Abstract
AIM
To assess blood chitinase 3-like 1 (CHi3L1) levels for 2 mo after minimally invasive colorectal resection (MICR) for colorectal cancer (CRC).
METHODS
CRC patients in an Institutional Review Board approved data/plasma bank who underwent elective MICR for whom preoperative (PreOp), early postoperative (PostOp), and 1 or more late PostOp samples [postoperative day (POD) 7-27] available were included. Plasma CHi3L1 levels (ng/mL) were determined in duplicate by enzyme linked immunosorbent assay.
RESULTS
PreOp and PostOp plasma sample were available for 80 MICR cancer patients for the study. The median PreOp CHi3L1 level was 56.8 CI: 41.9-78.6 ng/mL (n = 80). Significantly elevated (P < 0.001) median plasma levels (ng/mL) over PreOp levels were detected on POD1 (667.7 CI: 495.7, 771.7; n = 79), POD 3 (132.6 CI: 95.5, 173.7; n = 76), POD7-13 (96.4 CI: 67.7, 136.9; n = 62), POD14-20 (101.4 CI: 80.7, 287.4; n = 22), and POD 21-27 (98.1 CI: 66.8, 137.4; n = 20, P = 0.001). No significant difference in plasma levels were noted on POD27-41.
CONCLUSION
Plasma CHi3L1 levels were significantly elevated for one month after MICR. Persistently elevated plasma CHi3L1 may support the growth of residual tumor and metastasis.
Keywords: Colorectal cancer, Recurrence, Minimally inasive colorectal resection, Chitinase 3-like 1, Metastasis
Core tip: Colorectal cancer (CRC) resection surgery is well known to be associated with short lived immunosuppression and transient plasma protein changes. We have documented that a second set of blood protein alterations that last for 3 to 5 wk after CRC; interestingly, all of these proteins play a role in angiogenesis. This group of pro-angiogenic proteins includes vascular endothelial growth factor, placental growth factor, angiopoietin-2, monocyte chemo-attractant protein-1 and matrix metalloproteinase 2. Our published data further confirms that pro-angiogenic postoperative plasma from cancer patients stimulates in vitro endothelial cell proliferation, migration, and invasion. In this manuscript we are presenting data to demonstrate that a pro-angiogenic protein, chitinase 3-like 1, in CRC patients remain elevated for month after minimally invasive colorectal resection.
INTRODUCTION
Colorectal cancer (CRC) is the second most diagnosed cancer in the United States, with an expected 142820 cases and 50830 deaths in 2013[1]. Surgical resection is the primary treatment for the 80% of CRC patients who present without metastatic disease. While this procedure is considered curative for these patients, more than 40% who present with stage II or III disease will develop a recurrence[1,2].
It has been hypothesized that surgical resection of tumors may paradoxically contribute to the development of cancer recurrences. Murine studies have shown those laparotomy and bowel resections are associated with increased tumor growth and establishment vs results in anesthesia control mice[3-5]. In humans, numerous case reports have noted increased tumor growth soon after surgery in patients with residual cancer[6-9]. A number of mechanisms, including surgery-related immunosuppression and the removal of primary tumor generated anti-angiogenic factors, have been proposed to account for accelerated tumor growth after surgery[10,11]. Of interest, the last decade has seen the emergence of another possible mechanism, namely surgery-related proangiogenic plasma compositional changes that may support the establishment of metastases and the growth of already present tumor deposits during the early post-surgical period.
Angiogenesis or neo-vascularization is necessary for tumor growth greater than 1-2 mm[3,12,13]. During a nascent tumor’s initial avascular dormant stage, cells obtain nutrients by passive diffusion only. The activation of what has been called the “angiogenic switch” leads to the development of new vessels that infiltrate and extend beyond the tumor mass which permits growth and, later, metastasis. Similarly, further growth of established metastases requires new vessel formation.
It has been shown that colorectal resection is associated with continual elevations (2-5 wk) in the blood levels of a number of angiogenesis promoting proteins including vascular endothelial growth factor (VEGF), angiopoietin-2 (Ang-2), placental growth factor (PlGF), soluble vascular cell adhesion molecule-1 (sVCAM-1), monocyte chemotactic protein-1 (MCP-1), and matrix metalloproteinase-3 (MMP-3)[14-18]. Furthermore, human plasma from the second and third weeks after surgery has been shown to promote endothelial cell (EC) proliferation, invasion, and migration, which are key steps in angiogenesis[14,19]. It is possible, therefore, that the postoperative (PostOp) plasma composition may encourage the growth of residual tumor metastases after “curative” colorectal resection of a primary tumor. In an effort to better understand the impact of surgery the search goes on for other proteins with proangiogenic effects whose blood levels may be increased after surgery.
Chitinase 3-like 1 (CHi3L1), also named as YKL-40 and human cartilage glycoprotein-39 (HCgp-39), is a member of the glycosyl hydrolase 18 protein family[20]. The substrate for this family of proteins is chitin, a polymer of N-acetyl-glucosamine. Importantly, chitins are not found in mammals yet these proteins are produced by a number of mammalian cell types including EC’s neutrophils, macrophages, and vascular smooth muscle cells. CHi3L1 does not have any known enzymatic activity in mammals, however, it is believed that this protein binds to endogenous carbohydrates such as hyaluronic acid and heparin as well as specific receptors such as the interleukin (IL)-13 receptor α2 in macrophages[21]. CHi3L1 and other members of the glycosyl hydrolase 18 protein families are thought to play a role in innate and specific immune function, tissue remodeling, and the proliferation of fibroblasts, epithelial, synovial, and other cell types. CHi3L1 has also been shown to limit inflammation related organ injury and apoptosis in some settings[21].
There is also experimental evidence that CHi3L1 promotes tumor angiogenesis. Several investigators have demonstrated that CHi3L1, in vitro, increased EC (HUVEC) migration and tube formation which are essential initial steps in the process of angiogenesis[22,23]. Macrophages in tumor stroma have also been shown to express CHi3L1 which likely also promotes tumor angiogenesis[24].
Blood levels of CHi3L1 are negligible under normal physiologic conditions, however, elevated blood levels of CHi3L1 have been found in patients with chronic inflammatory conditions[25] and a wide variety of cancers including colorectal[22,26], breast[27], prostate[28], lung[29,30], thyroid[31], endometrial[32] pancreatic[33] hepatocellular[34], ovarian[35], gastric cancer[36], and malignant melanoma[37]. Additionally, for the majority of these malignancies, a correlation has been demonstrated between blood levels of CHi3L1 and a poor prognosis[26,29-44]. Also, increased expression of CHi3L1 in CRC has been shown to be strongly associated with increased microvascular density[22]. Although it has been shown that serum levels of CHi3L1 are elevated prior to surgery in CRC patients, the impact of surgical resection on blood levels after surgery are unknown. Because CHi3L1 involved in tissue remodeling and angiogenesis we hypothesized that following minimally invasive colorectal resection (MICR) for CRC blood levels of CHi3L1 might be increased. This study was carried out to investigate plasma levels of CHi3L1 during the first 4 to 6 wk following MICR for CRC.
MATERIALS AND METHODS
Patient selection for study
Patient populations who underwent elective MICR were selected for this study from an Institutional Review Board (IRB) approved multi center plasma and data bank that was organized by Columbia University and that included the following institutions: New York Presbyterian Hospital, Columbia University; the Ferguson Clinic, Grand Rapids, Michigan and Mount Sinai West Hospital Center, New York. The broadly stated purpose of this effort is to study the physiologic, immunologic, and oncologic ramifications of open and minimally invasive surgical methods. Prospective data including demographic, operative, and short term recovery statistics were collected for all patients. Patients who were immunosuppressed or transfused perioperatively were excluded. Patients undergoing urgent or emergent surgery were, likewise, excluded.
Blood sampling and processing
To be eligible for entry into this study preoperative (PreOp) and, at least, several PostOp plasma samples had to be available for CRC patients who underwent MICR. Of note, blood samples after postoperative day (POD) 7 were obtained at follow up office appointments but were not scheduled on a specific POD. Late post-operative samples were not available on the same day from all patients so the late samples were bundled into 7 d (or longer) time periods (POD 7-13, POD 14-20, POD 21-27, and POD 28-41). Samples were collected in tubes containing heparin. The samples were processed within 6 h of collection, and the plasma fraction stored in aliquots at -80 °C until the assay was performed.
Plasma CHi3L1 analysis
Plasma CHi3L1 levels were determined in duplicate using commercially available enzyme linked immunosorbent assay (ELISA) (R and D Systems, Minneapolis). CHi3L1 concentrations are reported as nanograms per milliliter (ng/mL).
Statistical analysis
For continuous variables, data are expressed as mean ± SD. Frequencies and percentages were determined for categorical variables. In regards to the CRC Pre vs PostOp CHi3L1 comparisons, the results are reported as the median and 95%CIs and the Wilcoxon signed rank test was used to analyze the data. Correlation between PostOp CHi3L1 plasma levels and incision size and length of surgery was evaluated by the Spearman’s rank correlation coefficient (rs) All data analysis was performed utilizing SPSS version 15.0 (SPSS, Inc., Chicago, IL). Because the sample size varies for the POD 7-13, POD 14-20, POD 21-27 and POD 28-41 time points a separate PreOp bar is included for each time point in the Figure 1.
Figure 1.
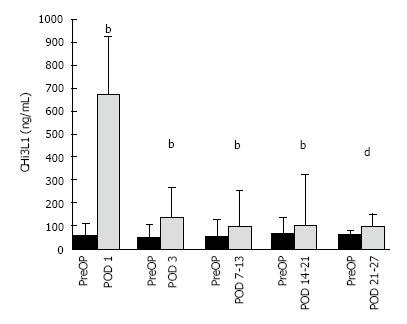
Enzyme linked immunosorbent assay determined preoperative and postoperative chitinase 3-like 1 levels of colorectal cancer patients. CHI3 L1 levels are expressed median and 75% quartile range: [PreOp vs POD1 (n = 79), PreOp vs POD3 (n = 76), PreOp vs POD7-13 (n = 62), PreOp vs POD14-20 (n = 22)] bP ≤ 0.001; PreOp vs POD 21-27 (n = 20), dP = 0.001. PreOp: Preoperative; CHI3L1: Chitinase 3-like 1; POD: Postoperative day.
RESULTS
A total of 80 MICR patients with CRC (42 male/38 female, age 65.66 ± 12.83 years) were included in the study. The majority of patients underwent right colectomy, lower anterior resection/anterior resection, or sigmoidectomy (Table 1). Laparoscopic-assisted methods were used in 59% while hand-assisted minimally invasive methods were utilized in 41% of the patients. There were 12 conversions (15%) to open methods (defined as final incision > 7 cm in laparoscopic-assisted cases and incision > 11 cm in hand-assisted cases). The mean surgical incision length was 7.8 ± 3.6 cm for the entire population, the mean operative time was 308.3 ± 124.2 min. The mean length of stay was 6.6 ± 4.3 d. Seven complications (8.8%) were noted including small bowel obstruction (3), wound infection (1), hernia (1), hyperkalemia (1), and hematoma (1). There were no perioperative deaths. The cancer pathological stage breakdown of the study population was as follows: Stage I, 20 (25.0%); stage II, 25 (31.3%); stage III, 32 (40%); stage IV, 3 (3.7%).
Table 1.
Demographic and clinical characteristics of the study population
| Age, years (mean ± SD) | 65.66 ± 12.83 |
| Sex, n (%) | |
| Male | 42 (52.5) |
| Female | 38 (47.5) |
| Incision length, cm (mean ± SD) | 7.78 ± 3.61 |
| Operative time, min (mean ± SD) | 308.3 ± 124.2 |
| Length of stay, d (mean ± SD) | 6.68 ± 4.30 |
| Type of resection, n (%) | |
| Right | 24 (30.0) |
| Transverse | 5 (6.2) |
| Left | 6 (7.5) |
| Sigmoid/rectosigmoid | 13 (16.3) |
| LAR/AR | 26 (32.5) |
| APR | 2 (2.5) |
| Subtotal/total | 4 (5.0) |
| Surgical method, n (%) | |
| Laparoscopic-assisted | 47 (59.0) |
| Hand-assisted/hybrid laparoscopic | 33(41.0) |
The median PreOp CHi3L1 level was 56.8 CI: 41.9, 78.6 ng/mL (n = 80). Significantly elevated median plasma levels were noted on POD 1 (667.7 CI: 495.7, 771.7 ng/mL, n = 79, P < 0.001), POD 3 (132.6 CI: 95.5, 173.7 ng/mL, n = 76, P < 0.001), POD 7-13 (96.4 CI: 67.7, 136.9 ng/mL, n = 62, P < 0.001), POD 14-20 (101.4 CI: 80.7, 287.4 ng/mL, n = 22, P < 0.001), and POD 21-27 (98.1 CI: 66.8, 137.4 ng/mL, n = 20, P = 0.001) when compared to PreOp levels. The percent increase from median baseline at each time point was 1068% at POD 1, 157% at POD 3, 88% at POD 7-13, 50% at POD 14-20, and 64.0% at POD 21-27. No significant difference found at the POD 28-41 time point.
To determine whether there was a statistical correlation between incision length and post surgery plasma CHi3L1 levels, the laparoscopic-assisted group (mean incision size 5.8 cm) and the hand-assisted group (mean incision, 10.7 ± 3.5 cm) were compared. While the mean incision size for the hand assisted group was higher, there was no statistically significant difference in PostOp plasma CHi3L1 levels between the groups. Furthermore, at 5 of the 6 PostOp time points there was no correlation between CHi3L1 levels and incision length. There was a statistically significant correlation between incision length and CHi3L1 levels at the POD 14-20 time point. There was no significant correlation between cancer stage and PreOp CHi3L1 plasma level.
DISCUSSION
We have demonstrated in several studies that elective surgery for CRC is associated with persistent plasma protein changes that render the blood proangiogenic for 2-3 wk as judged by in vitro invasion, migration and EC proliferation analysis[14-18]. This is in contradistinction to the vast majority of serum proteins that have been studied perioperatively whose levels are transiently elevated after major surgery (alterations resolving in < 1 wk such as IL-2, IL-6, CRP, etc). CHi3L1, which has been shown to promote EC proliferation and tube formation among its other effects, can now be added to the list of persistently elevated proangiogenic proteins.
In this study, mean plasma CHi3L1 levels of 80 CRC patients rose to a peak of 1068% over the PreOp baseline value on POD1, and remained significantly elevated at 50%-157% over baseline for four weeks after MICR until returning to baseline at the POD 28-34 time point. Of note, the percent changes in CHi3L1 levels over baseline were amongst the highest observed when compared to the results of the 9 other proteins previously shown to have long duration plasma increases post MICR.
Because CHi3L1 levels have been shown to be increased preoperatively in cancer patients when compared to tumor free patients, a fall in blood levels would be anticipated after the primary tumor is resected. Why then do plasma levels of CHi3L1 rise during the first month after surgery? Most likely there are several mechanisms. As regards the initial elevation during the first 3-4 d after MICR, although unproven, it is possible that macrophages and polymorphonuclear leukocytes (PMN’s), which play a vital role in the acute inflammatory response that follows surgery, generate the added CHi3L1. This hypothesis is based on the following observations. Both PMN’s and macrophages have been shown to generate CHi3L1 in the setting of chronic inflammation (e.g., IBD and rheumatoid arthritis) and blood levels of CHi3L1, known to be increased in these patients, have been shown to directly correlate with disease severity.
As regards the persistent plasma elevation noted during weeks 2 to 4, the authors believe, also admittedly without direct evidence, that the healing surgical wounds are the source of the added CHi3L1. Since CHi3L1 has been shown to play key role in tissue remodeling as well as angiogenesis it is certainly possible that it is involved with the wound healing process[9,26]. The hypothesis is that wound levels are very high such that the CHi3L1 spills over into the blood stream raising plasma levels. Support for this concept can be found in several human studies that measured VEGF levels in wound fluid and the blood of surgery patients.
In a Dutch study of MICR patients, on POD 4, wound VEGF fluid levels were found to be 7 times higher than serum concentrations which were elevated over PreOp baseline levels[45]. The same research team, in a study of mastectomy patients noted that on POD 4 wound VEGF levels were between 23 and 32 times greater than serum levels[46]. There is also strong preliminary unpublished data of the authors suggesting that wound levels of ANG2, and MMP2 are elevated and many times higher than plasma concentrations after MICR (plasma levels also elevated 3-4 wk). Further, the fact that the proteins shown to have markedly elevated wound levels play roles in neovascularization (like CHi3L1) indirectly suggests that the origin of the CHi3L1 during most of the first PostOp month is the wound. As mentioned above, the authors are currently conducting a study that is simultaneously assessing wound and plasma protein levels which will soon shed more light on this topic.
What are the potential clinical ramifications, if any, of the month long elevation in CHi3L1 levels? There is some research evidence that CHi3L1 may play a direct role in chronic inflammation related epithelial cancer development via up-regulation of β catenin[25]. As mentioned earlier, in addition to promoting angiogenesis in general there is strong in vivo evidence that CHi3L1 promotes tumor angiogenesis in particular. Macrophages are known to promote cancer progression by producing a number of growth and proangiogenic factors[47]. Tumor associated macrophages lack antigen presenting abilities and have been found to cluster in avascular areas of breast carcinomas[47-49]. Kawda et al[22] revealed that the CHi3L1 increased the secretion of inflammatory chemokines, IL-8 and monocyte chemoattractant protein-1 (MCP-1), from SW480 cells via mitogen-activated protein kinase (MAPK) pathway in vitro. Furthermore, CHi3L1 expressing colon cancer cells significantly increased macrophage recruitment in xenograft mice, as well as tumor growth and angiogenesis[22]. Persistently elevated levels of plasma CHi3L1 levels together with IL8 and MCP-1[17] may collectively enhance the blood angiogenic property after MICR.
The proangiogenic effects of CHi3L1 should be considered in the context of the other 9 proteins (all with proangiogenic effects) whose levels have also been shown to be persistently elevated after MICR. There is a concern that these plasma changes might promote tumor angiogenesis early after resection of the primary cancer in patients with established distant micro-metastases. Further, these compositional changes may encourage the establishment of new metastases by circulating viable tumor cells present in the blood stream after surgery. Is there any evidence of increased tumor growth after surgery in humans?
The medical literature contains numerous case reports of rapid growth of residual metastases after major surgery[7-9]. In particular, rapid growth of pre-existing metastatic cancer in the liver after resection of the primary colorectal tumor has been noted by several investigators[6,50-52]. Of note, in one investigation the vascular density of metastases has been noted to be significantly increased over the pre-resection baseline several months after primary resection; this suggests that tumor angiogenesis is stimulated after surgery[53]. Finally, there is strong experimental data showing that surgical trauma is associated with accelerated tumor growth[3-5].
The data from this study and others mentioned above suggest that the first month after surgery may be a precarious time for cancer patients who harbor residual tumor since the plasma composition is proangiogenic for up to a month. The fear is that these conditions will foster blood vessel formation in the tumor and, in so doing, will promote tumor growth. Because conventional adjuvant or palliative PostOp chemotherapy is usually started 4 to 8 wk post operatively the patient is left to their own during this time period. It is logical to administer some type of anti-cancer treatment in this unused time window. The challenge is to find effective anti-cancer agents that do not interfere with the process of wound healing. Presently, a phase I clinical trial is underway that is assessing perioperative treatment with polyphenon E (a green tea extract) and a milk thistle plant component called siliphos. Both agents have been shown to inhibit tumor growth while not inhibiting wound healing which makes them safe for the early PostOp period[54].
A weakness of this study was the relatively small number of late plasma samples collected. Most of the late samples were obtained during follow-up visits scheduled by the patients; unfortunately, many patients refused late PostOp blood draws. Thus, there were fewer late samples than at the PreOp, POD 1, and POD 3 time points. This made it necessary to bundle the late samples into 7 to 13 d blocks. Also, the timing of the late samples varied considerably. A larger study will allow a more meaningful correlation of PreOp CHi3L1 levels and cancer stage. Similarly, a follow up study with more uniform and comprehensive PostOp blood sampling should allow a better assessment of PostOp levels, in general, as well as the impact of surgical method, if any, on post surgery plasma levels.
The results of this study demonstrate that surgical stress, in particular MICR, is associated with notably increased plasma levels of CHi3L1 that persist for 1 mo after surgery. The source of this elevation is unclear. The clinical implications of these findings, if any, are uncertain. In theory, among other possible effects, this transient plasma alteration may promote tumor angiogenesis in patients with residual cancer after surgical resection of the primary tumor. The fact that plasma levels of 9 other proteins with proven proangiogenic effects have been shown to be elevated for 2 to 4 wk after MICR lends support to this hypothesis. Further study of perioperative plasma CHi3L1 levels and of the clinical ramifications of the surgery-related long duration proangiogenic plasma compositional changes appear to be warranted.
COMMENTS
Backgrounds
Major abdominal surgery is well known to be associated with a brief period of immunosuppression and short lived plasma protein changes. Recently, another group of blood protein alterations that lasts at least 3 to 5 wk after colorectal cancer (CRC) resection have been noted. All of these proteins play a role in angiogenesis. This set of proangiogenic proteins includes vascular endothelial growth factor (VEGF), placental growth factor (PlGF), angiopoietin-2, soluble vascular adhesion molecule-1 (sVCAM-1), monocyte chemo-attractant protein-1 (MCP-1) and matrix metalloproteinase-3. Proangiogenic chitinase 3-like 1 (Chi3L) protein promotes in vitro human endothelial cell (EC) migration and tube formation, however, the impact of minimally invasive colorectal resection (MICR) for CRC on plasma levels of CHi3L1 is unknown.
Research frontiers
CHi3L1, is a member of the Chitinase family of proteins and has chemotactic and proangiogenic properties similar to those of MCP-1 and IL8 that are mediated, in part, by MCP-1. Colon, breast, and hepatocellular carcinomas have been shown to express higher levels of CHi3L1. It has been shown that CHi3L1 may utilize its chitin binding ability to communicate with other signal transduction pathways to modulate inflammation, apoptosis, tissue remodeling, cell growth and angiogenesis. CHi3L1 has been shown to promote in vitro cancer cell proliferation, macrophage recruitment, human EC migration and tube formation, and contributes to wound healing. The authors analyzed preoperative (PreOp) and post-MICR CHi3L1 levels in CRC patients. Significantly elevated blood levels of CHi3L1 may promote tumor angiogenesis and therefore, the growth of residual tumor during the first month after MICR.
Innovations and breakthroughs
Persistently elevated plasma levels of the proangiogenic proteins including VEGF, Ang-2, PlGF, sVCAM-1 and MMP3 have been noted for 3-5 wk after CRC resection. Plasma from the 2nd and 3rd weeks after CRC resection has been shown to stimulate EC proliferation and migration in vitro when compared to EC culture results with PreOp plasma. This study reports that plasma CHi3L1 levels are significantly elevated over PreOp levels for a month after MICR for CRC. These persistent plasma compositional changes may promote tumor angiogenesis and growth in patients with residual cancer deposits in the 1st postoperative (PostOp) month.
Applications
This study results further support that persistent plasma compositional changes may promote tumor angiogenesis and growth in patients with residual cancer deposits in the first PostOp month. Thus, it is logical to give anti-cancer therapy perioperatively, for safe use in this period, in addition to having anti-cancer effects, candidate agents must not impair wound healing.
Terminology
The significantly increased blood proangiogenic protein levels during the early PostOp period after MICR and open colorectal resection may be associated with the short lived acute inflammatory response that occurs after surgery and resolves in the first week. In contrast, the later and persistent elevation noted during weeks 2-4 after surgery may be related to wound healing. Persistently elevated plasma CHi3L1 levels shown in this study, together with the similarly increased levels of the other proangiogenic proteins such as VEGF, ANG2, PlGF, sVCAM-1MCP-1and MMP3 may collectively promote tumor angiogenesis and therefore, the growth of residual tumor during the first month after MICR.
Peer-review
The paper is written in a good language, the logic is clear and the subject and results are discussed graphically and meaningfully.
Footnotes
Supported by Mr. Wade Thompson and family donation funds to the Divisions of Colon and Rectal surgery, Department of Surgery, Mount Sinai West Hospital, New York, NY 10019.
Institutional review board statement: This study was reviewed and approved by Mount Sinai Hospital, New York IRB.
Informed consent statement: All subjects in this study provided research study consent.
Conflict-of-interest statement: All authors have no conflicts of interest or financial ties to disclose.
Data sharing statement: No additional data available.
Manuscript source: Unsolicited manuscript
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Peer-review started: July 14, 2015
First decision: October 30, 2015
Article in press: June 3, 2016
P- Reviewer: Aglietta M, Sinagra E, Vizoso FJ S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Virgo KS, Vernava AM, Longo WE, McKirgan LW, Johnson FE. Cost of patient follow-up after potentially curative colorectal cancer treatment. JAMA. 1995;273:1837–1841. [PubMed] [Google Scholar]
- 3.Allendorf JD, Bessler M, Kayton ML, Oesterling SD, Treat MR, Nowygrod R, Whelan RL. Increased tumor establishment and growth after laparotomy vs laparoscopy in a murine model. Arch Surg. 1995;130:649–653. doi: 10.1001/archsurg.1995.01430060087016. [DOI] [PubMed] [Google Scholar]
- 4.Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs laparoscopic bowel resection in mice. Surg Endosc. 1998;12:1035–1038. doi: 10.1007/s004649900775. [DOI] [PubMed] [Google Scholar]
- 5.Carter JJ, Feingold DL, Kirman I, Oh A, Wildbrett P, Asi Z, Fowler R, Huang E, Whelan RL. Laparoscopic-assisted cecectomy is associated with decreased formation of postoperative pulmonary metastases compared with open cecectomy in a murine model. Surgery. 2003;134:432–436. doi: 10.1067/s0039-6060(03)00136-3. [DOI] [PubMed] [Google Scholar]
- 6.Peeters CF, de Waal RM, Wobbes T, Westphal JR, Ruers TJ. Outgrowth of human liver metastases after resection of the primary colorectal tumor: a shift in the balance between apoptosis and proliferation. Int J Cancer. 2006;119:1249–1253. doi: 10.1002/ijc.21928. [DOI] [PubMed] [Google Scholar]
- 7.Lange PH, Hekmat K, Bosl G, Kennedy BJ, Fraley EE. Acclerated growth of testicular cancer after cytoreductive surgery. Cancer. 1980;45:1498–1506. doi: 10.1002/1097-0142(19800315)45:6<1498::aid-cncr2820450633>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Crawford SE, Flores-Stadler EM, Huang L, Tan XD, Ranalli M, Mu Y, Gonzalez-Crussi F. Rapid growth of cutaneous metastases after surgical resection of thrombospondin-secreting small blue round cell tumor of childhood. Hum Pathol. 1998;29:1039–1044. doi: 10.1016/s0046-8177(98)90410-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee CG, Da Silva CA, Dela Cruz CS, Ahangari F, Ma B, Kang MJ, He CH, Takyar S, Elias JA. Role of chitin and chitinase/chitinase-like proteins in inflammation, tissue remodeling, and injury. Annu Rev Physiol. 2011;73:479–501. doi: 10.1146/annurev-physiol-012110-142250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allendorf JD, Bessler M, Horvath KD, Marvin MR, Laird DA, Whelan RL. Increased tumor establishment and growth after open vs laparoscopic surgery in mice may be related to differences in postoperative T-cell function. Surg Endosc. 1999;13:233–235. doi: 10.1007/s004649900952. [DOI] [PubMed] [Google Scholar]
- 11.O’Reilly MS, Holmgren L, Shing Y, Chen C, Rosenthal RA, Moses M, Lane WS, Cao Y, Sage EH, Folkman J. Angiostatin: a novel angiogenesis inhibitor that mediates the suppression of metastases by a Lewis lung carcinoma. Cell. 1994;79:315–328. doi: 10.1016/0092-8674(94)90200-3. [DOI] [PubMed] [Google Scholar]
- 12.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 13.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 14.Shantha Kumara HMC, Feingold D, Kalady M, Dujovny N, Senagore A, Hyman N, Cekic V, Whelan RL. Colorectal resection is associated with persistent proangiogenic plasma protein changes: postoperative plasma stimulates in vitro endothelial cell growth, migration, and invasion. Ann Surg. 2009;249:973–977. doi: 10.1097/SLA.0b013e3181a6cd72. [DOI] [PubMed] [Google Scholar]
- 15.Shantha Kumara HMC, Cabot JC, Yan X, Herath SA, Luchtefeld M, Kalady MF, Feingold DL, Baxter R, Whelan RL. Minimally invasive colon resection is associated with a persistent increase in plasma PlGF levels following cancer resection. Surg Endosc. 2011;25:2153–2158. doi: 10.1007/s00464-010-1514-z. [DOI] [PubMed] [Google Scholar]
- 16.Shantha Kumara HMC, Tohme ST, Herath SA, Yan X, Senagore AJ, Nasar A, Kalady MF, Baxter R, Whelan RL. Plasma soluble vascular adhesion molecule-1 levels are persistently elevated during the first month after colorectal cancer resection. Surg Endosc. 2012;26:1759–1764. doi: 10.1007/s00464-011-2112-4. [DOI] [PubMed] [Google Scholar]
- 17.Shantha Kumara HMC, Myers EA, Herath SA, Jang JH, Njoh L, Yan X, Kirchoff D, Cekic V, Luchtefeld M, Whelan RL. Plasma monocyte chemotactic protein-1 remains elevated after minimally invasive colorectal cancer resection. World J Gastrointest Oncol. 2014;6:413–419. doi: 10.4251/wjgo.v6.i10.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shantha Kumara HMC, Gaita DJ, Miyagaki H, Yan X, Herath SA, Cekic V, Whelan RL. Minimally invasive colorectal resection is associated with significantly elevated levels of plasma matrix metalloproteinase 3 (MMP-3) during the first month after surgery which may promote the growth of residual metastases. Surg Endosc. 2014;28:3322–3328. doi: 10.1007/s00464-014-3612-9. [DOI] [PubMed] [Google Scholar]
- 19.Shantha Kumara HMC, Kirchoff D, Naffouje S, Grieco M, Herath SA, Dujovny N, Kalady MF, Hyman N, Njoh L, Whelan RL. Plasma from the second and third weeks after open colorectal resection for cancer stimulates in vitro endothelial cell growth, migration, and invasion. Surg Endosc. 2012;26:790–795. doi: 10.1007/s00464-011-1953-1. [DOI] [PubMed] [Google Scholar]
- 20.Hakala BE, White C, Recklies AD. Human cartilage gp-39, a major secretory product of articular chondrocytes and synovial cells, is a mammalian member of a chitinase protein family. J Biol Chem. 1993;268:25803–25810. [PubMed] [Google Scholar]
- 21.He CH, Lee CG, Dela Cruz CS, Lee CM, Zhou Y, Ahangari F, Ma B, Herzog EL, Rosenberg SA, Li Y, et al. Chitinase 3-like 1 regulates cellular and tissue responses via IL-13 receptor α2. Cell Rep. 2013;4:830–841. doi: 10.1016/j.celrep.2013.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kawada M, Seno H, Kanda K, Nakanishi Y, Akitake R, Komekado H, Kawada K, Sakai Y, Mizoguchi E, Chiba T. Chitinase 3-like 1 promotes macrophage recruitment and angiogenesis in colorectal cancer. Oncogene. 2012;31:3111–3123. doi: 10.1038/onc.2011.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Malinda KM, Ponce L, Kleinman HK, Shackelton LM, Millis AJ. Gp38k, a protein synthesized by vascular smooth muscle cells, stimulates directional migration of human umbilical vein endothelial cells. Exp Cell Res. 1999;250:168–173. doi: 10.1006/excr.1999.4511. [DOI] [PubMed] [Google Scholar]
- 24.Libreros S, Garcia-Areas R, Iragavarapu-Charyulu V. CHI3L1 plays a role in cancer through enhanced production of pro-inflammatory/pro-tumorigenic and angiogenic factors. Immunol Res. 2013;57:99–105. doi: 10.1007/s12026-013-8459-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eurich K, Segawa M, Toei-Shimizu S, Mizoguchi E. Potential role of chitinase 3-like-1 in inflammation-associated carcinogenic changes of epithelial cells. World J Gastroenterol. 2009;15:5249–5259. doi: 10.3748/wjg.15.5249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cintin C, Johansen JS, Christensen IJ, Price PA, Sørensen S, Nielsen HJ. Serum YKL-40 and colorectal cancer. Br J Cancer. 1999;79:1494–1499. doi: 10.1038/sj.bjc.6690238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen BV, Johansen JS, Price PA. High levels of serum HER-2/neu and YKL-40 independently reflect aggressiveness of metastatic breast cancer. Clin Cancer Res. 2003;9:4423–4434. [PubMed] [Google Scholar]
- 28.Kucur M, Isman FK, Balci C, Onal B, Hacibekiroglu M, Ozkan F, Ozkan A. Serum YKL-40 levels and chitotriosidase activity as potential biomarkers in primary prostate cancer and benign prostatic hyperplasia. Urol Oncol. 2008;26:47–52. doi: 10.1016/j.urolonc.2007.07.020. [DOI] [PubMed] [Google Scholar]
- 29.Johansen JS, Drivsholm L, Price PA, Christensen IJ. High serum YKL-40 level in patients with small cell lung cancer is related to early death. Lung Cancer. 2004;46:333–340. doi: 10.1016/j.lungcan.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 30.Choi IK, Kim YH, Kim JS, Seo JH. High serum YKL-40 is a poor prognostic marker in patients with advanced non-small cell lung cancer. Acta Oncol. 2010;49:861–864. doi: 10.3109/02841861003631503. [DOI] [PubMed] [Google Scholar]
- 31.Huang Y, Prasad M, Lemon WJ, Hampel H, Wright FA, Kornacker K, LiVolsi V, Frankel W, Kloos RT, Eng C, et al. Gene expression in papillary thyroid carcinoma reveals highly consistent profiles. Proc Natl Acad Sci USA. 2001;98:15044–15049. doi: 10.1073/pnas.251547398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diefenbach CS, Shah Z, Iasonos A, Barakat RR, Levine DA, Aghajanian C, Sabbatini P, Hensley ML, Konner J, Tew W, et al. Preoperative serum YKL-40 is a marker for detection and prognosis of endometrial cancer. Gynecol Oncol. 2007;104:435–442. doi: 10.1016/j.ygyno.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 33.Chang ST, Zahn JM, Horecka J, Kunz PL, Ford JM, Fisher GA, Le QT, Chang DT, Ji H, Koong AC. Identification of a biomarker panel using a multiplex proximity ligation assay improves accuracy of pancreatic cancer diagnosis. J Transl Med. 2009;7:105. doi: 10.1186/1479-5876-7-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan JJ, Ge YS, Xu GL, Jia WD, Liu WF, Li JS, Liu WB. The expression of chitinase 3-like 1: a novel prognostic predictor for hepatocellular carcinoma. J Cancer Res Clin Oncol. 2013;139:1043–1054. doi: 10.1007/s00432-013-1415-3. [DOI] [PubMed] [Google Scholar]
- 35.Dupont J, Tanwar MK, Thaler HT, Fleisher M, Kauff N, Hensley ML, Sabbatini P, Anderson S, Aghajanian C, Holland EC, et al. Early detection and prognosis of ovarian cancer using serum YKL-40. J Clin Oncol. 2004;22:3330–3339. doi: 10.1200/JCO.2004.09.112. [DOI] [PubMed] [Google Scholar]
- 36.Bi J, Lau SH, Lv ZL, Xie D, Li W, Lai YR, Zhong JM, Wu HQ, Su Q, He YL, et al. Overexpression of YKL-40 is an independent prognostic marker in gastric cancer. Hum Pathol. 2009;40:1790–1797. doi: 10.1016/j.humpath.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 37.Schmidt H, Johansen JS, Gehl J, Geertsen PF, Fode K, von der Maase H. Elevated serum level of YKL-40 is an independent prognostic factor for poor survival in patients with metastatic melanoma. Cancer. 2006;106:1130–1139. doi: 10.1002/cncr.21678. [DOI] [PubMed] [Google Scholar]
- 38.Mitsuhashi A, Matsui H, Usui H, Nagai Y, Tate S, Unno Y, Hirashiki K, Seki K, Shozu M. Serum YKL-40 as a marker for cervical adenocarcinoma. Ann Oncol. 2009;20:71–77. doi: 10.1093/annonc/mdn552. [DOI] [PubMed] [Google Scholar]
- 39.Johansen JS, Christensen IJ, Riisbro R, Greenall M, Han C, Price PA, Smith K, Brünner N, Harris AL. High serum YKL-40 levels in patients with primary breast cancer is related to short recurrence free survival. Breast Cancer Res Treat. 2003;80:15–21. doi: 10.1023/A:1024431000710. [DOI] [PubMed] [Google Scholar]
- 40.Bergmann OJ, Johansen JS, Klausen TW, Mylin AK, Kristensen JS, Kjeldsen E, Johnsen HE. High serum concentration of YKL-40 is associated with short survival in patients with acute myeloid leukemia. Clin Cancer Res. 2005;11:8644–8652. doi: 10.1158/1078-0432.CCR-05-1317. [DOI] [PubMed] [Google Scholar]
- 41.Høgdall EV, Johansen JS, Kjaer SK, Price PA, Christensen L, Blaakaer J, Bock JE, Glud E, Høgdall CK. High plasma YKL-40 level in patients with ovarian cancer stage III is related to shorter survival. Oncol Rep. 2003;10:1535–1538. [PubMed] [Google Scholar]
- 42.Pelloski CE, Mahajan A, Maor M, Chang EL, Woo S, Gilbert M, Colman H, Yang H, Ledoux A, Blair H, et al. YKL-40 expression is associated with poorer response to radiation and shorter overall survival in glioblastoma. Clin Cancer Res. 2005;11:3326–3334. doi: 10.1158/1078-0432.CCR-04-1765. [DOI] [PubMed] [Google Scholar]
- 43.Hottinger AF, Iwamoto FM, Karimi S, Riedel E, Dantis J, Park J, Panageas KS, Lassman AB, Abrey LE, Fleisher M, et al. YKL-40 and MMP-9 as serum markers for patients with primary central nervous system lymphoma. Ann Neurol. 2011;70:163–169. doi: 10.1002/ana.22360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu CB, Chen LL, Tian JJ, Su L, Wang C, Gai ZT, Du WJ, Ma GL. Elevated serum YKL-40 level predicts poor prognosis in hepatocellular carcinoma after surgery. Ann Surg Oncol. 2012;19:817–825. doi: 10.1245/s10434-011-2026-3. [DOI] [PubMed] [Google Scholar]
- 45.Wu FP, Hoekman K, Sietses C, von Blomberg BM, Meijer S, Bonjer HJ, Cuesta MA. Systemic and peritoneal angiogenic response after laparoscopic or conventional colon resection in cancer patients: a prospective, randomized trial. Dis Colon Rectum. 2004;47:1670–1674. doi: 10.1007/s10350-004-0660-6. [DOI] [PubMed] [Google Scholar]
- 46.Wu FP, Hoekman K, Meijer S, Cuesta MA. VEGF and endostatin levels in wound fluid and plasma after breast surgery. Angiogenesis. 2003;6:255–257. doi: 10.1023/B:AGEN.0000029410.32264.b0. [DOI] [PubMed] [Google Scholar]
- 47.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 48.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 49.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 50.Peeters CF, de Geus LF, Westphal JR, de Waal RM, Ruiter DJ, Wobbes T, Oyen WJ, Ruers TJ. Decrease in circulating anti-angiogenic factors (angiostatin and endostatin) after surgical removal of primary colorectal carcinoma coincides with increased metabolic activity of liver metastases. Surgery. 2005;137:246–249. doi: 10.1016/j.surg.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 51.Yoshidome H, Kimura F, Shimizu H, Ohtsuka M, Kato A, Yoshitomi H, Furukawa K, Mitsuhashi N, Takeuchi D, Iida A, et al. Interval period tumor progression: does delayed hepatectomy detect occult metastases in synchronous colorectal liver metastases? J Gastrointest Surg. 2008;12:1391–1398. doi: 10.1007/s11605-008-0540-9. [DOI] [PubMed] [Google Scholar]
- 52.Kaibori M, Iwamoto S, Ishizaki M, Matsui K, Saito T, Yoshioka K, Hamada Y, Kwon AH. Timing of resection for synchronous liver metastases from colorectal cancer. Dig Dis Sci. 2010;55:3262–3270. doi: 10.1007/s10620-009-1124-6. [DOI] [PubMed] [Google Scholar]
- 53.Peeters CF, Westphal JR, de Waal RM, Ruiter DJ, Wobbes T, Ruers TJ. Vascular density in colorectal liver metastases increases after removal of the primary tumor in human cancer patients. Int J Cancer. 2004;112:554–559. doi: 10.1002/ijc.20374. [DOI] [PubMed] [Google Scholar]
- 54.Hoffman A, Baxter R, Nasar A, Gardner TR, Kumara S, Cordon-Cardo C, Ahmed A, Newman RA, Zmora O, Whelan RL. Perioperative polyphenon E, a green tea extract, does not affect the wound complication rate in mice after sham laparotomy yet has an inhibitory effect on wound healing. Surg Innov. 2012;19:399–406. doi: 10.1177/1553350612436565. [DOI] [PubMed] [Google Scholar]


