Abstract
Recent research has shown that the alteration of combinations in gene expression contributes to cellular phenotypic changes. Previously, it has been demonstrated that the combination of cadherin 1 and cadherin 2 expression can identify the diffuse-type and intestinal-type gastric cancers. Although the diffuse-type gastric cancer has been resistant to treatment, the precise mechanism and phenotypic involvement has not been revealed. It may be possible that stem cells transform into gastric cancer cells, possibly through the involvement of a molecule alteration and signaling mechanism. In this review article, we focus on the role of catenin beta 1 (CTNNB1 or β-catenin) and describe the regulation of CTNNB1 signaling in gastric cancer and stem cells.
Keywords: CTNNB1 signaling, β-catenin, Epithelial-mesenchymal transition, Gastric cancer, Stem cell
Core tip: CTNNB1 signaling is essential for revealing cancer mechanisms. The molecular dynamism in stem cells and cancer is illustrated with a pathway cascade. The CTNNB1 protein interacts with signaling molecules upon stimulation, leading to the transcription of genes related to cell proliferation. Mutations of signaling molecules are also important factors for cancer development. CTNNB1 signaling in stem cells and cancer are mainly described in the article.
INTRODUCTION
Transformed cells have dynamic molecular alterations, which can be identified via gene profiling[1]. Essential genes can be identified using advancing human genomic techniques, such as the clustered regularly interspaced short palindromic repeats (CRISPR) gene editing system[2]. In gastrointestinal cancers, various molecules, including catenin beta 1 (CTNNB1 or β-catenin), have important roles in phenotypic transitions[3-6]. Mutations in β-catenin or adenomatous polyposis coli (APC) induce β-catenin/T cell transcription factor (TCF) signaling in colon cancer[7,8]. Meanwhile, WNT/β-catenin signaling has a role in stem cell signaling[3,9]. The inhibition of glycogen synthase kinase 3 beta (GSK3β) promotes v-myc avian myelocytomatosis viral oncogene homolog (c-Myc or MYC) and β-catenin activity toward endoderm identification via forkhead box A2 (FoxA2) expression[9]. In cancer stem cells (CSCs), β-catenin signaling, which is downstream of the CSC marker prominin 1 (CD133 or PROM1), is required for CSC maintenance[10]. Although CD133-induced β-catenin signaling activation is cancer cell-type specific, β-catenin binds to the proximal promoter regions of integrin subunit beta 6 (ITGB6) and ITGB8 in gastric cancer cell lines[10]. This β-catenin signaling may be regulated by specific target genes[10].
Genomic rearrangements in the telomerase reverse transcriptase gene (TERT) and the up-regulation of TERT are important factors in high-risk neuroblastoma[11]. The detection of causative mutations in cancers has been facilitated since the completion of the Human Genome Project[12,13]. Because several individual mutations in gastrointestinal cancers have been identified as being targeted by tumor infiltrating lymphocytes, the mutations in cancer signaling cascades should be analyzed in the context of possible cancer immunotherapy[14]. Signaling molecules, including β-catenin and signal direction switch caused by misregulation of expression, are the main focus of this article.
CTNNB1 AND WNT SIGNALING
The canonical Wnt signaling pathway includes Wnt-Frizzled, dishevelled (DVL), Axin, GSK3 inactivation, and β-catenin dephosphorylation, stabilization and translocation into the nucleus[3]. The translocated β-catenin together with TCF transcriptionally regulates Wnt target genes, whereas the disruption of Wnt signaling caused by mutations in pathway genes can cause cancer[3]. The interaction of β-catenin and E-cadherin may be involved in cell-cell communication and signal transduction[15]. The disruption of VE-cadherin localization is involved in β-catenin phosphorylation and signaling via microparticles that are important for cell-cell communication in endothelial cells. However, this β-catenin activation is independent of Wnt/Frizzled[16]. Tubeimoside-1, which has anti-tumor properties, has been shown to inhibit the growth and invasion of colorectal cancer cells through inhibiting the Wnt/β-catenin signaling pathway[17]. The Wnt/β-catenin pathway is essential to the epithelial-mesenchymal transition (EMT) in breast cancer cells over-expressing C-X-C motif chemokine ligand 12 (CXCL12, or stromal cell-derived factor-1; SDF-1)[18]. The E6 region of high-risk human papillomavirus (HPV)-16, one of the possible causes of esophageal cancer, induces cell growth of esophageal cancer through activation of the Wnt/β-catenin signaling pathway and downregulation of miR-125b[19]. GSK3-mediated β-catenin phosphorylation is a key event in Wnt/β-catenin signaling[20]. GSK3 associates with AXIN to phosphorylate and regulate β-catenin[20]. These studies indicate the importance of Wnt/β-catenin signaling in tumorigenesis and EMT.
CTNNB1 SIGNALING IN GASTRIC CANCER
TERT activates Wnt/β-catenin signaling and promotes MYC expression[21]. The expression of TERT in gastric cancer is correlated with advanced TNM stages and lymphatic metastasis, which suggests TERT may be a therapeutic target for GC patients[21]. GC invasion and metastasis are associated with molecular mechanisms for TERT[21]. MYC expression is regulated by TERT via the Wnt/β-catenin pathway[21]. Dishevelled-Axin domain containing 1 (DIXDC1), a positive regulator of the Wnt pathway, is a significant prognostic indicator of intestinal-type gastric carcinoma[22]. DIXDC1 contains a DIX domain that is involved in the formation of a complex along with Axin, Dvl, APC, GSK3β, and β-catenin[22,23]. It has been shown that GSK3β-dependent phosphorylation of β-catenin is inhibited in the presence of Axin[23]. Axin regulates Wnt signaling as scaffold for the APC-glycogen synthase kinase-3β-β-catenin complex to down-regulate β-catenin, and Axin mutations in the DIX domain abolish JNK activity, whereas β-catenin signaling is not affected by Axin mutations[24]. MicroRNA-1225-5p (miR-1225-5p) has been reported to function as a tumor suppressor for gastric carcinoma, acting through inhibition of the insulin receptor substrate-1 (IRS1) and β-catenin signaling pathways to suppress gastric carcinoma proliferation and metastasis[25].
CTNNB1 SIGNALING IN STEM CELLS
Wnt/β-catenin signaling plays an important role in stem cell maintenance. The self-renewing mesenchymal cells with stem cell characteristics inhabit a niche for maintaining their stemness[26]. By modifying β-catenin in mouse osteoblasts, acute myeloid leukemias with common chromosomal alterations occur, and Notch signaling increases in hematopoietic cells[26,27]. The stem cell niche may be regulated by Wnt signaling and the nuclear accumulation of β-catenin[27]. Upon activation of β-catenin, the Notch ligand jagged 1 is up-regulated in osteoblasts, which leads to the activation of Notch signaling in hematopoietic stem cell progenitors, moving them towards malignant transformation[27].
In glioma stem cells, interleukin 17 receptor (IL-17R) expression is involved in self-renewal[28]. IL-17 up-regulates the expression of stemness/mesenchymal markers, such as fibronectin, CD44 and SOX2, in glioma stem cells[28]. IL-17 regulates signal transducer and activator of transcription 3 (STAT3), nuclear factor k-light-chain-enhancer of activated B cells (NF-κB), GSK3β and β-catenin in glioma stem cells[28].
In CXCL12 (or SDF-1)-overexpressed breast cancer cells, the Wnt/β-catenin pathway is required for the EMT, which induces cancer stem cell-like phenotype formation toward proliferation and metastasis in breast cancer cells[18,29]. Breast tumorigenesis can be suppressed by inhibition of β-catenin/LEF-1 signaling[30]. A synthesized peptide (TAT-NLS-BLBD-6) inhibits the nuclear interaction of β-catenin and LEF-1 in human breast cancer cells, suppressing Wnt/β-catenin signaling and resulting in inhibition of tumorigenesis[30]. Considering that TAT-NLS-BLBD-6 inhibits β-catenin/LEF-1 downstream target genes, including CDKN2A, CLDN1, ID6 and SOX2, Wnt/β-catenin signaling is likely to promote oncogenesis via LEF-1-targeted gene expression[30].
The Notch and Wnt/β-catenin signaling pathways play important roles in maintaining and promoting liver cancer stem cells[31]. Liver cancer stem cells expressing stemness markers, such as CD90, CD24, CD13 and CD133, with poor prognosis in patients are maintained by Notch and Wnt/β-catenin signaling[31]. Upon niche formation, WNT-SHH signaling modulates stem cell fates[32]. While canonical Wnt signaling mediated by β-catenin and LEF-1 is essential for placode formation, the combination of SHH and Wnt signals may be crucial for stem cell niche formation[32].
Interleukin-22 (IL-22) induces epithelial regeneration through intestinal stem cells (ISCs), whose niche provides Wnt, Notch and epidermal growth factor signals for normal epithelial maintenance[33]. Wnt/β-catenin signaling maintains these ISCs, whereas the IL-22 pathway may be involved in STAT3 signaling and cross-linked[33].
CTNNB1 AND THE EPITHELIAL-MESENCHYMAL TRANSITION
SNAI1 and CTNNB1 pathway in EMT
The loss of E-cadherin and the transformation of cells to the mesenchymal phenotype are involved in Smad signaling and the formation of β-catenin/LEF-1 complexes[34]. Transforming growth factor β 1 (TGFβ1) promotes the EMT via the Smad-independent Ras-Raf-MEK-ERK-AP-1 signaling pathway, which up-regulates the expression of the snail family zinc finger 1 (SNAI1) gene[34]. IL-8 plays a role in the maintenance of the tumor EMT through both autocrine and paracrine pathways[35]. The IL-8 pathway transduces the Ras-ERK and PI3K-AKT signals to induce IL-8 transcription through Snail and Twist, which activate the autocrine IL-8 pathway[35]. Moreover, IL-8 promotes E-cadherin transcription through Brachyury[35]. The paracrine IL-8 pathway consists of the recruitment of tumor–associated macrophages and neutrophils into tumor sites to promote EMT using cytokines[35].
The expression of EMT regulator SNAI1 is correlated with an increased risk of tumor relapse in breast cancer patients and the progression of colorectal cancer[36]. E-cadherin loss promotes the expression of EMT regulators, including β-catenin and NF-κB, which suggests that the pathway for SNAI1 and β-catenin may be crosslinked[36-39]. The expression of cadherin 1 (CDH1 or E-cadherin) is a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens[40]. The disease progression in patients with high-stage category cancers can be predicted with the expression of CDH1[40]. Decreased CDH1 may release β-catenin from the β-catenin complex, allowing it to translocate into the nucleus and activate transcription of target genes, such as MYC[40,41]. The cancer metastatic process is mediated via circulating tumor cells expressing EMT markers such as ETV5, NOTCH1, SNAI1, TGFB1, ZEB1 and ZEB2[42]. Considering that the expression of CTNNB1 is up-regulated in endometrial circulating tumor cells in endometrial cancer, CTNNB1 is a potential therapeutic target for endometrial cancer[42]. SNAI1, SNAI2, and SNAI3 expression in the ductal epithelium is up-regulated during development, and SNAI1 and SNAI2 are co-expressed with insulin[43]. CDH1 expression decreases during the EMT process in β-cell differentiation into islets. The expression of β-catenin is altered in process of β-cell clustering formation in islets[43].
TGFβ and CTNNB1 signaling in EMT
The TGFβ-induced EMT is regulated by phosphatase and tensin homologue deleted from chromosome 10 (PTEN), a tumor suppressor gene in lung cancer cells[44]. Upon stimulation with TGFβ, β-catenin translocates into nucleus. This activity is inhibited by the deletion of the phosphatase and C2 domains of unphosphorylated PTEN[44]. The expression of E-cadherin is down-regulated with TGFβ, which is inhibited by the phosphatase and C2 domains of unphosphorylated PTEN[44]. The isoflavone calycosin-7-O-β-D-glucopyranoside induces osteogenic differentiation through the BMP and WNT/β-catenin-signaling pathways[45]. Osteogenic differentiation is regulated by TGFβ signaling, which suggests some coordination of the β-catenin and TGFβ signaling pathways[45]. Jumonji domain-containing protein 2B (JMJD2B) may also be involved in TGFβ1-mediated β-catenin nuclear accumulation[46]. Nuclear translocation of β-catenin may be regulated by JMJD2B in the EMT process[46]. TGFβ1 down-regulates the canonical WNT signaling pathway and inhibits photoreceptor differentiation of adult human Müller stem cells[47]. In human Müller stem cells, TGFβ1 down-regulates WNT2B, dickkopf WNT signaling pathway inhibitor 1 (DKK1) and active β-catenin and up-regulates WNT5B to inhibit canonical Wnt signaling[47].
CTNNB1 IN CANCER STEM CELLS
Extrinsic factors are important when assessing cancer risk[48]. Stem cell division is related to cancer development, emphasizing the importance of understanding the molecular pathways involved in stem cell maintenance, gastric cancer and cell proliferation[48]. Ginsenoside Rh2, which inhibits growth of some types of cancer, decreases the number of CSC-like cells in hepatocellular carcinoma, possibly through β-catenin signaling[49]. Furthermore, the CSC markers CD133 and Epithelial cell adhesion molecule (EpCAM) are decreased by ginsenoside Rh2[49]. It is suggested that high levels of β-catenin are a signature of CSC-like cells[49]. Wnt/β-catenin signaling leads to the translocation of β-catenin into nucleus and the transcription of c-Myc, Axin2 and Brachyury[50]. Axin is stabilized with GSK3β and inhibits β-catenin signaling[50]. The proliferation of CSCs may also be regulated by Wnt/β-catenin signaling[50]. The periprostatic adipose tissue-derived adipocytes regulate migration of prostate cancer cells[51]. The chemokine CCL7 secretion of adipocytes stimulates the migration of CCR3-expressing prostate tumor cells[51]. This signaling that is mediated by chemokines in CSCs could be a future target for investigation.
MUTATIONS IN CTNNB1-RELATED SIGNALING AND CANCER
Mutations of SMAD family member 4 (Dpc4 or Smad4) and Apc in mice cause malignant intestinal tumors and stromal cell proliferation[52]. The DPC4 (SMAD4) gene is important for the TGFβ signaling pathway, which inhibits normal cell growth and promotes malignant cell growth[52]. APC mutations in papillary thyroid carcinoma are associated with familial adenomatous polyposis[53]. Through binding to 20-amino acid repeats, the APC/β-catenin signaling pathway is related to the development of thyroid cancer in patients with familial adenomatous polyposis[53]. Mutations in AXIN2 cause colorectal cancer, in which the mutations stabilize β-catenin and activate β-catenin/TCF signaling[54]. The AXIN2 mutations result in accumulated nuclear β-catenin[54]. CTNNB1 and APC mutations also occur in colorectal cancer with defective DNA mismatch repair[54]. For the treatment of cancer, identifying novel genome-wide therapeutic targets is essential, which suggests the importance of mutational studies in the cancer genome[55]. Main WNT/β-catenin pathway involved in cancer and stem cells is shown in cartoon (Figure 1). The network information source is mainly from Kyoto Encyclopedia of Genes and Genomes (KEGG) (http://www.genome.jp/kegg/).
Figure 1.
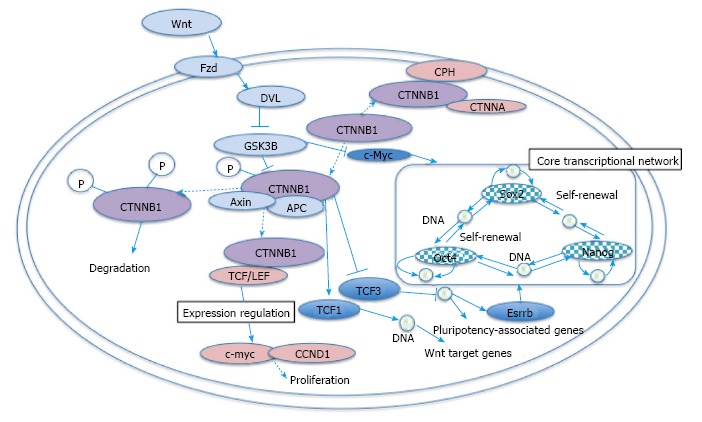
Main WNT/β-catenin pathway involved in cancer and stem cells. Wnt inhibits CTNNB1 phosphorylation by GSK3B, which leads to the transcription of pro-proliferation related genes via binding of CTNNB1 to TCF or LEF. P indicates phosphate. Red color shows molecules in pathways in cancer, whereas blue color shows molecules in signaling pathways regulating pluripotency of stem cells (KEGG). DVL: Dishevelled; TCF: T cell transcription factor; APC: Adenomatous polyposis coli.
CONCLUSION
Our knowledge is increasing due to recent advances in bioinformatics and computational capacity. How to efficiently utilize this new data and knowledge is an important issue for future development of the big data era. The WNT/β-catenin pathway is involved in cancer and pluripotent stem cell signaling, which may suggest the mechanism underlying cancer stem cells. As cancer therapeutics has different effects in different genomic condition, individual medicine may be predicted with genetic variants. One useful direction for the use of genomic information may be the identification of targets for the treatment of diseases with appropriate predictions.
Footnotes
Conflict-of-interest statement: The authors declare that no conflicts of interest exist.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: March 7, 2016
First decision: April 18, 2016
Article in press: May 27, 2016
P- Reviewer: Dawe GS, Yao CL S- Editor: Qi Y L- Editor: A E- Editor: Lu YJ
References
- 1.Nguyen LV, Pellacani D, Lefort S, Kannan N, Osako T, Makarem M, Cox CL, Kennedy W, Beer P, Carles A, et al. Barcoding reveals complex clonal dynamics of de novo transformed human mammary cells. Nature. 2015;528:267–271. doi: 10.1038/nature15742. [DOI] [PubMed] [Google Scholar]
- 2.Wang T, Birsoy K, Hughes NW, Krupczak KM, Post Y, Wei JJ, Lander ES, Sabatini DM. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–1101. doi: 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clevers H, Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149:1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 4.Song X, Xin N, Wang W, Zhao C. Wnt/beta-catenin, an oncogenic pathway targeted by H. pylori in gastric carcinogenesis. Oncotarget. 2015;6:35579–35588. doi: 10.18632/oncotarget.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Susman S, Barnoud R, Bibeau F, Borini F, Pocard M, Tomuleasa C, Sabourin JC. The Lauren classification highlights the role of epithelial-to-mesenchymal transition in gastric carcinogenesis: an immunohistochemistry study of the STAT3 and adhesion molecules expression. J Gastrointestin Liver Dis. 2015;24:77–83. doi: 10.15403/jgld.2014.1121.sus. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Deng Z, Wang Z, Wang D, Zhang L, Su Q, Lai Y, Li B, Luo Z, Chen X, et al. Zipper-interacting protein kinase promotes epithelial-mesenchymal transition, invasion and metastasis through AKT and NF-kB signaling and is associated with metastasis and poor prognosis in gastric cancer patients. Oncotarget. 2015;6:8323–8338. doi: 10.18632/oncotarget.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morin PJ, Sparks AB, Korinek V, Barker N, Clevers H, Vogelstein B, Kinzler KW. Activation of beta-catenin-Tcf signaling in colon cancer by mutations in beta-catenin or APC. Science. 1997;275:1787–1790. doi: 10.1126/science.275.5307.1787. [DOI] [PubMed] [Google Scholar]
- 8.Sparks AB, Morin PJ, Vogelstein B, Kinzler KW. Mutational analysis of the APC/beta-catenin/Tcf pathway in colorectal cancer. Cancer Res. 1998;58:1130–1134. [PubMed] [Google Scholar]
- 9.Morrison G, Scognamiglio R, Trumpp A, Smith A. Convergence of cMyc and beta-catenin on Tcf7l1 enables endoderm specification. EMBO J. 2016;35:356–368. doi: 10.15252/embj.201592116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Su YJ, Lin WH, Chang YW, Wei KC, Liang CL, Chen SC, Lee JL. Polarized cell migration induces cancer type-specific CD133/integrin/Src/Akt/GSK3beta/β-catenin signaling required for maintenance of cancer stem cell properties. Oncotarget. 2015;6:38029–38045. doi: 10.18632/oncotarget.5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peifer M, Hertwig F, Roels F, Dreidax D, Gartlgruber M, Menon R, Krämer A, Roncaioli JL, Sand F, Heuckmann JM, et al. Telomerase activation by genomic rearrangements in high-risk neuroblastoma. Nature. 2015;526:700–704. doi: 10.1038/nature14980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 13.Alioto TS, Buchhalter I, Derdak S, Hutter B, Eldridge MD, Hovig E, Heisler LE, Beck TA, Simpson JT, Tonon L, et al. A comprehensive assessment of somatic mutation detection in cancer using whole-genome sequencing. Nat Commun. 2015;6:10001. doi: 10.1038/ncomms10001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran E, Ahmadzadeh M, Lu YC, Gros A, Turcotte S, Robbins PF, Gartner JJ, Zheng Z, Li YF, Ray S, et al. Immunogenicity of somatic mutations in human gastrointestinal cancers. Science. 2015;350:1387–1390. doi: 10.1126/science.aad1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peifer M, McCrea PD, Green KJ, Wieschaus E, Gumbiner BM. The vertebrate adhesive junction proteins beta-catenin and plakoglobin and the Drosophila segment polarity gene armadillo form a multigene family with similar properties. J Cell Biol. 1992;118:681–691. doi: 10.1083/jcb.118.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al Thawadi H, Abu-Kaoud N, Al Farsi H, Hoarau-Véchot J, Rafii S, Rafii A, Pasquier J. VE-cadherin cleavage by ovarian cancer microparticles induces beta-catenin phosphorylation in endothelial cells. Oncotarget. 2016;7:5289–5305. doi: 10.18632/oncotarget.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bian Q, Liu P, Gu J, Song B. Tubeimoside-1 inhibits the growth and invasion of colorectal cancer cells through the Wnt/beta-catenin signaling pathway. Int J Clin Exp Pathol. 2015;8:12517–12524. [PMC free article] [PubMed] [Google Scholar]
- 18.Shan S, Lv Q, Zhao Y, Liu C, Sun Y, Xi K, Xiao J, Li C. Wnt/beta-catenin pathway is required for epithelial to mesenchymal transition in CXCL12 over expressed breast cancer cells. Int J Clin Exp Pathol. 2015;8:12357–12367. [PMC free article] [PubMed] [Google Scholar]
- 19.Zang B, Huang G, Wang X, Zheng S. HPV-16 E6 promotes cell growth of esophageal cancer via downregulation of miR-125b and activation of Wnt/beta-catenin signaling pathway. Int J Clin Exp Pathol. 2015;8:13687–13694. [PMC free article] [PubMed] [Google Scholar]
- 20.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–168. doi: 10.1016/j.tibs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang B, Xie R, Qin Y, Xiao YF, Yong X, Zheng L, Dong H, Yang SM. Human telomerase reverse transcriptase (hTERT) promotes gastric cancer invasion through cooperating with c-Myc to upregulate heparanase expression. Oncotarget. 2016;7:11364–11379. doi: 10.18632/oncotarget.6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Tan C, Qiao F, Wang W, Jiang X, Lian P, Chang B, Sheng W. Upregulated expression of DIXDC1 in intestinal-type gastric carcinoma: co-localization with β-catenin and correlation with poor prognosis. Cancer Cell Int. 2015;15:120. doi: 10.1186/s12935-015-0273-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kishida S, Yamamoto H, Hino S, Ikeda S, Kishida M, Kikuchi A. DIX domains of Dvl and axin are necessary for protein interactions and their ability to regulate beta-catenin stability. Mol Cell Biol. 1999;19:4414–4422. doi: 10.1128/mcb.19.6.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo W, Zou H, Jin L, Lin S, Li Q, Ye Z, Rui H, Lin SC. Axin contains three separable domains that confer intramolecular, homodimeric, and heterodimeric interactions involved in distinct functions. J Biol Chem. 2005;280:5054–5060. doi: 10.1074/jbc.M412340200. [DOI] [PubMed] [Google Scholar]
- 25.Zheng H, Zhang F, Lin X, Huang C, Zhang Y, Li Y, Lin J, Chen W, Lin X. MicroRNA-1225-5p inhibits proliferation and metastasis of gastric carcinoma through repressing insulin receptor substrate-1 and activation of beta-catenin signaling. Oncotarget. 2016;7:4647–4663. doi: 10.18632/oncotarget.6615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scadden DT. Nice neighborhood: emerging concepts of the stem cell niche. Cell. 2014;157:41–50. doi: 10.1016/j.cell.2014.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kode A, Manavalan JS, Mosialou I, Bhagat G, Rathinam CV, Luo N, Khiabanian H, Lee A, Murty VV, Friedman R, et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature. 2014;506:240–244. doi: 10.1038/nature12883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Parajuli P, Anand R, Mandalaparty C, Suryadevara R, Sriranga PU, Michelhaugh SK, Cazacu S, Finniss S, Thakur A, Lum LG, et al. Preferential expression of functional IL-17R in glioma stem cells: potential role in self-renewal. Oncotarget. 2016;7:6121–6135. doi: 10.18632/oncotarget.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu TH, Yao Y, Yu S, Han LL, Wang WJ, Guo H, Tian T, Ruan ZP, Kang XM, Wang J, et al. SDF-1/CXCR4 promotes epithelial-mesenchymal transition and progression of colorectal cancer by activation of the Wnt/beta-catenin signaling pathway. Cancer Lett. 2014;354:417–426. doi: 10.1016/j.canlet.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh TH, Hsu CY, Tsai CF, Chiu CC, Liang SS, Wang TN, Kuo PL, Long CY, Tsai EM. A novel cell-penetrating peptide suppresses breast tumorigenesis by inhibiting beta-catenin/LEF-1 signaling. Sci Rep. 2016;6:19156. doi: 10.1038/srep19156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang R, Sun Q, Wang P, Liu M, Xiong S, Luo J, Huang H, Du Q, Geller DA, Cheng B. Notch and Wnt/beta-catenin signaling pathway play important roles in activating liver cancer stem cells. Oncotarget. 2016;7:5754–5768. doi: 10.18632/oncotarget.6805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ouspenskaia T, Matos I, Mertz AF, Fiore VF, Fuchs E. WNT-SHH Antagonism Specifies and Expands Stem Cells prior to Niche Formation. Cell. 2016;164:156–169. doi: 10.1016/j.cell.2015.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindemans CA, Calafiore M, Mertelsmann AM, O’Connor MH, Dudakov JA, Jenq RR, Velardi E, Young LF, Smith OM, Lawrence G, et al. Interleukin-22 promotes intestinal-stem-cell-mediated epithelial regeneration. Nature. 2015;528:560–564. doi: 10.1038/nature16460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medici D, Hay ED, Goodenough DA. Cooperation between snail and LEF-1 transcription factors is essential for TGF-beta1-induced epithelial-mesenchymal transition. Mol Biol Cell. 2006;17:1871–1879. doi: 10.1091/mbc.E05-08-0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Long X, Ye Y, Zhang L, Liu P, Yu W, Wei F, Ren X, Yu J. IL-8, a novel messenger to cross-link inflammation and tumor EMT via autocrine and paracrine pathways (Review) Int J Oncol. 2016;48:5–12. doi: 10.3892/ijo.2015.3234. [DOI] [PubMed] [Google Scholar]
- 36.Deep G, Jain AK, Ramteke A, Ting H, Vijendra KC, Gangar SC, Agarwal C, Agarwal R. SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin. Mol Cancer. 2014;13:37. doi: 10.1186/1476-4598-13-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Whiteland H, Spencer-Harty S, Thomas DH, Davies C, Morgan C, Kynaston H, Bose P, Fenn N, Lewis PD, Bodger O, et al. Putative prognostic epithelial-to-mesenchymal transition biomarkers for aggressive prostate cancer. Exp Mol Pathol. 2013;95:220–226. doi: 10.1016/j.yexmp.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 38.Fan XJ, Wan XB, Yang ZL, Fu XH, Huang Y, Chen DK, Song SX, Liu Q, Xiao HY, Wang L, et al. Snail promotes lymph node metastasis and Twist enhances tumor deposit formation through epithelial-mesenchymal transition in colorectal cancer. Hum Pathol. 2013;44:173–180. doi: 10.1016/j.humpath.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 39.Moody SE, Perez D, Pan TC, Sarkisian CJ, Portocarrero CP, Sterner CJ, Notorfrancesco KL, Cardiff RD, Chodosh LA. The transcriptional repressor Snail promotes mammary tumor recurrence. Cancer Cell. 2005;8:197–209. doi: 10.1016/j.ccr.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 40.De Marzo AM, Knudsen B, Chan-Tack K, Epstein JI. E-cadherin expression as a marker of tumor aggressiveness in routinely processed radical prostatectomy specimens. Urology. 1999;53:707–713. doi: 10.1016/s0090-4295(98)00577-9. [DOI] [PubMed] [Google Scholar]
- 41.He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, Morin PJ, Vogelstein B, Kinzler KW. Identification of c-MYC as a target of the APC pathway. Science. 1998;281:1509–1512. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- 42.Alonso-Alconada L, Muinelo-Romay L, Madissoo K, Diaz-Lopez A, Krakstad C, Trovik J, Wik E, Hapangama D, Coenegrachts L, Cano A, et al. Molecular profiling of circulating tumor cells links plasticity to the metastatic process in endometrial cancer. Mol Cancer. 2014;13:223. doi: 10.1186/1476-4598-13-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole L, Anderson M, Antin PB, Limesand SW. One process for pancreatic beta-cell coalescence into islets involves an epithelial-mesenchymal transition. J Endocrinol. 2009;203:19–31. doi: 10.1677/JOE-09-0072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kusunose M, Hashimoto N, Kimura M, Ogata R, Aoyama D, Sakamoto K, Miyazaki S, Ando A, Omote N, Imaizumi K, et al. Direct regulation of transforming growth factor beta-induced epithelial-mesenchymal transition by the protein phosphatase activity of unphosphorylated PTEN in lung cancer cells. Cancer Sci. 2015;106:1693–1704. doi: 10.1111/cas.12831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jian J, Sun L, Cheng X, Hu X, Liang J, Chen Y. Calycosin-7-O-beta-d-glucopyranoside stimulates osteoblast differentiation through regulating the BMP/WNT signaling pathways. Acta Pharm Sin B. 2015;5:454–460. doi: 10.1016/j.apsb.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhao L, Li W, Zang W, Liu Z, Xu X, Yu H, Yang Q, Jia J. JMJD2B promotes epithelial-mesenchymal transition by cooperating with beta-catenin and enhances gastric cancer metastasis. Clin Cancer Res. 2013;19:6419–6429. doi: 10.1158/1078-0432.CCR-13-0254. [DOI] [PubMed] [Google Scholar]
- 47.Angbohang A, Wu N, Charalambous T, Eastlake K, Lei Y, Kim YS, Sun XH, Limb GA. Downregulation of the Canonical WNT Signaling Pathway by TGFbeta1 Inhibits Photoreceptor Differentiation of Adult Human Müller Glia with Stem Cell Characteristics. Stem Cells Dev. 2016;25:1–12. doi: 10.1089/scd.2015.0262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu S, Powers S, Zhu W, Hannun YA. Substantial contribution of extrinsic risk factors to cancer development. Nature. 2016;529:43–47. doi: 10.1038/nature16166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z, Zhao T, Liu H, Zhang L. Ginsenoside Rh2 inhibits hepatocellular carcinoma through beta-catenin and autophagy. Sci Rep. 2016;6:19383. doi: 10.1038/srep19383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim W, Kim M, Jho EH. Wnt/beta-catenin signalling: from plasma membrane to nucleus. Biochem J. 2013;450:9–21. doi: 10.1042/BJ20121284. [DOI] [PubMed] [Google Scholar]
- 51.Laurent V, Guérard A, Mazerolles C, Le Gonidec S, Toulet A, Nieto L, Zaidi F, Majed B, Garandeau D, Socrier Y, et al. Periprostatic adipocytes act as a driving force for prostate cancer progression in obesity. Nat Commun. 2016;7:10230. doi: 10.1038/ncomms10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takaku K, Oshima M, Miyoshi H, Matsui M, Seldin MF, Taketo MM. Intestinal tumorigenesis in compound mutant mice of both Dpc4 (Smad4) and Apc genes. Cell. 1998;92:645–656. doi: 10.1016/s0092-8674(00)81132-0. [DOI] [PubMed] [Google Scholar]
- 53.Kumamoto K, Ishida H, Ohsawa T, Ishibashi K, Ushiama M, Yoshida T, Iwama T. Germline and somatic mutations of the APC gene in papillary thyroid carcinoma associated with familial adenomatous polyposis: Analysis of three cases and a review of the literature. Oncol Lett. 2015;10:2239–2243. doi: 10.3892/ol.2015.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu W, Dong X, Mai M, Seelan RS, Taniguchi K, Krishnadath KK, Halling KC, Cunningham JM, Boardman LA, Qian C, et al. Mutations in AXIN2 cause colorectal cancer with defective mismatch repair by activating beta-catenin/TCF signalling. Nat Genet. 2000;26:146–147. doi: 10.1038/79859. [DOI] [PubMed] [Google Scholar]
- 55.Morrissy AS, Garzia L, Shih DJ, Zuyderduyn S, Huang X, Skowron P, Remke M, Cavalli FM, Ramaswamy V, Lindsay PE, et al. Divergent clonal selection dominates medulloblastoma at recurrence. Nature. 2016;529:351–357. doi: 10.1038/nature16478. [DOI] [PMC free article] [PubMed] [Google Scholar]


