Abstract
Children with Attention Deficit/Hyperactivity Disorder (ADHD) have poorer neuropsychological functioning relative to their typically-developing peers. However, it is unclear whether early neuropsychological functioning predicts later ADHD severity and/or the latter is longitudinally associated with subsequent neuropsychological functioning; and whether these relations are different in children with and without early symptoms of ADHD. This study aimed to examine the longitudinal associations between ADHD severity and neuropsychological functioning among children at high and low risk of developing ADHD. Hyperactive/Inattentive (H/I; N=140) and Typically-developing (TD; N=76) preschoolers (age 3 – 4 years) were recruited (BL) and followed annually for 3 years (F1, F2 and F3). Teachers rated the children’s ADHD severity and impairment using the Behavior Assessment System for Children-2 and the Children’s Problem Checklist, respectively. Parent reports of children’s ADHD severity were obtained using the Kiddie-Schedule for Affective Disorders and Schizophrenia – Present and Lifetime version. Neuropsychological functioning was assessed using the NEPSY. In the full sample, there were bi-directional longitudinal associations between neuropsychological functioning and ADHD severity between F1 and F3. Among H/I children, neuropsychological functioning at F1 and F2 predicted ADHD severity at F2 and F3, respectively. In contrast, among TD children the only significant relationship observed was that elevated ADHD symptoms at F2 were associated with poorer neuropsychological functioning at F3. Improved neuropsychological functioning may attenuate ADHD symptoms and associated impairment among H/I children during the early school years. Interventions designed to improve neuropsychological functioning among young H/I children may be beneficial in reducing their ADHD severity.
Keywords: ADHD, development, neuropsychological functioning, longitudinal research
Attention-Deficit/Hyperactivity Disorder (ADHD) is an early-emerging, chronic syndrome defined by developmentally inappropriate levels of inattention and hyperactivity/impulsivity with impairment in at least two settings (American Psychiatric Association, 2000). Research into the causes of ADHD provides compelling evidence for a substantial role of genes in the emergence (Faraone et al., 2005) and persistence (Larsson, Larsson, & Lichtenstein, 2004) of these maladaptive behaviors. However, simple genetic models cannot adequately account for the etiology of ADHD. Accumulating evidence suggests a complex interplay among genes, prenatal environment, and postnatal environment, which come together to influence brain development and produce the ADHD phenotype (Halperin, Bedard, & Curchack-Lichtin, 2012; Nigg, Nikolas, & Burt, 2010; Sonuga-Barke & Halperin, 2010). From this perspective, gene-by-environment interactions influence early brain development, such that by birth or shortly thereafter, the stage is set for ADHD phenotypes to emerge. Yet, the manner and extent to which such phenotypes are expressed throughout development is, to a considerable degree, determined by postnatal factors that continue to influence neural development (Halperin & Schulz, 2006; Halperin et al., 2012; Sonuga-Barke et al., 2010). As such, it is likely that variations in neural structure and function throughout development play a determining role in the extent to which symptoms worsen or diminish over time.
Consistent with this developmental perspective, remission of ADHD has been linked to the extent to which cortical thickening, which is developmentally delayed in ADHD children (Shaw et al., 2007), comes into alignment with that of typically-developing (TD) peers (Giedd & Rapoport, 2010; Proal et al., 2011; Shaw et al., 2007). Similarly, neuropsychological (Bedard, Trampush, Newcorn, & Halperin, 2010; Fischer, Barkley, Smallish, & Fletcher, 2005; Halperin, Trampush, Miller, Marks, & Newcorn, 2008) and functional neuroimaging (Schulz, Newcorn, Fan, Tang, & Halperin, 2005) data suggest that adolescents and young adults in whom childhood ADHD has largely remitted are more similar to their TD peers than those whose ADHD symptoms have persisted (but see van Lieshout, Luman, Buitelaar, Rommelse, & Oosterlaan, 2013; Vaughn et al., 2011). Most recently, cue-related functional connectivity was found to be greater between thalamus and prefrontal regions in ADHD-remitters relative to ADHD-persisters (Clerkin et al., in press). Further, using dimensional analyses, changes in global executive functioning and response inhibition, but not all executive function measures, predicted changes in ADHD symptom severity in women with childhood ADHD (Miller, Loya, & Hinshaw, 2013), and we found over a 4- to 5-year period, a close association between neuropsychological and behavioral trajectories in hyperactive/inattentive (H/I) preschool children (Rajendran et al., in press).
Thus, emerging evidence provides support for the notion that ADHD severity and brain development are linked, and that developmental variations in ADHD phenotypic presentation are associated with variability in early cognitive and neural development. Unfortunately, simultaneous tests of cross-lagged associations between ADHD severity and neuropsychological functioning over time among TD and H/I children have not been conducted, and existing correlational studies cannot provide data regarding the longitudinal relations between neural/neurocognitive development and behavioral change over time. While it has been postulated that enhanced neural development leads to a diminution of ADHD symptomatology (Giedd et al., 2010; Halperin et al., 2006), it is equally plausible that neuropsychological deficits (Carr, Nigg, & Henderson, 2006) and perhaps even deviant cortical thickness, are secondary to the presence of ADHD symptoms. From this latter perspective, children with ADHD may perform more poorly on cognitive tests because of their inattention and impulsiveness. Further, given that cortical development is highly sensitive to environmental stimulation (Farah et al., 2008; Halperin & Healey, 2011), diminished environmental stimulation due to inattentiveness among children with ADHD may be associated with attenuated cortical development. Thus, the extent to which variability in neural or neurocognitive development directly influences the trajectory of ADHD or vice versa has not been systematically evaluated. Yet, such knowledge could have a substantial impact on the development of early interventions, particularly those focusing on enhancing neural development (Halperin et al., 2012; Tamm, Nakonezny, & Hughes, 2012).
The primary aim of this study was to determine whether neuropsychological variations precede or follow symptom changes during early childhood. A sample of preschool children was recruited for being at high (H/I group) or low risk (TD group) of developing ADHD, and prospectively studied over a 3-year period. Cross-lagged panel analyses were conducted to elucidate whether neuropsychological development preceded or followed changes in ADHD severity. We also examined whether H/I and TD children differed in the association between neuropsychological functioning and ADHD severity over time. Given that ADHD symptoms exist on a continuum (Lubke, Hudziak, Derks, van Bijsterveldt, & Boomsma, 2009; Polderman et al., 2007) it is possible that changes in neuropsychological functioning and ADHD severity are associated in both H/I and TD children. However, children with ADHD show greater anomalies in cortical (Shaw et al., 2007), and neuropsychological (Frazier, Demaree, & Youngstrom, 2004; Willcutt, Doyle, Nigg, Faraone, & Pennington, 2005) development, and more behavioral impairments. Thus one may expect that relations between changes in behavioral and neuropsychological functioning would be easier to detect in the H/I group. In sum, this study explored competing hypotheses regarding the temporal relations between neuropsychological functioning and ADHD severity, and whether these associations would be evident both among H/I and TD children.
Methods and Materials
Participants
Children (N=216) from the New York metropolitan area were recruited via preschools and direct referrals into a longitudinal study when they were 3 – 4 years old (Baseline; BL) and followed up annually for three subsequent assessments (F1, F2, & F3). Parents and teachers completed the Attention-Deficit/Hyperactivity Disorder Rating Scale, Fourth Edition (ADHD-RS-IV; DuPaul, Power, Anastopoulos, & Reid, 1998). Children were accepted into the study as either TD (fewer than 3 items rated as Often or Very Often by both parents and teachers; N=76) or H/I (at least 6 symptoms rated as Often or Very Often by either parent or teacher; N=140). Children were excluded if they had a Full-Scale IQ less than 80, as measured by the Wechsler Preschool and Primary Scale of Intelligence, 3rd Edition (WPPSI-III; Wechsler, 2002), a pervasive developmental or neurological disorder, were taking systemic medications, including stimulants for ADHD, or if they or their parents were not fluent in English.
The BL sample [M (SD) age = 4.31 (0.47) years; 72.7% boys] was ethnically and racially diverse: White (N=126; 58.3%), Black (N=27, 12.5%), Asian (N=23; 10.6%), and mixed race (N=40, 18.6%); 68 (31.5%) were Hispanic.
Although all children were medication naïve at BL, 10, 21, and 23 children were taking ADHD medications at F1, F2, and F3, respectively. Parents were asked to rate children’s behavior while not on medication, either in the evening when it had worn off or over the weekend. Stimulant and non-stimulant medications for ADHD, but not neuroleptics or anti-depressants, were withheld the day of the assessment.
There were no differences between TD and H/I groups on gender, ethnicity, or race (all p >.10). However, although both groups were well within the middle class range, they differed on family SES (see measures below) at BL [Mean (SD) TD=69.16 (14.89); H/I= 61.28 (17.87); p=.001].
Procedure
Parent and teacher ratings of children’s ADHD severity and impairment were collected, and neuropsychological assessments of children were conducted at BL-F3. The Mean (SD) interval in months between BL and F1, F1 and F2, and F2 and F3 was 11.99 (1.21); 11.65 (1.69); 11.80 (1.51) months. This study was approved by the Institutional Review Board (IRB). Following a full description of the study, all parents signed IRB-approved informed-consent forms.
Measures
ADHD severity and impairment
Three different measures were used to assess ADHD severity and impairment. First, the Kiddie Schedule for Affective Disorders and Schizophrenia-Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, Rao, & Ryan, 1996), a semi-structured parent interview was used to assess ADHD severity in children. Evaluators were either Ph.D. level psychologists or doctoral students trained in psychopathology and supervised by a clinical psychologist. Scores on the 18 ADHD symptoms based on DSM-IV, were coded on a 3-point scale (not present, sub-threshold, or present with impairment) and summed to obtain an overall score with higher scores indicating greater severity. Cronbach’s alphas at BL, F1, F2, and F3 were .96, .95, .94, and .94 respectively.
Second, teacher reports on the Children’s Problem Checklist (CPC; Healey, Miller, Castelli, Marks, & Halperin, 2008) were used to assess impairment related to ADHD using six items (disrupts classroom, difficulty getting along with children at school, difficulty making or keeping friends, difficulty getting along with teachers and/or other adults, feeling bad about him/herself, and having many accidents). Each item was rated on a 4-point scale (no, mild, moderate, or severe problems). Internal consistency in the normative sample was .70 and stability at 6-month follow-up was .69 (Healey et al., 2008). In this sample, Cronbach’s alphas at BL, F1, F2, and F3 were .87, .80, .85, and .85, respectively.
Finally, the Behavior Assessment System for Children-2 (BASC-2; Reynolds & Kamphaus, 2002) was also used to assess ADHD severity in children. T-scores of teacher reports on the Hyperactivity and Attention Problems subscales were summed to derive a measure of ADHD severity. The BASC-2 has good reliability and validity for both clinical and normative samples (Reynolds & Kamphaus, 2002). In our sample, Cronbach’s alphas at BL, F1, F2, and F3 were .75, .75, .72, and .86, respectively.
Neuropsychological functioning
The Developmental Neuropsychological Assessment (NEPSY; Korkman, Kirk, & Kemp, 1998) was used to assess neuropsychological functioning in five domains: Attention/Executive functioning, Language, Visuospatial, Sensorimotor, and Memory. The NEPSY was used as a measure of neuropsychological functioning because at the time this study was initiated, it was the only broad-based standardized test battery of neuropsychological functioning that would allow for continuity of measurement between preschool and school-age. In addition, it provides age- and gender-specific norms that enable comparison to other samples of children. Test-retest reliability for the domains ranged from .70 to .91 (Korkman et al., 1998). In the present sample, Cronbach’s alphas of the five domains at BL, F1, F2, and F3 were .72, .78, .75, and .73, respectively.
Socioeconomic status
The Nakao-Treas Socioeconomic Prestige Index (SES; Nakao & Treas, 1994) was used to measure SES at BL. High scores on this index are indicative of higher SES. In two-parent families, scores for both parents were separately coded and the higher of the two scores adopted as the index of family SES.
Missing Data
Retention rates at F1, F2, and F3 were 87.04%, 81.50% and 73.61%, respectively. We conducted attrition analysis to test whether children at each time-point who did and did not have data differed. Out of 48 contrasts, only age of the child at BL was associated with the presence/absence of data on NEPSY at F3 (Mean[SD] with and without data, respectively: 4.18[.51] vs. 4.42[.39] years; F (1,214) =14.89; p<.001). Thus, data were considered to be missing at random (MAR). We employed a full information maximum likelihood (FIML) method, which may provide less biased estimates than list-wise or pair-wise deletion (Arbuckle, 1996; Enders & Bandalos, 2001). Additional data (23.61%) on NEPSY at F3 was missing as this measure was temporarily dropped from the study. A model-based stochastic regression imputation strategy was employed to assign values to these variables within the multi-group model framework. This form of imputation preserves the variability of the data and yields unbiased parameter estimates when the data are MAR (Little & Rubin, 2002; Enders, 2010).
Data Analysis
Univariate indices of normality revealed no significant skewness or kurtosis except for scores on the CPC for TD children (skewness and kurtosis ranged from 2.13–10.30). This variable was log transformed for subsequent analysis. Given the significant differences between groups on SES, this variable was included as a covariate in all analysis. Although IQ differed between the TD and H/I children, this was not included in the primary analyses because cognitive deficits may be a part of the profile of a neurodevelopmental disorder such as ADHD (Frazier et al., 2004) and adding it as a covariate may produce overcorrected and inconsistent findings (Dennis et al., 2009). After examining correlations we conducted structural equation modeling (SEM) fitting latent variables of the NEPSY (five domain scores) with latent variables of ADHD severity (parent report on K-SADS and teacher reports on BASC-2 and CPC) in a cross-lagged framework using the program AMOS 18. A non-significant chi square, Comparative Fit Index (CFI) >.95, and Root Mean Square Error of Approximation (RMSEA) <.08 were considered indices of good fit (Hu & Bentler, 1999; Kline, 2005). As the chi square statistic is known to be sensitive to minor deviations from a perfectly causal model (Byrne, 2001; Schumacker & Lomax, 2004) a χ2/df ratio below 3 was considered acceptable (Carmines & McIver, 1981). Measurement error of each variable at a given time-point was allowed to co-vary with the measurement error of that variable at every other time-point to account for shared variance associated with each indicator (Pitts, West, & Tein, 1996). After deriving an acceptable SEM model with the full sample, we ran a multi-group model to examine differences in longitudinal associations between H/I and TD groups. A fully constrained model with every path held constant across groups was used to formally test significance of difference in each path.
Results
The TD children had lower ADHD severity and better neuropsychological functioning (Table 1). There were several significant inverse correlations between NEPSY domains and ADHD symptoms and impairment (Table 2). Family SES was associated with ADHD severity and NEPSY scores at most time points in expected directions (r-range: .01 to .28; p-range .86 to <.001). Finally, there were several significant correlations between the same constructs over time (e.g., ADHD severity was associated over time; r ranging from .41 to .82; all p<.001).
Table 1.
Mean levels of Neuropsychological functioning, ADHD symptoms and Impairment
| Variable | TD Mean (SD) N=76 |
H/I Mean (SD) N=140 |
F | Cohen’s D |
|---|---|---|---|---|
| Baseline (BL) | ||||
| Attention/Executive | 107.01 (12.60) | 95.09 (11.83) | 46.63*** | 0.97 |
| Language | 105.40 (11.17) | 98.13 (12.45) | 17.73** | 0.61 |
| Sensorimotor | 98.15 (14.64) | 89.86 (14.45) | 15.53*** | 0.57 |
| Visuospatial | 111.64 (11.77) | 103.13 (12.43) | 23.34 *** | 0.70 |
| Memory | 97.93 (13.95) | 90.56 (15.09) | 12.06 *** | 0.51 |
| K-SADS | 4.62 (5.49) | 25.87(6.41) | 596.85*** | 3.58 |
| BASC-2 | 89.11 (12.75) | 128.02 (21.15) | 205.78*** | 2.23 |
| CPC | 0.50 (1.04) | 7.33 (5.54) | 110.07*** | 1.71 |
| Follow up 1 (F1) | ||||
| Attention/Executive | 105.59 (12.95) | 99.72 (14.59) | 7.62 ** | 0.42 |
| Language | 106.28 (15.73) | 99.78 (17.07) | 6.77* | 0.40 |
| Sensorimotor | 100.17 (13.93) | 90.72 (15.25) | 17.98 *** | 0.65 |
| Visuospatial | 113.27 (14.40) | 105.10 (14.05) | 14.59 *** | 0.57 |
| Memory | 102.42 (14.54) | 96.99 (16.80) | 5.08* | 0.35 |
| K-SADS | 5.80 (5.80) | 22.43 (8.74) | 202.59*** | 2.24 |
| BASC-2 | 90.58 (15.20) | 119.10 (22.46) | 78.28*** | 1.49 |
| CPC | 0.97 (1.89) | 4.37 (4.04) | 39.88*** | 1.08 |
| Follow up 2 (F2) | ||||
| Attention/Executive | 115.09 (14.12) | 109.74 (16.51) | 4.86 * | 0.35 |
| Language | 108.66 (17.93) | 100.92 (16.31) | 8.65** | 0.45 |
| Sensorimotor | 106.50 (13.44) | 93.54 (15.15) | 33.07 *** | 0.90 |
| Visuospatial | 116.66 (16.24) | 105.67 (15.81) | 19.60*** | 0.68 |
| Memory | 112.51 (15.97) | 106.29 (16.11) | 6.13* | 0.39 |
| K-SADS | 7.84 (7.36) | 22.37 (9.78) | 111.49*** | 1.68 |
| BASC-2 | 95.51 (16.91) | 118.70 (21.61) | 48.28 *** | 1.19 |
| CPC | 1.29 (2.76) | 5.01 (4.69) | 30.85 *** | 0.97 |
| Follow up 3 (F3) | ||||
| Attention/Executive | 122.73 (8.69) | 112.25(13.45) | 18.40 *** | 0.92 |
| Language | 113.11 (18.50) | 104.89(16.96) | 5.37* | 0.46 |
| Sensorimotor | 106.92 (15.37) | 95.06(14.22) | 16.01*** | 0.80 |
| Visuospatial | 118.49 (16.87) | 109.76(15.58) | 7.20** | 0.54 |
| Memory | 115.65(14.69) | 109.90(17.13) | 3.00† | 0.36 |
| K-SADS | 6.75 (7.08) | 22.44 (9.91) | 120.01*** | 1.82 |
| BASC-2 | 94.82 (13.88) | 117.53 (21.34) | 45.46 *** | 1.26 |
| CPC | 0.87 (1.63) | 4.85 (4.85) | 29.11*** | 1.10 |
Ns may vary due to missing data; SD= Standard Deviation; TD= Typically Developing; H/I= Hyperactive/Inattentive; K-SADS= Kiddie-Schedule for Affective Disorders; BASC-2= Behavioral Assessment System for Children-2; CPC= Children’s Problem Checklist Differences between TD and H/I group:
p<.001,
p<.01,
p<.05,
<.10.
Table 2.
Correlations between Neuropsychological functioning and ADHD symptoms and Impairment (N=216)
| K-SADS-PL | BASC-2 | CPC | K-SADS-PL | BASC-2 | CPC | K-SADS-PL | BASC-2 | CPC | K-SADS-PL | BASC-2 | CPC | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline (BL) | Follow up 1 (F1) | Follow up 2 (F2) | Follow up 3 (F3) | |||||||||
| Baseline (BL) | ||||||||||||
| Attention/Executive | −.44** | −.40** | −.35** | −.28** | −.27** | −.23** | −.40** | −.23** | −.23** | −.39** | −.21** | −.25** |
| Language | −.34** | −.33** | −28** | −.15* | −.26** | −.26** | −.26** | −.25** | −.19* | −.19* | −.22* | −.14† |
| Sensorimotor | −.32** | −.26** | −.26** | −.23** | −.17* | −.20* | −.24** | −.16† | −.13 | −.26** | −.24** | −.25** |
| Visuo-Spatial | −.37** | −.29** | −.29** | −.30** | −.27** | −.30** | −.32** | −.24** | −.22** | −.32** | −.28** | −.32** |
| Memory | −.31** | −.31** | −.24** | −.08 | −.23** | −.21** | −.27** | −.17* | −.16 | −.15 | −.10 | −.13 |
| Follow up 1 (F1) | ||||||||||||
| Attention/Executive | −.24** | −.23** | −.24** | −.23** | −.22** | −.26** | −.25** | −.21** | −.23** | −.25** | −.26** | −.31** |
| Language | −.23* | −.19* | −.20** | −.15* | −.20** | −.18* | −.20** | −.22* | −.22** | −.23** | −.20* | −.15 |
| Sensorimotor | −.34** | −.28** | −.31** | −.37** | −.27** | −.30** | −.34** | −.27** | −.37** | −.32** | −.36** | −.28** |
| Visuo-Spatial | −.35** | −.25** | −.20** | −.32** | −.21** | −.25** | −.27** | −.37** | −.37** | −.31** | −.37** | −.30** |
| Memory | −.23** | −.20** | −.21** | −.17* | −.12 | −.13 | −.20** | −.17* | −.19* | −.13 | −.05 | −.09 |
| Follow up 2 (F2) | ||||||||||||
| Attention/Executive | −.23** | −.24** | −.20** | −.24** | −.16† | −.09 | −.18* | −.22** | −.21** | −.28** | −.25** | −.28** |
| Language | −.25** | −.18** | −.20** | −.30** | −.21** | −.21* | −.22** | −.28** | −.28** | −.30** | −.27** | −.24** |
| Sensorimotor | −.40** | −.37** | −.34** | −.46** | −.36** | −.30** | −.39** | −.34** | −.35** | −.47** | −.44** | −.39** |
| Visuo-Spatial | −.36** | −.32** | −.28** | −.44** | −.34** | −.29** | −.33** | −.38** | −.42** | −.39** | −.34** | −.35** |
| Memory | −.18* | −.16* | −.17* | −.24** | −.17* | −.18* | −.11 | −.22** | −.21* | −.16 | −.08 | −.05 |
| Follow up 3 (F3) | ||||||||||||
| Attention/Executive | −.47** | −.48** | −.40** | −.48** | −.34** | −.29** | −.47** | −.32** | −.33** | −.56** | −.36** | −.33** |
| Language | −.32** | −.31** | −.27** | −.31** | −.27** | −.24** | −.28** | −.34** | −.27** | −.32** | −.28** | −.23** |
| Sensorimotor | −.35** | −.28** | −.30** | −.38** | −.26** | −.31** | −.37** | −.25** | −.44** | −.40** | −.27** | −.31** |
| Visuo-Spatial | −.35** | −.30** | −.28** | −.40** | −.31** | −.27** | −.28** | −.39** | −.40** | −.32** | −.30** | −.30** |
| Memory | −.20** | −.19** | −.15** | −.28** | −.17* | −.17* | −.18* | −.27** | −.23** | −.31** | −.15† | −.15† |
Ns may vary due to missing data. K-SADS-PL= Kiddie-Schedule for Affective Disorders; BASC-2= Behavioral Assessment System for Children-2; CPC= Children’s Problem Checklist
p<.01;
p<.05;
p<.10.
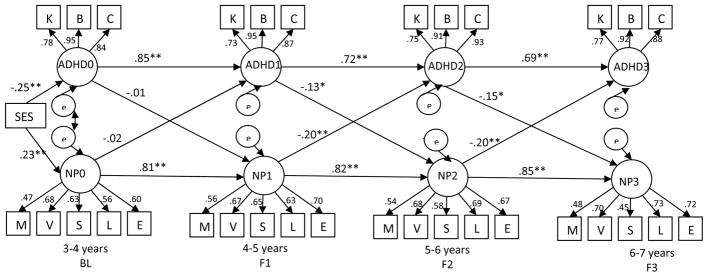
The cross-lagged model including the full sample (Figure 1) showed acceptable fit [χ2(df)= 785.05(437); p<.001; χ2/df ratio = 1.79; CFI= .92; RMSEA=.06]. An inspection of residuals showed no points of ill fit. There were significant cross-lagged relations between neuropsychological functioning and ADHD severity between F1 and F2, and between F2 and F3. However, BL ADHD severity and neuropsychological functioning were not significantly associated with F1 neuropsychological functioning and ADHD severity, respectively. Therefore, we trimmed the model to exclude BL ADHD severity and BL NEPSY scores. This model [χ2 (df)= 356.93(241); p<.001; χ2/df ratio = 1.48; CFI= .96; RMSEA=.05] showed significant cross-paths between ADHD symptom severity and neuropsychological functioning, and vice versa at each time-point.
Figure 1.
Cross-lagged model linking ADHD Symptoms and Impairment with Neuropsychological Functioning
Note: χ2(df)= 785.05(437); p<.001; χ2/df ratio = 1.79; CFI= .92; RMSEA=.06; K= K-SADS(parent), B= BASC-2(teacher), C= CPC (teacher), M=Memory, V=Verbal, S=Sensory Motor, L= Language; E=Attention/Executive;
ADHD0, ADHD1, ADHD2 and ADHD3 refer to ADHD symptoms and impairment; NP0, NP1, NP2 and NP3 refer to neuropsychological functioning on the NEPSY at each time point
All indicators load on to their respective factors at p<.001; ** p<.01 *p<.05
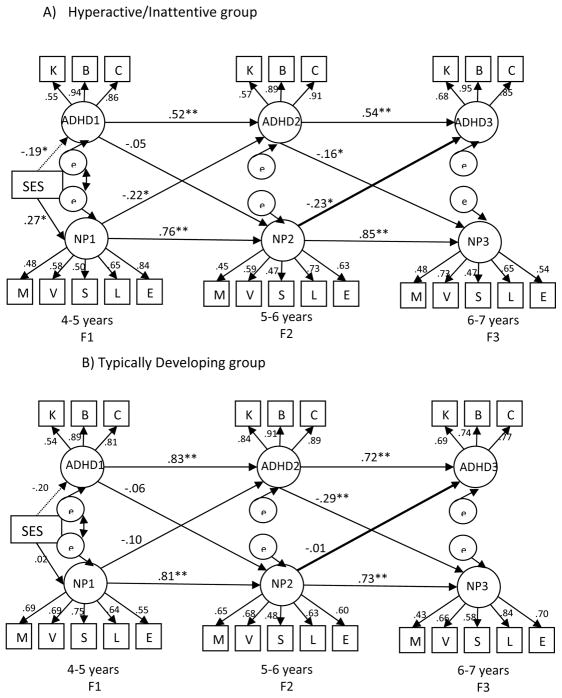
The multi-group model (Figure 2) exhibited good fit [χ2 (df)= 612.99(482); p<.001; χ2/df ratio = 1.27; CFI= .94; RMSEA=.04]. Among H/I children (Figure 2A), F1 and F2 neuropsychological functioning were associated with F2 and F3 ADHD severity (β=−.22 and −.23 respectively; p<.05), respectively. Elevated ADHD severity at F2 was associated with subsequently worse neuropsychological functioning at F3 (β= −.16; p<.05). Lower BL SES was significantly associated with poorer neuropsychological functioning and with more severe ADHD severity at F1. All paths leading-up to the time-point explained 7% (F1), 62% (F2), and 83% (F3) of the variance in neuropsychological functioning. At F2 and F3, this included the variance explained by neuropsychological functioning at F1 and F2 respectively. Similarly, paths leading up to ADHD severity explained 4% (F1), 37% (F2), and 42% (F3) of the variance in ADHD severity. At F2 and F3, this included the variance explained by ADHD severity at F1 and F2 respectively. Among TD children (Figure 2B), the only cross-lagged path that attained significance was that greater ADHD severity at F2 was associated with subsequently worse neuropsychological functioning at F3 (β= −.29; p<.01).
Figure 2.
Multi-group Model
Note: χ2(df) =612.99 (482); p<.001; χ2/df ratio =1.27; CFI= .94; RMSEA=.04;
Bold line between F2 neuropsychological functioning and F3 ADHD severity shows that the path was significantly different between the two groups.
K= K-SADS (parent), B= BASC-2 (teacher), C= CPC (teacher), M=Memory, V=Verbal, S=Sensory Motor, L= Language; E=Executive/Attention; ADHD1, ADHD2 and ADHD3 refer to ADHD symptoms and impairment; NP1, NP2, and NP3 refer to neuropsychological functioning; All indicators load on to their respective factors at p<.001; ** p<.01 *p<.05
Constrained Multi-Group Model and Comparison of Paths
To test whether the paths differed significantly between TD and H/I groups, we imposed invariance in the paths and factors across the two groups. The fit of this model was: χ2 (510) = 678.35; p<.001; χ2/df ratio=1.33; CFI= .93; RMSEA=.04. The chi square difference between the equal form model and the scale invariant model was statistically significant [Difference χ2 (df) = 65.36(28); p<.01]. This led us to reject the null hypothesis of equality between the paths in the TD and H/I children.
We then tested the statistical difference of each cross-lagged path by freeing one path at a time while holding all other paths and factor loadings invariant. The only significant difference in cross-lagged paths was between neuropsychological functioning at F2 and ADHD severity at F3 (p<.05). This path was significant among H/I children but not among TD children.
Post hoc analysis
An additional SEM model was run to test whether medication treatment was a potential confounder for the longitudinal relations between neuropsychological functioning and ADHD severity observed among H/I children. Two dichotomous variables (medication treatment at F1 and at F2) were included. Paths were tested from medication treatment at F1 leading up to both ADHD severity and neuropsychological functioning at F2; and from medication treatment at F2 to ADHD severity and neuropsychological functioning at F3. This model showed acceptable fit [Chi square (df) =404.97(284); p<.001; χ2/df ratio= 1.43; CFI=.92; RMSEA=.05]. While F1 ADHD severity was associated with greater likelihood of F2 medication treatment (β=.24; p<.01), and medication treatment at F1 was associated with medication treatment at F2 (β=.50; p<.001), no other paths from or to medication treatment were significant. Other paths in the model were consistent with what was observed in the model without medication treatment.
As a post hoc analysis, IQ was added into the multi-group model. This model showed acceptable fit to the data (Chi square[526]=692.76; p<.001; Chi square/df ratio=1.32; CFI=.93; RMSEA=.04). Among H/I children, BL IQ was significantly associated with Neuropsychological functioning at F1 (β =.60; p<.001) but not with ADHD severity (β =−.09; p=.35). As a result of inclusion of IQ, Neuropsychological functioning at F1 was not significantly associated with ADHD severity at F2 (β =−.17; p=.10) and SES was no longer significantly associated with ADHD or NEPSY. Other paths remained substantially similar to the model without IQ. Among TD children too, IQ was significantly associated with F1 Neuropsychological functioning (β=.61; p<.001) and marginally associated with ADHD severity (β= −.23; p=.06). Other paths did not change substantially.
Since a model-based stochastic regression imputation strategy was employed to impute data for the five domains of NEPSY at F3, we assessed the differences in paths between the model with imputation used in the study and a model in which there was no imputation. The mean change in the standardized paths in the model-based analyses as a result of imputation of NEPSY at F3 was <.02 (Table 3). Given that the results remained substantially the same with and without imputation, our analyses appear robust to the imputation of missing data.
Table 3.
Comparing cross-lagged paths in the model with stochastic imputation for NEPSY at F3, and with values not imputed
| Path | Model without imputation β ; p | Model with imputation β ; p | Difference β |
|---|---|---|---|
| H/I | |||
| Nepsy F1 to ADHD F2 | −.22;.03 | −.22; .04 | .01 |
| Nepsy F2 to ADHD F3 | −.25; .02 | −.23; .03 | .02 |
| ADHD F1 to Nepsy F2 | −.03; .73 | −.05; .60 | .02 |
| ADHD F2 to Nepsy F3 | −.12; .17 | −.16; .04 | .04 |
| TD | |||
| Nepsy F1 to ADHD F2 | −.13; .23 | −.10; .34 | .03 |
| Nepsy F2 to ADHD F3 | −.03; .81 | −.01; .99 | .02 |
| ADHD F1to Nepsy F2 | −.01; .94 | −.05;.61 | .04 |
| ADHD F2 to Nepsy F3 | −.24; .08 | −.29; .01 | .05 |
| Mean= .14/8=.017 | |||
β= Standardized paths in the model; p= p value; NEPSY and ADHD refer to latent variables in the model. F1: Follow up 1; F2: Follow up 2; F3: Follow up 3.
Discussion
To our knowledge, this is the first study to examine reciprocal longitudinal relations between neuropsychological functioning and dimensionally-assessed ADHD severity among children identified during preschool as being at high or low risk for developing ADHD. In the overall sample, there was an inverse, reciprocal, longitudinal association between ADHD severity and neuropsychological functioning after the age of 4–5 years. Further, there were significant differences between the H/I and TD groups, suggesting that the neurobehavioral trajectory in children at-risk for ADHD may be different from their TD peers. In the H/I group, improved neuropsychological functioning was associated with a subsequent diminution of ADHD severity, providing convincing support for the hypothesis that more optimal neural development is associated with a reduction of symptoms within that age-range; conversely, less optimal neurodevelopment may portend worse symptom presentation during early childhood (Giedd et al., 2010; Halperin et al., 2006; Shaw et al., 2007). Although of lesser magnitude, within that same group, greater ADHD severity at 5–6 years was associated with poorer neuropsychological functioning at 6–7 years, suggesting that entry into formal school may be the time that the association between neuropsychological functioning and ADHD severity becomes reciprocal, possibly leading to worse outcomes. This latter association between ADHD severity at 5–6 years and neuropsychological functioning a year later was also observed in TD children, suggesting that even modest expressions of ADHD among TD children might portend poorer neuropsychological functioning a year later. There were no associations between changes in neuropsychological functioning and later ADHD severity among TD children. This may be due to their consistently low and less variable levels of behavioral difficulties (see Table 1), as well as their greater temporal stability (see Figure 2B), which may have impeded the ability to test the impact of changes over time.
Notably, there were no cross-lagged associations between the ages of 3–4 and 4–5 years. It is possible that domains such as working memory, inhibitory control and executive functions, that are associated with ADHD symptoms, may not be adequately developed by the age of 3–4 years; or that they are not adequately assessed by the NEPSY in that age-range. NEPSY has poorer reliability in very young children and some subtests differ between preschoolers and school-aged children (Korkman et al., 1998); these differences may have impacted our results in unmeasured ways. Alternatively, early neuropsychological deficits may be significantly associated with escalation in symptoms only when children face demands from formal school settings.
It is notable that SES was significantly associated with neuropsychological functioning at age 4–5 years among H/I, but not TD children. However, given that the H/I group evidenced lower SES at BL than the TD group, it is not possible to make causal inferences about the impact of SES on neuropsychological functioning. We speculate that the lack of an association between SES and neuropsychological functioning in the TD group may be due to the moderating effect of SES on child functioning. Specifically, the influence of lower levels of SES could be more evident than the influence of middle and high levels of SES. Since there are more children with lower levels of SES in the H/I group, this association was statistically significant in this group. In support of this hypothesis, Turkheimer, Haley, Waldron, Onofrio, & Gottesman (2003) found that among twins in lower SES families, greater variance in IQ was accounted for by the environment rather than by genetics, whereas among higher SES families, greater variance was accounted for by genes, leading them to propose that SES modified the heritability of child IQ. Extending this to our study, given that there are more children with lower SES in H/I group, the association of SES with neurocognitive functioning may be more evident among H/I children.
An important strength of this study is that the H/I group was not restricted to those meeting diagnostic criteria for ADHD. This may have enhanced the generalizability of this group to a wider population of children who are symptomatic without necessarily meeting formal diagnostic criteria. Further, the use of dimensional measures of ADHD allowed for a more nuanced assessment of variations in symptom severity over time; this is important considering that these symptoms are observed along a continuum, with those who are diagnosed having the most symptoms (Lubke, Hudziak, Derks, van Bijsterveldt, & Boomsma, 2009).
Neuropsychological functioning was measured using a single latent variable that encompassed multiple domains for three main reasons. First, ADHD children show deficits across many domains of neuropsychological functioning including memory (Felton, Wood, Brown, Campbell, & Harter, 1987), motor (Blondis, 1999; Steger et al., 2001), language (Purvis & Tannock, 1997; Tirosh & Cohen, 1998), and visuomotor precision (Raggio, 1999). Second, though some research has suggested a variable number of factors of the NEPSY based on age (Mosconi, Nelson, & Hooper, 2008), other research has found that the NEPSY may be best conceptualized as single factor rather than as unique and uncorrelated domains (Stinnett, Oehler-Stinnett, Fuqua, & Palmer, 2002). Finally, neuroimaging data from ADHD children indicate delays throughout much of the neocortex, rather than a specific region (Shaw et al., 2006).
This study used prospective longitudinal data from multiple sources including parent and teacher report and laboratory assessment. It included a measure of impairment which is required for diagnosis but not often included in studies assessing changes in severity. Additionally, neuropsychological functioning was assessed using the NEPSY, which allowed for continuity of longitudinal measurement along with age- and gender-specific norms that enable comparison to other samples of children. The use of trained evaluators who were blind to clinical data, enhanced the validity of our findings and reducing the likelihood of reporter bias. Finally, SEM provided a stringent test of the longitudinal association between ADHD severity and neuropsychological functioning and made a statistical comparison of the paths between the two groups possible.
The present study also had limitations. First, it is not clear how missing data affected the results, though the FIML method is most recommended (Arbuckle, 1996; Enders & Bandalos, 2001), and supplementary analyses showed negligible impact of stochastic imputation for five variables of NEPSY at F3. Second, although we included multiple time-points across early childhood, it is unclear whether these relations hold-up in older children, adolescents and adults. Therefore, future research needs to test whether these associations are evident later in life. Further, it needs to be noted that the NEPSY does not measure all facets of neuropsychological functioning and facets which are not measured in the present study could be associated with ADHD severity over time. Future studies may also include comparisons to understand whether the executive and non-executive domains of neuropsychological functioning are differentially related to ADHD severity.
Developing an understanding of factors that lower the severity of ADHD and enhance cognitive functioning is highly beneficial considering the high cost of ADHD (Birnbaum et al., 2005; Marks et al., 2009) and the difficulties observed throughout the lifespan (Wilens, Biederman, & Spencer, 2002). Although further research needs to consider genetic and environmental factors that may bring about changes in neuropsychological functioning and ADHD severity, our findings suggest that interventions directed towards improving neuropsychological functioning in young children with elevated ADHD symptoms (Halperin et al., 2012; Tamm et al., 2012) may have the potential to mitigate the adverse long-term course of the disorder.
Acknowledgments
This research was supported by grant R01 MH068286 from NIMH.
Contributor Information
Khushmand Rajendran, Department of Psychology, Queens College, City University of New York
David Rindskopf, The Graduate Center, City University of New York
Sarah O’Neill, Department of Psychology, Queens College, City University of New York
David J. Marks, New York University, Langone Medical Center, Child Study Center, and Department of Psychology, Queens College, City University of New York
Yoko Nomura, Department of Psychology, Queens College and The Graduate Center, City University of New York
Jeffrey M. Halperin, Department of Psychology, Queens College and The Graduate Center, City University of New York
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 4. Washington, D.C: American Psychiatric Association; 2000. Text revision. [Google Scholar]
- Arbuckle JL. Full information estimation in the presence of incomplete data. In: Marcoulides GA, Schumacker RE, editors. Advanced structural equation modeling. Mahwah, NJ: Lawrence Erlbaum; 1996. pp. 243–277. [Google Scholar]
- Bedard ACV, Trampush JW, Newcorn JH, Halperin JM. Perceptual and motor inhibition in adolescents/young adults with childhood-diagnosed ADHD. Neuropsychology. 2010;24:424–434. doi: 10.1037/a0018752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum HG, Kessler RC, Lowe SW, Secnik K, Greenberg PE, Leong SA, Swensen AR. Costs of attention deficit-hyperactivity disorder (ADHD) in the US: Excess costs of persons with ADHD and their family members in 2000. Current Medical Research and Opinion. 2005;21:195–205. doi: 10.1185/030079904X20303. [DOI] [PubMed] [Google Scholar]
- Blondis TA. Motor disorders and attention-deficit/hyperactivity disorder. Pediatric Clinics of North America. 1999;46:899–913. doi: 10.1016/S0031-3955(05)70162-0. [DOI] [PubMed] [Google Scholar]
- Byrne BM. Structural equation modeling with AMOS. Mahwah, NJ: Lawrence Erlbaum; 2001. [Google Scholar]
- Carmines E, McIver J. Analyzing models with unobservable variables. In: Bohrnstedt GW, Borgotta EF, editors. Social measurement: Current issues. Beverly Hills: Sage; 1981. pp. 65–115. [Google Scholar]
- Carr LA, Nigg JT, Henderson JM. Attentional versus motor inhibition in adults with Attention-Deficit/Hyperactivity Disorder. Neuropsychology. 2006;20:430–441. doi: 10.1037/0894-4105.20.4.430. [DOI] [PubMed] [Google Scholar]
- Clerkin SM, Schulz KP, Berwid OG, Fan J, Newcorn JH, Tang CY, Halperin JM. Thalamo-cortical activation and connectivity during response preparation in adults with persistent and remitted ADHD. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2013.12070880. in press. [DOI] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, Fletcher JM. Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society. 2009;15:331–343. doi: 10.1017/S1355617709090481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPaul GJ, Power T, Anastopoulos A, Reid R. ADHD rating scales-IV: Checklists, norms and clinical interpretation. New York, NY: Guilford Press; 1998. [Google Scholar]
- Enders CK. Applied missing data analysis. The Guilford Press; New York: 2010. [Google Scholar]
- Enders CK, Bandalos DL. The relative performance of Full Information Maximum Likelihood Estimation for missing data in structural equation models. Structural Equation Modeling-A Multidisciplinary Journal. 2001;8:430–457. doi: 10.1207/S15328007SEM0803_5. [DOI] [Google Scholar]
- Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, Hurt H. Environmental stimulation, parental nurturance and cognitive development in humans. Developmental Science. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–1323. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Felton RH, Wood FB, Brown IS, Campbell SK, Harter MR. Separate verbal memory and naming deficits in attention deficit disorder and reading disability. Brain and Language. 1987;31:171–184. doi: 10.1016/0093-934X(87)90067-8. [DOI] [PubMed] [Google Scholar]
- Fischer M, Barkley RA, Smallish L, Fletcher K. Executive functioning in hyperactive children as young adults: Attention, inhibition, response perseveration, and the impact of comorbidity. Developmental Neuropsychology. 2005;27:107–133. doi: 10.1207/s15326942dn2701_5. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Demaree HA, Youngstrom EA. Meta-analysis of intellectual and neuropsychological test performance in attention-deficit/hyperactivity disorder. Neuropsychology. 2004;18:543–555. doi: 10.1037/0894-4105.18.3.543. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Rapoport JL. Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron. 2010;67:728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Bedard ACV, Curchack-Lichtin J. Preventive interventions for ADHD: A neurodevelopmental perspective. Neurotherapeutics. 2012;9:531–541. doi: 10.1007/s13311-012-0123-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Healey DM. The influences of environmental enrichment, cognitive enhancement, and physical exercise on brain development: Can we alter the developmental trajectory of ADHD? Neuroscience and Biobehavioral Reviews. 2011;35:621–634. doi: 10.1016/j.neubiorev.2010.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halperin JM, Schulz KP. Revisiting the role of the prefrontal cortex in the pathophysiology of attention-deficit/hyperactivity disorder. Psychological Bulletin. 2006;132:560–581. doi: 10.1037/0033-2909.132.4.560. [DOI] [PubMed] [Google Scholar]
- Halperin JM, Trampush JW, Miller CJ, Marks DJ, Newcorn JH. Neuropsychological outcome in adolescents/young adults with childhood ADHD: profiles of persisters, remitters and controls. Journal of Child Psychology and Psychiatry. 2008;49:958–966. doi: 10.1111/j.1469-7610.2008.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Healey DM, Miller CJ, Castelli KL, Marks DJ, Halperin JM. The impact of impairment criteria on rates of ADHD diagnoses in preschoolers. Journal of Abnormal Child Psychology. 2008;36:771–778. doi: 10.1007/s10802-007-9209-1. [DOI] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Structural Equation Modeling-A Multidisciplinary Journal. 1999;6:1–55. doi: 10.1080/10705519909540118. [DOI] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. The schedule for affective disorders and schizophrenia for school-age children present and lifetime version. 1. Pittsburg, PA: Department of Psychiatry, University of Pittsburg School of Medicine; 1996. [DOI] [PubMed] [Google Scholar]
- Kline RB. Principles and practice of structural equation modeling. 2. New York: The Guilford Press; 2005. [Google Scholar]
- Korkman M, Kirk U, Kemp S. NEPSY: A developmental neuropsychological assessment. San Antonio, Tx: The Psychological Corporation; 1998. [Google Scholar]
- Larsson JO, Larsson H, Lichtenstein P. Genetic and environmental contributions to stability and change of ADHD symptoms between 8 and 13 years of age: A longitudinal twin study. Journal of the American Academy of Child and Adolescent Psychiatry. 2004;43:1267–1275. doi: 10.1097/01.chi.0000135622.05219.bf. [DOI] [PubMed] [Google Scholar]
- Lubke GH, Hudziak JJ, Derks EM, van Bijsterveldt TC, Boomsma DI. Maternal ratings of Attention Problems in ADHD: Evidence for the existence of a continuum. Journal of the American Academy of Child and Adolescent Psychiatry. 2009;48:1085–1093. doi: 10.1097/CHI.0b013e3181ba3dbb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks DJ, Mlodnicka A, Bernstein M, Chacko A, Rose S, Halperin JM. Profiles of service utilization and the resultant economic impact in preschoolers with Attention Deficit/Hyperactivity Disorder. Journal of Pediatric Psychology. 2009;34:681–689. doi: 10.1093/jpepsy/jsn112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M, Loya F, Hinshaw SP. Executive functions in girls with and without childhood ADHD: Developmental trajectories and associations with symptom change. Journal of Child Psychology and Psychiatry and allied disciplines. 2013 doi: 10.1111/jcpp.12074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosconi M, Nelson L, Hooper SR. Confirmatory factor analysis of the NEPSY for younger and older school-age children. Psychological Reports. 2008;102(3):861–866. doi: 10.2466/pr0.102.3.861-866. [DOI] [PubMed] [Google Scholar]
- Nakao K, Treas J. Updating occupational prestige and socioeconomic scores: How the new measures measure up. Sociological Methodology. 1994;24:1–72. doi: 10.2307/270978. [DOI] [Google Scholar]
- Nigg J, Nikolas M, Burt SA. Measured gene-by-environment interaction in relation to Attention-Deficit/Hyperactivity Disorder. Journal of the American Academy of Child and Adolescent Psychiatry. 2010;49:863–873. doi: 10.1016/j.jaac.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts SC, West SG, Tein JY. Longitudinal measurement models in evaluation research: Examining stability and change. Evaluation and Program Planning. 1996;19:333–350. doi: 10.1016/S0149-7189(96)00027-4. [DOI] [Google Scholar]
- Polderman TJC, Derks EM, Hudziak JJ, Verhulst FC, Posthuma D, Boomsma DI. Across the continuum of attention skills: A twin study of the SWAN ADHD rating scale. Journal of Child Psychology and Psychiatry. 2007;48:1080–1087. doi: 10.1111/j.1469-7610.2007.01783.x. [DOI] [PubMed] [Google Scholar]
- Proal E, Reiss PT, Klein RG, Mannuzza S, Gotimer K, Ramos-Olazagasti MA, … Castellanos FX. Brain gray matter deficits at 33-year follow-up in adults with Attention-Deficit/Hyperactivity Disorder established in childhood. Archives of General Psychiatry. 2011;68:1122–1134. doi: 10.1001/archgenpsychiatry.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purvis KL, Tannock R. Language abilities in children with attention deficit hyperactivity disorder, reading disabilities, and normal controls. Journal of Abnormal Child Psychology. 1997;25:133–144. doi: 10.1023/a:1025731529006. [DOI] [PubMed] [Google Scholar]
- Raggio DJ. Visuomotor perception in children with attention deficit hyperactivity disorder-combined type. Perceptual and Motor Skills. 1999;88:448–450. doi: 10.2466/pms.1999.88.2.448. [DOI] [PubMed] [Google Scholar]
- Rajendran K, Trampush J, Rindskopf D, Marks DJ, O’Neill S, Halperin JM. Change in neuropsychological functioning is associated with the trajectory of ADHD severity and impairment in early childhood. American Journal of Psychiatry. doi: 10.1176/appi.ajp.2012.12101360. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds CR, Kamphaus RW. A clinician’s guide to the Behavior Assessment System for Children (BASC) New York: The Guilford Press; 2002. [Google Scholar]
- Schulz KP, Newcorn JH, Fan J, Tang CY, Halperin JM. Brain activation gradients in ventrolateral prefrontal cortex related to persistence of ADHD in adolescent boys. Journal of the American Academy of Child and Adolescent Psychiatry. 2005;44:47–54. doi: 10.1097/01.chi.0000145551.26813.f9. [DOI] [PubMed] [Google Scholar]
- Schumacker RE, Lomax RG. A beginner’s guide to structural equation modeling. New Jersey: Lawrence Erlbaum; 2004. [Google Scholar]
- Shaw P, Eckstrand K, Sharp W, Blumenthal J, Lerch JP, Greenstein D, … Rapoport JL. Attention-deficit/hyperactivity disorder is characterized by a delay in cortical maturation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:19649–19654. doi: 10.1073/pnas.0707741104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Lerch J, Greenstein D, Sharp W, Clasen L, Evans A, … Rapoport JL. Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Archives of General Psychiatry. 2006;63:540–549. doi: 10.1001/archpsyc.63.5.540. [DOI] [PubMed] [Google Scholar]
- Sonuga-Barke EJS, Halperin JM. Developmental phenotypes and causal pathways in attention deficit/hyperactivity disorder: potential targets for early intervention? Journal of Child Psychology and Psychiatry. 2010;51:368–389. doi: 10.1111/j.1469-7610.2009.02195.x. [DOI] [PubMed] [Google Scholar]
- Steger J, Imhof G, Coutts E, Gundelfinger R, Steinhausen EC, Brandeis D. Attentional and neuromotor deficits in ADHD. Developmental Medicine and Child Neurology. 2001;43:172–179. doi: 10.1017/S0012162201000330. [DOI] [PubMed] [Google Scholar]
- Stinnett TA, Oehler-Stinnett J, Fuqua DR, Palmer LS. Examination of the underlying structure of the NEPSY: A developmental neuropsychological assessment. Journal of Psychoeducational Assessment. 2002;20:66–82. doi: 10.1177/073428290202000105. [DOI] [Google Scholar]
- Tamm L, Nakonezny PA, Hughes CW. An open trial of a metacognitive executive functioning training for young children with ADHD. Journal of Attention Disorders. doi: 10.1177/1087054712445782. in press. [DOI] [PubMed] [Google Scholar]
- Tirosh E, Cohen A. Language deficit with attention-deficit disorder: A prevalent comorbidity. Journal of Child Neurology. 1998;13:493–497. doi: 10.1177/088307389801301005. [DOI] [PubMed] [Google Scholar]
- Turkheimer E, Haley A, Waldron M, D’Onofrio B, Gottesman II. Socioeconomic status modifies the heritability of IQ in young children. Psychological Science. 2003;14:623–628. doi: 10.1046/j.0956-7976.2003.psci_1475.x. [DOI] [PubMed] [Google Scholar]
- van Lieshout M, Luman M, Buitelaar J, Rommelse NNJ, Oosterlaan J. Does neurocognitive functioning predict future or persistence of ADHD? A systematic review. Clinical Psychology Review. 2013 doi: 10.1016/j.cpr.2013.02.003. [DOI] [PubMed] [Google Scholar]
- Vaughn AJ, Epstein JN, Rausch J, Altaye M, Langberg J, Newcorn JH, … Wigal T. Relation between outcomes on a Continuous Performance Test and ADHD symptoms over time. Journal of Abnormal Child Psychology. 2011;39:853–864. doi: 10.1007/s10802-011-9501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. The Wechsler Preschool and Primary Scale of Intelligence. 3. San Antonio, Tx: The Psychological Corporation; 2002. [Google Scholar]
- Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biological Psychiatry. 2005;57:1336–1346. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Wilens TE, Biederman J, Spencer TJ. Attention deficit/hyperactivity disorder across the lifespan. Annual Review of Medicine. 2002;53:113–131. doi: 10.1146/annurev.med.53.082901.103945. [DOI] [PubMed] [Google Scholar]