In an era when the development of new antimicrobial drugs is slow, vancomycin remains the preferred antimicrobial therapy for Clostridium difficile infection (CDI), the most important health care-related infection in the world today. The emergence of resistance to vancomycin would have significant consequences in relation to treating patients with CDI. In this paper, we describe for the first time a complete set of vancomycin resistance genes in C. difficile. The genes were very similar to genes found in vancomycin-resistant enterococci (VRE) that were associated with the emergence and global dissemination of this organism. Fortunately, the C. difficile strain did not show any reduced susceptibility to vancomycin in vitro (MIC, 1 mg/liter), possibly because of a small difference in one gene. However, this observation signals that we may be very close to seeing a fully vancomycin-resistant strain of C. difficile.
KEYWORDS: antimicrobial resistance, Clostridium difficile infection, mobile genetic element, vanB
ABSTRACT
In the last decade, Clostridium difficile infection (CDI) has reached an epidemic state with increasing incidence and severity in both health care and community settings. Vancomycin is an important first-line therapy for CDI, and the emergence of resistance would have significant clinical consequences. In this study, we describe for the first time a vanB2 vancomycin resistance operon in C. difficile, isolated from an Australian veal calf at slaughter. The operon was carried on an ~42-kb element showing significant homology and synteny to Tn1549, a conjugative transposon linked with the emergence and global dissemination of vancomycin-resistant enterococci (VRE). Notably, the C. difficile strain did not show any reduced susceptibility to vancomycin in vitro (MIC, 1 mg/liter), possibly as a result of an aberrant vanRB gene. As observed for other anaerobic species of the animal gut microbiota, C. difficile may be a reservoir of clinically important vancomycin resistance genes.
IMPORTANCE In an era when the development of new antimicrobial drugs is slow, vancomycin remains the preferred antimicrobial therapy for Clostridium difficile infection (CDI), the most important health care-related infection in the world today. The emergence of resistance to vancomycin would have significant consequences in relation to treating patients with CDI. In this paper, we describe for the first time a complete set of vancomycin resistance genes in C. difficile. The genes were very similar to genes found in vancomycin-resistant enterococci (VRE) that were associated with the emergence and global dissemination of this organism. Fortunately, the C. difficile strain did not show any reduced susceptibility to vancomycin in vitro (MIC, 1 mg/liter), possibly because of a small difference in one gene. However, this observation signals that we may be very close to seeing a fully vancomycin-resistant strain of C. difficile.
Observation
Since its first description as the causative agent of pseudomembranous colitis in 1978, Clostridium difficile has emerged as a major enteropathogen of humans and a significant burden to global health care systems (1). Vancomycin has been a first-line therapy for C. difficile infection (CDI) for almost 30 years, retaining good activity against C. difficile, including strains belonging to epidemic lineages and those with increased resistance to metronidazole (2). Despite sporadic reports of reduced susceptibility to vancomycin (MIC, ≥4 mg/liter; CLSI susceptibility breakpoint, ≤2 mg/liter), to date no underlying mechanisms have been identified (2). Sequencing of C. difficile genomes revealed the widespread presence of a vancomycin resistance operon (vanGCd) (3). Although it is often referred to as cryptic (phenotypically silent), transcriptional and biochemical studies showed that vanGCd was functional, conferring a modest increase in MIC in C. difficile (from 1 mg/liter to 2 mg/liter) (4). Here, we present the first description of a cryptic vanB2 operon in C. difficile, carried on an ~42-kb element showing significant homology and synteny to Tn1549, a conjugative transposon (CTn) linked with the emergence and global dissemination of vancomycin-resistant enterococci.
C. difficile strain AI0499 was recovered from the carcass of a calf (aged <7 days) in Victoria, Australia, in April 2013, identified as C. difficile by morphological and phenotypic traits as previously described (5), and confirmed by matrix-assisted laser desorption ionization–time of flight (MALDI-TOF) mass spectrometry. By PCR ribotyping, strain AI0499 was identified as ribotype (RT) 033 and thus negative for genes encoding large clostridial toxins A and B (tcdA− tcdB−) but positive for genes encoding binary toxin (cdtA+ cdtB+) (5).
Whole-genome sequencing (WGS) of AI0499 was performed in duplicate at two independent institutions. Genomic DNA (gDNA) was extracted using a Gentra Puregene kit (Qiagen, Hilden, Germany), and libraries were created using Nextera XT protocols (Illumina, Inc., San Diego, CA). The first sequence run was performed on an Illumina MiSeq sequencer with 250-bp paired-end (PE) chemistry, generating 406,204 reads and 36× coverage. The second was performed on an Illumina HiSeq sequencer with 100-bp PE chemistry, generating 3,684,407 reads and 131× coverage. Multilocus sequence typing (MLST) and antimicrobial gene profiling were performed using SRST2 (6). Genomes were assembled, annotated, and curated using a pipeline comprising SPAdes, Prokka, Artemis, and Easyfig (7–10). In vitro susceptibility to vancomycin was investigated in triplicate using the CLSI agar dilution methodology as previously described (11) and in triplicate using Etest methodology.
WGS and de novo assembly of the AI0499 genome revealed a single chromosome of 4,095,918 bp and 28.75% GC with 3,960 coding sequences (CDS) and an overall coverage of ~130×. Strain AI0499 was characterized as sequence type (ST) 11 (MLST clade 5) and harbored a complete binary toxin locus comprising cdtR, cdtA, and cdtB genes. Strain AI0499 possessed an uncommon pathogenicity locus identified as toxinotype XI and defined by the complete absence of tcdB, a fragmented and truncated tcdA gene (A2 fragment, 3,231 bp; A3 fragment, 915 bp), and a variant tcdC gene (allele tcdC-A1 as described by Curry et al.) (12, 13). SRST2 identified seven vancomycin resistance genes with >99% sequence identity to vanXB, vanB, vanHB, vanW, vanYB, vanSB, and vanRB. AI0499 was negative for vanGCd.
In Gram-positive bacteria, vancomycin resistance is mediated by several van operons and arises as a result of both (i) biosynthesis of modified peptidoglycan precursors ending in d-Ala-d-Lac or d-Ala-d-Ser to which vancomycin shows reduced binding and (ii) the elimination of high-affinity natural d-Ala-d-Ala precursors (13). Tn1549 (GenBank accession no. AF192329) is a member of the Tn916 family of conjugative transposons and harbors a vanB subtype 2 operon (vanB2) comprising genes encoding a dipeptidase (vanXB, 609 bp), a ligase (vanB, 1,029 bp), a dehydrogenase (vanHB, 972 bp), a putative hydrolase (vanW, 828 bp), and a carboxypeptidase (vanYB, 807 bp). Two further genes, vanSB (1,344 bp) and vanRB (663 bp), also colocated within the vanB2 operon, play a crucial role in the phenotypic expression of vancomycin resistance (14).
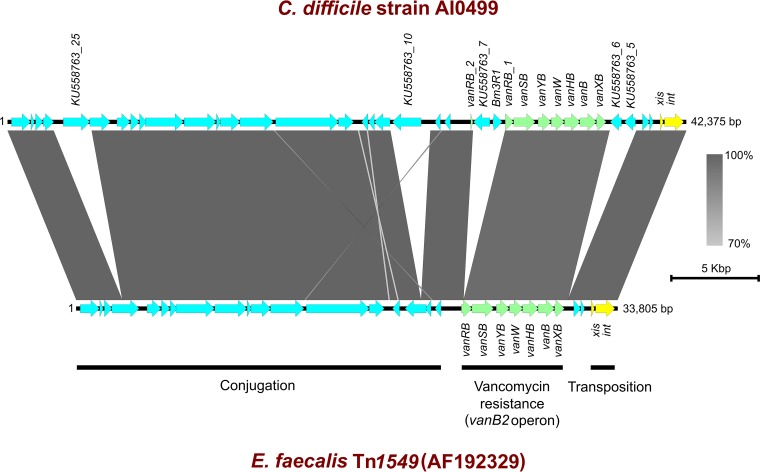
Sequence analysis of regions flanking the vanB2 gene cluster in AI0499 revealed an element of 42,375 bp showing significant sequence identity and synteny with the prototypical Tn1549 (GenBank sequence accession no. AF192329) (Fig. 1). Notably, this element differed markedly, particularly in its accessory region, from other putative Tn1549-like CTns, CTn2, CTn4, and CTn5, previously described in C. difficile (data not shown) (15, 16).
FIG 1 .
Comparative genomic analysis of Tn1549-like element in C. difficile strain AI0499 and prototypical Tn1549 of Enterococcus faecalis (GenBank accession no. AF192329). Arrows indicate open reading frames (ORFs) and direction of transcription. Excisionase (xis) and integrase (int) genes are shown in yellow, and genes comprising the vanB2 operon (vanXB, vanB, vanHB, vanW, vanYB, vanSB, and vanRB) are shown in green, with the remaining ORFs shown in blue. The figure was prepared using Easyfig (minimum blast hit length of 100 bp and a maximum E value of 0.001) (10). Vertical blocks between sequences indicate regions of homology with Blast nucleotide identity shown on a colored scale ranging from 70% (light gray) to 100% (dark gray). Bm3R1 and ORFs KU558763_5, KU558763_6, KU558763_7, KU558763_10, and KU558763_25 are shown to be present in strain AI0499 but absent from the sequence with accession no. AF192329 with Bm3R1 and KU558763_7 interrupting vanRB (vanRB_1 and vanRB_2 fragments shown). Overall sizes of elements in strain AI0499 and the sequence with accession no. AF192329 are 42,375 bp and 33,805 bp, respectively.
The element designated Tn1549-like contained 38 open reading frames (ORFs) and, like Tn1549, was organized into transposition, accessory (antimicrobial resistance), and conjugation regions (Fig. 1). Defining the left and right terminal ends of the element were 11-bp inverted repeats matching those found in Tn1549 and likely representing excision/integration sites (14). Comparing the vanB2 operon in AI0499 to that of Tn1549 revealed significant homology in vanXB, vanB, vanHB, vanW, vanYB, and vanSB (Fig. 1). However, in AI0499 vanRB was fragmented into a 525-bp fragment located adjacent to vanSB and a 134-bp fragment some 2.1 kb away (Fig. 1). Notably, two CDS present in strain AI0499 but absent in Tn1549 were found interrupting the vanRB gene. Bm3R1 (582 bp) and KU558763_7 (1,032 bp) encode a transcriptional repressor and decarboxylase originating from Bacillus megaterium and Bacillus cereus, respectively. The Tn1549-like element contained four additional CDS completely absent from Tn1549 (Fig. 1). KU558763_5 (684 bp) and KU558763_6 (702 bp), colocated between the transposition and vancomycin resistance regions, encode hypothetical proteins originating from Clostridium clostridioforme. KU558763_10 (1,803 bp), located ~3 kb into the conjugation region, and KU558763_25 (1,665 bp), located near the far left extremity, both encode group II introns originating from an unidentified Clostridiales member and C. clostridioforme, respectively.
Several clostridial species, including C. bolteae, C. hathewayi, C. innocuum, C. clostridioforme, and C. symbiosum, harbor vanB-like elements and demonstrate vancomycin resistance in vitro (17–19). Notably, strain AI0499 did not show any reduced susceptibility to vancomycin in vitro (MIC, 1 mg/liter), most likely due to the fragmentation of vanRB; however, this first description of a phenotypically silent vanB2 operon in C. difficile further confirms that anaerobes of the animal gut microbiota are a reservoir of clinically important vanB-like resistance operons.
Accession number(s).
The nucleotide sequence of the Tn1549-like element from strain AI0499 has been submitted to GenBank (accession no. KU558763). The HiSeq PE sequence reads have been deposited in the NCBI Short Read Archive under accession no. SRP067713.
ACKNOWLEDGMENTS
This study was supported by internal funding. D.R.K. and G.O.A. are funded by Australian Postgraduate Awards conferred by The University of Western Australia.
D.R.K., G.O.A., S.A.B., B.P.H., and T.V.R. declare no conflicts of interest relevant to this article.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors. This study was supported by internal funding. D.R.K. and G.O.A. are funded by Australian Postgraduate Awards conferred by The University of Western Australia.
REFERENCES
- 1.Kelly CP, LaMont JT. 2008. Clostridium difficile—more difficult than ever. N Engl J Med 359:1932–1940. doi: 10.1056/NEJMra0707500. [DOI] [PubMed] [Google Scholar]
- 2.Baines SD, Wilcox MH. 2015. Antimicrobial resistance and reduced susceptibility in Clostridium difficile: potential consequences for induction, treatment, and recurrence of C. difficile infection. Antibiotics 4:267–298. doi: 10.3390/antibiotics4030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammam F, Marvaud JC, Lambert T. 2012. Distribution of the vanG-like gene cluster in Clostridium difficile clinical isolates. Can J Microbiol 58:547–551. doi: 10.1139/w2012-002. [DOI] [PubMed] [Google Scholar]
- 4.Ammam F, Meziane-Cherif D, Mengin-Lecreulx D, Blanot D, Patin D, Boneca IG, Courvalin P, Lambert T, Candela T. 2013. The functional vanGCd cluster of Clostridium difficile does not confer vancomycin resistance. Mol Microbiol 89:612–625. doi: 10.1111/mmi.12299. [DOI] [PubMed] [Google Scholar]
- 5.Knight DR, Putsathit P, Elliott B, Riley TV. 2016. Contamination of Australian newborn calf carcasses at slaughter with Clostridium difficile. Clin Microbiol Infect 22:266.e1–266.e7. doi: 10.1016/j.cmi.2015.11.017. [DOI] [PubMed] [Google Scholar]
- 6.Inouye M, Dashnow H, Raven LA, Schultz MB, Pope BJ, Tomita T, Zobel J, Holt KE. 2014. SRST2: rapid genomic surveillance for public health and hospital microbiology labs. Genome Med 6:90. doi: 10.1186/s13073-014-0090-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bankevich A, Nurk S, Antipov D, Gurevich AA, Dvorkin M, Kulikov AS, Lesin VM, Nikolenko SI, Pham S, Prjibelski AD, Pyshkin AV, Sirotkin AV, Vyahhi N, Tesler G, Alekseyev MA, Pevzner PA. 2012. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol 19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seemann T. 2014. Prokka: rapid prokaryotic genome annotation. Bioinformatics 30:2068–2069. doi: 10.1093/bioinformatics/btu153. [DOI] [PubMed] [Google Scholar]
- 9.Carver T, Harris SR, Berriman M, Parkhill J, McQuillan JA. 2012. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28:464–469. doi: 10.1093/bioinformatics/btr703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sullivan MJ, Petty NK, Beatson SA. 2011. Easyfig: a genome comparison visualizer. Bioinformatics 27:1009–1010. doi: 10.1093/bioinformatics/btr039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knight DR, Giglio S, Huntington PG, Korman TM, Kotsanas D, Moore CV, Paterson DL, Prendergast L, Huber CA, Robson J, Waring L, Wehrhahn MC, Weldhagen GF, Wilson RM, Riley TV. 2015. Surveillance for antimicrobial resistance in Australian isolates of Clostridium difficile, 2013–14. J Antimicrob Chemother 70:2992–2999. doi: 10.1093/jac/dkv220. [DOI] [PubMed] [Google Scholar]
- 12.Rupnik M, Janezic S. 2016. An update on Clostridium difficile toxinotyping. J Clin Microbiol 54:13–18. doi: 10.1128/JCM.02083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Curry SR, Marsh JW, Muto CA, O’Leary MM, Pasculle AW, Harrison LH. 2007. tcdC genotypes associated with severe TcdC truncation in an epidemic clone and other strains of Clostridium difficile. J Clin Microbiol 45:215–221. doi: 10.1128/JCM.01599-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courvalin P. 2006. Vancomycin resistance in gram-positive cocci. Clin Infect Dis 42(Suppl 1):S25–S34. doi: 10.1086/491711. [DOI] [PubMed] [Google Scholar]
- 15.Brouwer MS, Warburton PJ, Roberts AP, Mullany P, Allan E. 2011. Genetic organisation, mobility and predicted functions of genes on integrated, mobile genetic elements in sequenced strains of Clostridium difficile. PLoS One 6:e23014. doi: 10.1371/journal.pone.0023014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brouwer MSM, Roberts AP, Mullany P, Allan E. 2012. In silico analysis of sequenced strains of Clostridium difficile reveals a related set of conjugative transposons carrying a variety of accessory genes. Mob Genet Elements 2:8–12. doi: 10.4161/mge.19297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ballard SA, Pertile KK, Lim M, Johnson PD, Grayson ML. 2005. Molecular characterization of vanB elements in naturally occurring gut anaerobes. Antimicrob Agents Chemother 49:1688–1694. doi: 10.1128/AAC.49.5.1688-1694.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marvaud JC, Mory F, Lambert T. 2011. Clostridium clostridioforme and Atopobium minutum clinical isolates with vanB-type resistance in France. J Clin Microbiol 49:3436–3438. doi: 10.1128/JCM.00308-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Launay A, Ballard SA, Johnson PD, Grayson ML, Lambert T. 2006. Transfer of vancomycin resistance transposon Tn1549 from Clostridium symbiosum to Enterococcus spp. in the gut of gnotobiotic mice. Antimicrob Agents Chemother 50:1054–1062. doi: 10.1128/AAC.50.3.1054-1062.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]