Abstract
SHP-2 is a tyrosine phosphatase expressed in most embryonic and adult tissues. SHP-2 regulates many cellular functions including growth, differentiation, migration, and survival. Genetic and biochemical evidence show that SHP-2 is required for rat sarcoma viral oncogene/extracellular signal-regulated kinases mitogen-activated protein kinase pathway activation by most tyrosine kinase receptors, as well as by G-protein–coupled and cytokine receptors. In addition, SHP-2 can regulate the Janus kinase/signal transducers and activators of transcription, nuclear factor-κB, phosphatidyl-inositol 3-kinase/Akt, RhoA, Hippo, and Wnt/β-catenin signaling pathways. Emerging evidence has shown that SHP-2 dysfunction represents a key factor in the pathogenesis of gastrointestinal diseases, in particular in chronic inflammation and cancer. Variations within the gene locus encoding SHP-2 have been associated with increased susceptibility to develop ulcerative colitis and gastric atrophy. Furthermore, mice with conditional deletion of SHP-2 in intestinal epithelial cells rapidly develop severe colitis. Similarly, hepatocyte-specific deletion of SHP-2 induces hepatic inflammation, resulting in regenerative hyperplasia and development of tumors in aged mice. However, the SHP-2 gene initially was suggested to be a proto-oncogene because activating mutations of this gene were found in pediatric leukemias and certain forms of liver and colon cancers. Moreover, SHP-2 expression is up-regulated in gastric and hepatocellular cancers. Notably, SHP-2 functions downstream of cytotoxin-associated antigen A (CagA), the major virulence factor of Helicobacter pylori, and is associated with increased risks of gastric cancer. Further compounding this complexity, most recent findings suggest that SHP-2 also coordinates carbohydrate, lipid, and bile acid synthesis in the liver and pancreas. This review aims to summarize current knowledge and recent data regarding the biological functions of SHP-2 in the gastrointestinal tract.
Keywords: PTPN11, inflammation, gastrointestinal cancer, epithelium
Abbreviations used in this paper: CagA, cytotoxin-associated gene A; ERK, extracellular signal-regulated kinases; FGF, fibroblast growth factor; GI, gastrointestinal; HCC, hepatocellular carcinoma; IBD, inflammatory bowel disease; IEC, intestinal epithelial cell; JMML, juvenile myelomonocytic leukemia; KO, knockout; MAPK, mitogen-activated protein kinase; NF-κB, nuclear factor-κB; PI3K, phosphatidyl-inositol 3-kinase; PTP, protein tyrosine phosphatase; RAS, rat sarcoma viral oncogene
Summary.
SHP-2 is a tyrosine phosphatase widely expressed and involved in multiple cell signaling processes. Accumulating evidence now is emerging whereby dysfunction in this protein tyrosine phosphatase also represents a key factor in the pathogenesis of gastrointestinal diseases, in particular in chronic inflammation and cancer.
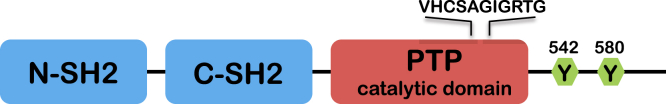
SHP-2 is a SH2 domain-containing protein tyrosine phosphatase (PTP) encoded by the PTPN11 gene.1, 2, 3, 4, 5 This PTP is expressed ubiquitously, sharing similar overall structure and high homology with SHP-1 phosphatase, which is expressed predominantly in hematopoietic cells.6 Both phosphatases contain 2 tandem SH2 domains at the N-terminus and 1 tyrosine phosphatase domain at the C-terminus (Figure 1). The SH2 domain is a sequence-specific phosphotyrosine-binding motif that mediates substrate recruitment and regulates phosphatase activity.7 In its inactive state, the N-terminal SH2 domain of SHP-2 binds the PTP domain, thus blocking access of substrates to the active site. Upon binding to phosphoproteins, the SH2 domain is released from the PTP domain, enabling SHP-2 to dephosphorylate its substrates.8, 9 In addition, a new regulatory mechanism based on SHP-2 dimerization recently was described. Indeed, 15% of SHP-2 in resting cells has been found to be in dimeric form, resulting in a decrease in catalytic activity of the phosphatase. Of note, the SH2 domains have no role in SHP-2 self-association.10 Importantly however, the dimer/monomer ratio is not static and is regulated by growth factors and the cell redox state.10 Given the significant regulatory role of SHP-2 in major signaling pathways (described later), keeping SHP-2 activity under control may be crucial for cell homeostasis.10
Figure 1.
Structure of SHP-2. Defined domains within SHP-2 are indicated. SHP-2 contains 2 tandem SH2 domains (blue), a single PTP domain (red), and a C-terminal hydrophobic tail that includes tyrosine phosphorylation sites (green).
Previous biochemical evidence has shown that SHP-2 enzymatic activity is required for its function in signal transduction.11, 12, 13, 14 The replacement of cysteine 459 by serine abolishes its enzymatic activity but not the capacity to bind to other signaling intermediates via its SH2 domains. This mutant thus functions as a dominant-negative molecule over the endogenous wild-type SHP-2.15 By using this Cys459Ser mutant, SHP-2 has been shown to positively regulate the signaling pathways of insulin, epidermal growth factor, platelet-derived growth factor, and fibroblast growth factor (FGF) in a number of studies, in both in vitro and in vivo models. More specifically, the introduction of this catalytically inactive SHP-2 mutant markedly inhibits the activation of mitogen-activated protein kinases (MAPKs) extracellular signal-regulated kinases (ERK)1/2 in response to epidermal growth factor, insulin, thrombin, and fibronectin.12, 14, 16, 17, 18, 19, 20 Further genetic and biochemical evidence also strongly shows that SHP-2 indeed is required for ERK/MAPK pathway activation by most, if not all, tyrosine kinase receptors, as well as by G-protein–coupled receptors, cytokine receptors, and integrins.8, 12, 14, 21, 22 SHP-2 binds directly to certain tyrosine kinase receptors or, more often, to scaffolds (Table 1), leading to its activation. Cells expressing dominant-negative SHP-214 or Ptpn11 gene exon 3–deleted mouse embryonic fibroblasts22 show defective rat sarcoma viral oncogene (RAS) activation, suggesting that SHP-2 acts upstream of RAS (Figure 2). However, other data have shown that a catalytically inactive mutant of SHP-2 (Cys459Ser) can perturb components of downstream signaling, even in the presence of a constitutively active RAS, suggesting that SHP-2 also may function either downstream and/or in parallel to RAS.13 In addition, SHP-2 was shown to functionally regulate other pathways including the Janus kinase/signal transducers and activators of transcription,23, 24 nuclear factor-κB (NF-κB),25, 26 phosphatidyl-inositol 3-kinase (PI3K)/Akt,27, 28, 29 Wnt/β-catenin,30, 31 Hippo,31 and RhoA32, 33 signaling (Figure 2).
Table 1.
Binding Partners of SHP-2 in Gastrointestinal Cells
| Organs | Proteins | Interactions | Cell types | Cell outcomes | References |
|---|---|---|---|---|---|
| Stomach | CagA | SH2 domain of SHP-2 and phosphorylated tyrosine in the EPIYA motif of CagA | AGS cells Human gastric epithelial cells |
Increased phosphatase activity of SHP-2 ↓ Induction of the hummingbird phenotype |
47, 59, 62, 117, 118 |
| FAK | SHP-2 in complex with CagA and FAK | AGS cells | Dephosphorylation of FAK on Y397, Y576, and Y577 by SHP-2 ↓ Induction of the hummingbird phenotype |
119 | |
| gp130 | SHP-2 in complex with gp130 | AGS cells | Activation of ERK1/2 by gp130 Inhibition of the Jak2/STAT3 pathway by SHP-2 |
59 | |
| Parafibromin | SH2 domain of SHP-2 and parafibromin | AGS cells | Dephosphorylation of parafibromin on Y290, Y293, and Y315 by SHP-2 ↓ Interaction between dephosphorylated parafibromin and β-catenin ↓ Nuclear accumulation of β-catenin and transcription of Wnt/β-catenin target genes |
30 | |
| YAP and TAZ | C-terminal tail of SHP-2 and WW domain and C-terminus PDZ-binding motif of TAZ | AGS cells | Dephosphorylation of YAP/TAZ by SHP-2 ↓ Nuclear accumulation of SHP-2 ↓ Interaction between dephosphorylated parafibromin ↓ Transcription of TEAD-regulated genes |
31 | |
| Intestine | IL22R1 | SHP-2 and phosphorylated IL22R1 (Y251, Y301) | SW480 cells | Activation of the ERK1/2 and Jak/STAT3 signaling pathways by IL22 | 120 |
| Bgp1 | N-SH2 domain of SHP-2 and phosphorylated Y in the ITIM motif of Bgp1 | CT51 cells | ND | 121 | |
| Liver | FRS2α | SH2 domain of SHP-2 and phosphorylated Y of FRS2α | Hep3B cells Mouse hepatic cells |
Activation of the ERK1/2 pathway by FGFR4 | 105 |
| Gab1 | SH2 domain of SHP-2 and phosphorylated Y of Gab1 | Hep3B cells Hep2G cells Mouse hepatic cells |
Activation of the ERK1/2 pathway | 90, 105, 122 | |
| Liver and pancreas | COP1 FASN |
N-SH2 domain of SHP-2 in a complex, association with FASN and COP1 | Mouse hepatic cells Mouse pancreatic cells |
FASN ubiquitination and degradation ↓ Impact on lipid metabolism and glucose homeostasis |
101 |
| Pancreas | p85/PI3K IRS2 |
SHP-2 in a complex with p85 and IRS2 | INS-1 832/13 pancreatic cells |
Glucose-induced activation of the Akt/FoxO1 pathway ↓ Insulin production |
98 |
| Sprouty 1 | ND | INS-1 832/13 cells |
Dephosphorylation of Sprouty 1 on Y by SHP-2 ↓ Glucose-induced activation of the ERK1/2 pathway ↓ Insulin production |
98 |
AGS, human gastric carcinoma cells; Bgp1, biliary glycoprotein 1; COP1, constitutive photomorphogenesis protein 1; CT51, mouse colonic carcinoma cells; EPIYA, Glu-Pro-Ile-Tyr-Ala; FAK, focal adhesion kinase; FASN, fatty acid synthase; FRS2α, fibroblast growth factor receptor substrate 2 α; Gab1, Grb2-associated binding protein; Hep, human hepatocellular carcinoma cells; IL22R1, interleukin-22 receptor 1; INS-1 832/13 cells, insulin-producing β-cells; IRS2, insulin receptor substrate 2; ITIM, immunoreceptor tyrosine-based inhibitor motif; ND, not determined; SW480, human colonic adenocarcinoma cells; TAZ, transcriptional coactivator with PDZ-binding motif; Y, tyrosine; gp130, glycoprotein 130; YAP, yes-associated protein.
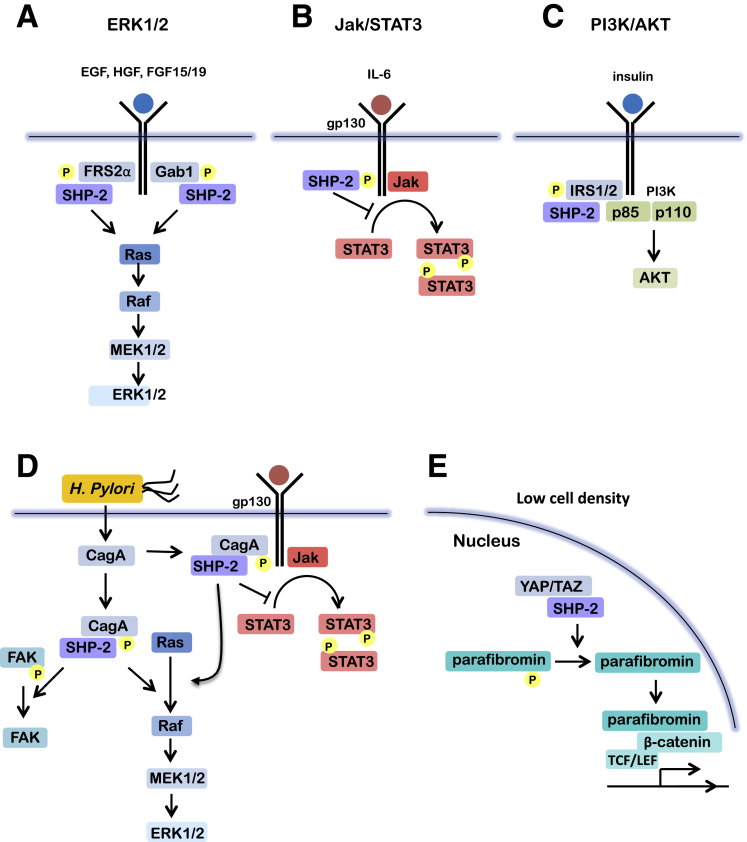
Figure 2.
Signaling pathways regulated by SHP-2 in the gastrointestinal tract. (A) In response to growth factors, SHP-2 binds via its SH2 domains either to autophosphorylated receptors (such as that for platelet-derived growth factor, not shown) or to docking proteins (such as the Grb2-associated proteins Gabs and fibroblast growth factor receptor substrate [FRS2]), which are tyrosine-phosphorylated by activated receptor tyrosine kinases or by Src family kinases. Such interactions result in the activation of SHP-2 and its consequent promotion of Ras activation, leading to cell growth. (B) In response to cytokines, the Janus kinases become phosphorylated and then phosphorylate STAT transcription factors, which dimerize and enter the nucleus. SHP-2 can inhibit this process by directly dephosphorylating either the Janus kinases or STAT proteins. (C) In response to insulin, SHP-2 assembles in a complex with insulin-receptor substrate (IRS)1/2 and the p85 subunit of PI3K, leading to Akt activation, leading to cell survival. (D) During H pylori infection, cagA is injected into the gastric epithelial cells and is phosphorylated by Src family kinases. SHP-2 then interacts with phosphorylated cagA, leading to the activation of the ERK1/2 MAPK pathway in a Ras-independent manner and to focal adhesion kinase (FAK) dephosphorylation and inactivation. CagA also can interact directly with STAT3 by binding or recruitment to gp130, thereby promoting hyperactivation and increased transcriptional activity. (E) At low cell density, nonphosphorylated YAP/TAZ promotes nuclear translocation of SHP-2, which in turn stimulates T-cell factor/lymphoid enhancer factor- and TEA domain family members-regulated genes via parafibromin dephosphorylation.
Despite extensive studies over the past decade, the mechanisms of SHP-2 action remain unclear. SHP-2 has been reported to interact with a number of diverse signaling components such as Gab1/2, fibroblast growth factor receptor substrate, insulin receptor substrate 1/2, p85, STAT1/3/5, Sprouty, and yes-associated protein/transcriptional coactivator with PSD-95, discs large, zona occludens 1-binding motif (Table 1 and Figure 2). As a result, SHP-2 has been shown to regulate numerous cellular functions, including progenitor cell development,34 growth,12, 35 differentiation,32, 36 and migration.37, 38 Notably, homozygous mice carrying a targeted mutation in the murine Shp2 gene, resulting in deletion of residues in the N-terminal SH2 domain, die around day 8.5–10.5 of gestation, with multiple defects in mesodermal patterning.39
PTPN11 Mutations and Phenotypes
In human beings, germline mutations in the Ptpn11 gene (which encodes SHP-2) have been identified in more than 50% of children with Noonan syndrome, a developmental disorder characterized by short stature, minor facial anomalies, and congenital heart defects.40 Of note, most children with Noonan syndrome show feeding and digestive disorders including vomiting, constipation, abdominal pain, and distension.41, 42 Noonan syndrome also frequently is associated with the development of juvenile myelomonocytic leukemia (JMML).40, 43 Conversely, somatic mutations in the PTPN11 gene also have been identified in 34% of JMML patients. Most of these somatic mutations are clustered within the PTP or the N-SH2 domain,44 altering the autoinhibition mechanism and resulting in hyperactivated SHP-2.43 Accordingly, expression of the most common and most active PTPN11 mutation (E76K) found in JMML and acute leukemias in pan hematopoietic cells in mice is sufficient to trigger the development of myeloproliferative disorder. Subsequently, these mice progress to acute leukemias.45 SHP-2 consequently has been identified as the first proto-oncogene to encode a tyrosine phosphatase.46
Accumulating evidence now is emerging whereby dysfunction in this PTP also represents a key factor in the pathogenesis of gastrointestinal (GI) diseases, in particular in chronic inflammation and cancer. The following sections provide an overview of current knowledge on the functions and roles of SHP-2 in the epithelia of stomach, intestine, pancreas, and liver.
SHP-2 in Gastric Carcinogenesis
A role for SHP-2 in gastric cancer was first suggested in 2002 when it was found that this phosphatase is an intracellular target of Helicobacter pylori cytotoxin-associated gene A (CagA) protein.47 CagA is the product of the cagA gene carried in virulent type I strains of H pylori, which infects approximately half of the world's population, causing gastric diseases ranging from peptic ulcer diseases to gastric adenocarcinoma.48, 49 CagA is introduced into gastric epithelial cells through a type IV secretion system and, once inside, Src family kinases phosphorylate the Glu-Pro-Ile-Tyr-Ala motif on tyrosine.50, 51 Tyrosine-phosphorylated cagA then specifically binds to the SH2 domains of SHP-2, relieving the autoinhibition mechanism and thereby increasing its phosphatase activity,47 resulting in activation of the downstream ERK/MAPK pathway.52 Activated ERK1/2 MAPK then promotes proliferation and survival gene programs.53, 54, 55 Furthermore, infection of gastric epithelial cells with cagA-positive H pylori has been shown to induce a unique elongated cell shape termed the hummingbird phenotype, which is dependent on cagA–SHP-2 interaction.47, 52 These studies hence provide a molecular basis for the pathogenic actions of cagA on gastric epithelial cells.
STAT3 also is activated in patients with infection with cagA-positive H pylori strains and with gastric adenocarcinoma.56 Because SHP-2 is a major negative regulator of STAT3 activation,23 sequestration or preferential binding by cagA may reduce the intracellular pool of SHP-2, thereby depleting this brake on STAT3 activation and resulting in increased transcription of STAT3 target genes, many of which promote proliferation, inflammation, angiogenesis, and inhibition of apoptosis.56, 57, 58 In addition, cagA may interact directly with STAT3 by binding or recruitment to gp130, thereby promoting hyperactivation and increased transcriptional activity.59 Of note, the STAT3, but not the ERK1/2, pathway can be activated to a lesser extent by cagA-negative H pylori strains, suggesting that STAT3 may be driven by other bacterial factors. The differential activation of these 2 signaling proteins may explain in part the increased predisposition of gastric epithelium to gastric cancer when infected with cagA+ H pylori strains compared with their cagA− counterparts.60 Nevertheless, because SHP-2, STAT3, and ERK1/2 MAPK are components of intracellular signaling of both growth factors and cytokines, it is likely that inappropriate activation of these proteins also may occur in gastric tumors, independently of infection.56
Of particular interest, transgenic mice expressing wild-type cagA show gastric epithelial hyperplasia, with some of the mice developing gastric polyps and adenocarcinoma. By contrast, these pathologic abnormalities are not observed in transgenic mice expressing the phosphorylation-resistant cagA, which is unable to bind SHP-2.61 These findings thus highlight the importance of cagA tyrosine phosphorylation, which enables cagA to deregulate SHP-2 in the development of H pylori–associated neoplasms. Of note, in vivo interaction between cagA and SHP-2 has been observed in patients with atrophic gastritis, but not in patients with intestinal metaplasia or cancer, suggesting that this mechanism is critical in the early phases of human gastric carcinogenesis.62
Different cagA types have been studied in relation to gastric cancer incidence. CagA-positive strains can be divided into East Asian and Western types based on differences in the sequences in the 3′ region of cagA,63 which contains a different number of copies of the Glu-Pro-Ile-Tyr-Ala tyrosine phosphorylation site motif. In particular, patients infected with the East Asian cagA-positive strains present higher disease severity compared with patients infected with the Western cagA-positive strains, the former being correlated with stronger SHP-2-binding activity.64
Of note, single-nucleotide polymorphisms in the PTPN11 gene have been associated with gastric atrophy in both Japanese (rs2301756)65, 66 and Chinese (rs12229892 and rs12423190)67, 68 populations. Although the impact of these polymorphisms currently is unknown, one could speculate that they may affect SHP-2 expression or activity or the interaction between cagA and SHP-2. Further studies in human specimens are needed to verify this hypothesis.
Interestingly, SHP-2 is overexpressed in gastric carcinomas but independently of H pylori infection.69, 70, 71 This suggests that SHP-2 could be modulated by different oncogenic pathways, emphasizing that this phosphatase may be crucial for gastric tumorigenesis. Although the precise mechanisms underlying the effect of SHP-2 on gastric carcinogenesis remain to be investigated further, targeting SHP-2 may represent a novel therapeutic approach for gastric cancer.
SHP-2 in Intestinal Inflammatory Bowel Diseases
A major advancement in understanding some of the in vivo functions of SHP-2 in the intestine recently was achieved by using conditional tissue-specific disruption of SHP-2 in mice (SHP-2intestinal epithelial cell (IEC)-knockout (KO) mice). Our group generated a mouse model with impaired SHP-2 expression by using the villin promoter villin-Cre, which ablates the floxed gene from all epithelial cells (including stem cells) of the small intestine and colon, but not in the mesenchymal compartment.72 Importantly, these mice rapidly develop severe inflammation 1 month after birth, with clinical and histopathologic features similar to ulcerative colitis.73 Two further studies also have reported that mice featuring a villinCre-mediated SHP-2 deletion rapidly develop colitis.74, 75 These findings thus clearly establish intestinal epithelial SHP-2 as a critical determinant for prevention of inflammation in the colon.
Of particular interest, intestinal permeability is increased markedly in mice with IEC-specific deletion of SHP-2.73 Furthermore, SHP-2IEC-KO mice feature reduced Goblet cell numbers associated with expansion of Paneth cells and increased lysozyme expression in their small intestine.74 These data hence suggest that SHP-2–dependent signaling directs cells to the Goblet cell fate while preventing Paneth cell expansion.74 Dysregulation of epithelial cell fate or differentiation, particularly in the colon, may have deleterious consequences for the host as exemplified by mucin 2–deficient mice that rapidly develop severe colitis.76 Conversely, Paneth cells that produce antimicrobial peptides such as α- and β-defensins and lysozyme also may participate in the development of colitis in SHP-2IEC-KO mice. Indeed, antimicrobial proteins shape the composition and abundance of microbiota, and segregate intestinal commensals from the mucosa.77, 78 Hence, one could speculate that loss of epithelial SHP-2 expression has a significant effect on key gut microbial groups that are important for homeostasis. Of note, the beneficial effect of antibiotics observed in the SHP-2IEC-KO mouse model73 suggest a role for microflora in the initiation of inflammation. In this respect, both clinical observations and animal studies support the involvement of intestinal microflora in the pathogenesis of inflammatory bowel diseases (IBDs).73 It therefore is tempting to speculate that a distinctive microbiota composition and a reduced functionality of the mucus barrier observed early in SHP-2–deficient mice are at the source of this inflammation.
Exactly how SHP-2 impacts cell fate is not totally clear but may rely on its ability to activate the Raf/MAPK pathway. Indeed, concomitant expression of activated KRas75 or MEK174 markedly promotes Goblet cell differentiation in the colon of SHP-2IEC-KO mice and prevents the development of colitis. In fact, Heuberger et al74 recently reported that SHP-2–dependent ERK signaling controls the choice between Goblet and Paneth cell fate in the small intestine. These investigators showed that inhibition of ERK signaling in small intestinal organoids and cultured cells promotes β-catenin transcriptional activity, which in turn induces a Paneth-cell maturation program.79 Taken together, these studies suggest that SHP-2–dependent ERK signaling directs Goblet cell differentiation after secretory progenitor specification, at the expense of Paneth cell differentiation in the intestine.
Importantly, intronic polymorphisms in the PTPN11 gene encoding for SHP-2 have notably been described in Japanese patients with ulcerative colitis.80 However, the impact of these polymorphisms on SHP-2 function was not elucidated. These investigators speculated that PTPN11 polymorphisms may affect the expression, activity, or affinity of SHP-2 to immunoreceptors in T and B cells. Accordingly, we found reduced SHP-2 gene transcripts in intestinal biopsy specimens from patients with active ulcerative colitis, emphasizing the inverse relationship between SHP-2 expression and colonic inflammatory phenotype.73 This could suggest that PTPN11 SNPs affect SHP-2 expression. However, additional biochemical and molecular studies conducted in a large number of human specimens are needed to verify this hypothesis.
Overall, these findings show that SHP-2 is required for homeostasis of the intestinal epithelium, in particular for proper differentiation of secretory progenitors. The next step will be to elucidate the exact molecular mechanisms by which SHP-2 exerts such protective effects on intestinal barrier function. Given that IBDs are very complex diseases, the identification of the specific SHP-2 targets may be one additional step in understanding the heterogeneity of these diseases.
SHP-2 in Colorectal Cancer
Recent data from our laboratory have shown that IEC-specific deletion of SHP-2 promotes chronic inflammation in the colon that results in regenerative hyperplasia and development of adenocarcinomas in aged mice (Gagné-Sansfaçon et al, unpublished data). These findings are reminiscent of those observed by Bard-Chapeau et al,81 who recently reported that hepatocyte-specific deletion of SHP-2 also promoted inflammation and tumorigenesis in the liver of aged mice. Over the past decade, many studies have linked chronic inflammation to cancer development, particularly in the GI tract (recently reviewed by Fichtner-Feigl et al,82 2015). For instance, chronic intestinal inflammation has been shown to support tumor initiation through oxidative stress–induced mutations.83 Therefore, these results suggest that by suppressing inflammation, SHP-2 prevents the development of tumors in the colon and the liver (see later for further discussion regarding the liver). Accordingly, SHP-2 levels are decreased significantly in colitis-associated tumors in mice.84 Likewise, in colorectal carcinomas, SHP-2 has been reported to be down-regulated at both the messenger RNA and protein levels, with lower SHP-2 expression being correlated with advanced stages.84, 85 In keeping with this, higher expression of SHP-2 in colorectal cancer patients has been related to better survival.86 Thus, these findings support the concept that, under specific contexts (such as inflammation), SHP-2 can act as a tumor suppressor.
Paradoxically, however, in human beings, gain-of-function mutations in the PTPN11 gene (SHP-2 gene) previously associated with certain forms of leukemias have been found in certain solid carcinomas including colorectal cancers.44 The somatic mutation specifically found in colorectal cancer specimens, the E76G mutation, disrupts the inhibitory intramolecular interaction with the PTP domain and leads to the hyperactivation of SHP-2.44 Mutations of this residue commonly are found in JMML.43, 44 In addition, in JMML, SHP-2 mutations are mutually exclusive with RAS or NF1 (a negative regulator of Ras), and several findings indicate that SHP-2 contributes to leukemia carcinogenesis through Ras activation.87 Of note, in colorectal cancer, KRAS or NRAS are mutated in nearly 50% of colorectal tumors at a relatively early stage of the carcinogenic process.88, 89 It therefore remains possible that the SHP-2 E76G mutation and/or SHP-2 overexpression/activation, by inducing the Ras/ERK signaling pathway, may be involved in early alterations leading to tumor formation in the colon in a sporadic context. It will be pertinent in future studies to determine whether gain-of-function mutations or overexpression of SHP-2 in intestinal epithelial cells also can cause or enhance colorectal tumorigenesis.
SHP-2 in Liver Inflammation and Cancer
A study conducted in hepatocyte-specific SHP-2 knocked-out mice has shown that SHP-2 ablation attenuates hepatocyte proliferation and liver regeneration after partial hepatectomy.90 However, these mice also develop hepatic inflammation and necrosis and, with age, develop hepatocellular adenomas. Moreover, hepatocyte-specific ablation of SHP-2 also drastically enhances liver tumor development in mice after injection of the chemical carcinogen diethylnitrosamine, reinforcing the tumor-suppressing function of SHP-2 in the liver.81 In keeping with these results, decreased SHP-2 expression has been detected in some human hepatocellular carcinoma (HCC) patient samples.81, 91, 92 However, SHP-2 expression recently was examined extensively in a large number of human HCCs and in which SHP-2 transcripts were significantly lower in 22% of HCCs relative to the paired noncancerous tissues, although higher SHP-2 expression surprisingly was found in 78% of HCCs.93 Immunoblot and immunostaining analyses also showed SHP-2 up-regulation in a majority of HCCs. Of particular note, SHP-2 expression was increased significantly in metastatic foci compared with the matched primary HCCs or adjacent normal tissues, thus indicating a potential role of SHP-2 in HCC metastasis. In addition, SHP-2 silencing in hepatoma cells suppressed their proliferation as well as their tumorigenic and invasive/metastatic potential, possibly by reducing the activation of RAS/ERK and PI3K/Akt signaling.93
Altogether, these data suggest that SHP-2 displays dual facets in liver cancer, either suppressing or promoting HCC development.93, 94 Such a dual signaling role shown by a single protein in tumorigenesis is not without precedent. NF-κB,95 STAT3,96 JNK,97 and parafibromin30 also can function as tumor suppressors or oncoproteins in a cell context–dependent manner. One could speculate that different binding partner proteins may direct the opposing cellular responses under physiological and pathologic conditions.
SHP-2 in Lipid and Glucose Metabolism
Accumulating evidence from mouse models has indicated that SHP-2 regulates glucose and lipid metabolism. For instance, Shp-2 deletion in the pancreas in mice causes defective glucose-stimulated insulin secretion and impaired glucose tolerance.98 In effect, Shp-2 deficiency impairs the expression of Pdx1 and insulin genes, leading to reduced insulin production by β-cells.98 On the other hand, mice lacking Shp-2 in the liver show increased hepatic insulin action and glucose tolerance, as well as enhanced systemic insulin sensitivity compared with control mice, indicating that SHP-2 is a negative regulator of insulin signaling in hepatocytes. Acute SHP-2 deletion by tail-vein injection of adenovirus-carrying Ad5-Cre in SHP-2flox/flox mice yields comparable results.99 SHP-2 has been proposed to putatively regulate liver insulin signaling by inhibiting insulin-receptor substrate 1/2 tyrosine phosphorylation, thereby attenuating PI3K association and Akt activation.99 Of note, SHP-2 protein expression in hepatocytes is regulated by nutritional status, increasing in mice fed a high-fat diet and decreasing during fasting.100 Thus, when challenged with high-fat feeding, mice with hepatic SHP-2 deficiency gain less weight and show decreased liver steatosis in comparison with control mice. In addition, hepatic SHP-2 deficiency attenuates the development of high-fat-diet–induced insulin resistance.
Lastly, in addition to the earlier-described observations, SHP-2 has been shown to regulate the degradation of fatty acid synthase, a key enzyme in fatty acid biosynthesis. Indeed, SHP-2 interacts with fatty acid synthase and induces its ubiquitination and degradation by forming a complex with ubiquitin E3 ligase COP1 and p38 MAPK. Accordingly, increased FAS protein levels have been observed in the liver and pancreas of SHP-2 conditional knocked-out mice.101
Studies in the past decade have provided evidence that bile acids are not simply biological detergents facilitating lipid absorption, but also key metabolic regulators of glucose and lipid homeostasis.102, 103 Interestingly, a novel function of SHP-2 in control of bile acid homeostasis has been found. Indeed, hepatocyte-specific deletion of SHP-2 results in bile acid accumulation and an enlarged bile acid pool in the liver. When fed a chow diet, these mice develop liver injury consistent with bile acid–induced damage to the hepatobiliary system.104 These phenotypic alterations can be explained by the fact that SHP-2 is required for FGF15/19 activation of FGFR4 and its downstream signaling pathways that repress bile acid biosynthesis in hepatocytes.105
Collectively, these studies indicate that hepatic SHP-2 is involved in the regulation of lipid and glucose metabolism, as well as in systemic energy balance.
Conclusion and Perspectives
Genome-wide association studies have associated the PTPN11 gene with gastric atrophy,67 gastric cancer,66 colitis, and serum lipid levels.106, 107 The mechanisms underlying these associations are still in the hypothesis stage, which stipulates that PTPN11 SNPs may change the expression of the gene and consequently influence the SHP-2 protein, which in turn regulates proliferation, differentiation, and/or metabolism in the cells. Conversely, somatic gain-of-function mutations in PTPN11 have been detected in colorectal cancer.44 Hence, these genetic alterations strongly suggest that SHP-2 may play a prominent role in various functions of the GI system. Accordingly, anti-inflammatory and antitumoral actions of SHP-2 furthermore have been shown with the generation of hepatocyte and intestinal epithelial cell–specific SHP-2– deficient mice.73, 81 The exact mechanism by which SHP-2 ablation in colonocytes and hepatocytes induces such effects is not totally clear. Notably, although the ERK pathway consistently was inhibited in colonocytes73 and hepatocytes81 of these mice, NF-κB and STAT3 conversely were hyperactivated. Importantly, both of these transcription factors have been identified as important contributors to inflammation-associated tumor development.108, 109, 110 Previous studies furthermore have documented a negative regulatory role of SHP-2 in the Janus kinase/signal transducers and activators of transcription23, 81 and NF-κB signaling.26 However, the context in which these major proinflammatory pathways specifically are regulated by SHP-2 remains unknown. Interestingly, IECs and hepatocytes have been found to be hypersensitive to lipopolysaccharide challenge (increased NF-κB activation and chemokine/cytokine production) after SHP-2 silencing.81 Hence, one could speculate that the phosphatase SHP-2 protects epithelial cells from aberrant Toll-like receptor signaling, which can lead to uncontrolled inflammation detrimental to the host. Abundant evidence also supports the pivotal role of pattern-recognition receptors including Toll-like receptors in gastric, intestinal, and liver carcinogenesis.111, 112, 113, 114, 115 Further study in this area nonetheless is needed to elucidate whether SHP-2 limits the innate immune response in gastrointestinal tract epithelia.
Lastly, nothing is known regarding the regulation of SHP-2 phosphatase activity in IBD or other gastrointestinal diseases. SHP-2 is inactivated during oxidative stress and in the presence of nitric oxide by-products.116 This suggests that loss of SHP-2 activity and of the cellular pathways regulated by SHP-2 may coincide with cell injury and oxidative stress occurring during inflammation.82 Fully characterizing not only the downstream effectors but also the upstream regulators of SHP-2 phosphatase will provide further insights in explaining the mechanistic link between inflammation and GI cancers.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding Geneviève Coulombe is a Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell student scholar, and Nathalie Rivard is the recipient of a Canadian Research Chair in colorectal cancer and inflammatory cell signaling.
References
- 1.Feng G.S., Hui C.C., Pawson T. SH2-containing phosphotyrosine phosphatase as a target of protein-tyrosine kinases. Science. 1993;259:1607–1611. doi: 10.1126/science.8096088. [DOI] [PubMed] [Google Scholar]
- 2.Freeman R.M., Jr., Plutzky J., Neel B.G. Identification of a human src homology 2-containing protein-tyrosine-phosphatase: a putative homolog of Drosophila corkscrew. Proc Natl Acad Sci U S A. 1992;89:11239–11243. doi: 10.1073/pnas.89.23.11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad S., Banville D., Zhao Z. A widely expressed human protein-tyrosine phosphatase containing src homology 2 domains. Proc Natl Acad Sci U S A. 1993;90:2197–2201. doi: 10.1073/pnas.90.6.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vogel W., Lammers R., Huang J. Activation of a phosphotyrosine phosphatase by tyrosine phosphorylation. Science. 1993;259:1611–1614. doi: 10.1126/science.7681217. [DOI] [PubMed] [Google Scholar]
- 5.Adachi M., Sekiya M., Miyachi T. Molecular cloning of a novel protein-tyrosine phosphatase SH-PTP3 with sequence similarity to the src-homology region 2. FEBS Lett. 1992;314:335–339. doi: 10.1016/0014-5793(92)81500-l. [DOI] [PubMed] [Google Scholar]
- 6.Yi T., Cleveland J.L., Ihle J.N. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991;78:2222–2228. [PubMed] [Google Scholar]
- 7.Koch C.A., Anderson D., Moran M.F. SH2 and SH3 domains: elements that control interactions of cytoplasmic signaling proteins. Science. 1991;252:668–674. doi: 10.1126/science.1708916. [DOI] [PubMed] [Google Scholar]
- 8.Neel B.G., Gu H., Pao L. The 'Shp'ing news: SH2 domain-containing tyrosine phosphatases in cell signaling. Trends Biochem Sci. 2003;28:284–293. doi: 10.1016/S0968-0004(03)00091-4. [DOI] [PubMed] [Google Scholar]
- 9.Hof P., Pluskey S., Dhe-Paganon S. Crystal structure of the tyrosine phosphatase SHP-2. Cell. 1998;92:441–450. doi: 10.1016/s0092-8674(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 10.Nardozza A.P., D'Orazio M., Trapannone R. Reactive oxygen species and epidermal growth factor are antagonistic cues controlling SHP-2 dimerization. Mol Cell Biol. 2012;32:1998–2009. doi: 10.1128/MCB.06674-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang T.L., Freeman R.M., Jr., O'Reilly A.M. The SH2-containing protein-tyrosine phosphatase SH-PTP2 is required upstream of MAP kinase for early Xenopus development. Cell. 1995;80:473–483. doi: 10.1016/0092-8674(95)90498-0. [DOI] [PubMed] [Google Scholar]
- 12.Rivard N., McKenzie F.R., Brondello J.M. The phosphotyrosine phosphatase PTP1D, but not PTP1C, is an essential mediator of fibroblast proliferation induced by tyrosine kinase and G protein-coupled receptors. J Biol Chem. 1995;270:11017–11024. doi: 10.1074/jbc.270.18.11017. [DOI] [PubMed] [Google Scholar]
- 13.Yamauchi K., Ribon V., Saltiel A.R. Identification of the major SHPTP2-binding protein that is tyrosine-phosphorylated in response to insulin. J Biol Chem. 1995;270:17716–17722. doi: 10.1074/jbc.270.30.17716. [DOI] [PubMed] [Google Scholar]
- 14.Noguchi T., Matozaki T., Horita K. Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol Cell Biol. 1994;14:6674–6682. doi: 10.1128/mcb.14.10.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qu C.K. The SHP-2 tyrosine phosphatase: signaling mechanisms and biological functions. Cell Res. 2000;10:279–288. doi: 10.1038/sj.cr.7290055. [DOI] [PubMed] [Google Scholar]
- 16.Milarski K.L., Saltiel A.R. Expression of catalytically inactive Syp phosphatase in 3T3 cells blocks stimulation of mitogen-activated protein kinase by insulin. J Biol Chem. 1994;269:21239–21243. [PubMed] [Google Scholar]
- 17.Bennett A.M., Hausdorff S.F., O'Reilly A.M. Multiple requirements for SHPTP2 in epidermal growth factor-mediated cell cycle progression. Mol Cell Biol. 1996;16:1189–1202. doi: 10.1128/mcb.16.3.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Manes S., Mira E., Gomez-Mouton C. Concerted activity of tyrosine phosphatase SHP-2 and focal adhesion kinase in regulation of cell motility. Mol Cell Biol. 1999;19:3125–3135. doi: 10.1128/mcb.19.4.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maegawa H., Hasegawa M., Sugai S. Expression of a dominant negative SHP-2 in transgenic mice induces insulin resistance. J Biol Chem. 1999;274:30236–30243. doi: 10.1074/jbc.274.42.30236. [DOI] [PubMed] [Google Scholar]
- 20.Inagaki K., Noguchi T., Matozaki T. Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene. 2000;19:75–84. doi: 10.1038/sj.onc.1203204. [DOI] [PubMed] [Google Scholar]
- 21.Qu C.K., Yu W.M., Azzarelli B. Genetic evidence that Shp-2 tyrosine phosphatase is a signal enhancer of the epidermal growth factor receptor in mammals. Proc Natl Acad Sci U S A. 1999;96:8528–8533. doi: 10.1073/pnas.96.15.8528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shi Z.Q., Yu D.H., Park M. Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol Cell Biol. 2000;20:1526–1536. doi: 10.1128/mcb.20.5.1526-1536.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu D., Qu C.K. Protein tyrosine phosphatases in the JAK/STAT pathway. Front Biosci. 2008;13:4925–4932. doi: 10.2741/3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yu C.L., Jin Y.J., Burakoff S.J. Cytosolic tyrosine dephosphorylation of STAT5. Potential role of SHP-2 in STAT5 regulation. J Biol Chem. 2000;275:599–604. doi: 10.1074/jbc.275.1.599. [DOI] [PubMed] [Google Scholar]
- 25.You M., Yu D.H., Feng G.S. Shp-2 tyrosine phosphatase functions as a negative regulator of the interferon-stimulated Jak/STAT pathway. Mol Cell Biol. 1999;19:2416–2424. doi: 10.1128/mcb.19.3.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu S., Liu X., Bao Y. Constitutive MHC class I molecules negatively regulate TLR-triggered inflammatory responses via the Fps-SHP-2 pathway. Nat Immunol. 2012;13:551–559. doi: 10.1038/ni.2283. [DOI] [PubMed] [Google Scholar]
- 27.Ivins Zito C., Kontaridis M.I., Fornaro M. SHP-2 regulates the phosphatidylinositide 3'-kinase/Akt pathway and suppresses caspase 3-mediated apoptosis. J Cell Physiol. 2004;199:227–236. doi: 10.1002/jcp.10446. [DOI] [PubMed] [Google Scholar]
- 28.Kwon M., Ling Y., Maile L.A. Recruitment of the tyrosine phosphatase Src homology 2 domain tyrosine phosphatase-2 to the p85 subunit of phosphatidylinositol-3 (PI-3) kinase is required for insulin-like growth factor-I-dependent PI-3 kinase activation in smooth muscle cells. Endocrinology. 2006;147:1458–1465. doi: 10.1210/en.2005-1115. [DOI] [PubMed] [Google Scholar]
- 29.Zhang S.Q., Tsiaras W.G., Araki T. Receptor-specific regulation of phosphatidylinositol 3′-kinase activation by the protein tyrosine phosphatase Shp2. Mol Cell Biol. 2002;22:4062–4072. doi: 10.1128/MCB.22.12.4062-4072.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi A., Tsutsumi R., Kikuchi I. SHP2 tyrosine phosphatase converts parafibromin/Cdc73 from a tumor suppressor to an oncogenic driver. Mol Cell. 2011;43:45–56. doi: 10.1016/j.molcel.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsutsumi R., Masoudi M., Takahashi A. YAP and TAZ, Hippo signaling targets, act as a rheostat for nuclear SHP2 function. Dev Cell. 2013;26:658–665. doi: 10.1016/j.devcel.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 32.Kontaridis M.I., Eminaga S., Fornaro M. SHP-2 positively regulates myogenesis by coupling to the Rho GTPase signaling pathway. Mol Cell Biol. 2004;24:5340–5352. doi: 10.1128/MCB.24.12.5340-5352.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schoenwaelder S.M., Petch L.A., Williamson D. The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr Biol. 2000;10:1523–1526. doi: 10.1016/s0960-9822(00)00831-9. [DOI] [PubMed] [Google Scholar]
- 34.Qu C.K., Shi Z.Q., Shen R. A deletion mutation in the SH2-N domain of Shp-2 severely suppresses hematopoietic cell development. Mol Cell Biol. 1997;17:5499–5507. doi: 10.1128/mcb.17.9.5499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aceto N., Sausgruber N., Brinkhaus H. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat Med. 2012;18:529–537. doi: 10.1038/nm.2645. [DOI] [PubMed] [Google Scholar]
- 36.He Z., Zhu H.H., Bauler T.J. Nonreceptor tyrosine phosphatase Shp2 promotes adipogenesis through inhibition of p38 MAP kinase. Proc Natl Acad Sci U S A. 2013;110:E79–E88. doi: 10.1073/pnas.1213000110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu D.H., Qu C.K., Henegariu O. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- 38.Hartman Z.R., Schaller M.D., Agazie Y.M. The tyrosine phosphatase SHP2 regulates focal adhesion kinase to promote EGF-induced lamellipodia persistence and cell migration. Mol Cancer Res. 2013;11:651–664. doi: 10.1158/1541-7786.MCR-12-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saxton T.M., Henkemeyer M., Gasca S. Abnormal mesoderm patterning in mouse embryos mutant for the SH2 tyrosine phosphatase Shp-2. EMBO J. 1997;16:2352–2364. doi: 10.1093/emboj/16.9.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tartaglia M., Mehler E.L., Goldberg R. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 41.Shah N., Rodriguez M., Louis D.S. Feeding difficulties and foregut dysmotility in Noonan's syndrome. Arch Dis Child. 1999;81:28–31. doi: 10.1136/adc.81.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Romano A.A., Allanson J.E., Dahlgren J. Noonan syndrome: clinical features, diagnosis, and management guidelines. Pediatrics. 2010;126:746–759. doi: 10.1542/peds.2009-3207. [DOI] [PubMed] [Google Scholar]
- 43.Tartaglia M., Niemeyer C.M., Fragale A. Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat Genet. 2003;34:148–150. doi: 10.1038/ng1156. [DOI] [PubMed] [Google Scholar]
- 44.Bentires-Alj M., Paez J.G., David F.S. Activating mutations of the noonan syndrome-associated SHP2/PTPN11 gene in human solid tumors and adult acute myelogenous leukemia. Cancer Res. 2004;64:8816–8820. doi: 10.1158/0008-5472.CAN-04-1923. [DOI] [PubMed] [Google Scholar]
- 45.Xu D., Liu X., Yu W.M. Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J Exp Med. 2011;208:1977–1988. doi: 10.1084/jem.20110450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan R.J., Feng G.S. PTPN11 is the first identified proto-oncogene that encodes a tyrosine phosphatase. Blood. 2007;109:862–867. doi: 10.1182/blood-2006-07-028829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Higashi H., Tsutsumi R., Muto S. SHP-2 tyrosine phosphatase as an intracellular target of Helicobacter pylori CagA protein. Science. 2002;295:683–686. doi: 10.1126/science.1067147. [DOI] [PubMed] [Google Scholar]
- 48.De Falco M., Lucariello A., Iaquinto S. Molecular mechanisms of Helicobacter pylori pathogenesis. J Cell Physiol. 2015;230:1702–1707. doi: 10.1002/jcp.24933. [DOI] [PubMed] [Google Scholar]
- 49.Blaser M.J., Perez-Perez G.I., Kleanthous H. Infection with Helicobacter pylori strains possessing cagA is associated with an increased risk of developing adenocarcinoma of the stomach. Cancer Res. 1995;55:2111–2115. [PubMed] [Google Scholar]
- 50.Selbach M., Moese S., Hauck C.R. Src is the kinase of the Helicobacter pylori CagA protein in vitro and in vivo. J Biol Chem. 2002;277:6775–6778. doi: 10.1074/jbc.C100754200. [DOI] [PubMed] [Google Scholar]
- 51.Stein M., Bagnoli F., Halenbeck R. c-Src/Lyn kinases activate Helicobacter pylori CagA through tyrosine phosphorylation of the EPIYA motifs. Mol Microbiol. 2002;43:971–980. doi: 10.1046/j.1365-2958.2002.02781.x. [DOI] [PubMed] [Google Scholar]
- 52.Higashi H., Nakaya A., Tsutsumi R. Helicobacter pylori CagA induces Ras-independent morphogenetic response through SHP-2 recruitment and activation. J Biol Chem. 2004;279:17205–17216. doi: 10.1074/jbc.M309964200. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W., Liu H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18. doi: 10.1038/sj.cr.7290105. [DOI] [PubMed] [Google Scholar]
- 54.Chambard J.C., Lefloch R., Pouyssegur J. ERK implication in cell cycle regulation. Biochim Biophys Acta. 2007;1773:1299–1310. doi: 10.1016/j.bbamcr.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 55.Cagnol S., Chambard J.C. ERK and cell death: mechanisms of ERK-induced cell death–apoptosis, autophagy and senescence. FEBS J. 2010;277:2–21. doi: 10.1111/j.1742-4658.2009.07366.x. [DOI] [PubMed] [Google Scholar]
- 56.Jackson C.B., Judd L.M., Menheniott T.R. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol. 2007;213:140–151. doi: 10.1002/path.2218. [DOI] [PubMed] [Google Scholar]
- 57.Kamimura D., Ishihara K., Hirano T. IL-6 signal transduction and its physiological roles: the signal orchestration model. Rev Physiol Biochem Pharmacol. 2003;149:1–38. doi: 10.1007/s10254-003-0012-2. [DOI] [PubMed] [Google Scholar]
- 58.Ihle J.N. STATs: signal transducers and activators of transcription. Cell. 1996;84:331–334. doi: 10.1016/s0092-8674(00)81277-5. [DOI] [PubMed] [Google Scholar]
- 59.Lee I.O., Kim J.H., Choi Y.J. Helicobacter pylori CagA phosphorylation status determines the gp130-activated SHP2/ERK and JAK/STAT signal transduction pathways in gastric epithelial cells. J Biol Chem. 2010;285:16042–16050. doi: 10.1074/jbc.M110.111054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang J.Q., Zheng G.F., Sumanac K. Meta-analysis of the relationship between cagA seropositivity and gastric cancer. Gastroenterology. 2003;125:1636–1644. doi: 10.1053/j.gastro.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 61.Ohnishi N., Yuasa H., Tanaka S. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc Natl Acad Sci U S A. 2008;105:1003–1008. doi: 10.1073/pnas.0711183105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yamazaki S., Yamakawa A., Ito Y. The CagA protein of Helicobacter pylori is translocated into epithelial cells and binds to SHP-2 in human gastric mucosa. J Infect Dis. 2003;187:334–337. doi: 10.1086/367807. [DOI] [PubMed] [Google Scholar]
- 63.Yamaoka Y., Kodama T., Kashima K. Variants of the 3' region of the cagA gene in Helicobacter pylori isolates from patients with different H. pylori-associated diseases. J Clin Microbiol. 1998;36:2258–2263. doi: 10.1128/jcm.36.8.2258-2263.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Azuma T., Yamazaki S., Yamakawa A. Association between diversity in the Src homology 2 domain–containing tyrosine phosphatase binding site of Helicobacter pylori CagA protein and gastric atrophy and cancer. J Infect Dis. 2004;189:820–827. doi: 10.1086/381782. [DOI] [PubMed] [Google Scholar]
- 65.Goto Y., Ando T., Yamamoto K. Association between serum pepsinogens and polymorphism of PTPN11 encoding SHP-2 among Helicobacter pylori seropositive Japanese. Int J Cancer. 2006;118:203–208. doi: 10.1002/ijc.21338. [DOI] [PubMed] [Google Scholar]
- 66.Hishida A., Matsuo K., Goto Y. Associations of a PTPN11 G/A polymorphism at intron 3 with Helicobacter pylori seropositivity, gastric atrophy and gastric cancer in Japanese. BMC Gastroenterol. 2009;9:51. doi: 10.1186/1471-230X-9-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang J., Jia Z.F., Kong F. Association of polymorphism of PTPN 11 encoding SHP-2 with gastric atrophy but not gastric cancer in Helicobacter pylori seropositive Chinese population. BMC Gastroenterol. 2012;12:89. doi: 10.1186/1471-230X-12-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He C., Tu H., Sun L. Helicobacter pylori-related host gene polymorphisms associated with susceptibility of gastric carcinogenesis: a two-stage case-control study in Chinese. Carcinogenesis. 2013;34:1450–1457. doi: 10.1093/carcin/bgt079. [DOI] [PubMed] [Google Scholar]
- 69.Dong S., Li F.Q., Zhang Q. Expression and clinical significance of SHP2 in gastric cancer. J Int Med Res. 2012;40:2083–2089. doi: 10.1177/030006051204000605. [DOI] [PubMed] [Google Scholar]
- 70.Jiang J., Jin M.S., Kong F. Increased expression of tyrosine phosphatase SHP-2 in Helicobacter pylori-infected gastric cancer. World J Gastroenterol. 2013;19:575–580. doi: 10.3748/wjg.v19.i4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kim J.S., Shin O.R., Kim H.K. Overexpression of protein phosphatase non-receptor type 11 (PTPN11) in gastric carcinomas. Dig Dis Sci. 2010;55:1565–1569. doi: 10.1007/s10620-009-0924-z. [DOI] [PubMed] [Google Scholar]
- 72.Madison B.B., Dunbar L., Qiao X.T. Cis elements of the villin gene control expression in restricted domains of the vertical (crypt) and horizontal (duodenum, cecum) axes of the intestine. J Biol Chem. 2002;277:33275–33283. doi: 10.1074/jbc.M204935200. [DOI] [PubMed] [Google Scholar]
- 73.Coulombe G., Leblanc C., Cagnol S. Epithelial tyrosine phosphatase SHP-2 protects against intestinal inflammation in mice. Mol Cell Biol. 2013;33:2275–2284. doi: 10.1128/MCB.00043-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heuberger J., Kosel F., Qi J. Shp2/MAPK signaling controls goblet/Paneth cell fate decisions in the intestine. Proc Natl Acad Sci U S A. 2014;111:3472–3477. doi: 10.1073/pnas.1309342111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamashita H., Kotani T., Park J.H. Role of the protein tyrosine phosphatase Shp2 in homeostasis of the intestinal epithelium. PLoS One. 2014;9:e92904. doi: 10.1371/journal.pone.0092904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Van der Sluis M., De Koning B.A., De Bruijn A.C. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131:117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 77.Salzman N.H., Hung K., Haribhai D. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 2010;11:76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaishnava S., Yamamoto M., Severson K.M. The antibacterial lectin RegIIIgamma promotes the spatial segregation of microbiota and host in the intestine. Science. 2011;334:255–258. doi: 10.1126/science.1209791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.van Es J.H., Jay P., Gregorieff A. Wnt signalling induces maturation of Paneth cells in intestinal crypts. Nat Cell Biol. 2005;7:381–386. doi: 10.1038/ncb1240. [DOI] [PubMed] [Google Scholar]
- 80.Narumi Y., Isomoto H., Shiota M. Polymorphisms of PTPN11 coding SHP-2 as biomarkers for ulcerative colitis susceptibility in the Japanese population. J Clin Immunol. 2009;29:303–310. doi: 10.1007/s10875-008-9272-6. [DOI] [PubMed] [Google Scholar]
- 81.Bard-Chapeau E.A., Li S., Ding J. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell. 2011;19:629–639. doi: 10.1016/j.ccr.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fichtner-Feigl S., Kesselring R., Strober W. Chronic inflammation and the development of malignancy in the GI tract. Trends Immunol. 2015;36:451–459. doi: 10.1016/j.it.2015.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Waldner M.J., Neurath M.F. Mechanisms of immune signaling in colitis-associated cancer. Cell Mol Gastroenterol Hepatol. 2015;1:6–16. doi: 10.1016/j.jcmgh.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cai P., Guo W., Yuan H. Expression and clinical significance of tyrosine phosphatase SHP-2 in colon cancer. Biomed Pharmacother. 2014;68:285–290. doi: 10.1016/j.biopha.2013.10.012. [DOI] [PubMed] [Google Scholar]
- 85.Chang W., Gao X., Han Y. Gene expression profiling-derived immunohistochemistry signature with high prognostic value in colorectal carcinoma. Gut. 2014;63:1457–1467. doi: 10.1136/gutjnl-2013-305475. [DOI] [PubMed] [Google Scholar]
- 86.Yu S.J., Yu J.K., Ge W.T. SPARCL1, Shp2, MSH2, E-cadherin, p53, ADCY-2 and MAPK are prognosis-related in colorectal cancer. World J Gastroenterol. 2011;17:2028–2036. doi: 10.3748/wjg.v17.i15.2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loh M.L., Vattikuti S., Schubbert S. Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood. 2004;103:2325–2331. doi: 10.1182/blood-2003-09-3287. [DOI] [PubMed] [Google Scholar]
- 88.Rajagopalan H., Bardelli A., Lengauer C. Tumorigenesis: RAF/RAS oncogenes and mismatch-repair status. Nature. 2002;418:934. doi: 10.1038/418934a. [DOI] [PubMed] [Google Scholar]
- 89.Nagasaka T., Sasamoto H., Notohara K. Colorectal cancer with mutation in BRAF, KRAS, and wild-type with respect to both oncogenes showing different patterns of DNA methylation. J Clin Oncol. 2004;22:4584–4594. doi: 10.1200/JCO.2004.02.154. [DOI] [PubMed] [Google Scholar]
- 90.Bard-Chapeau E.A., Yuan J., Droin N. Concerted functions of Gab1 and Shp2 in liver regeneration and hepatoprotection. Mol Cell Biol. 2006;26:4664–4674. doi: 10.1128/MCB.02253-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jiang C., Hu F., Tai Y. The tumor suppressor role of Src homology phosphotyrosine phosphatase 2 in hepatocellular carcinoma. J Cancer Res Clin Oncol. 2012;138:637–646. doi: 10.1007/s00432-011-1143-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhao X., Hu S., Wang L. Functional short tandem repeat polymorphism of PTPN11 and susceptibility to hepatocellular carcinoma in Chinese populations. PLoS One. 2014;9:e106841. doi: 10.1371/journal.pone.0106841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Han T., Xiang D.M., Sun W. PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol. 2015;63:651–660. doi: 10.1016/j.jhep.2015.03.036. [DOI] [PubMed] [Google Scholar]
- 94.Li S., Hsu D.D., Wang H. Dual faces of SH2-containing protein-tyrosine phosphatase Shp2/PTPN11 in tumorigenesis. Front Med. 2012;6:275–279. doi: 10.1007/s11684-012-0216-4. [DOI] [PubMed] [Google Scholar]
- 95.Perkins N.D. NF-kappaB: tumor promoter or suppressor? Trends Cell Biol. 2004;14:64–69. doi: 10.1016/j.tcb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 96.Zhang H.F., Lai R. STAT3 in cancer-friend or foe? Cancers (Basel) 2014;6:1408–1440. doi: 10.3390/cancers6031408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tournier C. The 2 faces of JNK signaling in cancer. Genes Cancer. 2013;4:397–400. doi: 10.1177/1947601913486349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zhang S.S., Hao E., Yu J. Coordinated regulation by Shp2 tyrosine phosphatase of signaling events controlling insulin biosynthesis in pancreatic beta-cells. Proc Natl Acad Sci U S A. 2009;106:7531–7536. doi: 10.1073/pnas.0811715106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Matsuo K., Delibegovic M., Matsuo I. Altered glucose homeostasis in mice with liver-specific deletion of Src homology phosphatase 2. J Biol Chem. 2010;285:39750–39758. doi: 10.1074/jbc.M110.153734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nagata N., Matsuo K., Bettaieb A. Hepatic Src homology phosphatase 2 regulates energy balance in mice. Endocrinology. 2012;153:3158–3169. doi: 10.1210/en.2012-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu J., Deng R., Zhu H.H. Modulation of fatty acid synthase degradation by concerted action of p38 MAP kinase, E3 ligase COP1, and SH2-tyrosine phosphatase Shp2. J Biol Chem. 2013;288:3823–3830. doi: 10.1074/jbc.M112.397885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Thomas C., Pellicciari R., Pruzanski M. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678–693. doi: 10.1038/nrd2619. [DOI] [PubMed] [Google Scholar]
- 103.Russell D.W. The enzymes, regulation, and genetics of bile acid synthesis. Annu Rev Biochem. 2003;72:137–174. doi: 10.1146/annurev.biochem.72.121801.161712. [DOI] [PubMed] [Google Scholar]
- 104.Calkin A.C., Tontonoz P. Transcriptional integration of metabolism by the nuclear sterol-activated receptors LXR and FXR. Nat Rev Mol Cell Biol. 2012;13:213–224. doi: 10.1038/nrm3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li S., Hsu D.D., Li B. Cytoplasmic tyrosine phosphatase Shp2 coordinates hepatic regulation of bile acid and FGF15/19 signaling to repress bile acid synthesis. Cell Metab. 2014;20:320–332. doi: 10.1016/j.cmet.2014.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jamshidi Y., Gooljar S.B., Snieder H. SHP-2 and PI3-kinase genes PTPN11 and PIK3R1 may influence serum apoB and LDL cholesterol levels in normal women. Atherosclerosis. 2007;194:e26–e33. doi: 10.1016/j.atherosclerosis.2006.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia Z.F., Cao X.Y., Cao D.H. Polymorphisms of PTPN11 gene could influence serum lipid levels in a sex-specific pattern. Lipids Health Dis. 2013;12:72. doi: 10.1186/1476-511X-12-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bollrath J., Greten F.R. IKK/NF-kappaB and STAT3 pathways: central signalling hubs in inflammation-mediated tumour promotion and metastasis. EMBO Rep. 2009;10:1314–1319. doi: 10.1038/embor.2009.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bollrath J., Phesse T.J., von Burstin V.A. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 110.Greten F.R., Eckmann L., Greten T.F. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 111.Fukata M., Abreu M.T. Role of Toll-like receptors in gastrointestinal malignancies. Oncogene. 2008;27:234–243. doi: 10.1038/sj.onc.1210908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tye H., Jenkins B.J. Tying the knot between cytokine and toll-like receptor signaling in gastrointestinal tract cancers. Cancer Sci. 2013;104:1139–1145. doi: 10.1111/cas.12205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kennedy C.L., Najdovska M., Tye H. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis. Oncogene. 2014;33:2540–2546. doi: 10.1038/onc.2013.205. [DOI] [PubMed] [Google Scholar]
- 114.Szabo G., Billiar T.R., Machida K. Toll-like receptor signaling in liver diseases. Gastroenterol Res Pract. 2010;2010:971270. doi: 10.1155/2010/971270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fukata M., Shang L., Santaolalla R. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 2011;17:1464–1473. doi: 10.1002/ibd.21527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chen C.Y., Willard D., Rudolph J. Redox regulation of SH2-domain-containing protein tyrosine phosphatases by two backdoor cysteines. Biochemistry. 2009;48:1399–1409. doi: 10.1021/bi801973z. [DOI] [PubMed] [Google Scholar]
- 117.Higashi H., Tsutsumi R., Fujita A. Biological activity of the Helicobacter pylori virulence factor CagA is determined by variation in the tyrosine phosphorylation sites. Proc Natl Acad Sci U S A. 2002;99:14428–14433. doi: 10.1073/pnas.222375399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hashi K., Murata-Kamiya N., Varon C. Natural variant of the Helicobacter pylori CagA oncoprotein that lost the ability to interact with PAR1. Cancer Sci. 2014;105:245–251. doi: 10.1111/cas.12342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsutsumi R., Takahashi A., Azuma T. Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol Cell Biol. 2006;26:261–276. doi: 10.1128/MCB.26.1.261-276.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Meng S., Gui Q., Xu Q. Association of Shp2 with phosphorylated IL-22R1 is required for interleukin-22-induced MAP kinase activation. J Mol Cell Biol. 2010;2:223–230. doi: 10.1093/jmcb/mjq017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huber M., Izzi L., Grondin P. The carboxyl-terminal region of biliary glycoprotein controls its tyrosine phosphorylation and association with protein-tyrosine phosphatases SHP-1 and SHP-2 in epithelial cells. J Biol Chem. 1999;274:335–344. doi: 10.1074/jbc.274.1.335. [DOI] [PubMed] [Google Scholar]
- 122.Takahashi-Tezuka M., Yoshida Y., Fukada T. Gab1 acts as an adapter molecule linking the cytokine receptor gp130 to ERK mitogen-activated protein kinase. Mol Cell Biol. 1998;18:4109–4117. doi: 10.1128/mcb.18.7.4109. [DOI] [PMC free article] [PubMed] [Google Scholar]