean (range), mm | Procedure length, mean (range), min |
| Sattianayagam et al[21] | 2008-2012 | Australia | 10 | 75 (43-86) | NA | 12 | 35 (15-65) | NA |
| Cardoso et al[22] | 2005-2007 | Brazil | 12 | 71.2 (27-91) | 50 | 15 | 16.8 (8-20) | 140 |
| Chaves et al[23] | 2007-2009 | Brazil | 15 | 67.1 (32-81) | 20 | 16 | 16.2 (6-35) | 85 (20-150) |
| Santos et al[24] | 2010-2011 | Brazil | 9 | 65 (58-73) | 0 | 9 | 28.6 (20-45) | 103 (60-240) |
| Xu et al[25] | 2006-2009 | China | 120 | 51.5 (26-75) | 40 | 120 | 18.8 (8-30) | 64.6 (30-120) |
| He et al[26] | 2008-2012 | China | 144 | 55.8 (18-78) | 72 | 145 | 15.14 | 63.4 (20-180) |
| Zhang et al[27] | 2008-2011 | China | 18 | 65.3 (30-71) | 61 | 18 | 26 (10-35) | 90 (50-120) |
| Probst et al[28] | 2003-2010 | Germany | 83 | 68.6 (41-87) | 40 | 91 | NA | 142 (60-420) |
| Schumacher et al[29] | 2008-2010 | Germany | 30 | 61 (35-93) | 43 | 30 | 25 (20-70) | 74 (15-402) |
| Catalano et al[30] | 2005-2007 | Italy | 12 | 68 (38-83) | 100 | 12 | NA | 111 (62-150) |
| Coda et al[31] | 2007-2009 | Italy | 7 | 72 (61-83) | 43 | 7 | 26 (15-50) | 123 (50-360) |
| Hirasaki et al[32] | 2000-2004 | Japan | 144 | 70 (45-91) | NA | 144 | 13 | 73 |
| Yokoi et al[33] | 1999-2003 | Japan | 46 | 67 (45-89) | 9 | 46 | NA | NA |
| Ono et al[34] | 2000-2007 | Japan | 408 | 67 | NA | 444 | NA | NA |
| Hirasawa et al[35] | 2000-2009 | Japan | 58 | 69.3 | 21 | 58 | 20.3 (3-50) | 82 (22-275) |
| Yoshinaga et al[36] | 2001-2006 | Japan | 24 | 61.7 (37-85) | 8 | 25 | 16.5 (3-60) | NA |
| Takenaka et al[37] | 2001-2005 | Japan | 275 | NA | NA | 306 | NA | NA |
| Miyahara et al[38] | 2001-2010 | Japan | 1082 | 71.7 (36-92) | 29 | 1190 | NA | 99.8 (10-675) |
| Ohnita et al[39] | 2001-2010 | Japan | 1209 | 72 (33-95) | 27 | 1322 | NA | NA |
| Oka et al[40] | 2002-2004 | Japan | 185 | NA | NA | 195 | 19.4 (5-100) | 84.4 |
| Shimura et al[41] | 2002-2005 | Japan | 55 | 71.4 (46-91) | 22 | 59 | 15.5 | 58 (7-640) |
| Hirasaki et al[42] | 2002-2006 | Japan | 112 | 70 (45-89) | NA | 112 | 19 | 69 |
| Ohta et al[43] | 2002-2010 | Japan | 1500 | NA | NA | 1795 | NA | NA |
| Kamada et al[44] | 2002-2010 | Japan | 46 | 65.5 (29-90) | 48 | 46 | NA | NA |
| Toyonaga et al[45] | 2002-2007 | Japan | 821 | 71 (31-93) | 34 | 1136 | 13 (1-105) | NA |
| Kosaka et al[46] | 2002-2007 | Japan | 438 | 69.4 | 26 | 438 | 14.6 | 47 (8-345) |
| Yamaguchi et al[47] | 2003-2005 | Japan | 54 | NA | NA | 54 | 19.1 (30-70) | 129 (29-440) |
| 1Akasaka et al[48] | 2003-2008 | Japan | 1188 | 71 | 27 | 1188 | 20 (2-105) | 90 (6-750) |
| Ono et al[49] | 2003-2011 | Japan | 80 | 69.6 | 20 | 80 | NA | 83.7 |
| 1Toyokawa et al[50] | 2003-2010 | Japan | 967 | NA | 32 | 1123 | 18 | 98 |
| Tanabe et al[51] | 2003-2007 | Japan | 421 | 69 (41-91) | 23 | 421 | NA | 67 (7-360) |
| Shimamura et al[52] | 2004-2012 | Japan | 521 | NA | NA | 616 | NA | NA |
| Takahashi et al[53] | 2004-2013 | Japan | 459 | 71.4 | 25 | 459 | 17.2 | NA |
| Yamamoto et al[54] | 2005-2011 | Japan | 1430 | 69.6 | 28 | 1520 | 15.3 | 101 |
| Higashimaya et al[55] | 2005-2011 | Japan | 891 | 69.1 | 27 | 1027 | 18.3 | NA |
| Hoteya et al[56] | 2005-2010 | Japan | 1224 | 68 | 24 | 1463 | 21 | 89 |
| Matsumura et al[57] | 2005-2014 | Japan | 413 | 72.1 | 30 | 425 | 18.4 | NA |
| Sohara et al[58] | 2006-2011 | Japan | 681 | 70.9 (45-91) | 40 | 850 | 20.8 (2-150) | 42 (4-360) |
| 1Nishimura et al[59] | 2006-2012 | Japan | 669 | 71 | 27 | 750 | NA | NA |
| Tsuji et al[60] | 2007-2009 | Japan | 328 | 68 | 29 | 398 | 43 | 69 |
| Akahoshi et al[61] | 2007-2009 | Japan | 35 | 72 (52-85) | 34 | 35 | 15.6 | 104 (33-264) |
| Mukai et al[62] | 2007-2010 | Japan | 142 | 72.4 | 32 | 161 | NA | 81 |
| Tanaka et al[63] | 2008-2011 | Japan | 32 | 71 (56-84) | 63 | 33 | 17 (4-67) | 111 (23-399) |
| Okamoto et al[64] | 2009-2010 | Japan | 45 | 69 (49-83) | 29 | 45 | 14 (10-35) | 80 |
| Watari et al[65] | 2010-2012 | Japan | 94 | 70.9 (48-87) | 24 | 98 | NA | NA |
| Sumiyama et al[66] | 2010-2012 | Japan | 100 | NA | 18 | 105 | 18 (3-53) | 34 (4-151) |
| Kusano et al[67] | 2011-2012 | Japan | 10 | 69.2 | 20 | 10 | 16.3 | 130.5 |
| Kawamura et al[68] | NA | Japan | 4 | NA | 25 | 4 | 24 (14-36) | 50.5 (28-72) |
| Lee et al[69] | 2003-2008 | South Korea | 461 | 62 | 30 | 487 | NA | NA |
| Kim et al[70] | 2003-2006 | South Korea | 337 | NA | 23 | 337 | 16 | 49 |
| 1Shin et al[71] | 2003-2010 | South Korea | 1105 | 65 (27-87) | 32 | 1105 | NA | NA |
| Jang et al[72] | 2004-2007 | South Korea | 402 | 60 (34-84) | 37 | 402 | NA | NA |
| Kim et al[73] | 2004-2007 | South Korea | 142 | 62 | 34 | 142 | NA | NA |
| Kang et al[74] | 2005-2008 | South Korea | 456 | 62.4 | 23 | 456 | 20.6 | NA |
| Goh et al[75] | 2005-2009 | South Korea | 210 | NA | NA | 210 | NA | NA |
| Ahn et al[76] | 2005-2008 | South Korea | 889 | 62.8 | 23 | 916 | 21.5 | 37.5 |
| Yoo et al[77] | 2005-2010 | South Korea | 729 | 64 (55-70) | 26 | 823 | 18 (12-25) | 52 (33-84) |
| Lim et al[78] | 2005-2011 | South Korea | 24 | 63 (56-75) | 21 | 24 | 16 (4-52) | 42 (16-103) |
| Park et al[79] | 2005-2011 | South Korea | 916 | 62 | 73 | 931 | NA | NA |
| Chung et al[80] | 2005-2010 | South Korea | 76 | 61.1 | 42 | 76 | NA | NA |
| Kim et al[81] | 2007-2012 | South Korea | 126 | 55 (28-85) | 44 | 126 | 12 (1-50) | NA |
| Min et al[82] | 2007-2011 | South Korea | 1527 | 63 (27-87) | 21 | 1577 | 16 (1-110) | NA |
| Kim et al[83] | 2008-2010 | South Korea | 440 | 64 | 29 | 450 | 19 | 48 |
| Yoon et al[84] | 2008-2010 | South Korea | 1319 | 63 | 34 | 1443 | 15.7 | 61.8 |
| Choi et al[85] | 2008-2012 | South Korea | 616 | NA | 26 | 616 | 12.9 | 27.7 |
| Chun et al[86] | 2009-2012 | South Korea | 35 | 54.15 | NA | 35 | 18 | 32.3 (7-84) |
| 1Chung et al[87] | 2010-2012 | South Korea | 76 | 64 | 36 | 76 | NA | 44 |
| Kim et al[88] | 2012-2013 | South Korea | 446 | NA | 34 | 446 | NA | NA |
| Bialek et al[89] | 2007-2010 | Poland | 37 | 63 (24-86) | 62 | 37 | 25 (10-60) | NA |
| Dinis-Ribeiro et al[90] | 2005-2008 | Portugal | 19 | 74 | NA | 19 | NA | 90 (40-300) |
| Lee et al[91] | 2004-2006 | Taiwan | 25 | 69 (36-82) | 44 | 25 | 19 | NA |
| 1Chang et al[92] | 2004-2007 | Taiwan | 70 | 66.5 (35-84) | 36 | 70 | 18.5 (8-40) | 92.4 (25-210) |
| Chu et al[93] | 2009-2011 | Taiwan | 16 | 51.9 (35-65) | 63 | 16 | 26.1 (20-42) | 52 (30-120) |
| González et al[94] | NA | Uruguay | 5 | NA | NA | 5 | 25.2 | 85 (30-180) |
Multicenter studies. NA: Not available.
Figure 2.
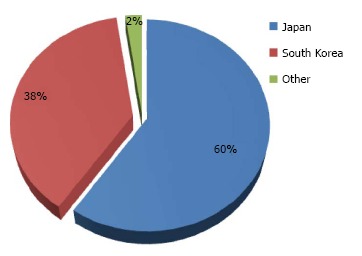
Percentage distribution of 27155 patients who underwent gastric endoscopic submucosal dissection between 1999 and 2014 in 11 countries. Others include China, Taiwan, Australia, Germany, Italy, Poland, Portugal, Brazil and Uruguay that contributed ≤ 1% each.
Efficacy
R0 resection rate was reported in 53 studies across which meta-analysis yielded a pooled estimate of 90% (95%CI: 87%-92%) (Figure 3). There was significant between-study heterogeneity (P < 0.001) which was partly explained by difference in region (P = 0.02) and sample size (P = 0.04), but not by any of the other pre-specified variables. Specifically, R0 resection rate was higher in Asia compared to the western world, and an increase in number of tumors operated by 100 is associated with 0.7% higher rate. Although significant asymmetry in the funnel plot was apparent (P = 0.001) (Figure 4), further exploration with a cumulative meta-analysis suggests that this asymmetry is not likely due to publication bias (Figure 5): The result from high-precision studies (e.g., first 25 studies in Figure 5) did not substantially differ from the overall estimate. In addition, lower estimates were reported in the low-precision studies which is the reverse of what we would expect for a publication bias. Rather, our analysis suggests that the asymmetry is due to true heterogeneity based on sample size. This notion is further supported by finding of sample size as a source of heterogeneity, and lack of asymmetry across quartile of sample size (Figure 6)[12].
Figure 3.
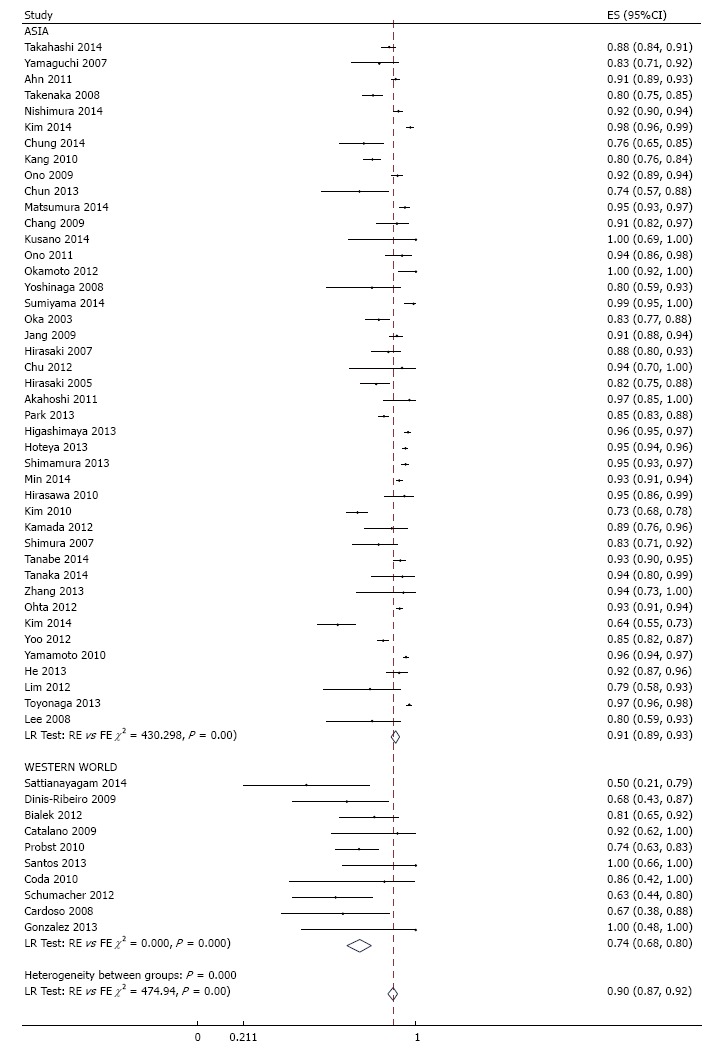
Meta-analysis of histologic en bloc resection rate in 53 studies involving 18017 tumors in 16472 patients that underwent gastric endoscopic submucosal dissection, stratified by region. Each dot and the horizontal line through them correspond to the point estimate and confidence interval from each study respectively while the center and width of the diamond corresponds to the pooled estimate and its confidence interval respectively. Even though weighting (not shown) was done, it is not explicit because an iterative procedure was used in parameter estimation. ES: Estimate.
Figure 4.
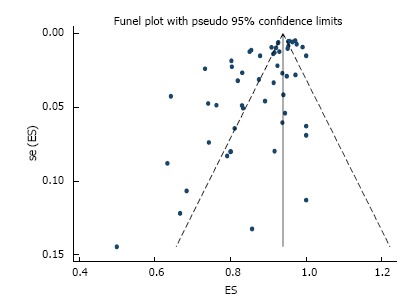
Funnel plot of histologically confirmed en bloc (R0) resection rate in 53 studies involving 18017 tumors in 16472 patients that underwent gastric endoscopic submucosal dissection. Each dot represents the R0 resection rate. Asymmetry in the distribution of study estimates around the center of the funnel suggests a potential publication bias. P value for egger’s test < 0.001. ES: Estimate; se (ES): Standard error of estimate.
Figure 5.
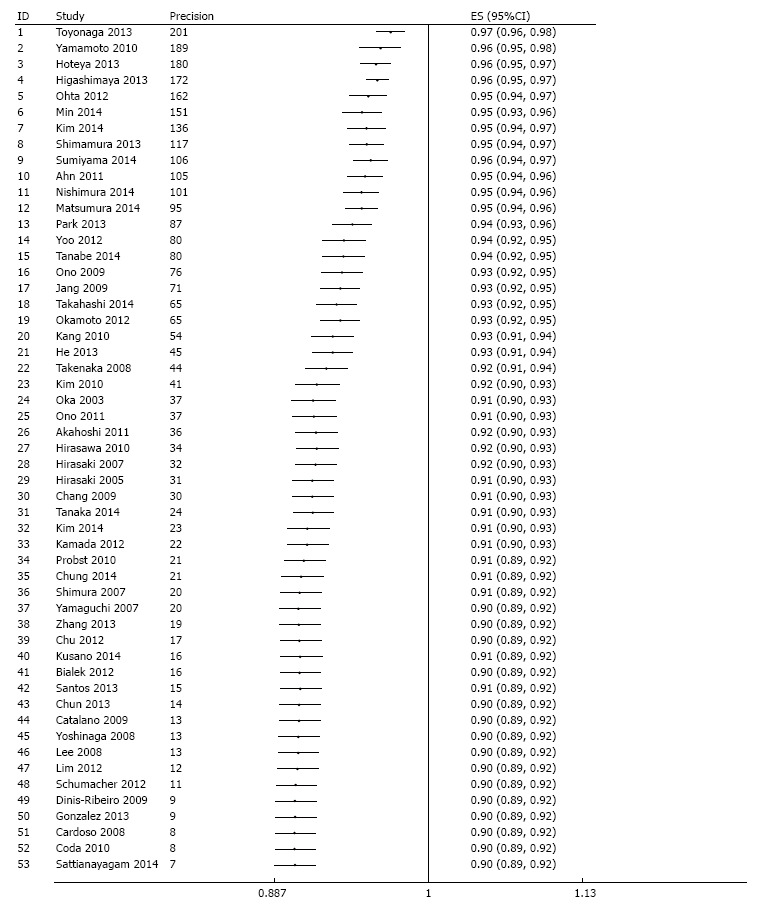
Evaluation of potential publication bias via a cumulative meta-analysis plotted as a function of study precision. The dots and the error bars correspond to the cumulative estimates and associated 95%CI respectively. After sorting by precision (calculated as inverse of standard error) from most precise to least precise study, a variance - weighted method was used to obtain cumulative meta-analysis estimates by adding one study at a time. Analysis begins with the most precise study; thereafter, effect estimate from the next study in order of decreasing precision are added at each step in the analysis and cumulative estimate and 95%CI is recalculated until the least precise study is added.
Figure 6.
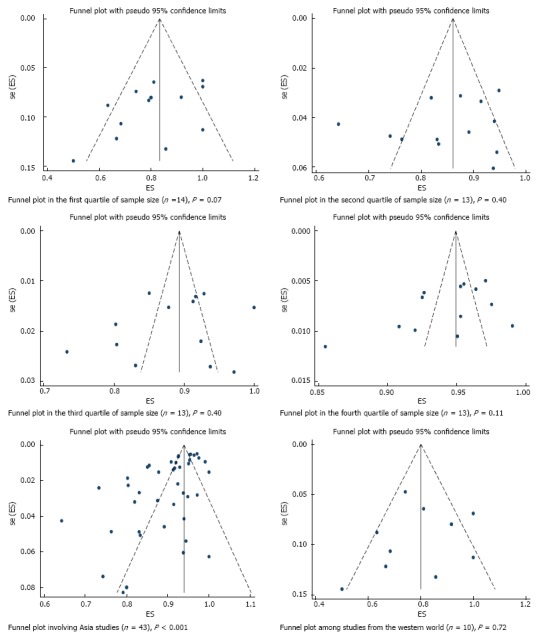
Funnel plot of histologically confirmed en bloc (R0) resection rate in 53 studies involving 18017 tumors in 16472 patients that underwent gastric endoscopic submucosal dissection, stratified based on sources of heterogeneity. Each dot represents the R0 resection rate. Lack of asymmetry in the funnel plot within quartile of study precision (calculated as inverse of standard error) indicates that the asymmetry in the overall plot (Figure 4) is most likely due to true heterogeneity by sample size rather than a publication bias. P values were calculated based on egger’s test. ES: Estimate; se (ES): Standard error of estimate.
Endoscopic en bloc and curative resection rates were reported in 60 and 20 studies respectively. Across studies, meta-analysis yielded a pooled estimate of 94% (95%CI: 93%-96%) (Figure 7) for endoscopic en bloc resection rate and 86% (95%CI: 83%-89%) (Figure 8) for curative resection rate. Evaluation for heterogeneity, publication bias, and the result of a cumulative meta-analysis for the secondary endpoints were generally similar to those of R0 resection.
Figure 7.
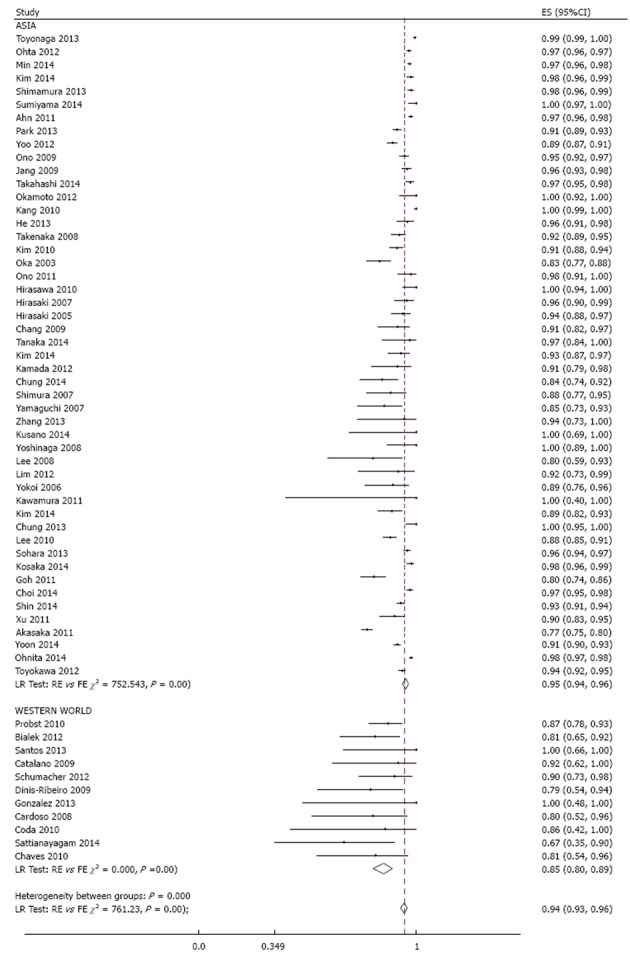
Meta-analysis of endoscopic en bloc resection rate in 60 studies involving 21511 tumors in 19935 patients that underwent gastric endoscopic submucosal dissection, stratified by region. Each dot and the horizontal line through them correspond to the point estimate and confidence interval from each study respectively while the center and width of the diamond corresponds to the pooled estimate and its confidence interval respectively. Even though weighting (not shown) was done, it is not explicit because an iterative procedure was used in parameter estimation. ES: Estimate.
Figure 8.
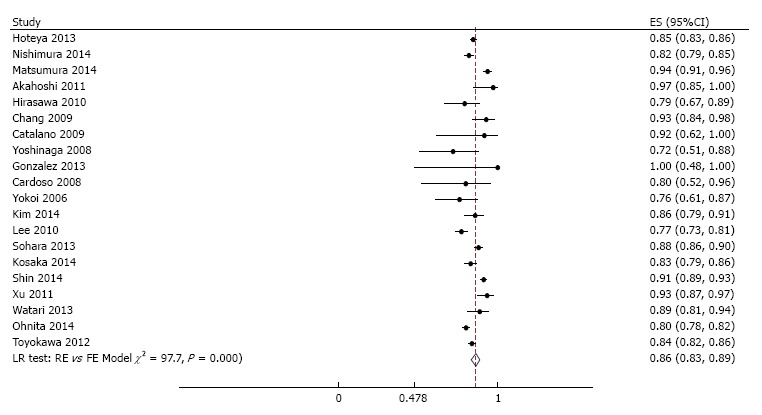
Meta-analysis of curative resection rate in 20 studies involving 8589 tumors in 7785 patients that underwent gastric endoscopic submucosal dissection. Each dot and the horizontal line through them correspond to the point estimate and confidence interval from each study respectively while the center and width of the diamond corresponds to the pooled estimate and its confidence interval respectively. Even though weighting (not shown) was done, it is not explicit because an iterative procedure was used in parameter estimation. All studies except one (Emura 2014, Colombia) were from Asia. ES: Estimate.
Adverse outcomes
Perforation and major bleeding requiring intervention were the most common peri-operative complications reported (Table 3). Immediate and delayed perforation rates were 2.7% (95%CI: 2.1%-3.3%) and 0.39% (95%CI: 0.06%-2.4%) respectively while rates of immediate and delayed major bleeding were 2.9% (95%CI: 1.3-6.6) and 3.6% (95%CI: 3.1%-4.3%). Evaluation for potential sources of heterogeneity showed that the rate (95%CI) of immediate perforation was significantly lower with epithelial [2.7% (2.2%-3.6%)] compared with subepithelial tumors [8.9% (2.7-15%)] (P = 0.02) and has declined by 0.29% (0.05%-0.54%) per year over the duration of study (P = 0.02). Similarly, the rate (95%CI) of immediate bleeding has declined by 2.3% (0.72%-3.9%) per year over the duration of study (P = 0.007). Lastly, we found that the rate (95%CI) of delayed bleeding increases by 1.3% (0.07%-2.5%) for every 10 years increase in age.
Table 3.
Rates of adverse outcomes in patients undergoing gastric endoscopic submucosal dissection between 1998 and 2014
| Adverse outcomes | Studies, n | Patients, n | Tumor, n | Rate (95%CI), %1 |
| Immediate2 | ||||
| Perforation3 | 66 | 24855 | 27118 | 2.7 (2.1, 3.3) |
| Major bleeding4 | 19 | 3815 | 3943 | 2.9 (1.3, 6.6) |
| Delayed5 | ||||
| Perforation | 13 | 2570 | 2852 | 0.39 (0.06, 2.4) |
| Major bleeding6 | 63 | 21612 | 23338 | 3.6 (3.1, 4.3) |
| Recurrence7 | ||||
| Among tumors with R0 | 17 | - | 2027 | 0.02 (0.001, 1.4) |
| Among tumors without R0 | 13 | - | 203 | 7.7 (3.6, 16) |
| Irrespective of R0 status8 | 33 | 11256 | 12398 | 0.75 (0.42, 1.3) |
The rates are calculated as a percentage of the total number of tumors operated;
Immediate refers to adverse outcomes occurring within 24 h of the procedure;
The rate (95%CI) of immediate perforation was significantly lower with epithelial [2.7% (2.2%-3.6%)] compared with subepithelial tumors [8.9% (2.7%-15%)] (P = 0.02) and declined by 0.29% (0.05%-0.54%) per year over the duration of study (P = 0.02);
The rate (95%CI) of major immediate bleeding declined by 2.3% (0.72%-3.9%) per year over the duration of study (P = 0.007);
Delayed refers to adverse outcome occurring 24 h after the procedure;
The rate (95%CI) of delayed bleeding increases by 1.3% (0.07%-2.5%) for every 10 year increase in age;
Average follow-up was 26, 28 and 32 mo for assessment of recurrence among tumors with R0, without R0, and irrespective of R0 status respectively;
The rate (95%CI) of recurrence decreases by 0.4% (0.1%-0.7%) for every 10 year increase in age (P = 0.01) and there was a trend towards higher rate in Western countries [5.1% (0.5%-11%)] compared with Asia [0.5% (0.3%-0.6%)] (P = 0.06). R0: Histologically-confirmed en bloc resection.
After an average follow up of about 30 mo post-operative, the rate of tumor recurrence was 0.02% (95%CI: 0.001-1.4) among those with R0 resection and 7.7% (95%CI: 3.6%-16%) among those without R0 resection (Table 3). Overall, irrespective of the resection status, recurrence rate was 0.75% (95%CI: 0.42%-1.3%). The rate (95%CI) of recurrence decreases by 0.4% (0.1%-0.7%) for every 10 year increase in age (P = 0.01) and there was a trend towards higher rate in Western countries [5.1% (0.5%-11%)] compared with Asia [0.5% (0.3%-0.6%), P = 0.06].
Our estimates were generally comparable to those of subgroup analysis restricting to studies reporting outcomes exclusively among patients with cancer although with slightly higher risk of recurrence (Table 4).
Table 4.
Clinical outcomes among patients with gastric cancers who underwent endoscopic submucosal dissection
| Outcomes | Studies, n | Tumor, n | Rate (95%CI)1 |
| Efficacy measures | |||
| R0 resection | 24 | 8520 | 87 (84-90) |
| Endoscopic en bloc resection | 29 | 9652 | 94 (91-96) |
| Curative resection | 10 | 5234 | 83 (80-86) |
| Safety measures | |||
| Immediate perforation2 | 31 | 12076 | 3.1 (2.4-3.9) |
| Immediate major bleeding2 | 6 | 303 | 2.9 (0.24-27) |
| Delayed perforation3 | 6 | 1486 | 0.15 (0.01-3.8) |
| Delayed bleeding3 | 29 | 11925 | 3.8 (3.0-4.7) |
| Recurrence (if R0)4 | 8 | 724 | 0.14 (0.004-4.6) |
| Recurrence (if not R0)4 | 7 | 152 | 8.5 (3.6-19) |
| Recurrence (irrespective of R0 status)4 | 18 | 7681 | 0.77 (0.39-1.5) |
The rates are calculated as a percentage of the total number of tumors operated;
Immediate refers to adverse outcomes occurring within 24 h of the procedure;
Delayed refers to adverse outcome occurring 24 h after the procedure;
Average follow-up was about 26, 24 and 37 mo for assessment of recurrence among tumors with R0, without R0, and irrespective of R0 status respectively. R0: Histologically-confirmed en bloc resection.
DISCUSSION
Our meta-analysis showed that, across multiple studies in 11 countries, ESD demonstrated an excellent treatment success in patients with gastric tumors. Perioperatively, perforation and major bleeding were the most commonly reported serious adverse outcomes but their risk is modest. In addition, the risk of tumor recurrence in patients with treatment success after a moderate duration of follow-up is very low. These findings provide evidence that ESD is effective and offers a reasonable safety profile across a wide range of patients.
Treatment success was assessed in three ways: R0, endoscopic en bloc and curative resection rates. In this study, we considered R0 resection as primary endpoint. Across studies, there were excellent results based on this endpoint. However, there was significant heterogeneity in study estimates that was partly explained by two main factors: First, the estimates vary by region, with higher rates of clinical success being reported by studies from Asia compared to the western world. This, in a way, was expected since the procedure was developed in Asia and has been used for a long time in this part of the world allowing for the development of expert skill needed for the procedure as well as development of better techniques. On the other hand, experience in the procedure had been low in other parts of the world. Second, lower rates of treatment success were reported in the smaller studies compared to the large ones. Since the number of tumor operated is expected to correlate with level of expertise, we presume this is an indicator of better outcome with increasing level of expertise or experience.
Perioperatively, major bleeding and perforation were the most common serious adverse events. However, most of these adverse events were successfully managed endoscopically with only very few ones requiring surgical intervention. The relatively low risk of recurrence has been the attractive feature of ESD. After a moderate follow up, tumor recurrence was present in only 8 in 1000 tumors after the procedure, and this rate was majorly influenced by those without R0 resection, i.e., patients with positive lateral or vertical tumor margins. In patients with R0 resection, the risk of recurrence is negligible: 2 in 10000 tumors. Overall, our estimates were comparable to those of subgroup analysis involving studies exclusively among patients with cancer, although with slightly higher risk of recurrence in this subgroup.
Before the invention of ESD in the late 1990s in Japan, EMR was the most widely used minimally invasive option for non-invasive gastric tumors in the world; and it’s still the most widely used in many Western countries. However, the superior benefit of ESD in terms of complete resection and tumor recurrence as compared to EMR had been demonstrated in a few meta-analysis[13-15]. Although the risk of bleeding and perforation tends to be higher with ESD, most cases of such adverse event were amenable to endoscopic management; thus, making the benefit to outweigh the risk[16]. Absolute indications for endoscopic resection had included moderately or well-differentiated elevated cancers ≤ 20 mm in diameter; and small (≤ 10 mm), flat and depressed lesions without ulceration or scarring. In addition, these lesions must be intra-mucosal and with no lymphovascular involvement. However, the success of ESD has led to the extension of this criteria to include intra-mucosal cancer without ulceration > 20 mm or with ulcerations ≤ 30 mm, and upper submucosal cancer ≤ 30 mm. Overall, ESD remains the best endoscopic option for cancers ≥ 20 mm while EMR is an option for those < 20 mm. Endoscopic resection is however not indicated in tumors with poorly differentiated component or signet ring cell[17]. Furthermore, the proficiency of the ESD procedure takes some time to acquire as prior studies have suggested that it takes at least 30 procedures for a beginner to overcome the learning curve[18,19].
Our study has several strengths. Notably, a guideline-driven approach ensures that our analysis was systematic and comprehensive. In addition, we made attempt to gather all available data by placing no restriction on language, date of publication, location, etc. Our moderately large number of studies enabled us to shed more light on potential sources of heterogeneity in clinical outcomes after ESD.
Limitations of this study should also be considered. First, due to rapidly evolving techniques in ESD procedures, the rates of each outcome may vary slightly by technique and our rates of adverse outcomes might have been over-estimated compared to new technique. This is particularly apparent with the finding of declining rates of immediate perforation and bleeding over the study period. Second, the recurrence rates were assessed after variable follow-up between and within study, and since the rate of recurrence is time-dependent, cautious interpretation of average follow-up reported is warranted when applied to individual cases. Third, there was significant asymmetry in the funnel plot of histologic en bloc resection rate indicating potential selective reporting of outcomes by authors. However, further exploration with cumulative meta-analysis indicates that this asymmetry is not likely due to publication bias since lower estimates were reported in the low precision studies[20]. Rather, we presume that the asymmetry is probably due to chance or better expertise among the high precision studies since precision is proportional to the number of tumors operated, which in turn is expected to correlate with level of expertise. In addition, we mitigated against publication bias in our methodology by placing no restriction on publication language and excluding all overlapping studies[20].
In conclusion, gastric ESD is a safe and effective technique based on the large and broad body of current medical literature. It compares favorably with EMR and warrants consideration as first-line therapy when an expert operator is available.
COMMENTS
Background
Advances in endoscopic techniques have led to the development of endoscopic submucosal dissection (ESD) for en-bloc resection of gastrointestinal tumors. The authors systematically reviewed the medical literature to evaluate the safety and efficacy of gastric ESD.
Research frontiers
Accumulating evidence from Asia suggests that ESD is safe and more effective than other minimally invasive alternative such as endoscopic mucosal resection. However, the procedure is still not popular in the West and the available results (even from Asia) are mixed. The authors therefore performed a systematic review and meta-analysis to analyze available evidence and explore for potential sources of heterogeneity.
Innovations and breakthroughs
This meta-analysis represents the largest assessment of gastric ESD to date. The authors were able to show that gastric ESD is safe and effective when an expert operator is available. More importantly, they were also able to explore for sources of heterogeneity among the available results in the literature.
Applications
The authors believe that with proper training in the techniques of gastric ESD, this procedure can become the first line therapy for gastric tumor in Western countries.
Terminology
ESD is an advanced endoscopic technique used to remove gastrointestinal tumors. The procedure involves passage of endoscopic tube through the throat in order to assess the tumor in the stomach. Thereafter, the tumor dissection is performed by injecting fluid below the lesion at the submucosal layer in order to elevate the tumor. The procedure is completed by dissecting through the surrounding mucosa to the submucosal layer beneath the tumor. Meta-analysis is a statistical method used to combine results from multiple similar studies in order to achieve a greater statistical power and evaluate for potential sources of heterogeneity.
Peer-review
The article is very interesting and well written. The number of studies and patients included is also very satisfactory.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest.
Data sharing statement: Dataset and statistical code available from the first author at eakintoy@med.wayne.edu.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: March 27, 2016
First decision: May 13, 2016
Article in press: July 13, 2016
P- Reviewer: Giannopoulos GA, Lee CL, Mentes O S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
References
- 1.Watanabe K, Ogata S, Kawazoe S, Watanabe K, Koyama T, Kajiwara T, Shimoda Y, Takase Y, Irie K, Mizuguchi M, et al. Clinical outcomes of EMR for gastric tumors: historical pilot evaluation between endoscopic submucosal dissection and conventional mucosal resection. Gastrointest Endosc. 2006;63:776–782. doi: 10.1016/j.gie.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 2.Barbieri LA, Hassan C, Rosati R, Romario UF, Correale L, Repici A. Systematic review and meta-analysis: Efficacy and safety of POEM for achalasia. United European Gastroenterol J. 2015;3:325–334. doi: 10.1177/2050640615581732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA. 1998;279:1200–1205. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 4.Puli SR, Kakugawa Y, Saito Y, Antillon D, Gotoda T, Antillon MR. Successful complete cure en-bloc resection of large nonpedunculated colonic polyps by endoscopic submucosal dissection: a meta-analysis and systematic review. Ann Surg Oncol. 2009;16:2147–2151. doi: 10.1245/s10434-009-0520-7. [DOI] [PubMed] [Google Scholar]
- 5.Kim JS, Kim BW, Shin IS. Efficacy and safety of endoscopic submucosal dissection for superficial squamous esophageal neoplasia: a meta-analysis. Dig Dis Sci. 2014;59:1862–1869. doi: 10.1007/s10620-014-3098-2. [DOI] [PubMed] [Google Scholar]
- 6.Park CH, Kim EH, Kim HY, Roh YH, Lee YC. Clinical outcomes of endoscopic submucosal dissection for early stage esophagogastric junction cancer: a systematic review and meta-analysis. Dig Liver Dis. 2015;47:37–44. doi: 10.1016/j.dld.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Tanimoto MA, Guerrero ML, Morita Y, Aguirre-Valadez J, Gomez E, Moctezuma-Velazquez C, Estradas-Trujillo JA, Valdovinos MA, Uscanga LF, Fujita R. Impact of formal training in endoscopic submucosal dissection for early gastrointestinal cancer: A systematic review and a meta-analysis. World J Gastrointest Endosc. 2015;7:417–428. doi: 10.4253/wjge.v7.i4.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bang CS, Baik GH, Shin IS, Kim JB, Suk KT, Yoon JH, Kim YS, Kim DJ, Shin WG, Kim KH, et al. Endoscopic submucosal dissection for early gastric cancer with undifferentiated-type histology: A meta-analysis. World J Gastroenterol. 2015;21:6032–6043. doi: 10.3748/wjg.v21.i19.6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nyaga VN, Arbyn M, Aerts M. Metaprop: a Stata command to perform meta-analysis of binomial data. Arch Public Health. 2014;72:39. doi: 10.1186/2049-3258-72-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Leimu R, Koricheva J. Cumulative meta-analysis: a new tool for detection of temporal trends and publication bias in ecology. Proc Biol Sci. 2004;271:1961–1966. doi: 10.1098/rspb.2004.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 13.Cao Y, Liao C, Tan A, Gao Y, Mo Z, Gao F. Meta-analysis of endoscopic submucosal dissection versus endoscopic mucosal resection for tumors of the gastrointestinal tract. Endoscopy. 2009;41:751–757. doi: 10.1055/s-0029-1215053. [DOI] [PubMed] [Google Scholar]
- 14.Lian J, Chen S, Zhang Y, Qiu F. A meta-analysis of endoscopic submucosal dissection and EMR for early gastric cancer. Gastrointest Endosc. 2012;76:763–770. doi: 10.1016/j.gie.2012.06.014. [DOI] [PubMed] [Google Scholar]
- 15.Facciorusso A, Antonino M, Di Maso M, Muscatiello N. Endoscopic submucosal dissection vs endoscopic mucosal resection for early gastric cancer: A meta-analysis. World J Gastrointest Endosc. 2014;6:555–563. doi: 10.4253/wjge.v6.i11.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Park YM, Cho E, Kang HY, Kim JM. The effectiveness and safety of endoscopic submucosal dissection compared with endoscopic mucosal resection for early gastric cancer: a systematic review and metaanalysis. Surg Endosc. 2011;25:2666–2677. doi: 10.1007/s00464-011-1627-z. [DOI] [PubMed] [Google Scholar]
- 17.Hoppo T, Jobe BA. Endoscopy and role of endoscopic resection in gastric cancer. J Surg Oncol. 2013;107:243–249. doi: 10.1002/jso.23126. [DOI] [PubMed] [Google Scholar]
- 18.Gotoda T, Friedland S, Hamanaka H, Soetikno R. A learning curve for advanced endoscopic resection. Gastrointest Endosc. 2005;62:866–867. doi: 10.1016/j.gie.2005.07.055. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto S, Uedo N, Ishihara R, Kajimoto N, Ogiyama H, Fukushima Y, Yamamoto S, Takeuchi Y, Higashino K, Iishi H, et al. Endoscopic submucosal dissection for early gastric cancer performed by supervised residents: assessment of feasibility and learning curve. Endoscopy. 2009;41:923–928. doi: 10.1055/s-0029-1215129. [DOI] [PubMed] [Google Scholar]
- 20.van Enst WA, Ochodo E, Scholten RJ, Hooft L, Leeflang MM. Investigation of publication bias in meta-analyses of diagnostic test accuracy: a meta-epidemiological study. BMC Med Res Methodol. 2014;14:70. doi: 10.1186/1471-2288-14-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sattianayagam PT, Desmond PV, Jayasekera C, Chen RY. Endoscopic submucosal dissection: experience in an Australian tertiary center. Ann Gastroenterol. 2014;27:212–218. [PMC free article] [PubMed] [Google Scholar]
- 22.Cardoso DM, Campoli PM, Yokoi C, Ejima FH, Barreto PA, de Brito AM, Mota ED, de Fraga Júnior AC, da Mota OM. Initial experience in Brazil with endoscopic submucosal dissection for early gastric cancer using insulation-tipped knife: a safety and feasibility study. Gastric Cancer. 2008;11:226–232. doi: 10.1007/s10120-008-0489-0. [DOI] [PubMed] [Google Scholar]
- 23.Chaves DM, Maluf Filho F, de Moura EG, Santos ME, Arrais LR, Kawaguti F, Sakai P. Endoscopic submucosal dissection for the treatment of early esophageal and gastric cancer--initial experience of a western center. Clinics (Sao Paulo) 2010;65:377–382. doi: 10.1590/S1807-59322010000400005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Santos JO, Miyajima N, Carvalho R, Leal RF, Ayrizomo Mde L, Coy CS. Feasibility of endoscopic submucosal dissection for gastric and colorectal lesions: Initial experience from the Gastrocentro--UNICAMP. Clinics (Sao Paulo) 2013;68:141–146. doi: 10.6061/clinics/2013(02)OA04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu LH, Jun Bo Q, Liu Gen G, Fei L, Ya Min W, Yu Ming L, Hua Sheng L. Treatment of gastric epithelial tumours by endoscopic submucosal dissection using an insulated-tip diathermic knife. Can J Gastroenterol. 2011;25:97–101. doi: 10.1155/2011/135060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He Z, Sun C, Wang J, Zheng Z, Yu Q, Wang T, Chen X, Liu W, Wang B. Efficacy and safety of endoscopic submucosal dissection in treating gastric subepithelial tumors originating in the muscularis propria layer: a single-center study of 144 cases. Scand J Gastroenterol. 2013;48:1466–1473. doi: 10.3109/00365521.2013.845796. [DOI] [PubMed] [Google Scholar]
- 27.Zhang S, Chao GQ, Li M, Ni GB, Lv B. Endoscopic submucosal dissection for treatment of gastric submucosal tumors originating from the muscularis propria layer. Dig Dis Sci. 2013;58:1710–1716. doi: 10.1007/s10620-013-2559-3. [DOI] [PubMed] [Google Scholar]
- 28.Probst A, Pommer B, Golger D, Anthuber M, Arnholdt H, Messmann H. Endoscopic submucosal dissection in gastric neoplasia - experience from a European center. Endoscopy. 2010;42:1037–1044. doi: 10.1055/s-0030-1255668. [DOI] [PubMed] [Google Scholar]
- 29.Schumacher B, Charton JP, Nordmann T, Vieth M, Enderle M, Neuhaus H. Endoscopic submucosal dissection of early gastric neoplasia with a water jet-assisted knife: a Western, single-center experience. Gastrointest Endosc. 2012;75:1166–1174. doi: 10.1016/j.gie.2012.02.027. [DOI] [PubMed] [Google Scholar]
- 30.Catalano F, Trecca A, Rodella L, Lombardo F, Tomezzoli A, Battista S, Silano M, Gaj F, de Manzoni G. The modern treatment of early gastric cancer: our experience in an Italian cohort. Surg Endosc. 2009;23:1581–1586. doi: 10.1007/s00464-009-0350-5. [DOI] [PubMed] [Google Scholar]
- 31.Coda S, Trentino P, Antonellis F, Porowska B, Gossetti F, Ruberto F, Pugliese F, D’Amati G, Negro P, Gotoda T. A Western single-center experience with endoscopic submucosal dissection for early gastrointestinal cancers. Gastric Cancer. 2010;13:258–263. doi: 10.1007/s10120-010-0544-5. [DOI] [PubMed] [Google Scholar]
- 32.Hirasaki S, Tanimizu M, Nasu J, Shinji T, Koide N. Treatment of elderly patients with early gastric cancer by endoscopic submucosal dissection using an insulated-tip diathermic knife. Intern Med. 2005;44:1033–1038. doi: 10.2169/internalmedicine.44.1033. [DOI] [PubMed] [Google Scholar]
- 33.Yokoi C, Gotoda T, Hamanaka H, Oda I. Endoscopic submucosal dissection allows curative resection of locally recurrent early gastric cancer after prior endoscopic mucosal resection. Gastrointest Endosc. 2006;64:212–218. doi: 10.1016/j.gie.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 34.Ono S, Fujishiro M, Niimi K, Goto O, Kodashima S, Yamamichi N, Omata M. Technical feasibility of endoscopic submucosal dissection for early gastric cancer in patients taking anti-coagulants or anti-platelet agents. Dig Liver Dis. 2009;41:725–728. doi: 10.1016/j.dld.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 35.Hirasawa K, Kokawa A, Oka H, Yahara S, Sasaki T, Nozawa A, Tanaka K. Superficial adenocarcinoma of the esophagogastric junction: long-term results of endoscopic submucosal dissection. Gastrointest Endosc. 2010;72:960–966. doi: 10.1016/j.gie.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 36.Yoshinaga S, Gotoda T, Kusano C, Oda I, Nakamura K, Takayanagi R. Clinical impact of endoscopic submucosal dissection for superficial adenocarcinoma located at the esophagogastric junction. Gastrointest Endosc. 2008;67:202–209. doi: 10.1016/j.gie.2007.09.054. [DOI] [PubMed] [Google Scholar]
- 37.Takenaka R, Kawahara Y, Okada H, Hori K, Inoue M, Kawano S, Tanioka D, Tsuzuki T, Yagi S, Kato J, et al. Risk factors associated with local recurrence of early gastric cancers after endoscopic submucosal dissection. Gastrointest Endosc. 2008;68:887–894. doi: 10.1016/j.gie.2008.03.1089. [DOI] [PubMed] [Google Scholar]
- 38.Miyahara K, Iwakiri R, Shimoda R, Sakata Y, Fujise T, Shiraishi R, Yamaguchi K, Watanabe A, Yamaguchi D, Higuchi T, et al. Perforation and postoperative bleeding of endoscopic submucosal dissection in gastric tumors: analysis of 1190 lesions in low- and high-volume centers in Saga, Japan. Digestion. 2012;86:273–280. doi: 10.1159/000341422. [DOI] [PubMed] [Google Scholar]
- 39.Ohnita K, Isomoto H, Shikuwa S, Yajima H, Minami H, Matsushima K, Akazawa Y, Yamaguchi N, Fukuda E, Nishiyama H, et al. Early and long-term outcomes of endoscopic submucosal dissection for early gastric cancer in a large patient series. Exp Ther Med. 2014;7:594–598. doi: 10.3892/etm.2014.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oka S, Tanaka S, Kaneko I, Mouri R, Hirata M, Kawamura T, Yoshihara M, Chayama K. Advantage of endoscopic submucosal dissection compared with EMR for early gastric cancer. Gastrointest Endosc. 2006;64:877–883. doi: 10.1016/j.gie.2006.03.932. [DOI] [PubMed] [Google Scholar]
- 41.Shimura T, Sasaki M, Kataoka H, Tanida S, Oshima T, Ogasawara N, Wada T, Kubota E, Yamada T, Mori Y, et al. Advantages of endoscopic submucosal dissection over conventional endoscopic mucosal resection. J Gastroenterol Hepatol. 2007;22:821–826. doi: 10.1111/j.1440-1746.2006.04505.x. [DOI] [PubMed] [Google Scholar]
- 42.Hirasaki S, Kanzaki H, Matsubara M, Fujita K, Ikeda F, Taniguchi H, Yumoto E, Suzuki S. Treatment of over 20 mm gastric cancer by endoscopic submucosal dissection using an insulation-tipped diathermic knife. World J Gastroenterol. 2007;13:3981–3984. doi: 10.3748/wjg.v13.i29.3981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ohta T, Ishihara R, Uedo N, Takeuchi Y, Nagai K, Matsui F, Kawada N, Yamashina T, Kanzaki H, Hanafusa M, et al. Factors predicting perforation during endoscopic submucosal dissection for gastric cancer. Gastrointest Endosc. 2012;75:1159–1165. doi: 10.1016/j.gie.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 44.Kamada K, Tomatsuri N, Yoshida N. Endoscopic submucosal dissection for undifferentiated early gastric cancer as the expanded indication lesion. Digestion. 2012;85:111–115. doi: 10.1159/000334681. [DOI] [PubMed] [Google Scholar]
- 45.Toyonaga T, Man-i M, East JE, Nishino E, Ono W, Hirooka T, Ueda C, Iwata Y, Sugiyama T, Dozaiku T, et al. 1,635 Endoscopic submucosal dissection cases in the esophagus, stomach, and colorectum: complication rates and long-term outcomes. Surg Endosc. 2013;27:1000–1008. doi: 10.1007/s00464-012-2555-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kosaka T, Endo M, Toya Y, Abiko Y, Kudara N, Inomata M, Chiba T, Takikawa Y, Suzuki K, Sugai T. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a single-center retrospective study. Dig Endosc. 2014;26:183–191. doi: 10.1111/den.12099. [DOI] [PubMed] [Google Scholar]
- 47.Yamaguchi Y, Katusmi N, Aoki K, Toki M, Nakamura K, Abe N, Morozumi K, Sugiyama M, Ishida H, Takahashi S. Resection area of 15 mm as dividing line for choosing strip biopsy or endoscopic submucosal dissection for mucosal gastric neoplasm. J Clin Gastroenterol. 2007;41:472–476. doi: 10.1097/01.mcg.0000247987.02677.b3. [DOI] [PubMed] [Google Scholar]
- 48.Akasaka T, Nishida T, Tsutsui S, Michida T, Yamada T, Ogiyama H, Kitamura S, Ichiba M, Komori M, Nishiyama O, et al. Short-term outcomes of endoscopic submucosal dissection (ESD) for early gastric neoplasm: multicenter survey by osaka university ESD study group. Dig Endosc. 2011;23:73–77. doi: 10.1111/j.1443-1661.2010.01062.x. [DOI] [PubMed] [Google Scholar]
- 49.Ono S, Kato M, Nakagawa M, Imai A, Yamamoto K, Shimizu Y. Outcomes and predictive factors of “not self-completion” in gastric endoscopic submucosal dissection for novice operators. Surg Endosc. 2013;27:3577–3583. doi: 10.1007/s00464-013-2929-0. [DOI] [PubMed] [Google Scholar]
- 50.Toyokawa T, Inaba T, Omote S, Okamoto A, Miyasaka R, Watanabe K, Izumikawa K, Horii J, Fujita I, Ishikawa S, et al. Risk factors for perforation and delayed bleeding associated with endoscopic submucosal dissection for early gastric neoplasms: analysis of 1123 lesions. J Gastroenterol Hepatol. 2012;27:907–912. doi: 10.1111/j.1440-1746.2011.07039.x. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe S, Ishido K, Higuchi K, Sasaki T, Katada C, Azuma M, Naruke A, Kim M, Koizumi W. Long-term outcomes of endoscopic submucosal dissection for early gastric cancer: a retrospective comparison with conventional endoscopic resection in a single center. Gastric Cancer. 2014;17:130–136. doi: 10.1007/s10120-013-0241-2. [DOI] [PubMed] [Google Scholar]
- 52.Shimamura Y, Ishii N, Nakano K, Ikeya T, Nakamura K, Takagi K, Fukuda K, Suzuki K, Fujita Y. Repeat endoscopic submucosal dissection for recurrent gastric cancers after endoscopic submucosal dissection. World J Gastrointest Endosc. 2013;5:600–604. doi: 10.4253/wjge.v5.i12.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Takahashi F, Yoshitake N, Akima T, Kino H, Nakano M, Tsuchida C, Tsuchida K, Tominaga K, Sasai T, Masuyama H, et al. A second-look endoscopy may not reduce the bleeding after endoscopic submucosal dissection for gastric epithelial neoplasm. BMC Gastroenterol. 2014;14:152. doi: 10.1186/1471-230X-14-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yamamoto Y, Fujisaki J, Ishiyama A, Hirasawa T, Igarashi M. Current status of training for endoscopic submucosal dissection for gastric epithelial neoplasm at Cancer Institute Hospital, Japanese Foundation for Cancer Research, a famous Japanese hospital. Dig Endosc. 2012;24 Suppl 1:148–153. doi: 10.1111/j.1443-1661.2012.01278.x. [DOI] [PubMed] [Google Scholar]
- 55.Higashimaya M, Oka S, Tanaka S, Sanomura Y, Yoshida S, Hiyama T, Arihiro K, Shimamoto F, Chayama K. Outcome of endoscopic submucosal dissection for gastric neoplasm in relationship to endoscopic classification of submucosal fibrosis. Gastric Cancer. 2013;16:404–410. doi: 10.1007/s10120-012-0203-0. [DOI] [PubMed] [Google Scholar]
- 56.Hoteya S, Matsui A, Iizuka T, Kikuchi D, Yamada A, Yamashita S, Furuhata T, Domon K, Nakamura M, Mitani T, et al. Comparison of the clinicopathological characteristics and results of endoscopic submucosal dissection for esophagogastric junction and non-junctional cancers. Digestion. 2013;87:29–33. doi: 10.1159/000343934. [DOI] [PubMed] [Google Scholar]
- 57.Matsumura T, Arai M, Maruoka D, Okimoto K, Minemura S, Ishigami H, Saito K, Nakagawa T, Katsuno T, Yokosuka O. Risk factors for early and delayed post-operative bleeding after endoscopic submucosal dissection of gastric neoplasms, including patients with continued use of antithrombotic agents. BMC Gastroenterol. 2014;14:172. doi: 10.1186/1471-230X-14-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sohara N, Hagiwara S, Arai R, Iizuka H, Onozato Y, Kakizaki S. Can endoscopic submucosal dissection be safely performed in a smaller specialized clinic? World J Gastroenterol. 2013;19:528–535. doi: 10.3748/wjg.v19.i4.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimura J, Nishikawa J, Hamabe K, Nakamura M, Goto A, Okamoto T, Miura O, Sakaida I. Efficacy of endoscopic submucosal dissection for cancer of the operated stomach. J Gastrointest Cancer. 2014;45:27–33. doi: 10.1007/s12029-013-9544-0. [DOI] [PubMed] [Google Scholar]
- 60.Tsuji Y, Ohata K, Ito T, Chiba H, Ohya T, Gunji T, Matsuhashi N. Risk factors for bleeding after endoscopic submucosal dissection for gastric lesions. World J Gastroenterol. 2010;16:2913–2917. doi: 10.3748/wjg.v16.i23.2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Akahoshi K, Honda K, Motomura Y, Kubokawa M, Okamoto R, Osoegawa T, Nakama N, Kashiwabara Y, Higuchi N, Tanaka Y, et al. Endoscopic submucosal dissection using a grasping-type scissors forceps for early gastric cancers and adenomas. Dig Endosc. 2011;23:24–29. doi: 10.1111/j.1443-1661.2010.01037.x. [DOI] [PubMed] [Google Scholar]
- 62.Mukai S, Cho S, Kotachi T, Shimizu A, Matuura G, Nonaka M, Hamada T, Hirata K, Nakanishi T. Analysis of delayed bleeding after endoscopic submucosal dissection for gastric epithelial neoplasms. Gastroenterol Res Pract. 2012;2012:875323. doi: 10.1155/2012/875323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tanaka S, Toyonaga T, Morita Y, Fujita T, Yoshizaki T, Kawara F, Wakahara C, Obata D, Sakai A, Ishida T, et al. Endoscopic submucosal dissection for early gastric cancer in anastomosis site after distal gastrectomy. Gastric Cancer. 2014;17:371–376. doi: 10.1007/s10120-013-0283-5. [DOI] [PubMed] [Google Scholar]
- 64.Okamoto K, Okamura S, Muguruma N, Kitamura S, Kimura T, Imoto Y, Miyamoto H, Okahisa T, Takayama T. Endoscopic submucosal dissection for early gastric cancer using a cross-counter technique. Surg Endosc. 2012;26:3676–3681. doi: 10.1007/s00464-012-2364-7. [DOI] [PubMed] [Google Scholar]
- 65.Watari J, Tomita T, Toyoshima F, Sakurai J, Kondo T, Asano H, Yamasaki T, Okugawa T, Ikehara H, Oshima T, et al. Clinical outcomes and risk factors for perforation in gastric endoscopic submucosal dissection: A prospective pilot study. World J Gastrointest Endosc. 2013;5:281–287. doi: 10.4253/wjge.v5.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sumiyama K, Toyoizumi H, Ohya TR, Dobashi A, Hino S, Kobayashi M, Goda K, Imazu H, Kawakita Y, Kato T, et al. A double-blind, block-randomized, placebo-controlled trial to identify the chemical assistance effect of mesna submucosal injection for gastric endoscopic submucosal dissection. Gastrointest Endosc. 2014;79:756–764. doi: 10.1016/j.gie.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 67.Kusano T, Etoh T, Akagi T, Ueda Y, Shiroshita H, Yasuda K, Satoh M, Inomata M, Shiraishi N, Kitano S. Evaluation of 0.6% sodium alginate as a submucosal injection material in endoscopic submucosal dissection for early gastric cancer. Dig Endosc. 2014;26:638–645. doi: 10.1111/den.12268. [DOI] [PubMed] [Google Scholar]
- 68.Kawamura M, Sekine H, Kikuchi T, Sakai Y, Nagasaki F, Naganuma H, Shibuya R, Ando M. Endoscopic submucosal dissection for gastric neoplasms by using a novel attachment device-a one-sided, expandable balloon. Gastrointest Endosc. 2011;74:415–418. doi: 10.1016/j.gie.2011.03.1247. [DOI] [PubMed] [Google Scholar]
- 69.Lee TH, Cho JY, Chang YW, Kim JO, Lee JS, Cho WY, Kim HG, Kim WJ, Park YS, Jin SY. Appropriate indications for endoscopic submucosal dissection of early gastric cancer according to tumor size and histologic type. Gastrointest Endosc. 2010;71:920–926. doi: 10.1016/j.gie.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 70.Kim BJ, Chang TH, Kim JJ, Min BH, Lee JH, Son HJ, Rhee PL, Rhee JC, Kim KM, Park CK. Efficacy and safety of endoscopic submucosal dissection for early gastric cancer in patients with comorbid diseases. Gut Liver. 2010;4:186–191. doi: 10.5009/gnl.2010.4.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shin KY, Jeon SW, Cho KB, Park KS, Kim ES, Park CK, Chung YJ, Kwon JG, Jung JT, Kim EY, et al. Clinical outcomes of the endoscopic submucosal dissection of early gastric cancer are comparable between absolute and new expanded criteria. Gut Liver. 2015;9:181–187. doi: 10.5009/gnl13417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jang JS, Choi SR, Qureshi W, Kim MC, Kim SJ, Jeung JS, Han SY, Noh MH, Lee JH, Lee SW, et al. Long-term outcomes of endoscopic submucosal dissection in gastric neoplastic lesions at a single institution in South Korea. Scand J Gastroenterol. 2009;44:1315–1322. doi: 10.3109/00365520903254304. [DOI] [PubMed] [Google Scholar]
- 73.Kim DY, Hong SJ, Cho GS, Jeong GA, Kim HK, Han JP, Lee YN, Ko BM, Lee MS. Long-term efficacy of endoscopic submucosal dissection compared with surgery for early gastric cancer: a retrospective cohort study. Gut Liver. 2014;8:519–525. doi: 10.5009/gnl13061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509–516. doi: 10.1007/s00464-009-0614-0. [DOI] [PubMed] [Google Scholar]
- 75.Goh PG, Jeong HY, Kim MJ, Eun HS, Kim HJ, Kim ES, Kim YJ, Lee SY, Moon HS, Lee ES, et al. Clinical outcomes of endoscopic submucosal dissection for undifferentiated or submucosal invasive early gastric cancer. Clin Endosc. 2011;44:116–122. doi: 10.5946/ce.2011.44.2.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ahn JY, Choi KD, Choi JY, Kim MY, Lee JH, Choi KS, Kim DH, Song HJ, Lee GH, Jung HY, et al. Procedure time of endoscopic submucosal dissection according to the size and location of early gastric cancers: analysis of 916 dissections performed by 4 experts. Gastrointest Endosc. 2011;73:911–916. doi: 10.1016/j.gie.2010.11.046. [DOI] [PubMed] [Google Scholar]
- 77.Yoo JH, Shin SJ, Lee KM, Choi JM, Wi JO, Kim DH, Lim SG, Hwang JC, Cheong JY, Yoo BM, et al. Risk factors for perforations associated with endoscopic submucosal dissection in gastric lesions: emphasis on perforation type. Surg Endosc. 2012;26:2456–2464. doi: 10.1007/s00464-012-2211-x. [DOI] [PubMed] [Google Scholar]
- 78.Lim CH, Park JM, Park CH, Cho YK, Lee IS, Kim SW, Choi MG, Chung IS. Endoscopic submucosal dissection of gastric neoplasia involving the pyloric channel by retroflexion in the duodenum. Dig Dis Sci. 2012;57:148–154. doi: 10.1007/s10620-011-1863-z. [DOI] [PubMed] [Google Scholar]
- 79.Park CH, Shin S, Park JC, Shin SK, Lee SK, Lee YC, Lee H. Long-term outcome of early gastric cancer after endoscopic submucosal dissection: expanded indication is comparable to absolute indication. Dig Liver Dis. 2013;45:651–656. doi: 10.1016/j.dld.2013.01.014. [DOI] [PubMed] [Google Scholar]
- 80.Chung MW, Jeong O, Park YK, Lee KH, Lee JH, Lee WS, Joo YE, Choi SK, Cho SB. [Comparison on the long term outcome between endoscopic submucosal dissection and surgical treatment for undifferentiated early gastric cancer] Korean J Gastroenterol. 2014;63:90–98. doi: 10.4166/kjg.2014.63.2.90. [DOI] [PubMed] [Google Scholar]
- 81.Kim MN, Kim HK, Shim CN, Lee HJ, Lee H, Park JC, Shin SK, Lee SK, Lee YC. Tumour size is related to the curability of signet ring cell early gastric cancer with endoscopic submucosal dissection: a retrospective single centre study. Dig Liver Dis. 2014;46:898–902. doi: 10.1016/j.dld.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 82.Min BH, Kim KM, Park CK, Lee JH, Rhee PL, Rhee JC, Kim JJ. Outcomes of endoscopic submucosal dissection for differentiated-type early gastric cancer with histological heterogeneity. Gastric Cancer. 2015;18:618–626. doi: 10.1007/s10120-014-0378-7. [DOI] [PubMed] [Google Scholar]
- 83.Kim HH, Park SJ, Park MI, Moon W. Clinical impact of second-look endoscopy after endoscopic submucosal dissection of gastric neoplasms. Gut Liver. 2012;6:316–320. doi: 10.5009/gnl.2012.6.3.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yoon JY, Shim CN, Chung SH, Park W, Chung H, Lee H, Shin SK, Lee SK, Lee YC, Park JC. Impact of tumor location on clinical outcomes of gastric endoscopic submucosal dissection. World J Gastroenterol. 2014;20:8631–8637. doi: 10.3748/wjg.v20.i26.8631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Choi CW, Kim HW, Kang DH, Hong YM, Kim SJ, Park SB, Cho M, Kim DJ, Hong JB. Clinical outcomes of second-look endoscopy after gastric endoscopic submucosal dissection: predictive factors with high risks of bleeding. Surg Endosc. 2014;28:2213–2220. doi: 10.1007/s00464-014-3457-2. [DOI] [PubMed] [Google Scholar]
- 86.Chun SY, Kim KO, Park DS, Lee IJ, Park JW, Moon SH, Baek IH, Kim JH, Park CK, Kwon MJ. Endoscopic submucosal dissection as a treatment for gastric subepithelial tumors that originate from the muscularis propria layer: a preliminary analysis of appropriate indications. Surg Endosc. 2013;27:3271–3279. doi: 10.1007/s00464-013-2904-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chung WC, Kim BW, Lim CH, Kim TH, Park JM, Kim JS. Grasper type scissors for endoscopic submucosal dissection of gastric epithelial neoplasia. World J Gastroenterol. 2013;19:6221–6227. doi: 10.3748/wjg.v19.i37.6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim JS, Chung MW, Chung CY, Park HC, Ryang DY, Myung DS, Cho SB, Lee WS, Joo YE. The need for second-look endoscopy to prevent delayed bleeding after endoscopic submucosal dissection for gastric neoplasms: a prospective randomized trial. Gut Liver. 2014;8:480–486. doi: 10.5009/gnl13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Białek A, Wiechowska-Kozłowska A, Pertkiewicz J, Polkowski M, Milkiewicz P, Karpińska K, Ławniczak M, Starzyńska T. Endoscopic submucosal dissection for treatment of gastric subepithelial tumors (with video) Gastrointest Endosc. 2012;75:276–286. doi: 10.1016/j.gie.2011.08.029. [DOI] [PubMed] [Google Scholar]
- 90.Dinis-Ribeiro M, Pimentel-Nunes P, Afonso M, Costa N, Lopes C, Moreira-Dias L. A European case series of endoscopic submucosal dissection for gastric superficial lesions. Gastrointest Endosc. 2009;69:350–355. doi: 10.1016/j.gie.2008.08.035. [DOI] [PubMed] [Google Scholar]
- 91.Lee IL, Wu CS, Tung SY, Lin PY, Shen CH, Wei KL, Chang TS. Endoscopic submucosal dissection for early gastric cancers: experience from a new endoscopic center in Taiwan. J Clin Gastroenterol. 2008;42:42–47. doi: 10.1097/01.mcg.0000225696.54498.ff. [DOI] [PubMed] [Google Scholar]
- 92.Chang CC, Lee IL, Chen PJ, Wang HP, Hou MC, Lee CT, Chen YY, Cho YP, Lin JT. Endoscopic submucosal dissection for gastric epithelial tumors: a multicenter study in Taiwan. J Formos Med Assoc. 2009;108:38–44. doi: 10.1016/S0929-6646(09)60030-9. [DOI] [PubMed] [Google Scholar]
- 93.Chu YY, Lien JM, Tsai MH, Chiu CT, Chen TC, Yang KC, Ng SC. Modified endoscopic submucosal dissection with enucleation for treatment of gastric subepithelial tumors originating from the muscularis propria layer. BMC Gastroenterol. 2012;12:124. doi: 10.1186/1471-230X-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.González N, Parra-Blanco A, Villa-Gómez M, Gamba A, Taullard A, Silveira A, Sanguinetti A, Olano C, Cohen H. Gastric endoscopic submucosal dissection: from animal model to patient. World J Gastroenterol. 2013;19:8326–8334. doi: 10.3748/wjg.v19.i45.8326. [DOI] [PMC free article] [PubMed] [Google Scholar]


