Graphical abstract
Keywords: Isopropyl myristate (IPM), Immobilized lipase B, Packed bed reactor (PBR), Green synthesis
Highlights
-
•
Continuous synthesis of isopropyl myristate was studied in a single phase medium using packed bed reactor (PBR).
-
•
Two step synthesis with intermediate dehydration step.
-
•
98.5% conversion with a residence time of 30 min.
-
•
Immobilized lipase stable for more than 50 days (1000 hrs).
Abstract
Isopropyl myristate is a useful functional molecule responding to the requirements of numerous fields of application in cosmetic, pharmaceutical and food industry. In the present work, lipase-catalyzed production of isopropyl myristate by esterification of myristic acid with isopropyl alcohol (molar ratio of 1:15) in the homogenous reaction medium was performed on a bench-scale packed bed reactors, in order to obtain suitable reaction performance data for upscaling. An immobilized lipase B from Candida antartica was used as the biocatalyst based on our previous study. The process intensification resulted in a clean and green synthesis process comprising a series of packed bed reactors of immobilized enzyme and water dehydrant. In addition, use of the single phase reaction system facilitates efficient recovery of the product with no effluent generated and recyclability of unreacted substrates. The single phase reaction system coupled with a continuous operating bioreactor ensures a stable operational life for the enzyme.
1. Introduction
There has been great demand of long chain fatty acid esters in the last few decades owing to their increasing market shares. Isopropyl myristate (IPM) is one such fatty acid esters having great interest in food, cosmetic and pharmaceutical industries as an emollient, thickening agent, or lubricant. It is an essential ingredient or carrier of active ingredient in cosmetic and pharmaceutical industries. The chemical methods used for IPM synthesis have inherent disadvantages thus diverting towards the ‘green’ synthesis route. The competence lies in the cost of biocatalyst and therefore it is very essential to develop processes which will successfully replace the existing chemical processes. In this aspect, many examples of Packed Bed Reactor (PBR) biocatalytic synthesis have been documented [1], [2], [3], [4].
Several researchers have discussed biotransformation reactions in PBR system to evaluate their technical and economical feasibility [5], [6], [7]. PBR provides a lower ratio of substrate to enzyme than the conventional batch reactors resulting in a higher reaction performance. Stirred-tank reactors with immobilized enzymes are not suitable for long term industrial-scale production and are susceptible to breaking by the stirring shear force in a stirred – tank reactor. The PBR is commonly employed in industries as (i) it facilitates use of catalyst at high density and subsequent efficient separation; (ii) it permits the use of a continuous mode of operation; (iii) it allows reuse of the enzyme without the need for prior separation; and (iv) it is more cost effective than batch operation [8], [9], [10]. Overall, PBR has many advantages for large-scale process, such as a high yield with low cost, and ease of design, operation and maintenance [11]. The immobilized form of enzyme has great advantages such as ease of recovery, high stability and reuse of catalyst. In addition, a novel single phase created by isopropyl alcohol enhanced the stability and activity of an enzyme used. One such enzyme is Novozym 435 which has been used in PBR mode and reported in literature [5]. In case of isopropyl myristate synthesis, several authors have reported Space Time Yield (STY) for batch mode of reaction (Table 1). However, no attempt has been made, best to our knowledge, for the continuous PBR mode synthesis of isopropyl myristate.
Table 1.
Various STY for Isopropyl myristate synthesis.
| Author | Catalyst | Reaction conditions | STY (mM of IPM produced/g of catalyst/h) |
|---|---|---|---|
| Verma et al. [12] | Lipase | 65 °C temperature, n-Heptane as solvent | 1.56 |
| Chun-hua et al. [13] | Lipase | 55 °C temperature, petroleum ether as solvent | 62.2 |
| Deng Bin et al. [14] | Lanthanum oxide | Microwave Radiation with power of 450 W, cyclohexane as the water-carrying agent | 322.46 |
In the present work, continuous enzymatic synthesis of an isopropyl myristate using packed bed reactor with an immobilized enzyme Novozym 435. The robust, commercial immobilized enzyme, Novozym 435, was selected based on our previous studies [15] and it has shown high activity, selectivity and high conversion. Water (formed as a by-product) has several effects on the reaction rate, enzyme activity and deterred complete conversion of myristic acid to isopropyl myristate. Thus the efficient removal of water becomes essential to get the high productivity. The molecular sieves packed in column were used so to as to prevent such inhibitory effects of water in the esterification reaction. Further, a novel single phase reaction medium with isopropyl alcohol as the major solvent enhances the stability and activity of the enzyme used. Thus the present research explores the possibility of use of PBRs for the synthesis of isopropyl myristate.
2. Materials and methods
2.1. Materials
The immobilized lipase B from C. antarctica (Novozym 435 with specific activity of 10,000 PLU/g, Particle size 0.3–0.9 mm, Bulk density – 0.43 g/cm3) was a brought from Novo Nordisk Ltd. (Bagsvaerd, Denmark). The same initial batch of enzyme (10 g) was used throughout the research work. Myristic acid, isopropyl alcohol (with 0.2% water), Molecular sieves 4 Å, Potassium hydroxide was obtained from SD Fine Chemicals (Mumbai, India). Standard sample of isopropyl myristate was a kind gift from Anshul Life Sciences (Mumbai, India). All the other chemicals and reagents were of analytical grade.
2.2. Methods
2.2.1. Product analysis
Sample withdrawn from reaction mixture was suitably diluted in tert-butanol and filtered through 0.2 micron syringe filter. A 10 μl aliquot was injected into an Agilent Zorbax C-18 reverse phase HPLC column (4.6 mm I.D., 250 mm length and 5 μm particle size) at 30 °C. The mobile phase consisted of 80% acetonitrile (solvent A) and 20% of 30 mM phosphoric acid in water (solvent B) with a flow rate of 1 mL/min. The gradient started at 50% A followed by a linear increase to 90% A in 15 min. 100% A was then held for 10 min followed by returning to initial condition in next 10 min. Detection was carried out at 205 nm by UV detector. The concentration of the sample was determined from the standard curve for myristic acid and isopropyl myristate. The conversion (%) was calculated as follows
2.2.2. Isopropyl myristate synthesis in a packed bed reactor (PBR)
A continuous esterification of myristic acid with isopropyl alcohol using immobilized lipase was studied in a packed bed reactor. A 2.5 × 30 cm (diameter × length) glass jacketed column was employed for the synthesis of isopropyl myristate. The enzyme bed was held in the jacketed column between two adjustable steel adaptors. The reactor set up was operated as an up flow system. A re-circulating water bath was used to maintain the desired temperature of the reactor bed (60 °C). A stirrer was used to thoroughly mix the substrate. A peristaltic pump was used to pump the substrate (feed solution) to the reactor system.
2.2.3. Optimization of isopropyl myristate synthesis in PBR
2.2.3.1. Effect of residence time
10 g of immobilized lipase (Novozym 435) was mixed with isopropyl alcohol and the slurry was packed into a jacketed glass column maintained at 60 °C. The bed porosity of packed bed reactor was 0.4. The substrate mixture (myristic acid and isopropyl alcohol, molar ratio – 1:15) was continuously agitated by a magnetic stirrer and passed through the column at varying flow rates (1.4–6.2 mL/min) to achieve desirable residence time. The esterification reaction (as % conversion) was monitored by HPLC analysis.
2.2.3.2. Effect of substrate molar concentration (acid/alcohol molar ratio)
To study the effect of isopropyl alcohol concentration, reaction mixtures were prepared by fixing myristic acid concentration and varying the amount of isopropyl alcohol in a molar ratio from 1:1 to 1:15 while holding constant the rest of the parameters in the reaction mixture. After reaching a steady state, the outlet sample was analyzed by HPLC and thus % conversion was calculated.
2.2.3.3. Effect of reaction temperature
Isopropyl myristate synthesis was carried out at varying temperatures in a jacketed column packed with 50 mL of an enzyme. The temperature range used was 30–70 °C. The reaction mixture (myristic acid and isopropyl alcohol, molar ratio – 1:15) was passed through the column at the flow rate of 3 mL/min with a residence time of 16 min. After reaching a steady state, the outlet sample was analyzed by HPLC and thus % conversion was calculated.
2.2.3.4. Multicolumn studies
Three tubular packed-bed reactor (2.5 cm ID × 30 cm length) were used which consisted of a jacketed glass tube. The first and third reactor was packed with 10 g of immobilized lipase and the second reactor was packed with 20 g of molecular sieve particle. 500 mL of reaction mixture containing myristic acid dissolved in isopropyl alcohol (molar ratio 1:15) was prepared as feed solution and introduced into the reactor in an up-flow configuration at a flow rate of 3 mL/min. After reaching a steady state, the outlet sample was analyzed by HPLC and thus % conversion was calculated.
2.2.3.5. Reactor stability
The operational stability of Novozym 435 in PBR was studied by carrying out synthesis of isopropyl myristate at flow rate of 3 mL/min and 60 °C temperature with acid to alcohol molar ratio of 1:15 for period of 50 days.
3. Results and discussion
3.1. Isopropyl myristate synthesis in a packed bed reactor (PBR)
The isopropyl myristate synthesis in a PBR was attempted based on the optimized parameters obtained from our previous studies [15]; Lipase catalyst – Novozym 435, Temperature of the reaction– 60 °C, Acid to alcohol molar ratio – 1:15, Time of reaction – 4 h, Conversion obtained – 87.65%. The parameters needed for efficient continuous synthesis for isopropyl myristate thus systematically studied to achieve the high productivity.
3.2. Effect of residence time
The residence time is one of the most crucial parameters responsible for the higher yields in the packed bed bioreactor. In order to achieve higher yield of ester, a sufficient residence time was needed. In this study, the effect of residence time was investigated in the range of 8–35 min by adjusting the volumetric flow rate from 1.4 to 6.2 mL/min. The results of residence time effects on the synthesis of isopropyl myristate at steady state condition are shown in Fig. 1. These experimental results revealed that by increasing residence time (i.e. decreasing flow rate) the conversion of isopropyl myristate was increased and reached to the highest conversion of 90% with the residence time of 16 min. This is because the longer residence time would lengthen the enzyme substrate contact time and create higher esterification activity. While lower residence time reduces mass transfer of substrate into the supported-enzyme interface, thus the less distribution and short contact time of the substrate and the enzyme active site, yielding lower conversion of substrate. This was contractedly to literature where Aziah Serri et al. [16] reported conversion to 90% of citronellyl malonate at a higher feed flow rate into the packed bed reactor system. They have reasoned out that with lower flow rate (high residence time), there were high chances of inhibition occurring by the malonic acid in the reaction. The fluid behavior at low flow rate (1.4 mL/min) was found to be laminar in nature (Reynolds number = 850) while at high flow rate (6.2 mL/min) it was transient in nature (Reynolds number = 3760).
Fig. 1.
Effect of residence time on isopropyl myristate synthesis. [Molar ratio of myristic acid to isopropyl alcohol = 1:15, temperature of reaction = 60 °C, enzyme: Novozym 435].
3.3. Effect of substrate molar ratio (acid/alcohol molar ratio)
The substrate molar ratio decides the fate of reaction rate and activity of lipase catalyzed reaction and hence optimum acid to alcohol molar ratio for isopropyl myristate synthesis was investigated. Fig. 3 showed the effects of acid to alcohol molar ratio on the conversion during the esterification of isopropyl myristate. The highest conversion (90%) was achieved when the molar ratio of isopropyl alcohol to myristic acid was more than 10. For a lower acid to alcohol ratio (1:1 to 1:3), the isopropyl myristate conversion achieved was at a range 60–90%. At low acid to alcohol ratio, higher viscosity of the reaction medium was expected, which reduces the access of substrate molecules to the biocatalyst. This can be improved by increasing the temperature of the reaction, but at the same time it affects the stability of the enzyme. The present results can be used to deduce that isopropyl alcohol will not inhibit the esterification reaction when used in excess. Indeed, it acts as a solvent providing homogenous phase for catalysis and enhances the reaction rate as well as stability of an enzyme. Previously, Aziah Serri et al. [17] reported that increasing the concentration of citronellol (an alcohol component) increased the reaction rate for citronellyl laurate. A similar result was also found by Yadav et al. [18] where excess used of citronellol leads to a higher conversion of citronellyl laurate. The optimized acid to alcohol molar ratio of 1:15 was taken for the next set of experiments.
Fig. 3.
Effect of acid to alcohol molar ratio on isopropyl myristate synthesis. [Temperature of reaction = 60 °C, enzyme: Novozym 435].
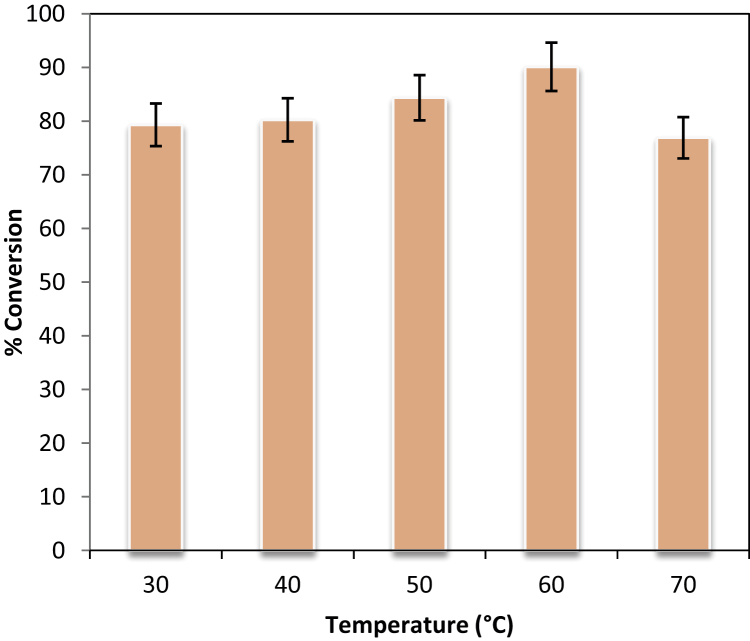
3.4. Effect of reaction temperature
The temperature plays an essential role in the activity and stability of an immobilized lipase. When continuous synthesis is carried out in the PBR, the uniform heating is essential parameters as uneven heating leads to variation in%conversion. Therefore, it was essential to access the temperature dependency for Novozym 435 in a PBR. The Fig. 2 shows the effects of various temperatures (30–70 °C) on the enzymatic synthesis of isopropyl myristate at steady state. Increase in temperature is seen to favour the esterification reaction as this helped to increase the frequency of collision between molecules. Additionally, there is a reduction of viscosity of the reaction mixture which improved the accessibility of substrate to biocatalyst microenvironment. At temperature of 70 °C, lower conversion was obtained because of deactivation of an enzyme. The different lipases show variation in thermal stability based on their structure and source. Sabeder et al. [19] highlighted that Novozym 435 stability was greatly reduced at higher temperature (>60 °C) while Dahlan et al. [20] reported that an immobilized Candida rugosalipase show decrease in activity above 50 °C during synthesis of citronellyl butyrate in n-hexane. The present enzyme (Novozym 435) activity was found to be highest and stable at 60 °C. Hence, it was selected as the optimum temperature for further experimental studies.
Fig. 2.
Effect of temperature on isopropyl myristate synthesis. [Molar ratio of myristic acid to isopropyl alcohol = 1:15, residence time = 16 min, enzyme: Novozym 435].
3.5. Multicolumn studies
Product inhibition is often found in the lipase catalyzed reaction which is one of the major obstacles, that lower conversions and make the enzyme reactor incompetent with the chemical process. There are many ways of overcoming this inhibitory effect of the product in the enzyme reactor. Removal of the product from the reaction medium is one of the most efficient and commonly used methods [21]. Here, we studied the effect of coupling the enzyme PBR with by-product removal (water dehydration). the operation was split into three step process based on the above concept. The first PBR composed of immobilized lipase, which gives 90% conversion with the generation of by-product (2% water content) in residence time of 16 min (Fig. 4). The accumulation of this water formed in the reaction reduces lipase activity. Thus, control of water is needed to attain high conversion and activity. So the second PBR consisting of molecular sieves was integrated. This selectively removes byproducts (water) formed. The product stream from second PBR with low water content (0.3%) was then fed into the third PBR comprising of an immobilized lipase. The residence time of 14 min was optimized for the highest conversion (98.5%) of esterification (Fig. 5). Hence, the present studies show that the packed-bed immobilized lipase reactors with a separate molecular sieve column (for water dehydration) is an effective strategy for the continuous synthesis of isopropyl myristate. The main advantage of the present process is that regeneration of molecular sieve column can be independently carried out upon saturation without disrupting the immobilized enzyme reactor. Earlier, the use of an external molecular sieve column in a pilot reaction test has been reported [22], where the water formed during the reaction was effectively removed yielding a high sugar ester conversion. Also, Paul et al. [23] reported the use of dry Dowex HCR-W2 resin in Na-form as a selective water adsorbent, packed together with the immobilized enzyme particles in a column which result in a substantial reduction in the accumulation of water on the biocatalyst improving cycle times and productivity.
Fig. 4.
Esterification reaction profile for First PBR. [Molar ratio of myristic acid to isopropyl alcohol = 1:15, temperature of reaction = 60 °C, enzyme: Novozym 435].
Fig. 5.
Esterification reaction profile in third PBR. [Molar ratio of myristic acid to isopropyl alcohol = 1:15, temperature of reaction = 60 °C, enzyme: Novozym 435].
3.6. Reactor stability
The success of any developed bioreactor is dependent largely upon the maintenance of biocatalytic activity over operational time. The operational stability of immobilized lipase reactor (first PBR) was studied for a prolonged period under the optimized conditions. A continuous synthesis 90% of isopropyl myristate at a flow rate of 3 mL/min (residence time = 16 min) was monitored at a column temperature of 60 °C by measuring the% conversion (Fig. 6). This indicates that there is no deactivation of the lipase enzyme over the tested period of time. Previously, Novozym 435 has been reported [24] for high activity (91.9%) after nine recycle for synthesis of oleyl oleate. Similarly, the same enzyme has shown high stability for five recycles during the enzymatic synthesis of mannosyl myristate in ionic liquid [25]. This present result demonstrates the possible use of a packed bed reactor with Novozym 435 for continous large-scale enzymatic synthesis of isopropyl myristate.
Fig. 6.
Operational stability of Novozym 435 enzyme reactor. [Molar ratio of myristic acid to isopropyl alcohol = 1:15, residence time = 16 min, temperature of reaction = 60 °C, enzyme: Novozym 435].
4. Conclusion
The present work was a comprehensive study on the reaction parameters influencing the enzymatic synthesis of isopropyl myristate in a continuous packed bed reactor. In order to improve the esterification yield, packed-bed reactors were combined with provision of water removal. The developed process scheme resulted in 98.5% conversion with a residence time of 30 min. The immobilized lipase B from C. antarctica stably operated for at least fifty days and STY of 26 mM/g/h for isopropyl myristate was achieved. The long-term stability of the biocatalyst in the homogenous phase reaction system confirmed the suitability and applicability of the process scheme for scale-up of synthesis of isopropyl myristate.
Acknowledgements
This study was supported by the Department of Biotechnology, Ministry of India and Institute of Chemical Technology, Mumbai. We wish to express our gratitude to the DBT-ICT-Centre for Energy Biosciences for providing facilities for the experimental work.
Contributor Information
Rajeshkumar N. Vadgama, Email: rajesh.vdgm@gmail.com.
Annamma A. Odaneth, Email: a.dbtceb@gmail.com, a.annamma@ictmumbai.edu.in.
Arvind M. Lali, Email: arvindmlali@gmail.com.
References
- 1.Khaled N., Montet D., Pina M., Graille J. Biotechnol. Lett. 1991;13:167–172. [Google Scholar]
- 2.Xu J.H., Kato Y., Asano Y. Biotechnol. Bioeng. 2001;73:493–499. doi: 10.1002/bit.1084. [DOI] [PubMed] [Google Scholar]
- 3.Petzelbauer I., Kuhna B., Splechtna B., Kuble K.D., Nidetzky B. Biotechnol. Bioeng. 2002;77:619–631. doi: 10.1002/bit.10110. [DOI] [PubMed] [Google Scholar]
- 4.Xi W.W., Xi J.H. Process Biochem. 2005;40:2161–2166. [Google Scholar]
- 5.Watanabe Y., Shimaida Y., Sughara A., Tominaga Y. J. Am. Oil Chem. Soc. 2001;78:703–707. [Google Scholar]
- 6.Nie K., Xie F., Wang F., Tan T. J. Mol. Catal. B: Enzym. 2006;43:142–147. [Google Scholar]
- 7.Royan E., Minander N., Hahn-Hagerdal B. Biotechnol. Bioeng. 1996;51:317–326. doi: 10.1002/(SICI)1097-0290(19960805)51:3<317::AID-BIT7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 8.Shimada Y., Suenaga M., Sugihara A., Nakai S., Tominaga Y. J. Am. Oil Chem. Soc. 1999;76:189–193. [Google Scholar]
- 9.Xu X., Balchen S., Hoy C.E., Adler-Nissen J. J. Am. Oil Chem. Soc. 1998;75:1573–1579. [Google Scholar]
- 10.Knez Z., Habulin M. J. Supercrit. Fluids. 2002;23:29–42. [Google Scholar]
- 11.Roca E., Meinander N., Hahn-Hagerdal B. Biotechnol. Bioeng. 1996;51:317–326. doi: 10.1002/(SICI)1097-0290(19960805)51:3<317::AID-BIT7>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 12.Verma M.L., Chauhan G.S., Kanwar S.S. Acta Microbiol. Immune. Hung. 2008;55:327–342. doi: 10.1556/AMicr.55.2008.3.4. [DOI] [PubMed] [Google Scholar]
- 13.Chun-hua Y., Ming G., Jiang-fan L. China Surfactant Deterg Cosmet. 2009:06–09. [Google Scholar]
- 14.Deng Bin, Zhang Ai-hua, Xu An-wu J. Xiangnan Univ. 2008:05. [Google Scholar]
- 15.Vadgama R.N., Odaneth A.A., Lali A.M. Green synthesis of isopropyl myristate in novel single phase medium Part I: Batch optimization studies. Biotechnol. Rep. 2015 doi: 10.1016/j.btre.2015.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aziah S.N., Kamaruddin A.H., Tau Len K.Y. Food Bioprod. Process. 2010;88:327–332. [Google Scholar]
- 17.Aziah S.N., Kamaruddin A.H., Long W.S. Bioprocess Biosyst. Eng. 2006;29:253–260. doi: 10.1007/s00449-006-0074-z. [DOI] [PubMed] [Google Scholar]
- 18.Yadav G.D., Lathi P.S. J. Mol. Catal. B Enzym. 2004;27:113–119. [Google Scholar]
- 19.S. Sabeder, M. Habulin, Z. Krenz, Proceeding of the meeting in Calmar (France) (2005) 10th European meeting on supercritical Fulids: reactions, materials and natural products processing.
- 20.Dahlan A., Kamaruddin H., Najafpou G.D. Int. J. Eng. 2005;18:153–164. [Google Scholar]
- 21.Sekyoo J., Hwang B.Y., Kim J., Kim B.G. J. Mol. Catal. B Enzym. 2010;10:597–604. [Google Scholar]
- 22.In Sang Y., Park S.J., Yoon H.H. J. Ind. Eng. Chem. 2007;13:1–6. [Google Scholar]
- 23.Paul M., John L.G., Giorgio C. Biotechnol. Bioeng. 1998;60:445–453. [Google Scholar]
- 24.Salina M.R., Mahiran B., Abu B.S., Arbakariya A., Rosfarizan M., Basyaruddin A.R., Raja N.Z.R.A.R. Electron. J. Biotechnol. 2005;8:291–297. [Google Scholar]
- 25.Nadine G., Gaetan R., Magali D., Katherine N., Christine J., Marie-Laure F. Biotechnol. Agron. Soc. Environ. 2013;17:556–562. [Google Scholar]