Abstract
Beside its poor prognosis and its late diagnosis, pancreatic cancer remains one of the most painful malignancies. Optimal management of pain in this cancer represents a real challenge for the oncologist whose objective is to ensure a better quality of life to his patients. We aimed in this paper to review all the treatment modalities incriminated in the management of pain in pancreatic cancer going from painkillers, chemotherapy, radiation therapy and interventional techniques to agents under investigation and alternative medicine. Although specific guidelines and recommendations for pain management in pancreatic cancer are still absent, we present all the possible pain treatments, with a progression from medical multimodal treatment to radiotherapy and chemotherapy then interventional techniques in case of resistance. In addition, alternative methods such as acupuncture and hypnosis can be added at any stage and seems to contribute to pain relief.
Keywords: Pain management, Interventional, Medical, Treatment, Pancreatic cancer
Core tip: This paper presents a road map for the pain management of pancreatic cancer. All treatment modalities incriminated in the management of pain in pancreatic cancer going from painkillers, chemotherapy, radiation therapy and interventional techniques to agents under investigation and alternative medicine are reviewed and their indications are discussed.
INTRODUCTION
Pancreatic cancer (PC) is one of the most aggressive tumors with less than 5% survival at 5 years[1]. More than 80% of these tumors are detected at an advanced stage because of the difficulty of diagnosis[2]. At early stages, PC remains an asymptomatic disease. Many signs and symptoms appear gradually with advancing disease as jaundice, abdominal and back pain, weight loss and poor appetite, digestive problems, diabetes and blood clots. Abdominal and back pain has always been one of the major symptoms in patients with PC with more than 50% of patients with PC suffering from pain[3]. There is no pathognomonic pain description, but often, over time, the pain of PC may radiate more through the abdomen to the back area. Due to the preponderance and the relevance of this symptom, in some clinical trials[4,5], pain control was considered as one of the criteria of response to the treatment.
Many physiopathology mechanisms are incriminated in generating pain in this disease. Understanding the mechanism of pain will help the oncologists adapt the treatment panel to ensure a good quality of life for their patients.
We aimed in this review to describe first the different hypotheses of pain physiopathology in pancreatic cancer, to discuss medical and interventional options and finally to present a road map for oncologists leading to the optimum pain management in pancreatic cancer.
RESEARCH
Search strategy for the identification of studies
Electronic searches of the literature, published till September 2015, were conducted using PubMed database to identify articles reporting the pain management of pancreatic cancer.
Only trials describing pancreatic cancer pain management modalities were included. The year of publication of the article was not among the exclusion criteria. Search strategies employed included key words and Boolean operators described as follows: “Pancreatic cancer” and “pain management”. This search was augmented by a hand search of the reference lists of relevant articles included in the literature review. Two different investigators performed the search ML and HK and the abstracts were independently reviewed for possible inclusion.
Types of studies: Review articles, peer review articles and some ongoing trials evaluating pancreatic cancer pain management modalities were included in the review. The language of publication of eligible studies was restricted to English.
Selection criteria
The articles to be included were first selected individually on the basis of their titles by 2 of the authors. Then, the abstracts of the available articles were examined. Our searches yielded 24 study reports from the PubMed database. These selected articles were then evaluated by 2 authors (ML and HK). Finally, 18 reports published till September 2015 were considered eligible by both authors and were retrieved for data extraction. Some of the remaining studies were used in the introduction and the discussion sections.
PHYSIOPATHOLOGY OF PAIN IN PC
Pain in PC may be the result of three components: Visceral, somatic, and neuropathic pain. Visceral nociceptive influxes are produced by ductal obstruction, damage and inflammation in the upper abdominal viscera. Somatic pain is caused by cancer extension into the peritoneum and bones. The signals reach the celiac plexus nerves, at T12-L1 vertebral levels, via the sympathetic system. Primary sensory neurons involved in pain sensation release predominantly substance P and glutamate in the dorsal horn of the spinal cord. From there, they synapse through the splanchnic nerves with T5-T12 dorsal root ganglia, then to the central nervous system[6]. The most characteristic aspect of pancreatic neoplasia is an extrapancreatic nerve plexus invasion, which consists of the first route of metastasis. This may explain the neuropathic pain sensation. Pour et al[7] highlighted the histogenetic similarity between neuronal cells and the pancreatic cancer cells. The two types of cells may share identical growth factor receptors and surface adhesion molecules and have an affinity to neural tissues. Although PC cells damage nerves[8], several studies showed a harmony with cell-to-cell adhesion and migration of pancreatic cancer cells along the dorsal root ganglia neuritis inducing a mutual trophic nerve-cancer cell interaction[9-11]. Nerve cells neurotrophic factors and chemokines are capable of boosting PC cell invasiveness, multiplication, and locomotion. On the other hand, PC cells secrete neuromodulatory agents, which cause neuroplasticity and neuropathic pain. An increase of density of calcitonin gene-related peptide (CGRP) and tyrosine hydroxylase was noted, connoting a proliferation of nerve growth factors[12]. Studies have shown that growing sensory fibers are associated with necrotic damage of the nerve fiber endings and increased pain sensation[13].
Another described mechanism of pain is the neovascularization and increased density of sensory and sympathetic nerve fibers within these newly formed vessels[12]. Molecules secreted by tumor cells such as vascular endothelial growth factor, artemin, interleukin-1 and prostaglandins play a role in the interaction between the vascular system and the sensory neurons[14,15].
In addition to the local nerve invasion, a high density of macrophages is noted within the tumor, which induces excessive expression and secretion of nerve growth factors[16]. The pancreas is innervated by sensory neurons that express CGRP and tyrosine kinase receptor A, which are the receptors of nerve growth factors. The high density of macrophage-secreted nerve growth factors activates the sensory nerves and generates pain afferents. Thus inflammation via macrophages and other inflammatory cells contributes to the pain sensation.
The NGF also activates the transient receptor potential vanilloid receptor subtype 1 (TRPV1)[17]. The TRPV1 is a nonselective cation channel, which increases the permeability for sodium and calcium ions when activated and causes neuronal depolarization with burning sensation and release of substance P. In fact, an upregulation of substance P receptors, neurokinin receptors-1 (NK1-R), was described in pancreatic cancer. NK-R1 antagonist MEN 11467 inhibited pancreatic cancer cell growth[18].
Of interest, the presence of pain is a predictor of survival in pancreatic adenocarcinoma[5,15,19]. This is not the case in other pancreatic malignancies where tumoral invasion mechanisms are different and do not imply neural invasion. In addition, the localization of the tumor plays a role in the pain pattern, pancreatic body presenting the highest incidence cancer localization in pancreas[20]. This is probably due to its proximity to the celiac plexus.
In conclusion, pain in pancreatic cancer has a complex physiopathology. It eminently implies a neuronal invasion and a neurogenic inflammation.
PAIN MANAGEMENT MODALITIES IN PC
Medical approach
Opiods and derviates: Most cancer related pain is controlled by pharmacological oral treatments. The optimal management is based on the World Health Organization (WHO)’s analgesic ladder, with a progressive administration of nonopioids (aspirin and paracetamol); then, as necessary, mild opioids (codeine); then strong opioids, until reaching pain relief. Morphine, the standard “step 3” opioid, has been widely used for the control of chronic cancer pain, especially in moderate to severe pain[21], and is the first-line medical therapy for pancreatic cancer[22]. Oral route is the gold standard with two types of molecules: Normal release with rapid onset for breakthrough pain and modified release marked by a long acting effect, used for maintenance treatment[21]. The second route of administration, if patient is unable of oral intake is subcutaneous. The oral, sublingual and nebulized routes of administration of morphine are not common because there is no evident superiority over the oral route[21].
Fentanyl is an alternative to morphine for cancer pain management in its different administration routes. Transdermal patches are suitable for patients whose opioids requirements are stable[23,24]. Sublingual fentanyl tablets and nasal spray may be an option for breakthrough pain[25,26].
Oxycodon showed a superior analgesic effect on visceral pain in a tissue-differentiated experimental pain model[27]. This hypothesis was not clinically confirmed. Similar analgesia is provided with oral morphine and oxycodon[28] and particularly in PC[29].
Buprenorphine is another strong opioid available as a prescription choice. A systemic review by Schmidt-Hansen et al[30] ranked Buprenorphine as a “fourth-line option” after morphine, oxycodone and fentanyl in cancer pain management.
At the end-of-life stage in advanced PC, thoracic epidural opioid in combination with local anesthetics could be considered[31].
Opioids must be employed with regards to their considerable potential for side effects including sedation and respiratory depression, pruritus, nausea and constipation. The prescription of a stool softener and laxatives for constipation, diphenhydramine for pruritus, and antiemetics for nausea are recommended.
Apart from the classically known opioids side effects, several experimental studies performed on mouse models showed that morphine can interfere with regulation of cancer cell growth by inhibiting apoptosis and by stimulating angiogenesis and metastasis[32,33]. So far, the results of both in vitro and in vivo studies are contradictory and not conclusive. The effect appears to vary depending on the cancer cell type. Prospective randomized controlled trials are currently conducted to find an answer to this dilemma[34]. In the meanwhile, opioids are still a central cornerstone in the management of pancreatic cancer pain.
Antiepileptics: Considering the multidimensional PC pain mechanisms, adjuvant medications and multimodal approach are recommended. Gabapentin and Pregabalin are anti-epileptic agents used as first line treatment in neuropathic pain, including diabetic neuropathy[35], post herpetic pain[36], and neuropathic pain of central origin[37]. Their effectiveness is also demonstrated in cancer-related neuropathic pain[38]. Their target is voltage dependent calcium channel. They block calcium influx into presynaptic nerve terminals, thus reducing the release of excitatory neurotransmitters on spinal neurons. Upstream transmission of neuropathic pain in the central nervous system and neuronal excitability are consecutively inhibited[39]. These molecules may have beneficial effects as adjuvants and opioids - sparing for coeliac plexus pain[40,41].
Corticosteroids: Steroids have been proved particularly useful as adjuvant therapy for visceral pain[42]. Glucocorticoids inhibit prostaglandin synthesis, a precursor of inflammation cascade, and reduce vascular permeability decreasing tissue edema. Steroid receptors are also localized in the central and peripheral nervous systems and are responsible for neuron plasticity, decreasing discharge in an injured nerve and in neuropathic pain[43]. Moreover, perioperative dexamethasone administration has been associated with improved survival after pancreatic adenocarcinoma resection[44].
Approaches under investigation
As mentioned before, neurokinin receptors-1 receptor and its ligand substance P are activated in PC, which contribute to cancer cell growth and neuronal invasion[18]. A promising treatment approach is to target this pathway by NK-1R antagonists MEN 11467, inhibiting nerves alteration and neuropathic pain. Table 1 summarizes the molecules under investigation[17,18,45-47].
Table 1.
Potential molecules that were under investigation
| Molecule | Mechanisms | Ref. |
| Capsaicin/resiniferatoxin | Activation of vanilloid receptors on cancer cells, inducing apoptosis | Hartel et al[17] |
| MEN 11467 | NK-1R antagonists, inhibiting cell growth and neuronal invasion | Friess et al[18] |
| HSV-Enk viral vector | Increased met-enkephalin production in the peripheral nerve terminal endings and in dorsal root ganglion, reducing pain | Lu et al[45] Yang et al[46] |
| Phentolamine | Alpha-adrenergic blockade of sympathetic induced pain | Yasukawa[47] |
NK-1R: Neurokinin-1 receptors; HSV-enk: Herpes simplex virus carrying the human preproenkephalin.
Chemotherapy and radiation therapy
Pain control is largely implemented as secondary end point in the trials evaluating chemotherapeutical regimen in PC. In 1997, the approval of gemcitabine as the standard of care in the treatment of advanced PC was not only based on the small benefit in survival (less than 2 mo) but also on the better pain control. Twenty-three point eight percent of patients receiving Gemcitabine in advanced PC were classified as positive in pain category (pain intensity or/and analgesic use was reduced), while only 4.8% of patients receiving 5-FU were considered in the same category. FOLFIRINOX also showed a better quality of life and pain control in the management of metastatic PC[4,5].
Radiation therapy is frequently used in PC for many purposes going from local control of the disease when associated to chemotherapy in adjuvant or locally advanced setting, to pain management and control in palliative and metastatic disease. Radiotherapy is particularly effective in controlling and relieving pain caused by large tumors compressing other organs or structures, such as nerves or the spine. The effect of radiation therapy is usually late, and it occurring many weeks after the initiation of treatment. The radiation therapy can shrink the tumor, which may help in relieving the pain. The radiation therapy can also be effective in targeting some metastatic lesions.
Interventional therapies
Celiac plexus block and neurolysis: Despite all the available analgesics and the possibility of combination of different molecules, treatment is often suboptimal, and many side effects are observed[48,49]. Thus, more efficient forms of pain management are essential for such patients. An alternative approach is the use of celiac plexus block (CPB) or neurolysis (CPN).
The major component of pancreatic carcinoma pain is mediated by sympathetic fibers from the pancreas and is relayed through the celiac plexus to the splanchnic nerves. A local anesthetic, mainly bupivacaine, can be used in combination with steroids to temporary inhibit the celiac plexus[50]. Celiac plexus neurolysis (CPN) represents the prolonged interruption of the plexus by the injection of alcohol or phenol[51]. Formerly, it was managed by anesthesiologists and radiologists via a posterior approach. However, complications occurred in 1% of cases, secondary to displacement of the needle, causing paraplegia or pneumothorax when entering the spinal artery or diaphragm respectively[52-54]. Anterior approach evolved lately via the guidance of transcutaneous ultrasound, X-ray fluoroscopy, computed tomography, or recently endoscopic ultrasonography[55-57]. Endoscopic ultrasonography which permits direct access to the celiac plexus, is an attractive technique compared to percutaneous CPN, because complications can be avoided. Furthermore, it allows ultrasound guided fine needle aspiration sampling and tumor staging[58-60].
Sympathetic inhibition can be as well done at the level of the splanchnic nerves. Splanchnicectomy consists in sectioning the roots of the splanchnic nerves from T5 to T10. The approach by thoracotomy was first described in 1942 by Mallet-Guy[61]. Thoracoscopic splanchnicectomy, less invasive, was introduced in 1993[62]. Good results were noted with thoracoscopic unilateral left splanchnicectomy[63,64]. It can be repeated for right side in case of pain recurrence or done bilaterally from the beginning[63]. With splanchnicectomy, analgesic effects last for approximately 2 mo, even for 3 mo of partial to complete relief[52].
Intrathecal therapy
Intrathecal therapy can be proposed for end-of-life stages or in refractory pain[30]. The implantable intrathecal drug delivery systems are an alternative with reduced drug toxicities and improvement of pain scores[65]. Various molecules can be used including morphine, fentanyl, local anesthetics, baclofen and/or clonidine[66,67].
Alternative medicine
Acupuncture: The developments of effective and safe therapeutic strategies that complement pharmaceutical treatment are interesting in the management of PC pain. Acupuncture analgesia has been widely used to treat cancer pain[68]. A systemic review published in 2012 including a total of 15 randomized controlled trials, showed that acupuncture is an effective adjunctive method in cancer pain management and pain relief was superior compared to drug therapy alone[69]. Chen et al[70] showed the effect of acupuncture on PC pain. Acupuncture’s effects can be explained as viscero-cutaneous, cutaneo-visceral, cutaneo-muscular, and viscero-muscular reflexes[71]. Jiaji points, which correlate to segmental dispersion of the sympathetic and parasympathetic systems, were chosen on the nerve segments of the pancreas.
Hypnosis
Hypnosis is an increasingly used approach to pain control in patients with cancer. There is significant evidence that hypnosis is useful at reducing cancer-related pain[72,73]. Hypnosis modulates the pain sensation by a functional disconnection between the prefrontal cortex, center of decision-making and the anterior cingulate, zone playing a role in attention and motivation. This will make therapeutic suggestions easily integrated[74].
DISCUSSION
With the presence of a large panel of strategies and modalities in the pain management of PC and the absence of clear recommendations and guidelines, every oncologist is treating pain in PC according to their own experience. The choice of pain management modality depends of the pain characteristics and physiopathology, the stage and the prognosis of the disease, the performance status of the patient, his comorbidities, his past medical history and his future therapeutic options (Figure 1).
Figure 1.
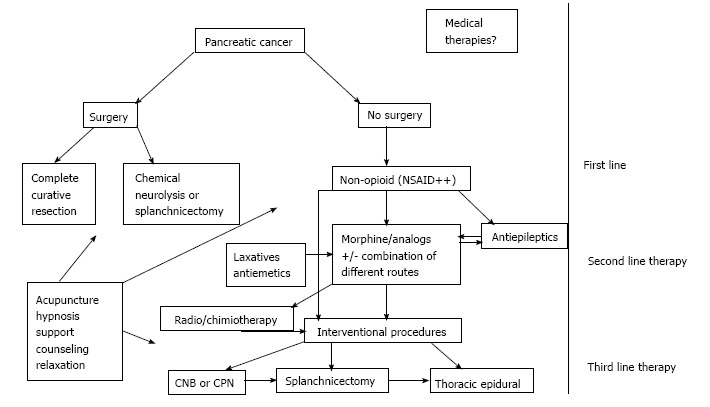
Algorithm of pancreatic cancer pain management. CPN: Celiac plexus neurolysis: NSAID: Non-steroidal anti-inflammatory drug.
Owing to the fact that PC pain involves visceral, somatic and neuropathic components, the main therapeutic approach is a multimodal analgesia. A combination of drugs and/or techniques with distinct mechanisms of action leads to a synergistic effect and allows a better analgesia with decreased consumption of opioids, and hence lesser opioid-related adverse events. A standardized multimodal analgesia protocol based on available evidence is hard to be proposed in PC patients. The essential points remain the addition of antiepileptics for neuropathic pain, the frequent use of corticosteroids and the referral to an interventional procedure after a pluridisciplinary case study. The patients with metastatic disease will benefit more from systemic therapies if the pain is multi-localized and generalized. However, local treatments such as radiation therapy can play a role in controlling pain by targeting some well-defined metastasis generating pain. Patient with a poor prognosis (survival less than three months) will not benefit from an interventional therapeutic approach as patients with better prognosis, because of the risk of the intervention, especially in patients with bad performance status, compared to the time of benefice.
The presence of comorbidities or the past medical history can guide the pain management in PC. Chronic use of corticoids should be careful because of the increased risk of gastric bleeding, especially when combined to non-steroidal anti-inflammatory drugs, which amplify the risk by 15-folds[75]. Gastric protection should be considered. Patients presenting long-term constipation or ileus paralytic should receive morphine associated to laxatives with close surveillance. Patients with PC have a higher risk of developing thrombosis, and are usually under prophylactic or therapeutic anti-coagulants. Special considerations should be taken to discontinue anticoagulant therapy in interventional therapeutic approach (celiac plexus block, intra-thecal treatment), if the patient is under chronic thromboprophylactic prevention for primary or secondary risk. The discontinuation of anticoagulation is sometimes a reasonable cause to choose less invasive analgesic strategies in high-risk patients.
In PC patients who have not received chemotherapeutical agents, transitory painkillers and not a definitive pain management approach will be a logical option, while waiting for the response to the treatment.
Finally, the choice of pain management modality can be influenced by the team expertise and abilities. Celiac plexus neurolysis under endoscopic ultrasonography requires specialized equipment and expert specialists, which are not always available. CT scan or X-ray fluoroscopy guided posterior approach remains an equivalent technique with good results. Thoracotomic bilateral splanchnicectomy is mainly reserved for patients who require abdominal surgery (occlusion, biliary and/or intestinal bypass), making it a two-in-one procedure with no additional surgical risk for the patient[76].
CONCLUSION
Developing pain management recommendations in pancreatic cancer seems to be an interesting and practical target for oncologists and palliative care physicians, since more than the half of these patients will develop severe pain without any detailed and structured road map. We exposed all the pain treatments that are available and proven efficient and safe, medical multimodal treatment, radiotherapy, chemotherapy and finally interventional techniques as a last resort (Table 2). Team expertise and competence remain the major factors in the treatment choice.
Table 2.
Summarizing all treatment approaches, administration route or techniques and indications
| Treatment approaches | Medications and modalities | Administration route/techniques | Indications |
| Medical treatment | Opioids and derivates | Per os | Moderate to severe pain |
| S/C | Impossible oral intake (occlusion) | ||
| Patch (fentanyl) | Breakthrough pain | ||
| Nasal | Breakthrough pain | ||
| Antiepileptics | Per os | Neuropathic pain | |
| corticosteroids | Per os or IV | Adjuvant, especially in metastatic bone pain | |
| Interventional treatment | Celiac plexus block (LA) or neuolysis | Transcutaneous guidance1 or endoscopic ultrasonography | Refractory pain |
| Splanchnicectomy | Thoracotomy, thoracoscopy | Intractable pain in non resectable tumor | |
| Intrathecal therapy | Implantable intrathecal drug delivery systems | End-of-life stage | |
| Alternative medicine | Acupuncture | Jiaji points | adjuvant to drug therapy and/or interventional techniques |
| Hypnosis | Sessions with an expertise | Any stage of pain | |
| Chemotherapy and radiation therapy | FOLFIRINOX | IV | Locally advanced tumor |
| Gemcitabine |
Ultrasound, X-ray fluoroscopy or computed tomography. S/C: Subcutaneous; IV: Intravenous; LA: Local anesthetics.
Footnotes
Conflict-of-interest statement: Authors confirm that they do not have any conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: Lebanon
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: February 12, 2016
First decision: March 23, 2016
Article in press: May 27, 2016
P- Reviewer: Ramia J, Sperti C S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Seufferlein T, Bachet JB, Van Cutsem E, Rougier P. Pancreatic adenocarcinoma: ESMO-ESDO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2012;23 Suppl 7:vii33–vii40. doi: 10.1093/annonc/mds224. [DOI] [PubMed] [Google Scholar]
- 3.D’Haese JG, Hartel M, Demir IE, Hinz U, Bergmann F, Büchler MW, Friess H, Ceyhan GO. Pain sensation in pancreatic diseases is not uniform: the different facets of pancreatic pain. World J Gastroenterol. 2014;20:9154–9161. doi: 10.3748/wjg.v20.i27.9154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burris HA, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, Portenoy RK, Storniolo AM, Tarassoff P, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 5.Conroy T, Desseigne F, Ychou M, Bouché O, Guimbaud R, Bécouarn Y, Adenis A, Raoul JL, Gourgou-Bourgade S, de la Fouchardière C, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 6.Furness JB, Callaghan BP, Rivera LR, Cho HJ. The enteric nervous system and gastrointestinal innervation: integrated local and central control. Adv Exp Med Biol. 2014;817:39–71. doi: 10.1007/978-1-4939-0897-4_3. [DOI] [PubMed] [Google Scholar]
- 7.Pour PM, Weide L, Liu G, Kazakoff K, Scheetz M, Toshkov I, Ikematsu Y, Fienhold MA, Sanger W. Experimental evidence for the origin of ductal-type adenocarcinoma from the islets of Langerhans. Am J Pathol. 1997;150:2167–2180. [PMC free article] [PubMed] [Google Scholar]
- 8.Bockman DE, Büchler M, Beger HG. Interaction of pancreatic ductal carcinoma with nerves leads to nerve damage. Gastroenterology. 1994;107:219–230. doi: 10.1016/0016-5085(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 9.Eibl G, Reber HA. A xenograft nude mouse model for perineural invasion and recurrence in pancreatic cancer. Pancreas. 2005;31:258–262. doi: 10.1097/01.mpa.0000175176.40045.0f. [DOI] [PubMed] [Google Scholar]
- 10.Ceyhan GO, Demir IE, Altintas B, Rauch U, Thiel G, Müller MW, Giese NA, Friess H, Schäfer KH. Neural invasion in pancreatic cancer: a mutual tropism between neurons and cancer cells. Biochem Biophys Res Commun. 2008;374:442–447. doi: 10.1016/j.bbrc.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Gil Z, Cavel O, Kelly K, Brader P, Rein A, Gao SP, Carlson DL, Shah JP, Fong Y, Wong RJ. Paracrine regulation of pancreatic cancer cell invasion by peripheral nerves. J Natl Cancer Inst. 2010;102:107–118. doi: 10.1093/jnci/djp456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindsay TH, Jonas BM, Sevcik MA, Kubota K, Halvorson KG, Ghilardi JR, Kuskowski MA, Stelow EB, Mukherjee P, Gendler SJ, et al. Pancreatic cancer pain and its correlation with changes in tumor vasculature, macrophage infiltration, neuronal innervation, body weight and disease progression. Pain. 2005;119:233–246. doi: 10.1016/j.pain.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 13.Grelik C, Allard S, Ribeiro-da-Silva A. Changes in nociceptive sensory innervation in the epidermis of the rat lower lip skin in a model of neuropathic pain. Neurosci Lett. 2005;389:140–145. doi: 10.1016/j.neulet.2005.07.056. [DOI] [PubMed] [Google Scholar]
- 14.Ceyhan GO, Giese NA, Erkan M, Kerscher AG, Wente MN, Giese T, Büchler MW, Friess H. The neurotrophic factor artemin promotes pancreatic cancer invasion. Ann Surg. 2006;244:274–281. doi: 10.1097/01.sla.0000217642.68697.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ceyhan GO, Bergmann F, Kadihasanoglu M, Altintas B, Demir IE, Hinz U, Müller MW, Giese T, Büchler MW, Giese NA, et al. Pancreatic neuropathy and neuropathic pain--a comprehensive pathomorphological study of 546 cases. Gastroenterology. 2009;136:177–186.e1. doi: 10.1053/j.gastro.2008.09.029. [DOI] [PubMed] [Google Scholar]
- 16.Friess H, Zhu ZW, di Mola FF, Kulli C, Graber HU, Andren-Sandberg A, Zimmermann A, Korc M, Reinshagen M, Büchler MW. Nerve growth factor and its high-affinity receptor in chronic pancreatitis. Ann Surg. 1999;230:615–624. doi: 10.1097/00000658-199911000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartel M, di Mola FF, Selvaggi F, Mascetta G, Wente MN, Felix K, Giese NA, Hinz U, Di Sebastiano P, Büchler MW, et al. Vanilloids in pancreatic cancer: potential for chemotherapy and pain management. Gut. 2006;55:519–528. doi: 10.1136/gut.2005.073205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friess H, Zhu Z, Liard V, Shi X, Shrikhande SV, Wang L, Lieb K, Korc M, Palma C, Zimmermann A, et al. Neurokinin-1 receptor expression and its potential effects on tumor growth in human pancreatic cancer. Lab Invest. 2003;83:731–742. doi: 10.1097/01.lab.0000067499.57309.f6. [DOI] [PubMed] [Google Scholar]
- 19.Müller MW, Friess H, Köninger J, Martin D, Wente MN, Hinz U, Ceyhan GO, Blaha P, Kleeff J, Büchler MW. Factors influencing survival after bypass procedures in patients with advanced pancreatic adenocarcinomas. Am J Surg. 2008;195:221–228. doi: 10.1016/j.amjsurg.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 20.Eyigor C, Karaca B, Kuzeyli-Yildirim Y, Uslu R, Uyar M, Coker A. Does the tumor localization in advanced pancreatic cancer have an influence on the management of symptoms and pain? J BUON. 2010;15:543–546. [PubMed] [Google Scholar]
- 21.Hanks GW, Conno F, Cherny N, Hanna M, Kalso E, McQuay HJ, Mercadante S, Meynadier J, Poulain P, Ripamonti C, et al. Morphine and alternative opioids in cancer pain: the EAPC recommendations. Br J Cancer. 2001;84:587–593. doi: 10.1054/bjoc.2001.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hemati K, Zaman B, Hassani V, Imani F, Dariaie P. Efficacy of fentanyl transdermal patch in the treatment of chronic soft tissue cancer pain. Anesth Pain Med. 2015;5:e22900. doi: 10.5812/aapm.22900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heim M. Noninterventional study of transdermal fentanyl (fentavera) matrix patches in chronic pain patients: analgesic and quality of life effects. Pain Res Treat. 2015;2015:198343. doi: 10.1155/2015/198343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takakuwa O, Oguri T, Maeno K, Murase H, Asano T, Ichikawa H, Kawaguchi Y, Uemura T, Ohkubo H, Takemura M, et al. Long-term use of a once-a-day fentanyl citrate transdermal patch in lung cancer patients. Oncol Lett. 2015;9:2105–2108. doi: 10.3892/ol.2015.3022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thronæs M, Popper L, Eeg M, Jaatun E, Kvitberg M, Kaasa S. Efficacy and tolerability of intranasal fentanyl spray in cancer patients with breakthrough pain. Clin Ther. 2015;37:585–596. doi: 10.1016/j.clinthera.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Staahl C, Christrup LL, Andersen SD, Arendt-Nielsen L, Drewes AM. A comparative study of oxycodone and morphine in a multi-modal, tissue-differentiated experimental pain model. Pain. 2006;123:28–36. doi: 10.1016/j.pain.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt-Hansen M, Bennett MI, Arnold S, Bromham N, Hilgart JS. Oxycodone for cancer-related pain. Cochrane Database Syst Rev. 2015;2:CD003870. doi: 10.1002/14651858.CD003870.pub5. [DOI] [PubMed] [Google Scholar]
- 29.Mercadante S, Tirelli W, David F, Arcara C, Fulfaro F, Casuccio A, Gebbia V. Morphine versus oxycodone in pancreatic cancer pain: a randomized controlled study. Clin J Pain. 2010;26:794–797. doi: 10.1097/ajp.0b013e3181ecd895. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt-Hansen M, Bromham N, Taubert M, Arnold S, Hilgart JS. Buprenorphine for treating cancer pain. Cochrane Database Syst Rev. 2015;3:CD009596. doi: 10.1002/14651858.CD009596.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sloan PA. Thoracic epidural analgesics provide excellent cancer pain relief at the end of life. J Opioid Manag. 2010;6:161. doi: 10.5055/jom.2010.0105. [DOI] [PubMed] [Google Scholar]
- 32.Gupta K, Kshirsagar S, Chang L, Schwartz R, Law PY, Yee D, Hebbel RP. Morphine stimulates angiogenesis by activating proangiogenic and survival-promoting signaling and promotes breast tumor growth. Cancer Res. 2002;62:4491–4498. [PubMed] [Google Scholar]
- 33.Singleton PA, Moss J. Effect of perioperative opioids on cancer recurrence: a hypothesis. Future Oncol. 2010;6:1237–1242. doi: 10.2217/fon.10.99. [DOI] [PubMed] [Google Scholar]
- 34.Juneja R. Opioids and cancer recurrence. Curr Opin Support Palliat Care. 2014;8:91–101. doi: 10.1097/SPC.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 35.Rosenstock J, Tuchman M, LaMoreaux L, Sharma U. Pregabalin for the treatment of painful diabetic peripheral neuropathy: a double-blind, placebo-controlled trial. Pain. 2004;110:628–638. doi: 10.1016/j.pain.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Stacey BR, Barrett JA, Whalen E, Phillips KF, Rowbotham MC. Pregabalin for postherpetic neuralgia: placebo-controlled trial of fixed and flexible dosing regimens on allodynia and time to onset of pain relief. J Pain. 2008;9:1006–1017. doi: 10.1016/j.jpain.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 37.Vranken JH, Dijkgraaf MG, Kruis MR, van der Vegt MH, Hollmann MW, Heesen M. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain. 2008;136:150–157. doi: 10.1016/j.pain.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 38.Caraceni A, Zecca E, Martini C, De Conno F. Gabapentin as an adjuvant to opioid analgesia for neuropathic cancer pain. J Pain Symptom Manage. 1999;17:441–445. doi: 10.1016/s0885-3924(99)00033-0. [DOI] [PubMed] [Google Scholar]
- 39.Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Göthert M. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–236. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 40.Pelham A, Lee MA, Regnard CB. Gabapentin for coeliac plexus pain. Palliat Med. 2002;16:355–356. doi: 10.1191/0269216302pm548xx. [DOI] [PubMed] [Google Scholar]
- 41.Olesen SS, Graversen C, Bouwense SA, van Goor H, Wilder-Smith OH, Drewes AM. Quantitative sensory testing predicts pregabalin efficacy in painful chronic pancreatitis. PLoS One. 2013;8:e57963. doi: 10.1371/journal.pone.0057963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vyvey M. Steroids as pain relief adjuvants. Can Fam Physician. 2010;56:1295–1297, e415. [PMC free article] [PubMed] [Google Scholar]
- 43.Mensah-Nyagan AG, Meyer L, Schaeffer V, Kibaly C, Patte-Mensah C. Evidence for a key role of steroids in the modulation of pain. Psychoneuroendocrinology. 2009;34 Suppl 1:S169–S177. doi: 10.1016/j.psyneuen.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 44.Call TR, Pace NL, Thorup DB, Maxfield D, Chortkoff B, Christensen J, Mulvihill SJ. Factors associated with improved survival after resection of pancreatic adenocarcinoma: a multivariable model. Anesthesiology. 2015;122:317–324. doi: 10.1097/ALN.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 45.Lu Y, McNearney TA, Lin W, Wilson SP, Yeomans DC, Westlund KN. Treatment of inflamed pancreas with enkephalin encoding HSV-1 recombinant vector reduces inflammatory damage and behavioral sequelae. Mol Ther. 2007;15:1812–1819. doi: 10.1038/sj.mt.6300228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang H, McNearney TA, Chu R, Lu Y, Ren Y, Yeomans DC, Wilson SP, Westlund KN. Enkephalin-encoding herpes simplex virus-1 decreases inflammation and hotplate sensitivity in a chronic pancreatitis model. Mol Pain. 2008;4:8. doi: 10.1186/1744-8069-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yasukawa M, Yasukawa K, Kamiizumi Y, Yokoyama R. Intravenous phentolamine infusion alleviates the pain of abdominal visceral cancer, including pancreatic carcinoma. J Anesth. 2007;21:420–423. doi: 10.1007/s00540-007-0528-8. [DOI] [PubMed] [Google Scholar]
- 48.Schmulewitz N, Hawes R. EUS-guided celiac plexus neurolysis--technique and indication. Endoscopy. 2003;35:S49–S53. doi: 10.1055/s-2003-41530. [DOI] [PubMed] [Google Scholar]
- 49.Tran QN, Urayama S, Meyers FJ. Endoscopic ultrasound-guided celiac plexus neurolysis for pancreatic cancer pain: a single-institution experience and review of the literature. J Support Oncol. 2006;4:460–462, 464; discussion 463-464. [PubMed] [Google Scholar]
- 50.Collins D, Penman I, Mishra G, Draganov P. EUS-guided celiac block and neurolysis. Endoscopy. 2006;38:935–939. doi: 10.1055/s-2006-944734. [DOI] [PubMed] [Google Scholar]
- 51.Penman ID, Gilbert D. Basic technique for celiac plexus block/neurolysis. Gastrointest Endosc. 2009;69:S163–S165. doi: 10.1016/j.gie.2008.12.036. [DOI] [PubMed] [Google Scholar]
- 52.Eisenberg E, Carr DB, Chalmers TC. Neurolytic celiac plexus block for treatment of cancer pain: a meta-analysis. Anesth Analg. 1995;80:290–295. doi: 10.1097/00000539-199502000-00015. [DOI] [PubMed] [Google Scholar]
- 53.Davies DD. Incidence of major complications of neurolytic coeliac plexus block. J R Soc Med. 1993;86:264–266. doi: 10.1177/014107689308600507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdalla EK, Schell SR. Paraplegia following intraoperative celiac plexus injection. J Gastrointest Surg. 1999;3:668–671. doi: 10.1016/s1091-255x(99)80091-2. [DOI] [PubMed] [Google Scholar]
- 55.Caratozzolo M, Lirici MM, Consalvo M, Marzano F, Fumarola E, Angelini L. Ultrasound-guided alcoholization of celiac plexus for pain control in oncology. Surg Endosc. 1997;11:239–244. doi: 10.1007/s004649900334. [DOI] [PubMed] [Google Scholar]
- 56.Wang PJ, Shang MY, Qian Z, Shao CW, Wang JH, Zhao XH. CT-guided percutaneous neurolytic celiac plexus block technique. Abdom Imaging. 2006;31:710–718. doi: 10.1007/s00261-006-9153-5. [DOI] [PubMed] [Google Scholar]
- 57.Puli SR, Reddy JB, Bechtold ML, Antillon MR, Brugge WR. EUS-guided celiac plexus neurolysis for pain due to chronic pancreatitis or pancreatic cancer pain: a meta-analysis and systematic review. Dig Dis Sci. 2009;54:2330–2337. doi: 10.1007/s10620-008-0651-x. [DOI] [PubMed] [Google Scholar]
- 58.Buscail L, Faure P, Bournet B, Selves J, Escourrou J. Interventional endoscopic ultrasound in pancreatic diseases. Pancreatology. 2006;6:7–16. doi: 10.1159/000090022. [DOI] [PubMed] [Google Scholar]
- 59.Prasad P, Wittmann J, Pereira SP. Endoscopic ultrasound of the upper gastrointestinal tract and mediastinum: diagnosis and therapy. Cardiovasc Intervent Radiol. 2006;29:947–957. doi: 10.1007/s00270-005-0184-z. [DOI] [PubMed] [Google Scholar]
- 60.Si-Jie H, Wei-Jia X, Yang D, Lie Y, Feng Y, Yong-Jian J, Ji L, Chen J, Liang Z, De-Liang F. How to improve the efficacy of endoscopic ultrasound-guided celiac plexus neurolysis in pain management in patients with pancreatic cancer: analysis in a single center. Surg Laparosc Endosc Percutan Tech. 2014;24:31–35. doi: 10.1097/SLE.0000000000000032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mallet-Guy P. La splanchnicectomie gauche dans le traitement des pancreatites chronique. Presse med. 1943;51:145–146. [PubMed] [Google Scholar]
- 62.Worsey J, Ferson PF, Keenan RJ, Julian TB, Landreneau RJ. Thoracoscopic pancreatic denervation for pain control in irresectable pancreatic cancer. Br J Surg. 1993;80:1051–1052. doi: 10.1002/bjs.1800800842. [DOI] [PubMed] [Google Scholar]
- 63.Tomulescu V, Grigoroiu M, Stănescu C, Kosa A, Merlusca G, Vasilescu C, Ionescu M, Popescu I. [Thoracoscopic splanchnicectomy--a method of pain palliation in non-resectable pancreatic cancer and chronic pancreatitis] Chirurgia (Bucur) 2005;100:535–540. [PubMed] [Google Scholar]
- 64.Leksowski K. Thoracoscopic splanchnicectomy for control of intractable pain due to advanced pancreatic cancer. Surg Endosc. 2001;15:129–131. doi: 10.1007/s004640090009. [DOI] [PubMed] [Google Scholar]
- 65.Burton AW, Rajagopal A, Shah HN, Mendoza T, Cleeland C, Hassenbusch SJ, Arens JF. Epidural and intrathecal analgesia is effective in treating refractory cancer pain. Pain Med. 2004;5:239–247. doi: 10.1111/j.1526-4637.2004.04037.x. [DOI] [PubMed] [Google Scholar]
- 66.Smith HS, Deer TR, Staats PS, Singh V, Sehgal N, Cordner H. Intrathecal drug delivery. Pain Physician. 2008;11:S89–S104. [PubMed] [Google Scholar]
- 67.Nielsen JB, Sjøgren P. [Clonidine in the treatment of cancer pain] Ugeskr Laeger. 2008;170:3650–3653. [PubMed] [Google Scholar]
- 68.Lu W, Dean-Clower E, Doherty-Gilman A, Rosenthal DS. The value of acupuncture in cancer care. Hematol Oncol Clin North Am. 2008;22:631–648, viii. doi: 10.1016/j.hoc.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Choi TY, Lee MS, Kim TH, Zaslawski C, Ernst E. Acupuncture for the treatment of cancer pain: a systematic review of randomised clinical trials. Support Care Cancer. 2012;20:1147–1158. doi: 10.1007/s00520-012-1432-9. [DOI] [PubMed] [Google Scholar]
- 70.Chen H, Liu TY, Kuai L, Zhu J, Wu CJ, Liu LM. Electroacupuncture treatment for pancreatic cancer pain: a randomized controlled trial. Pancreatology. 2013;13:594–597. doi: 10.1016/j.pan.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 71.Cabioglu MT, Arslan G. Neurophysiologic basis of Back-Shu and Huatuo-Jiaji points. Am J Chin Med. 2008;36:473–479. doi: 10.1142/S0192415X08005916. [DOI] [PubMed] [Google Scholar]
- 72.Montgomery GH, Schnur JB, Kravits K. Hypnosis for cancer care: over 200 years young. CA Cancer J Clin. 2013;63:31–44. doi: 10.3322/caac.21165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jensen MP. Hypnosis for chronic pain management: a new hope. Pain. 2009;146:235–237. doi: 10.1016/j.pain.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 74.Taylor AG, Goehler LE, Galper DI, Innes KE, Bourguignon C. Top-down and bottom-up mechanisms in mind-body medicine: development of an integrative framework for psychophysiological research. Explore (NY) 2010;6:29–41. doi: 10.1016/j.explore.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piper JM, Ray WA, Daugherty JR, Griffin MR. Corticosteroid use and peptic ulcer disease: role of nonsteroidal anti-inflammatory drugs. Ann Intern Med. 1991;114:735–740. doi: 10.7326/0003-4819-114-9-735. [DOI] [PubMed] [Google Scholar]
- 76.Masuda T, Kuramoto M, Shimada S, Ikeshima S, Yamamoto K, Nakamura K, Baba H. Splanchnicectomy for pancreatic cancer pain. Biomed Res Int. 2014;2014:941726. doi: 10.1155/2014/941726. [DOI] [PMC free article] [PubMed] [Google Scholar]


