Abstract
Tourette disorder (TD) is characterized by motor and vocal tics, and children with TD tend to present a lower quality of life than neurotypical children. This study applied a manualized treatment for childhood tics disorder, Facotik, to a consecutive case series of children aged 8–12 years. The Facotik therapy was adapted from the adult cognitive and psychophysiological program validated on a range of subtypes of tics. This approach aims to modify the cognitive–behavioral and physiological processes against which the tic occurs, rather than only addressing the tic behavior. The Facotik therapy lasted 12–14 weeks. Each week 90-min session contained 20 min of parental training. The therapy for children followed 10 stages including: awareness training; improving motor control; modifying style of planning; cognitive and behavioral restructuring; and relapse prevention. Thirteen children were recruited as consecutive referrals from the general population, and seven cases completed therapy and posttreatment measures. Overall results showed a significant decrease in symptom severity as measured by the YGTSS and the TSGS. However, there was a discrepancy between parent and child rating, with some children perceiving an increase in tics, possibly due to improvement of awareness along therapy. They were also individual changes on adaptive aspects of behavior as measured with the BASC-2, and there was variability among children. All children maintained or improved self-esteem posttreatment. The results confirm the conclusion of a previous pilot study, which contributed to the adaptation of the adult therapy. In summary, the Facotik therapy reduced tics in children. These results underline that addressing processes underlying tics may complement approaches that target tics specifically.
Keywords: Tourette disorder, tics, children, treatment, cognitive–behavioral therapy, psychophysiological
Introduction
Definition and Symptoms
Tourette disorder (TD) is considered a motor disorder in the neurodevelopmental disorders section of the DSM-5 (1). TD is diagnosed by multiple motor tics and at least one vocal tic present for at least 1 year. This child-onset disorder appears to be a complex condition with the changing nature of tics evolving over time in frequency, intensity, localization, and complexity (2). Children and adolescents are the most affected by TD with a prevalence rate between 0.3 and 0.9% (3).
Studies report that children with TD are more likely to experience daily struggles in several spheres of activities (4). Cutler et al. (5) showed that 66% of 57 young participants reported some physical consequences associated with tics (e.g., pain, aches, physical discomfort). In a school setting, tics may interfere with academic performance and produce difficulty concentrating, writing, or reading (6). Children with TD may also experience relationship problems because they can be victimized when their tics are severe and complex, and they can be stigmatized or have more conflicts with their parents and teachers than other children (7–9). Hoekstra and colleagues (10) reported an increase in emotional problems over time in TD children and a higher rate of cognitive difficulties than children in the general population (p < 0.05) (11). Consequently, children with TD tend to present a lower quality of life than neurotypical children (5, 12, 13).
About 85% of individuals with TD report at least one comorbid disorder (14, 15). The most frequent comorbidity in children with TD is attention deficit hyperactivity disorder and oppositional defiant disorder (16, 17), but they can also show obsessive–compulsive disorder, anxiety disorder, and depressive disorder (18, 19). The severity of the comorbidity seems to worsen the quality of life of these children often more than tics. The variety of symptoms then interferes in daily functioning, leading to several impairments in children with TD and comorbidity (20, 21).
Behavioral Therapies
Canadian, American, and European clinical guidelines recommend medication plus a cognitive–behavioral treatment for reducing tics (22–24). Behavioral therapies are recommended as evidence-based interventions to manage tics, and behavioral approaches have taken several forms depending on whether the tic is conceptualized.
The comprehensive behavioral intervention for tics (CBIT) proposed by Woods and colleagues (25) is mainly based on the habit reversal treatment [HRT, Ref. (26)], which was reported to be effective for both children and adults (27–31). In addition to HRT components, such as awareness training, relaxation, competing response, contingency management, and generalization training, CBIT emphasizes the importance of addressing environmental factors that can influence tic manifestations. This 8-week treatment also uses strategies such as psychoeducation about tics and function-based interventions. CBIT appears to be effective for tic reduction in children and adults with TD (32–34). However, the premonitory urge remained unchanged across therapy (35), whereas, in theory, it should decrease with the negative reinforcement process. Therefore, the mechanisms underlying tics and therapeutic processes remain unclear.
In treatment by exposure and response prevention [ERP, Ref. (36)], the aim is to reduce tics by breaking the negative reinforcement loop between the premonitory urge and the tic itself. The individual learns to tolerate the premonitory urge and resists the appearance of tics for longer and longer periods (response prevention). A study comparing two treatment protocols ERP/HR in 43 participants with TD (aged 7 to 55 years old) showed no significant difference between groups in reduction of tics, where 58% of the participant in the ERP group and 28% of the participant in the HR group showed a decrease of at least 30% on the YGTSS (37). However, some children are unable to feel and detect the premonitory urges (38), and the therapy to resist the tic may be sometimes emotionally difficult for the child because of the pressure to succeed.
Cognitive Psychophysiological Treatment
An elaboration of the functional role of tics in sensorimotor regulation is found in O’Connor’s (39) cognitive psychophysiological model. This model conceives of tics as serving a function of sensorimotor autoregulation, while decreasing tension in muscles inappropriately contracted. Tension in TD seems characterized by a cycle where the muscle is inappropriately prepared prior to execution (40). For example, during an activity, the individual with a tic is preparing too quickly for an immediate response, but, at the same time, preparing more muscles with more effort than necessary. This preparation is inappropriate so the tic action relieves, in part, through local tension release. Electromyographic (EMG) recordings of tic-affected muscles show that these muscles are rarely associated with zero tension and have a greater difficulty compared to non-affected muscles achieving different degrees of tension rather just an all or nothing state of tension [(41) and replication is in preparation]. People suffering from tics also subjectively report chronic tension, and Hoogduin et al. (36) reported high overall muscle tension as a consistent feeling in all patients, when identifying premonitory urges. The originality of this approach is its targeting of excessive overall sensorimotor activations by addressing cognitions, behaviors, and physiological strategies, which engender excessive tension leading to and maintaining tics, rather than learning a competitive response to the tic or to the urge to tic.
The cognitive psychophysiological [CoPs; Ref. (40)] treatment for tics was developed in order to focus on the processes influencing thoughts and behaviors underlying tics, rather than working exclusively on the tic per se. Several cognitive factors are targeted in the CoPs treatment, such as anticipation, rigid beliefs (e.g., about action and organization), a judgmental style of thinking, attentional focus, and a perfectionistic style of planning action involving over-activity and over-preparation. This thinking can encourage the tendency to complete several tasks rapidly and at the same time (a style termed over-activity), together with an over-investment in preparation for action by recruiting redundant muscles and employing more effort than necessary (a style termed over-preparation). People with tics frequently experienced rigid thinking about how they should act and appear, resulting in inflexible black and white thoughts, which impair adaptation (42). In addition, meta-cognitive factors, as defined by O’Connor as thoughts about performing the tic and expectations or beliefs about tic onset, are targeted along with how people with tics evaluate and judge situations at high risk for eliciting tics (42). These cognitive factors also interact with physiological factors such as an increased sensorimotor activation, leading to hypersensitivity and over-reactivity and so, as a circular linking, to tic onset (42). A behavioral target of this therapy is to break the negative reinforcement cycle between the tic onset and the immediate relief of the accumulation of muscular tensions caused by the heightened sensorimotor activation (40). There is evidence of tension building up, prior to ticking, and subjective reports of relief, post-ticking (40). The aim of the CoPs treatment is to help the individual in understanding how these cognitive–behavioral and physiological factors lead to tension and how gradually addressing and modifying them can prevent tension build-up and tic onset, while increasing self-control.
An open trial showed the efficacy of CoPs treatment in adults with tics compared to waitlist with a 6:1 ratio (43). Results showed that 10 of the 85 participants completely reduced tic onset after therapy (gains maintained at 6-month follow-up). Prior results also showed efficacy in tic reduction in adults with or without medication, following CoPs treatment (44, 45). The therapy was applied to five adolescents with TD, in a pilot study (46). Results showed a decrease in tic frequency and intensity and improvement in social functioning for the five participants. The CoPs treatment has also been adapted for children with TD addressing explosive outbursts (EO). Results showed a decrease of EO frequency of at least 34% for four participants out of six. Another participant showed a 75% decrease in posttreatment, but did not complete the follow-up assessment. The last participant showed a 67% decrease between the beginning of therapy and follow-up, despite an increase of EO frequency at baseline assessment (47). Finally, a single-case design study of the CoPs treatment addressing tic severity in childhood was conducted with 11 children aged 8–12 years old (48). A decrease of 29.8% of tic onset was observed posttreatment (p < 0.001, d = 0.97), and the decrease was monitored over 1 year. Results showed a decrease of at least 1 SD in measures, post 12 months.
After this pilot study, a manualized version of the treatment protocol in children termed Facotik has been finalized (49), and the aim of the current study is to evaluate its efficacy in a larger consecutive case series. Based on previous research, a decrease of tic severity was expected after treatment. The efficacy of the treatment adapted for children will have important implications for the intervention in TD and whether addressing the underlying sensorimotor processes is sufficient to reduce tics.
Materials and Methods
Participants
The recruitment was carried out through the Centre d’études troubles obsessionnels-compulsifs et tics – Institut universitaire en santé mentale de Montréal. Consecutive referrals were evaluated according to the inclusion criteria: 8–12 years old, a primary TD diagnosis, and medication stable at least 1 month before treatment and stable for the duration of the therapy. Exclusion criteria were: a diagnosis of autism spectrum disorder or intellectual disability, receiving another behavioral treatment for tics during the study, and a problem of geographical location to assure treatment adherence. Thirteen children were originally recruited and seven children completed therapy and posttreatment measures (one retracted before therapy, four abandoned during therapy, and one completed the therapy, but did not complete the follow-up). Table 1 summarizes age, sex, medication intake, and number of days between the first and the last therapy session for each participant that completed the therapy. The mean age of the seven participants was 10.29 years (six boys, one girl). Mean age of the non-completers was 9.4 years (four boys, two girls). There was no statistical difference between completers and non-completers over all measures of tic severity in the pre-treatment assessment as shown in Table 2.
Table 1.
Age, sex, medication intake, and length of the therapy for each participant.
| Participant | Age | Sex | Medication intake | Days between first and last therapy session |
|---|---|---|---|---|
| 1 | 11 | Girl | Valerian, atomoxetine | 91 |
| 2 | 10 | Boy | – | 98 |
| 3 | 10 | Boy | – | 115 |
| 4 | 12 | Boy | – | 98 |
| 5 | 11 | Boy | Melatonin | 104 |
| 6 | 9 | Boy | Methylphenidade, risperidone | 106 |
| 7 | 9 | Boy | – | 105 |
Table 2.
Tests of tic severity differences between completers and non-completers on the YGTSS and on the TSGS.
| Scale | Median score for completers (participants) | Median score for non-completers | Asymptotic Wilcoxon–Mann–Whitney Test |
|---|---|---|---|
| YGTSS | |||
| Global | 37.00 | 29.50 | Z = −0.857, p = 0.39 |
| Tic severity | 23.00 | 19.50 | Z = −0.714, p = 0.48 |
| Deterioration | 10.00 | 10.00 | Z = −0.158, p = 0.87 |
| TSGS | |||
| Global | 25.50 | 21.08 | Z = −0.286, p = 0.78 |
| Tic domain | 13.00 | 10.00 | Z = −0.644, p = 0.52 |
| Social functioning domain | 10.00 | 10.00 | Z = 0.443, p = 0.66 |
Assessment Measures
Yale Global Tic Severity Scale
The Yale Global Tic Severity Scale [YGTSS, Ref. (50)] is used to assess a global scale based on a tic severity subscale with five dimensions (number, frequency, intensity, complexity, and interference of tics) and an impairment subscale. Inter-rater agreement ranges from 0.52 to 0.99 and 0.85 for the global severity score. Factor loadings on the items in factor analyses revealed two separated factors, one for motor tics and overall impairment and one for phonic tics, although the two factors account only for 8% of the variance showing a low-factor validity. The YGTSS is completed by the children with the help of an independent evaluator. Scores for the YGTSS ranged from 0 to 100.
Tourette’s Syndrome Global Scale
The Tourette’s Syndrome Global Scale [TSGS, Ref. (51)] is used to assess a global scale based on a tics domain and a social functioning domain. The tics domain evaluates frequency and disruption of different subtypes of tics (motor/phonic and simple/complex). Social functioning domain included the assessment of learning, motor restlessness, and occupational problems. There is a good inter-rater agreement (0.89) for the global scale, and the criterion validity was demonstrated as a correlation between TSGS’s global scale and severity of TD symptomatology ranked by four raters ranging from 0.46 to 0.99. The TSGS highly correlates with the YGTSS for motor, phonic, and total tics (from 0.86 to 0.91), but the correlation is moderate for the global score. The TSGS is completed by the children with the help of an independent evaluator and by one of their parents. Scores for the TSGS ranged from 0 to 100.
Behavior Assessment System for Children – Second Edition
The Behavior Assessment System for Children – Second Edition [BASC-2, Ref. (52)] is a multidimensional and multimodal assessment for adaptive and clinical aspects of behavior and personality in children. Two tests were used to assess participants on secondary outcomes of the therapy, one by the parents and one by the children. The Parent Rating Scale (PRS) assesses nine clinical scales (hyperactivity, aggression, conduct problems, anxiety, depression, somatization, atypicality, withdrawal, and attention problems), five adaptive scale (adaptability, social skills, leadership, activities of daily living, and functional communication), three clinical composite scale (externalizing problems composite, internalizing problems composite, and behavioral symptoms index), and one adaptive composite scale (Adaptive skills composite), over 160 items. The self-reported personality (SRP) for children assesses 10 clinical scales (attitude to school, attitude to teachers, atypicality, locus of control, social stress, anxiety, depression, sense of inadequacy, attention problems, and hyperactivity), four adaptive scales (Relations with parents, Interpersonal relations, Self-esteem and Self-reliance), 4 clinical composite scales (school problems composite, internalizing problems composite, inattention/hyperactivity composite, and emotional symptoms index), and 1 adaptive composite scale (personal adjustment composite), over 139 items.
For both tests, scores were converted to T-score based on the age of the participant. Intervals of T-scores indicating thresholds for “normal,” “at risk,” and “clinically significant” ranges are presented in Table 3, for the clinical scales and for the adaptive scales. Internal consistency of scales and composite scales for the PRS were all above α = 0.80, and test–retest reliability were all above 0.77. For the SRP, internal consistency of scales and composite scales ranged from α = 0.71 to α = 0.96 and test–retest reliability ranged from 0.66 to 0.83. Change in time on the SRP could be attributed to low reliability. Confirmatory factor analysis for the PRS showed a comparative fit index of 0.88 and a root mean square error of approximation of 0.13, both indicating near good validity of the test. Confirmatory factor analysis results were equivalent on the SRP, with a comparative fit index of 0.90 and a root mean square error of approximation of 0.11, indicating good and near good validity of the test.
Table 3.
BASC-2 T-scores indicating thresholds scores for clinical and adaptive scales.
| Type of scales |
T-scores |
||||
|---|---|---|---|---|---|
| <30 | 40 | 50 | 60 | >70 | |
| Clinical | Normal | At-risk | Clinical | ||
| Adaptive | Clinical | At-risk | Normal | ||
The gray shade are visual indicator of the At-risk and Clinical score range for the BASC-2.
Culture-Free Self-esteem Inventory
The Culture-Free Self-Esteem Inventory – second edition form B [CFSEI, Ref. (53)] was used to evaluate change in self-esteem in children between pre- and posttreatment as a secondary benefit of the therapy. The CFSEI form B included 30 yes or no items assessing five subscales (general, social, academic, parents, and defensiveness) extracted from form A. Correlation between the two forms was 0.86. Test–retest reliability was ranging from 0.79 to 0.92 for the total score and was ranging from 0.49 to 0.80 for subscales. Concurrent validity was obtained with the self-esteem inventory (54), ranging from 0.71 to 0.80.
Treatment Material
The Facotik treatment is a manualized therapy (therapist and child manual), including a self-monitoring diary and a token economy motivational board. The therapist’s manual includes an explicit protocol for every exercise and instructions for the participants and their parents for each session of the therapy with time estimation. The child’s manual contains information on each topic of the treatment with colorful examples, activities named “challenges,” and exercises to practice between therapy sessions named “missions of the week.” A particular concern was to adapt the CoPs exercises to a child’s cognitive level of functioning. For this purpose, a narrative approach was proposed in Facotik where two characters named Lea and Nico accompanied the child over the treatment. To improve understanding, new elements have been added in the children adaptation of the therapy, such as concrete language, practical examples, metaphors, visual analogies, and pictures. Also, behavioral restructuring precedes cognitive restructuring, unlike the adult version. The self-monitoring diary is used for assessing frequency of tics, conducting a functional analysis (antecedents, consequences), and clinical awareness training. Each participant notes the frequency of a targeted tic for a 15-min period, once a day, in a predetermined high-risk tic onset situation. The child also estimates the intensity of the tics (low, medium, or high) and his/her principal activity at this time. The token economy motivational system works on a three-point award for each therapy session, one for participating in the challenges during the session, one for completing the self-monitoring diary every day and one for completing the weekly exercises or missions between sessions. Children could exchange nine points for a specific reward (not necessarily tangible, e.g., a specific activity), determined with their parents at the second session.
Procedure
Participants and one of their parents completed the pre-treatment assessment with a trained specialized evaluator, including YGTSS, TSGS, BASC-2, and CFSEI. The certified evaluator was independent of the therapy process and research protocol. The evaluator completed the scoring of the YGTSS and the TSGS, after semi-structured interviews with the parents and the children separately. Afterward, each participant followed the Facotik therapy with one of the two trained psychotherapists: a licensed psychologist and a certified final year graduate student. The Facotik therapy lasted 12 to 14 sessions depending on the understanding and on the success of the steps by the child. Each 90-min session began by reviewing the content previously discussed and ended with 20 min of parental training (information on the clinical objective of the session, supportive coping strategies, and how to give positive reinforcement for home exercises to their child). Information was also given to the parents on the theoretical approach to enable them to act as a collaborator in the therapy process based on a psychoeducation method (55, 56). Psychotherapists wrote a progress report at the end of each therapy session, indicating children’s progress and difficulties.
The Facotik treatment is progressive and passes through progressive therapeutic steps with a “one tic at a time” approach. Table 4 presents a schema of the clinical objectives and the therapeutic components of each therapy session. The clinical objective distributed over 14 sessions are: awareness training, muscle discrimination, relaxation, reduced sensorimotor activation, modifying style of planning action, cognitive restructuration of anticipation and appraisals, behavioral restructuration, generalizations, and preventing relapse. The first clinical objective (awareness training) is spread over several sessions, while, from the 9th therapy session, several clinical objectives are addressed in the same sessions. Between each therapy session, the child completed the self-monitoring diary and the weekly exercises. Three participants completed treatment in 13 sessions, and four others completed therapy in 14 sessions (the total duration of the therapy was an average of 102.49 days between the first and the last session, all children skipped at least 1 week between two sessions due to sickness or scheduling constraints). At posttreatment, each participant and one of their parents completed all assessments on the YGTSS, the TSGS, the BASC-2, and the CFSEI.
Table 4.
Procedure, therapeutic components, and clinical objectives of each Facotik session.
| Clinical objectives | Session | Procedure and therapeutic components |
|---|---|---|
| Awareness training | 1 |
|
| 2 |
|
|
| 3 |
|
|
| 4 |
|
|
| 5 |
|
|
| Muscle discrimination | 6 |
|
| Relaxation | 7 |
|
| Sensory-motor activation | 8 |
|
| Style of planning action | 9–10 |
|
| Cognitive restructuring | 11–13 |
|
| Behavioral restructuring | 11–13 |
|
| Global restructuring | 11–13 |
|
| Generalization | 14 |
|
| Relapse prevention | 14 |
|
Ethics
This study was approved by the local ethic review board of the Institut universitaire en santé mentale de Montréal in accordance with the ethical standards of the Canadian Tri-Council Policy Statement of Ethical Conduct for Research Involving Humans. The parents of the participants (or the legal guardian) gave their signed consent for the participation of their child to the study (assessments and therapy), and the child himself gave his or her approval.
Data Analyses
Two analysis procedures were planned. For statistical analyses, one-sided exact Wilcoxon signed-rank test was conducted due to the small sample on the children and parents’ assessments to evaluate global symptoms decrease after treatment, as measured by the YGTSS global scale and the TSGS global scale. Additional one-sided exact Wilcoxon signed-rank tests were conducted with the tic severity subscale and the impairment subscale of the YGTSS and with the tics domain and the social functioning domain of the TSGS, using a Pratt correction in the case of tied ranks. Person’s correlations were computed between parents and children for pre-treatment scores, posttreatment scores, and difference scores on all scales and subscales. Difference scores were computed as pre-treatment score minus posttreatment score for each parents and children. All analyses were calculated with n = 7 based on a complete dataset. All statistical analyses were computed using R statistical software (57) and the coin package (58). For clinical results, changes of at least 1 SD on the BASC-2 subscales and on the CFSEI were reported.
Results
Statistical Results
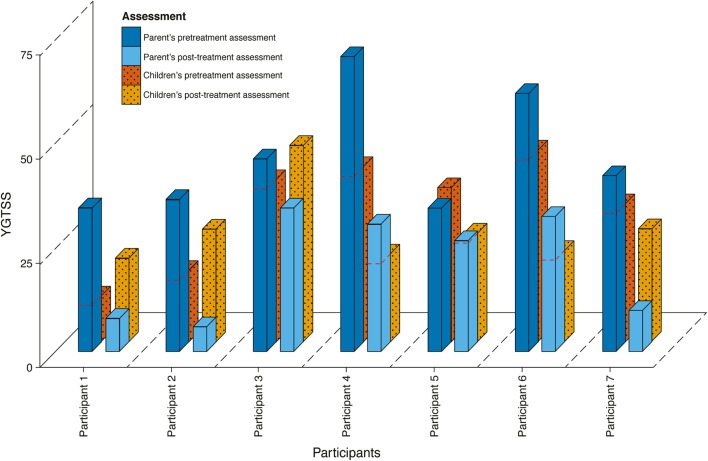
Results of the parents’ assessments on the YGTSS global scale showed a general and significant symptom decrease at posttreatment (from Mdn = 43.00 to Mdn = 27.00, Z = −2.37, p = 0.008, r = −0.63). This decrease was not perceived by the children themselves, as they estimated no significant symptoms decrease (from Mdn = 37.00 to Mdn = 26.00, Z = −0.85, p = 0.234). Figure 1 shows the global scale on the YGTSS for pre- and posttreatment as assessed by the children and their parent. Four participants showed a decrease on the YGTSS global scale for both child and parent, while the other three reported discrepant results. Correlations between parents and children showed good agreement for pre-treatment scores (r = 0.70, p = 0.005), but poor agreement for posttreatment scores (r = 0.34, p = 0.234) and for difference scores (r = 0.30, p = 0.300).
Figure 1.
Results on the YGTSS for parents and children in pre- and posttreatment.
Analysis of the YGTSS subscales showed a significant decrease in both the tic severity subscale (Mdn = 27.00 to Mdn = 15.00, Z = −2.37, p = 0.008, r = −0.63) and the impairment subscale (Mdn = 20.00 to Mdn = 10.00, Z = −2.19, p = 0.031, r = −0.69), as observed by parents. Children reported a significant decrease on the tic severity subscale (Mdn = 23.00 to Mdn = 16.00, Z = −2.29, p = 0.016, r = −0.66), but not on the impairment subscale (Mdn = 10.00 to Mdn = 10.00, Z = 0.81, p = 0.813). Correlations between parents and children on the tic severity subscale were moderate for pre-treatment scores (r = 0.60, p = 0.023), negative for posttreatment scores (r = -0.35, p = 0.220), and poor for difference scores (r = 0.29, p = 0.315). Correlations between parents and children on the impairment subscale were poor for pre-treatment scores (r = 0.61, p = 0.021), good for posttreatment scores (r = 0.75, p = 0.002), and moderate for difference scores (r = 0.50, p = 0.069).
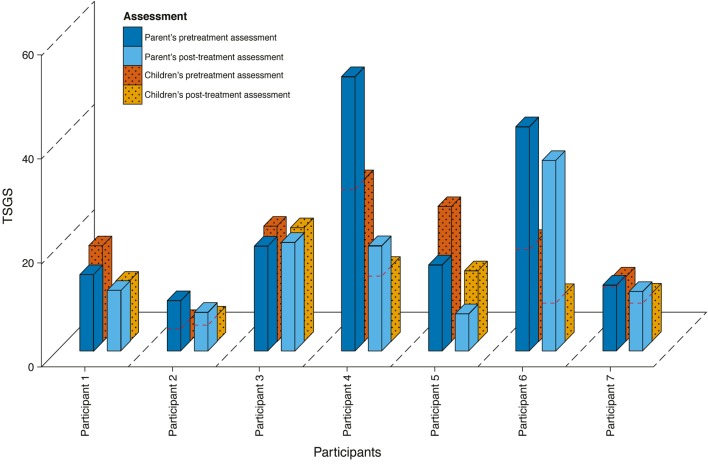
In contrast to YGTSS, results on the TSGS global scale showed a significant symptom decrease after treatment, as assessed by children (from Mdn = 25.50 to Mdn = 11.67, Z = −2.37, p = 0.008, r = −0.59), and by the parents (from Mdn = 16.83 to Mdn = 12.00, Z = −2.20, p = 0.016, r = −0.59). Figure 2 shows scores on the TSGS global scale for pre- and posttreatment as assessed by children and parents for each participant. Five participants showed a decrease in tic symptoms on the TSGS global scale, while a further two reported discrepant results. Correlations between parents and children showed good agreement for pre-treatment scores (r = 0.74, p = 0.002), poor agreement for posttreatment scores (r = 0.19, p = 0.515), and moderate agreement for difference scores (r = 0.47, p = 0.090).
Figure 2.
Results on the TSGS for parents and children in pre- and posttreatment.
Analysis of the TSGS domains as reported by parents showed a significant decrease in the tics domain (Mdn = 13.00 to Mdn = 4.00, Z = −2.37, p = 0.008, r = −0.63), but not on the social functioning domain (Mdn = 10.00 to Mdn = 10.00, Z = −0.71, p = 0.750). Children reported a significant decrease on tics domain (Mdn = 13.00 to Mdn = 6.00, Z = −2.37, p = 0.008, r = −0.63), but not on the social functioning domain (Mdn = 10.00 to Mdn = 6.67, Z = 0.78, p = 0.281). Correlations between parents and children on the tics domain were good for pre-treatment scores (r = 0.75, p = 0.002), moderate for posttreatment scores (r = −0.41, p = 0.145), and good for difference scores (r = 0.77, p = 0.001). Correlations between parents and children on the social functioning domain were moderate for pre-treatment scores (r = 0.55, p = 0.042), poor for posttreatment scores (r = 0.20, p = 0.493), and negative for difference scores (r = −0.11, p = 0.708).
Clinical Results
The BASC-2 and the CFSEI were used to detect if the Facotik therapy brought secondary benefits to develop adaptive and clinical aspects of behaviors and self-esteem. Table 5 showed clinical changes of at least 1 SD on the BASC-2 SRP and on the BASC-2 Parent rating scale (PRS). There were no globally significant changes over participants even if there were some changes at the individual level. For all participants and all clinical subscales together, parents reported improvements in 13 subscales and decreases in 6 subscales, while the children report 9 improvements and 9 decreases. An overall decrease is observed for participant 1 (as noted by the parent) and participant 7 (as noted by the child). Participant 6 is the only one to present only slight increases observed by the parent (atypicality) and the child (anxiety, attention problems). However, children scoring shows that the attitude toward school, teachers, and the school problems composite increased slightly for three participants. All other participants showed decreases and increases in some subscales without general trend. For the adaptive scales, improvements are observed by parents in five subscales for the seven participants (adaptability for two participants, leadership, functional communication, and adaptive skills). Children have noted improvements in three subscales (self-esteem for two participants and self-reliance) and one decrease (interpersonal relations). All these results are not significant, but showed clinical changes of at least 1 SD.
Table 5.
Clinical change between pre- and posttreatment on the BASC-2a.
| Participant 1 |
Participant 2 |
Participant 3 |
Participant 4 |
Participant 5 |
Participant 6 |
Participant 7 |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||
| (A) Data from the Parent Rating Scale (PRS) | |||||||||||||||
| Clinical scales | Conduct problems | 65 | 51 | – | – | – | – | – | – | – | – | – | – | – | – |
| Externalizing problems | 62 | 52 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Anxiety | 72 | 49 | – | – | – | – | 57 | 72 | – | – | – | – | – | – | |
| Depression | 68 | 52 | 11 | 49 | – | – | 67 | 54 | – | – | – | – | – | – | |
| Somatization | – | – | 67 | 47 | 53 | 36 | – | – | 44 | 56 | – | – | 44 | 70 | |
| Internalizing problems | 65 | 46 | – | – | 53 | 40 | – | – | – | – | – | – | – | – | |
| Atypicality | 65 | 52 | – | – | – | – | 44 | 54 | – | – | 49 | 65 | – | – | |
| Withdrawal | 69 | 56 | – | – | – | – | 65 | 54 | – | – | – | – | – | – | |
| Behavioral symptoms index | 68 | 56 | – | – | – | – | – | – | – | – | – | – | – | – | |
| Adaptive scales | Adaptability | – | – | – | – | 32 | 53 | – | – | – | – | 16 | 28 | – | – |
| Leadership | – | – | – | – | 38 | 51 | – | – | – | – | – | – | – | – | |
| Functional communication | – | – | 30 | 55 | – | – | – | – | – | – | – | – | – | – | |
| Adaptive skills | – | – | – | – | 40 | 53 | – | – | – | – | – | – | – | – | |
| (B) Data from the self-reported personality (SRP) | |||||||||||||||
| Clinical scales | Attitude to school | – | – | – | – | – | – | – | – | 45 | 61 | – | – | – | – |
| Attitude to teachers | 49 | 71 | – | – | – | – | 36 | 49 | – | – | – | – | – | – | |
| School problems composite | 52 | 68 | – | – | – | – | – | – | 42 | 52 | – | – | – | – | |
| Atypicality | – | – | – | – | – | – | – | – | – | – | – | – | 59 | 45 | |
| Locus of control | – | – | – | – | 53 | 42 | – | – | 51 | 37 | – | – | 58 | 46 | |
| Social stress | 13 | 48 | – | – | 50 | 64 | – | – | – | – | – | – | 52 | 37 | |
| Anxiety | – | – | – | – | – | – | – | – | – | – | 39 | 51 | 62 | 47 | |
| Depression | – | – | – | – | – | – | – | – | – | – | – | – | 61 | 45 | |
| Internalizing problems composite | – | – | – | – | – | – | – | – | – | – | – | – | 57 | 42 | |
| Attention problems | – | – | – | – | – | – | – | – | – | – | 40 | 51 | – | – | |
| Emotional symptoms index | – | – | – | – | – | – | – | – | – | – | – | – | 54 | 40 | |
| Adaptive scales | Interpersonal relations | – | – | – | – | 50 | 38 | – | – | – | – | – | – | – | – |
| Self-esteem | – | – | – | – | 41 | 58 | – | – | – | – | – | – | 47 | 58 | |
| Self-reliance | – | – | – | – | 47 | 59 | – | – | – | – | – | – | – | – | |
a(A) data from the Parent Rating Scale (PRS); (B) data from the self-reported personality (SRP). Only scores that changed for at least 1 SD (10 T-score) are shown. Clinical scales: scores ≥ 60 are “at-risk”; scores ≥ 70 are “clinically significant.” Adaptive scales: scores ≤ 40 are “at-risk”; scores ≤ 30 are “clinically significant.”
One child showed improvement on self-esteem as measured by the CFSEI, with an increase on the total score of 2 SD, the global subtest of 1.5 SD, the parent subtest of 1 SD and the academic subtest of 2.7 SD. All other participants maintained medium to high levels of self-esteem from pre- to posttreatment. Table 6 shows data for all participants on the CFSEI.
Table 6.
T-score on the CFSEI for each participant in pre- and posttreatment on each scale.
| Total score |
Global subtest |
Parent subtest |
Academic subtest |
Social subtest |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| Part 1 | 63 | 60 | 65 | 65 | 50 | 50 | 63 | 63 | 55 | 46 |
| Part 2 | 63 | 65 | 60 | 65 | 60 | 60 | 63 | 63 | 55 | 55 |
| Part 3 | 55 | 52 | 55 | 55 | 60 | 60 | 54 | 54 | 46 | 38 |
| Part 4 | 63 | 63 | 60 | 60 | 60 | 60 | 63 | 63 | 55 | 46 |
| Part 5 | 65 | 68 | 65 | 65 | 60 | 60 | 63 | 63 | 55 | 55 |
| Part 6 | 60 | 63 | 60 | 60 | 60 | 60 | 63 | 63 | 46 | 46 |
| Part 7 | 45 | 65a | 50 | 65a | 50 | 60a | 36 | 63a | 46 | 46 |
aChange in T-score of at least 1 SD.
Discussion
Principal Results
The purpose of the current study was to evaluate the efficiency of the Facotik treatment to decrease the severity of tics in children aged 8–12 years old. Secondary benefits to improve adaptive and clinical aspects of behaviors and self-esteem were also anticipated.
The overall results showed a significant decrease in tics as assessed by the parents of children with TD. The results as assessed by children were discrepant; tics decreased significantly for all children as measured with the TSGS and four participants on seven reported a non-significant decrease on the YGTSS. However, children and parents, all reported a significant decrease in tic severity when the subscales of the two questionnaires were analyzed. What is interesting is that, even considering this change in tics, children and parents generally perceive no changes in the impairment subscale. This could be explained by the presence of comorbidity symptoms, which was not controlled in this study or by the subjective experience of the impairment. The correlations between the child/parents’ rating showed a good agreement regarding the tic severity in pre-treatment, but not in posttreatment, neither for difference scores (pre-minus posttreatment), suggesting a disagreement about the perception of change. There are two possible explanations for the preceding results. First, the difference between the child/parents’ rating on the YGTSS and the TSGS may highlight the sensitivity of the TSGS, which is multidimensional and is rated on a scale rather than in categories as in the YGTSS. Second, the tic decrease might not always be detected by the children themselves and discrepancies between the child/parents’ rating may be explained by one of the therapy components termed “awareness training” (59). Children are more aware of their tics after the therapy and they can detect and report them more accurately than at pre-treatment, while the parents noticed a decrease of tics because they were already conscious of the tics. The self-monitoring diaries are a key component of the tic awareness training (60). The focus on a single tic may help children to acknowledge the difference between a situation with high risk of tic onset versus low-risk situations. Some situations may be perceived as a high risk in the first place, but may become low risk following the self-monitoring diary. Thus, the mixed results may be more of an indication of the therapy process than an absence of progress in tic reduction.
Adaptive and clinical aspects of behaviors in children, as measured by the BASC-2, showed no significant changes, but improvements and clinical changes were reported individually, suggesting a regular fluctuation over time. There are further improvements to clinical subscales than deterioration as reported by children and parents. As an example, internalizing problems showed punctual improvement. Improvements have also been noticed in general for the adaptive scales. This highlights positive results although there are no significant differences. All the participants maintained or reached medium to high levels of self-esteem from pre- to posttreatment. However, attitude toward school or teacher appear to have increased for three participants after therapy. This could be explained by the fact that the therapy ended concurrently with or after the end of the school year, and posttreatment assessment took place (particularly for participants 4 and 5) just before the return to school period (in August).
In terms of experiential factors, all children benefited from the therapy, and no adverse effects were reported by the participants or their parents. The participants reported to the therapists that theoretical concepts and exercises were presented in a clear and colorful way, which made them comprehensive for all, even for participant 4 who had language issues; some activities took more time, but without causing a significant delay. Some children had a little trouble to identify their irrational thoughts during high-risk tic onset situations, and all participants reported that completing their self-monitoring diary and relaxation exercises were most helpful to them. According to the therapists, the set of strategies formed a coherent whole, and children were open-minded to the complementary elements of the therapy; they were particularly interested when the style of action planning was addressed.
Limitations
The limitations of the present study are those inherent in a consecutive case series without baseline or control group and a limited number of participants. The attrition rate was around 40%, but there were no clinical or demographical differences between participants and those who abandoned. Personal motivation and difficulty scheduling therapy sessions appear to account for attrition. This protocol had a confounding variable, considering that the posttreatment was concomitant with the preparation of a new school year. This situation could have an impact on tics and on clinical aspects of behaviors. A 6- and 12-month follow-up assessment is planned. The participants were prescribed a variety of medications, and comorbidities were not controlled. Nonetheless, the statistical and clinical significance of the tic reduction indicates potential efficacy of the Facotik treatment.
Future Research
The main strength of the current study is the demonstration of the effect of the Facotik treatment for the decrease of tic severity in children as a first step of the validation procedure. These findings, with a manualized treatment and a structured protocol, highlight the clinical importance of working on the cognitive and central processes underlying tics in children as in adults (40). CoPs treatment in adults has been shown to produce neurocognitive changes in style of action and concomitant cerebral functioning (61, 62). Such physiological changes (activation of the pre-motor and motor cortex) related to the intervention remain to be validated in children with tics (61, 62). In conclusion, this study has important implications for the conceptualization of interventions in TD; namely to know if tics are the necessary and sufficient target for effective interventions or if the processes underlying tics should also be addressed to obtain greater symptom reduction and wider behavioral impact. Future research will include a randomized clinical trial design where the efficacy of Facotik treatment as well as CoPs treatment in adults is compared to CBIT (2015–2020). Follow-up data and the effect of the therapy on quality of life for all the participants of the present study are still pending. Finally, the Facotik therapy manual will be published as a workbook for therapists and specialized training will be offered to clinicians to facilitate knowledge transfer.
Author Contributions
JL created a new therapy for children with tics (Facotik). She oversaw the project (e.g., method, ethics, supervision) and coordinated the writing of the article with a focus on the results analysis and the discussion. KO is the author of the conceptual model that led to the new therapy for children presented in this article. He is the principal researcher on the grant that supported this study. He revised the text and helped with the data analysis. GJ-N contributed to the writing of the manuscript, especially the introduction and the review of the literature. PV contributed to the writing and the text formatting and was in charge of the statistical analysis. ML revised the manuscript and was on the funding grant that supported this study. His research focus is on psychophysiological data and on event-related potentials [see other article in the same topic: Morand-Beaulieu et al. (62)].
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer EJ and handling Editor declared their shared affiliation, and the handling Editor states that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
This research was supported by a grant from the “Fonds de la Recherche en Santé du Québec (FRSQ); Regroupement multidisciplinaire de la recherche clinique sur le spectre du trouble obsessionnel compulsif” (Research group #20573). The authors would like to acknowledge the Obsessive–Compulsive Disorder and Tic Disorder Studies Centre team for their participation with assessment and treatment.
References
- 1.American Psychiatric Association , editor. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: American Psychiatric Association; (2013). [Google Scholar]
- 2.Cohen SC, Leckman JF, Bloch MH. Clinical assessment of Tourette syndrome and tic disorders. Neurosci Biobehav Rev (2013) 37(6):997–1007. 10.1016/j.neubiorev.2012.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord (2015) 30(2):221–8. 10.1002/mds.26089 [DOI] [PubMed] [Google Scholar]
- 4.Cavanna AE, David K, Bandera V, Termine C, Balottin U, Schrag A, et al. Health-related quality of life in Gilles de la Tourette syndrome: a decade of research. Behav Neurol (2013) 27(1):83–93. 10.3233/BEN-120296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cutler D, Murphy T, Gilmour J, Heyman I. The quality of life of young people with Tourette syndrome. Child Care Health Dev (2009) 35(4):496–504. 10.1111/j.1365-2214.2009.00983.x [DOI] [PubMed] [Google Scholar]
- 6.Packer LE. Tic-related school problems: impact on functioning, accommodations, and interventions. Behav Modif (2005) 29(6):876–99. 10.1177/0145445505279383 [DOI] [PubMed] [Google Scholar]
- 7.Rivera-Navarro J, Cubo E, Almazán J. The impact of Tourette’s syndrome in the school and the family: perspectives from three stakeholder groups. Int J Adv Couns (2014) 36(1):96–113. 10.1007/s10447-013-9193-9 [DOI] [Google Scholar]
- 8.Storch E, Murphy T, Chase R, Keeley M, Goodman W, Murray M, et al. Peer victimization in youth with Tourette’s syndrome and chronic tic disorder: relations with tic severity and internalizing symptoms. J Psychopathol Behav Assess (2007) 29(4):211–9. 10.1007/s10862-007-9050-4 [DOI] [Google Scholar]
- 9.Zinner SH, Conelea CA, Glew GM, Woods DW, Budman CL. Peer victimization in youth with tourette syndrome and other chronic tic disorders. Child Psychiatry Hum Dev (2012) 43(1):124–36. 10.1007/s10578-011-0249-y [DOI] [PubMed] [Google Scholar]
- 10.Hoekstra PJ, Lundervold AJ, Lie SA, Gillberg C, Plessen K. Emotional development in children with tics: a longitudinal population-based study. Eur Child Adolesc Psychiatry (2013) 22(3):185–92. 10.1007/s00787-012-0337-y [DOI] [PubMed] [Google Scholar]
- 11.Liu S, Zheng L, Zheng X, Zhang X, Yi M, Ma X. The subjective quality of life in young people with tourette syndrome in China. J Atten Disord (2014). 10.1177/1087054713518822 [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez-Colina AM, Eaton CK, Lee JL, LaMotte J, Blount RL. Health-related quality of life and psychosocial functioning in children with Tourette syndrome: parent-child agreement and comparison to healthy norms. J Child Neurol (2015) 30(3):326–32. 10.1177/0883073814538507 [DOI] [PubMed] [Google Scholar]
- 13.Storch EA, Merlo LJ, Lack C, Milsom VA, Geffken GR, Goodman WK, et al. Quality of life in youth with Tourette’s syndrome and chronic tic disorder. J Clin Child Adolesc Psychol (2007) 36(2):217–27. 10.1080/15374410701279545 [DOI] [PubMed] [Google Scholar]
- 14.Eapen V, Robertson MM. Are there distinct subtypes in Tourette syndrome? Pure-Tourette syndrome versus Tourette syndrome-plus, and simple versus complex tics. Neuropsychiatr Dis Treat (2015) 11:1431–6. 10.2147/NDT.S72284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirschtritt ME, Lee PC, Pauls DL, Dion Y, Grados MA, Illmann C, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in tourette syndrome. JAMA Psychiatry (2015) 72(4):325–33. 10.1001/jamapsychiatry.2014.2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freeman RD, Tourette Syndrome International Database Consortium . Tic disorders and ADHD: answers from a world-wide clinical dataset on Tourette syndrome. Eur Child Adolesc Psychiatry (2007) 16(1):15–23. 10.1007/s00787-007-1003-7 [DOI] [PubMed] [Google Scholar]
- 17.Lebowitz ER, Motlagh MG, Katsovich L, King RA, Lombroso PJ, Grantz H, et al. Tourette syndrome in youth with and without obsessive compulsive disorder and attention deficit hyperactivity disorder. Eur Child Adolesc Psychiatry (2012) 21(8):451–7. 10.1007/s00787-012-0278-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freeman RD, Fast DK, Burd L, Kerbeshian J, Robertson MM, Sandor P. An international perspective on Tourette syndrome: selected findings from 3500 individuals in 22 countries. Dev Med Child Neurol (2000) 42(7):436–47. 10.1017/S0012162200000839 [DOI] [PubMed] [Google Scholar]
- 19.Ludolph AG, Roessner V, Münchau A, Müller-Vahl K. Tourette syndrome and other tic disorders in childhood, adolescence and adulthood. Dtsch Arztebl Int (2012) 109(48):821. 10.3238/arztebl.2012.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eddy CM, Rizzo R, Gulisano M, Agodi A, Barchitta M, Calì P, et al. Quality of life in young people with Tourette syndrome: a controlled study. J Neurol (2011) 258(2):291–301. 10.1007/s00415-010-5754-6 [DOI] [PubMed] [Google Scholar]
- 21.Elstner K, Selai CE, Trimble MR, Robertson MM. Quality of life (QOL) of patients with Gilles de la Tourette’s syndrome. Acta Psychiatr Scand (2001) 103(1):52–9. 10.1111/j.1600-0447.2001.00147.x [DOI] [PubMed] [Google Scholar]
- 22.Pringsheim T, Doja A, Gorman D, McKinlay D, Day L, Billinghurst L, et al. Canadian guidelines for the evidence-based treatment of tic disorders: pharmacotherapy. Can J Psychiatry (2012) 57(3):133–43. [DOI] [PubMed] [Google Scholar]
- 23.Murphy TK, Lewin AB, Storch EA, Stock S, American Academy of Child and Adolescent Psychiatry (AACAP) Committee on Quality Issues (CQI) . Practice parameter for the assessment and treatment of children and adolescents with tic disorders. J Am Acad Child Adolesc Psychiatry (2013) 52(12):1341–59. 10.1016/j.jaac.2013.09.015 [DOI] [PubMed] [Google Scholar]
- 24.Verdellen C, van de Griendt J, Hartmann A, Murphy T, ESSTS Guidelines Group . European clinical guidelines for Tourette Syndrome and other tic disorders. Part III: behavioural and psychosocial interventions. Eur Child Adolesc Psychiatry (2011) 20(4):197–207. 10.1007/s00787-011-0167-3 [DOI] [PubMed] [Google Scholar]
- 25.Woods DW, Piacentini J, Chang S, Deckersbach T, Ginsburg G, Peterson A, et al. Managing Tourette Syndrome: A Behavioral Intervention for Children and Adults Therapist Guide: A Behavioral Intervention for Children and Adults Therapist Guide. New York: Oxford University Press; (2008). [Google Scholar]
- 26.Azrin NH, Nunn RG. Habit-reversal: a method of eliminating nervous habits and tics. Behav Res Ther (1973) 11(4):619–28. 10.1016/0005-7967(73)90119-8 [DOI] [PubMed] [Google Scholar]
- 27.Azrin NH, Peterson AL. Habit reversal for the treatment of Tourette syndrome. Behav Res Ther (1988) 26(4):347–51. 10.1016/0005-7967(88)90089-7 [DOI] [PubMed] [Google Scholar]
- 28.Bate KS, Malouff JM, Thorsteinsson ET, Bhullar N. The efficacy of habit reversal therapy for tics, habit disorders, and stuttering: a meta-analytic review. Clin Psychol Rev (2011) 31(5):865–71. 10.1016/j.cpr.2011.03.013 [DOI] [PubMed] [Google Scholar]
- 29.Cook CR, Blacher J. Evidence-based psychosocial treatments for tic disorders. Clin Psychol Sci Pract (2007) 14(3):252–67. 10.1111/j.1468-2850.2007.00085.x [DOI] [Google Scholar]
- 30.Hwang GC, Tillberg CS, Scahill L. Habit reversal training for children with Tourette syndrome: update and review. J Child Adolesc Psychiatr Nurs (2012) 25(4):178–83. 10.1111/jcap.12002 [DOI] [PubMed] [Google Scholar]
- 31.Himle MB, Woods DW, Piacentini JC, Walkup JT. Brief review of habit reversal training for Tourette syndrome. J Child Neurol (2006) 21(8):719–25. 10.1177/08830738060210080101 [DOI] [PubMed] [Google Scholar]
- 32.Himle MB, Freitag M, Walther M, Franklin SA, Ely L, Woods DW. A randomized pilot trial comparing videoconference versus face-to-face delivery of behavior therapy for childhood tic disorders. Behav Res Ther (2012) 50(9):565–70. 10.1016/j.brat.2012.05.009 [DOI] [PubMed] [Google Scholar]
- 33.Piacentini J, Woods DW, Scahill L, Wilhelm S, Peterson AL, Chang S, et al. Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA (2010) 303(19):1929–37. 10.1001/jama.2010.607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilhelm S, Peterson AL, Piacentini J, Woods DW, Deckersbach T, Sukhodolsky DG, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry (2012) 69(8):795–803. 10.1001/archgenpsychiatry.2011.1528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Specht MW, Woods DW, Nicotra CM, Kelly LM, Ricketts EJ, Conelea CA, et al. Effects of tic suppression: ability to suppress, rebound, negative reinforcement, and habituation to the premonitory urge. Behav Res Ther (2013) 51(1):24–30. 10.1016/j.brat.2012.09.009 [DOI] [PubMed] [Google Scholar]
- 36.Hoogduin K, Verdellen C, Cath D. Exposure and response prevention in the treatment of Gilles de la Tourette’s syndrome: four case studies. Clin Psychol Psychother (1997) 4(2):125–35. [DOI] [Google Scholar]
- 37.Verdellen CWJ, Keijsers GPJ, Cath DC, Hoogduin CAL. Exposure with response prevention versus habit reversal in Tourettes’s syndrome: a controlled study. Behav Res Ther (2004) 42(5):501–11. 10.1016/S0005-7967(03)00154-2 [DOI] [PubMed] [Google Scholar]
- 38.Banaschewski T, Woerner W, Rothenberger A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol (2003) 45(10):700–3. 10.1017/S0012162203001294 [DOI] [PubMed] [Google Scholar]
- 39.O’Connor K. A cognitive-behavioral/psychophysiological model of tic disorders. Behav Res Ther (2002) 40(10):1113–42. 10.1016/S0005-7967(02)00048-7 [DOI] [PubMed] [Google Scholar]
- 40.O’Connor KP. Cognitive-Behavioral Management of Tic Disorders. New York: Wiley; (2005). [Google Scholar]
- 41.O’Connor K, Gareau D, Borgeat F. Muscle control in chronic tic disorders. Biofeedback Self Regul (1995) 20(2):111–22. 10.1007/BF01720968 [DOI] [PubMed] [Google Scholar]
- 42.O’Connor K, St-Pierre-Delorme ME, Leclerc J, Lavoie M, Blais MT. Meta-cognitions in Tourette syndrome, tic disorders, and body-focused disorder. Can J Psychiatry (2014) 59(8):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O’Connor K, Lavoie M, Blanchet P, St-Pierre-Delorme MÈ. Evaluation of a cognitive psychophysiological model for management of tic disorders: an open trial. Br J Psychiatry (2016) 209(1):76–83. 10.1192/bjp.bp.114.154518 [DOI] [PubMed] [Google Scholar]
- 44.O’Connor KP, Brault M, Robillard S, Loiselle J, Borgeat F, Stip E. Evaluation of a cognitive-behavioural program for the management of chronic tic and habit disorders. Behav Res Ther (2001) 39(6):667–81. 10.1016/S0005-7967(00)00048-6 [DOI] [PubMed] [Google Scholar]
- 45.O’Connor KP, Laverdure A, Taillon A, Stip E, Borgeat F, Lavoie M. Cognitive behavioral management of Tourette’s syndrome and chronic tic disorder in medicated and unmedicated samples. Behav Res Ther (2009) 47(12):1090–5. 10.1016/j.brat.2009.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bellavance J. Évaluation des effets d’un programme d’entraînement à l’autogestion des tics sur le fonctionnement social d’adolescents atteints du syndrome de Gilles de la Tourette. (2010). Available from: http://www.svs.ulaval.ca/index.php?pid=651etall=1etid=3888
- 47.Leclerc J, O’Connor KP, Forget J, Lavoie ME. Évaluation de l’effet d’un programme d’entraînement à l’autogestion des épisodes explosifs chez des enfants atteints du syndrome de Gilles de la Tourette. Prat Psychol. (2012) 18(3):221–44. 10.1016/j.prps.2010.07.001 [DOI] [Google Scholar]
- 48.Leclerc BJ, Valois P, J-Nolin G, Bombardier M, Ouellette S, O’Connor KP. A cognitive-behavioral and psychophysiological treatment for tics in children; managing underlined processes. J Dev Phys Disabil (2016) 28(4):581–93. 10.1007/s10882-016-9496-y [DOI] [Google Scholar]
- 49.Leclerc J, Goulet G, Hamel N, O’Connor KP. Façotik; Léa et Nico font face aux tics. Guides méthodologiques (enfant et thérapeute). Montreal: Centre d’études pour les troubles obsessionnels et les tics; (2013). [Google Scholar]
- 50.Leckman JF, Riddle MA, Hardin MT, Ort SI, Swartz KL, Stevenson J, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry (1989) 28(4):566–73. 10.1097/00004583-198907000-00015 [DOI] [PubMed] [Google Scholar]
- 51.Harcherik DF, Leckman JF, Detlor J, Cohen DJ. A new instrument for clinical studies of Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry (1984) 23(2):153–60. 10.1097/00004583-198403000-00006 [DOI] [PubMed] [Google Scholar]
- 52.Reynolds CR, Kamphaus RW. Behavior assessment system for children: parent rating scales. Circle Pines. MN: American Guidance Service; (1992). [Google Scholar]
- 53.Battle J. Culture-Free Self-Esteem Inventories. 2th ed Austin: PRO-ED; (1992). [Google Scholar]
- 54.Coopersmith S. The Antecedents of Self-Esteem. Palo Alto, CA: Consulting Psychologists Press; (1967). [Google Scholar]
- 55.Greene RW, Ablon JS, Goring JC. A transactional model of oppositional behavior: underpinnings of the collaborative problem solving approach. J Psychosom Res (2003) 55(1):67–75. 10.1016/S0022-3999(02)00585-8 [DOI] [PubMed] [Google Scholar]
- 56.Diamond G, Josephson A. Family-based treatment research: a 10-year update. J Am Acad Child Adolesc Psychiatry (2005) 44(9):872–87. 10.1097/01.chi.0000169010.96783.4e [DOI] [PubMed] [Google Scholar]
- 57.Core Team R. R: A Language and Environment for Statistical Computing. 3.2.2. Vienna, Austria: R Foundation for Statistical Computing; (2015). [Google Scholar]
- 58.Hothorn T, Hornik K, van de Wiel MA, Zeileis A. Implementing a class of permutation tests: the coin package. J Stat Softw (2008) 28(8):1–23. 10.18637/jss.v028.i08 [DOI] [Google Scholar]
- 59.Miltenberger RG, Fuqua RW, Woods DW. Applying behavior analysis to clinical problems: review and analysis of habit reversal. J Appl Behav Anal (1998) 31:447–69. 10.1901/jaba.1998.31-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Woods DW, Koch M, Miltenberger RG, Lumley VA. Sequential application of major habit reversal components to treat motor tics in children. J Appl Behav Anal (1996) 29:483–93. 10.1901/jaba.1996.29-483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lavoie ME, Imbriglio TV, Stip E, O’Connor KP. Neurocognitive changes following cognitive-behavioral treatment in Tourette syndrome and chronic tic disorder. Int J Cogn Ther (2011) 4(1):34–50. 10.1521/ijct.2011.4.1.34 [DOI] [Google Scholar]
- 62.Morand-Beaulieu SM, O’Connor KP, Sauvé G, Blanchet PJ, Lavoie ME. Cognitive behavioural therapy induces sensorimotor and specific electrocortical changes in chronic tic and Tourette’s disorder. Neuropsychologia (2015) 79(B):310–21. 10.1016/j.neuropsychologia.2015.05.024 [DOI] [PubMed] [Google Scholar]