Abstract
Background & Aims
Hirschsprung disease (HSCR) is caused by failure of cells derived from the neural crest (NC) to colonize the distal bowel in early embryogenesis, resulting in absence of the enteric nervous system (ENS) and failure of intestinal transit postnatally. Treatment is by distal bowel resection, but neural cell replacement may be an alternative. We tested whether aneuronal (aganglionic) colon tissue from patients may be colonized by autologous ENS-derived cells.
Methods
Cells were obtained and cryopreserved from 31 HSCR patients from the proximal resection margin of colon, and ENS cells were isolated using flow cytometry for the NC marker p75 (nine patients). Aneuronal colon tissue was obtained from the distal resection margin (23 patients). ENS cells were assessed for NC markers immunohistologically and by quantitative reverse-transcription polymerase chain reaction, and mitosis was detected by ethynyl-2′-deoxyuridine labeling. The ability of human HSCR postnatal ENS-derived cells to colonize the embryonic intestine was demonstrated by organ coculture with avian embryo gut, and the ability of human postnatal HSCR aneuronal colon muscle to support ENS formation was tested by organ coculture with embryonic mouse ENS cells. Finally, the ability of HSCR patient ENS cells to colonize autologous aneuronal colon muscle tissue was assessed.
Results
ENS-derived p75-sorted cells from patients expressed multiple NC progenitor and differentiation markers and proliferated in culture under conditions simulating Wnt signaling. In organ culture, patient ENS cells migrated appropriately in aneural quail embryo gut, and mouse embryo ENS cells rapidly spread, differentiated, and extended axons in patient aneuronal colon muscle tissue. Postnatal ENS cells derived from HSCR patients colonized autologous aneuronal colon tissue in cocultures, proliferating and differentiating as neurons and glia.
Conclusions
NC-lineage cells can be obtained from HSCR patient colon and can form ENS-like structures in aneuronal colonic muscle from the same patient.
Keywords: Hirschsprung Disease, Aganglionosis, Cell Therapy, Megacolon, Enteric Nervous System
Abbreviations used in this paper: CHIR-99021, 6-[2-[[4-(2,4-dichlorophenyl)-5-(5-methyl-1H-imidazol-2-yl)pyrimidin-2-yl]amino]ethylamino]pyridine-3-carbonitrile; EdU, ethynyl-2′-deoxyuridine; eGFP, enhanced green fluorescent protein; ENC, enteric neural crest; ENS, enteric nervous system; FBS, fetal bovine serum; GFAP, glial fibrillary acidic protein; GSK3, glycogen synthase kinase 3; HNK1, human natural killer-1; HSCR, Hirschsprung disease; MTR, MitoTracker Red; NC, neural crest; nNOS, neuronal nitric oxide synthase; nTCM, neural tissue culture medium; PBS, phosphate-buffered saline; PFA, paraformaldehyde; qRT-PCR, quantitative reverse transcription and polymerase chain reaction; RCH, Royal Children’s Hospital; SMA, smooth muscle actin; SOX10, sex-determining region Y–box 10; TUJ1, neuron-specific class III β-tubulin
Summary.
We show that enteric neural cells isolated from Hirschsprung disease patients can colonize aneuronal colon tissue to generate neurons and glia. Our findings establish the therapeutic potential of using patient's own neural cells to form an enteric nervous system in autologous tissue.
The enteric nervous system (ENS) is a huge ganglionated network that coordinates bowel motility.1 Hirschsprung disease (HSCR) is a congenital condition in which the ENS is absent from the distal bowel.2 This aneuronal (often termed aganglionic) bowel segment prevents passage of intestinal contents and causes distension (megacolon) with potentially fatal outcome. Treatment for HSCR is surgical resection of the aganglionic bowel and anastomosis of the neuronal (ganglionated) proximal bowel to the anorectum, but constipation and fecal soiling are common postoperative complications.3 Cell-based therapies have been proposed to repopulate the aneuronal bowel by the transplantation of stem or progenitor cells.4, 5, 6
The ENS is derived from embryonic neural crest (NC) cells, mostly from the vagal level of the neural tube.7 Vagal NC cells enter the proximal gastrointestinal tract and as enteric neural crest (ENC) cells migrate along the entire tract in a distal-directed wave over the 4th to 7th weeks of gestation in humans.8, 9, 10 Experiments in animal models indicate that the sacral level of the NC also provides neurons to the gut,11, 12 but these are numerically less capable13 and cannot compensate for a lack of vagal NC-derived cells.14, 15 Recently a late-arising population of ENS neurons has been identified in mice derived from Schwann cell intermediaries; these are a minority source and give a restricted range of neuron types.16 HSCR results when this wave does not complete colonization of the entire gut. Cell-replacement therapy postnatally after HSCR diagnosis for a defect of early embryonic origin faces difficulties. ENC stem or progenitor cells would have to be obtained and expanded before transplantation; then they would have to be introduced into the aneuronal gut without immune rejection. They must then migrate, colonize, differentiate, and establish connections to mediate bowel function at later stages and in a far larger tissue than would occur during normal ENS development.
Rodent and avian embryonic ENC progenitor cells can colonize embryonic aneural gut and form ENS in vitro.17, 18, 19, 20 Further, rodent embryo NC cells can colonize the aneuronal gut and differentiate into neurons and glia of postnatal HSCR model mice in vivo.21, 22 In addition, it has been shown that postnatal mouse ENC cells surgically placed in the wall of the colon of postnatal normal and HSCR-model syngeneic mice are able to migrate, proliferate, assemble into correctly placed ganglia and differentiate as both glia and neurons that extend axons, make and receive synapses, and show electrical activity.23 This is a key step to answering the therapeutic questions of whether ENS formation can be accomplished when both ENS-lineage donors and colon tissue recipients are postnatal. A further question is, can this be accomplished when both ENC and colon are not only of postnatal human origin, but when (to avoid immune rejection) they are of autologous HSCR patient origin?
We obtained human ENS-derived cells from the proximal margin of HSCR patient colon using flow cytometry for the NC marker p75. These cells were assessed for additional NC markers by antibody labeling and quantitative reverse-transcription polymerase chain reaction (qRT-PCR), and for proliferation with ethynyl-2′-deoxyuridine (EdU). We tested the ability of these cells to migrate in embryonic aneuronal gut in organ culture by combining the human ENS-derived cells with quail embryo intestine,24 a tissue known to support cell colonization. We then ascertained the ability of human postnatal HSCR aneuronal distal colon muscle tissue to support ENS formation by, in organ culture, transplanting into it genetically labeled ENC-derived cells from embryonic mice, cells with proven ENS-forming ability.23 We then labeled human p75-sorted cells using fluorescent tags and implanted them into distal colonic muscle tissue from the same HSCR patients and maintained the combinations in organ culture. The results show that NC-lineage ENS cells can be isolated from neuronal regions of HSCR patient postnatal colon, and that these ENS-derived cells can spread, proliferate, and differentiate in receptive intestinal tissues including aneuronal HSCR colon muscle from the same patient.
Materials and Methods
Human Tissues
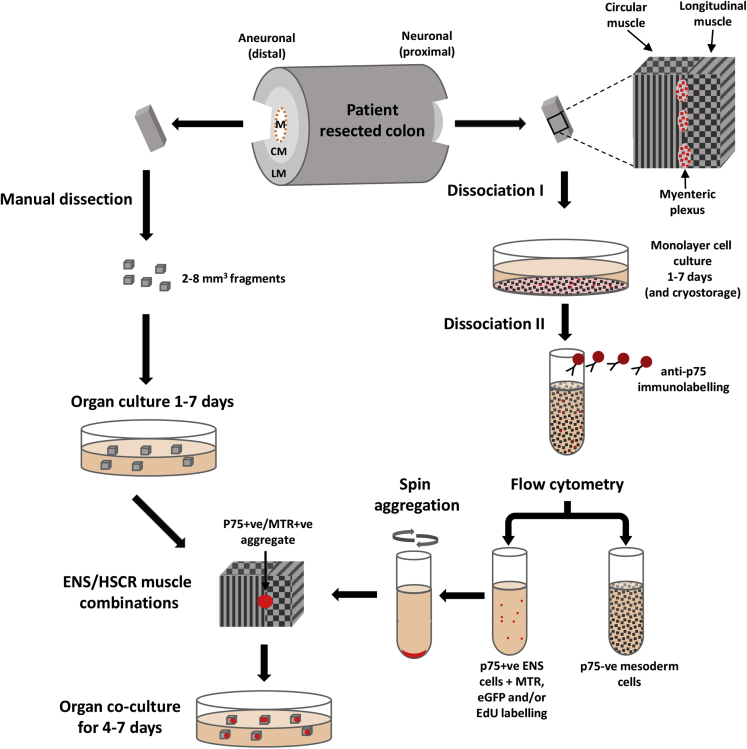
Colon tissue was obtained immediately after surgery for HSCR at the Royal Children’s Hospital (RCH). In most cases (24 of 31), the patients were younger than 4 months at surgery, the length of resected bowel was less than 12 cm (27 of 31), and no other clinical conditions were identified (Table 1). Familial forms of HSCR (patients with first- and second-degree relatives affected) were reported in a small number of cases (4 of 31). After obtaining tissue from the proximal (neuronal) and distal (aneuronal) ends, the remainder of resected bowel tissue was histologically examined at RCH Anatomical Pathology. Samples of the muscle layers of both ends of all specimens were fixed in 4% paraformaldehyde (PFA), embedded in optimal cutting temperature (OCT) compound (Tissue-Tek, Sakura Finetek Europe, Alphen aan den Rijn, Netherlands), frozen, and sectioned (CM1900 cryostat; Leica Microsystems, Wetzlar, Germany) for immunolabeling. The procedures for resected tissues are described here, and a flow chart is shown in Figure 1.
Table 1.
Source of Human Gut Samples: Demographic and Clinical Characteristics of Children With Hirschsprung Disease and Length of the Colon-Rectum Resected During the Surgical Procedure
| Type of Surgical Procedure | No. of Children (n = 31) | Age at Surgical Procedure (mo) | Length of Colon-Rectum Resected (cm) |
|---|---|---|---|
| Laparoscopic-assisted and transanal pull-through | 16 | 3.7 ± 2.2 | 15.4 ± 7.5 |
| Transanal endorectal pull-through (de La Torre-Soave) | 10 | 4.0 ± 2.0 | 11.8 ± 5.2 |
| Duhamel | 3 | 24 ± 22.3 | Total colonic |
| Swenson | 1 | 169 | 67 |
| Colectomy (Re-do) | 1 | 102 | 11 |
Note: Age and length values are mean ± standard deviation.
Figure 1.
Transplant of enteric nervous system (ENS) cells to autologous colon explants from Hirschsprung disease (HSCR) patients. ENS cells from the proximal region of resected colon were sorted by flow cytometry after immunolabeling for p75. Aneuronal smooth muscle fragments were obtained from the distal region. Cocultures of these were established to test the ENS-forming ability of p75+ cells in autologous HSCR patient aneuronal colon muscle. CM, circular muscle; EdU, 5-ethynyl-2′-deoxyuridine; eGFP, enhanced green fluorescent protein; LM, longitudinal muscle; M, mucosa; MTR, MitoTracker Red.
Human tissue was collected under approval from the Royal Children’s Hospital (Parkville, Melbourne, Australia) Human Research Ethics Committee (HREC 30014A). Parent/guardian consent was obtained for all participants. Animal tissues were obtained under the conditions of the Murdoch Children’s Research Institute Animal Ethics Committee (AEC651).
Human Colon Tissue Dissociation
To obtain human ENS cells from myenteric plexuses between proximal colon muscle layers, the mucosa was removed by scraping, and the muscle layer was cut into 1–2 mm3 fragments. Dissociation was achieved in Ham’s F-12 media (GIBCO/Invitrogen, Grand Island, NY) supplemented with 0.5% w/v Dispase II (Roche, Basel, Switzerland) and 0.1% w/v CLSAFA collagenase (Worthington Biochemical, Lakewood, NJ) at 37°C with orbital shaking and mechanical trituration. After 1 hour, the initial cell suspension was decanted, and the remaining tissue pieces were dissociated for an additional hour. We added 1 mg/mL hyaluronidase Type I-S (Sigma-Aldrich, St. Louis, MO), 0.1 mg/mL DNAase I (Roche), and 1 mM EDTA (Sigma-Aldrich Australia, Sydney, Australia) to the pooled cell suspension for 15 minutes. The cell suspension was centrifuged at 400g in a bench centrifuge for 5 minutes, the supernatant was removed, and the pellet was resuspended. The cell suspension was washed in Ham’s F-12 medium with 5% v/v heat-inactivated fetal bovine serum (FBS) (Thermo Fisher Scientific, Waltham, MA) and filtered through a 40-μm cell-strainer (Falcon/BD Biosciences, Bedford, MA).
Culture of Human Hirschsprung Disease Colon Cells
Dissociated cells from the muscle layers, including ENS cells, were grown on tissue culture plates (Fisher Scientific, Roskilde, Denmark) pretreated with 20 μg/mL human fibronectin (Roche, Switzerland). To maximize spontaneous aggregation, fibronectin was reduced or omitted in some cultures. Cells were grown in a 1:1 mixture of Dulbecco’s modified Eagle medium (Thermo Fisher Scientific) and Ham’s F-12 (GIBCO/Invitrogen) with l-glutamine, B27 and N2 (GIBCO/Invitrogen), 10 ng/mL human recombinant fibroblast growth factor 2 (basic) (FGF2) and 10 ng/mL human epidermal growth factor (both R&D Systems, Minneapolis, MN), and 10 U/mL penicillin, 100 μg/mL streptomycin (GIBCO/Invitrogen), 50 μg/mL gentamicin (Sigma-Aldrich Australia), and 50 μg/mL metronidazole (Sigma-Aldrich Australia), this is referred to as nTCM. In some cases nTCM was supplemented with 3 μM glycogen synthase kinase 3 (GSK3) inhibitor CHIR-99021 (6-[2-[[4-(2,4-dichlorophenyl)-5-(5-methyl-1H-imidazol-2-yl)pyrimidin-2-yl]amino]ethylamino]pyridine-3-carbonitrile; Tocris Bioscience/R&D Systems, Minneapolis, MN), which simulates Wnt signaling.25
Cell Sorting and Labeling of Human Enteric Nervous System-Derived Cells
After 1–7 days of culture, adherent cells were lifted with 0.025% w/v trypsin (Sigma-Aldrich) and stained for 45 minutes with mouse p75/CD271:Alexa 647 antibody (BD Biosciences/Pharmingen, San Diego, CA) (see Figure 1). Cells were washed in 5% FBS in Ham’s F-12, and 10 μg/mL propidium iodide (Sigma-Aldrich) was added to detect dead cells. Cells staining positive for p75 (647: bandpass filter 660/20) and negative for propidium iodide (bandpass filter 692/40) were isolated by flow cytometry using a 1-drop pure mode (MoFlo, Beckman Coulter, Brea, CA; or Influx, BD Biosciences, San Jose, CA) and a sheath pressure of 22 psi with a 100-μm diameter nozzle and Dulbecco’s phosphate-buffered saline (PBS) sheath fluid (prepared in house).
To observe human cells in human colon explants, we incubated the p75+ cells for 20 minutes in 10 nM MitoTracker Red (MTR) CMXRos (Life Technologies, Carlsbad, CA) at 37°C, then washed twice in nTCM. Microscopy confirmed that all cells contained MTR. In some cases the p75+ cells were transduced with lentiviral particles carrying a constitutive enhanced green fluorescent protein (eGFP) reporter construct (PLL3; CMG-eGFP).
To generate lentiviral particles, human embryonic kidney 293 (HEK293) cells were transfected by Fugene HD (Promega, Madison, WI) with constructs pBR8.91 and pMDG and pLL3. The supernatant containing viral particles was collected after 2 days and then applied to p75+ cells for 24 hours. We found that the addition of 10% fetal calf serum (Thermo Fisher Scientific) to the cell culture medium aided in the survival of p75+ cells after lentiviral infection. After 4 days of culture, the cells that were positive for both p75+ and eGFP (p75+/eGFP+) were isolated by flow cytometry as described earlier (eGFP: bandpass filter 530/40). Both p75+/MTR and p75+/eGFP+ cells were spin aggregated, as we will describe herein.
Cell Sorting for Mouse Enteric Nervous System–Derived Cells
Mouse ENS Kikume+ cells were obtained by flow cytometry (Kikume: bandpass filter 530/40) from embryonic day E13.5–E14.5 EdnrbKik mice on a C57Bl/6 background.23, 26 In these mice, all ENS cells express the fluorescent protein Kikume under the control of an enteric-specific region of the Ednrb promoter. Matings were conducted as described elsewhere.23 The gut from the stomach to the anus was dissected and screened by fluorescence microscopy to confirm the presence of labeled ENS. To collect mouse ENS cells, the gut was dissociated in 0.1% trypsin/EDTA (GIBCO/Invitrogen) at 37°C for 20 minutes, with gentle pipetting.
Generation of Spun Enteric Nervous System Cell Aggregates
After flow cytometry ENS cells (human: 5000; mouse: 10,000) were deposited into round-bottom, low cell-adherence plastic 96-well plates (Corning Life Sciences, Tewksbury, MA). The cells were aggregated by centrifugation at 400g for 5 minutes, and maintained for 1–2 days in normal culture conditions before use in coculture experiments. In most cases ENS cells formed spheres after overnight culture in low-adherence conditions.
Avian Aneural Intestinal Organ Culture
Fertilized quail (Coturnix japonica) eggs were obtained from Lago Game Supplies (Victoria, Australia) and were incubated at 38°C. Postumbilical gut was dissected from 4-day embryos to act as recipients for ENS cell immigration in organ culture. At this stage, this gut region is not invaded by ENC cells27 and is therefore aneural. Human ENS p75+ cell aggregates were applied to the proximal end of the gut, and the ensemble was maintained in culture in Ham’s F-12 medium supplemented with 10% FBS, l-glutamine, and 10 U penicillin and 100 μg/mL streptomycin. After 4 days, the explants were fixed and underwent immunohistochemical staining as whole mounts.
Human Aneuronal Colon Explant Coculture With Enteric Nervous System Cells
Human aneuronal colon muscle was obtained from patients as already described, but from the distal end of the resected bowel. The submucosa was removed, and the muscle was cut into 2–8 mm3 fragments containing both longitudinal and circular muscle layers; these layers were clearly distinguishable with a dissecting microscope (Leica MZ6; Leica Microsystems). These could be maintained for at least 12 days in culture in nTCM supplemented with 5% FBS at 37°C and 5% CO2 without alterations in cell nuclear morphology or in mesodermal smooth muscle, detected with Hoechst 33342 (5 μg/mL) and smooth muscle actin (SMA) antibody (see below), respectively. For combination explants, these human muscle tissues were cocultured with either EdnrbKik mouse or human ENS cell aggregates. These aggregates were inserted in a pocket created between the two muscle layers. These were maintained in culture for up to 7 days and monitored for Kikume expression for mouse/human combinations and MTR or eGFP for human/human combinations. Explants were fixed for 1 hour at 4°C in 4% PFA for whole-mount immunolabeling or tissue sectioning.
Ethynyl-2′-Deoxyuridine Labeling and Cell Proliferation Analysis
Human colon monolayer cultures and p75-sorted ENS cells before aggregation were treated with 5 μM EdU (GIBCO/Invitrogen) for 12 hours at 37°C and washed twice in PBS. To quantify the proliferation of cells positive for sex-determining region Y–box 10 (SOX10) in the nTCM and CHIR-99021 groups, the EdU-labeled human colon monolayer cultures were fixed in 4% PFA for 10 minutes and stained for SOX10 (see below), and EdU-labeled cells were detected by the Click-iT EdU Imaging Kit (GIBCO/Invitrogen) according to the manufacturer’s instructions. Ten microscopic fields were visualized from each experimental group (nTCM with and without CHIR-99021 with cells from two HSCR patients), and the proportion of SOX10+/EdU+ cells was expressed as the ratio of total SOX10+ cells counted in each field. Human p75-sorted cell aggregates were used for coculture with human aneuronal colon, where incorporated EdU also served as a cell tracker. Mouse ENS-derived cell aggregates (Kikume+) in coculture with human aneuronal colon muscle explants were pretreated for 8 hours with 5 μM EdU during days 1–2 of the culture period.
Gene Expression Analysis
Total RNA was isolated from cells using RNeasy mini kit (Qiagen, Valencia, CA), and contaminant genomic DNA was removed with DNA-free reagents (Ambion/Life Technologies, Austin, TX). Primer sequences were designed using Primer3 (http://frodo.wi.mit.edu/primer3/) and are listed in Table 2. Where possible, the primers sets were designed to span introns to discriminate between amplification of genomic DNA and cDNA. For qRT-PCR, oligo-dT primed cDNA was synthesized from 200 ng of total RNA using Murine Moloney Leukaemia Virus reverse transcriptase (Promega). We performed the qRT-PCR on an ABI Prism 7500 Real-Time PCR System using SYBR green master mix (Applied Biosystems, Foster City, CA) according to the manufacturer’s protocols. Relative gene expression values were obtained by normalization to the reference gene L32 using the −2ΔΔCt method, where −2ΔΔCt = ΔCt sample − ΔCt calibrator as described elsewhere.28
Table 2.
Oligonucleotide Primers Used in Quantitative Reverse-Transcription Polymerase Chain Reaction Amplification of Human Genes
| Gene | Sequence | Amplicon Size (bp) | Primer Design | Gene Role |
|---|---|---|---|---|
| SOX10 | Sense: GCTGCTGAACGAAAGTGACAAG Antisense: TCTTGTAGTGGGCCTGGATG |
197 | Spans exons 3–4 (intron 3) | Early NC development and marks migrating ENC cells, Glia |
| AP2 | Sense: ACTCGGAGACCTCTCGATCC Antisense: GGACACGGGGCCTTTCTTAAT |
115 | Spans exons 2–4 (introns 2 and 3) | Specifier gene for early induction of ENC fate |
| FOXD3 | Sense: CCCAAGAACAGCCTAGTGAAGC Antisense: TTCTCCCTGTAGTAGGGGAAGC |
140 | Monoexonic transcript (non-intron spanning) | Maintenance of ENC progenitor |
| P75 | Sense: ATCCCTGTCTATTGCTCCATCC Antisense: TGTGGAGTTTTTCTCCCTCTGG |
154 | Spans exons 4–5 (intron 4) | Nerve growth factor receptor, labels all ENS |
| RPL32 | Sense: CATCTCCTTCTCGGCATCA Antisense: ACCCTGTTGTCAATGCCTC |
153 | Spans exons 1–3 (introns 1 and 2) | Non ENS, gene for qRT-PCR normalization |
ENC, enteric neural crest; ENS, enteric nervous system; NC, neural crest; qRT-PCR, quantitative reverse-transcription polymerase chain reaction.
Immunolabeling
Cells and tissues were fixed in 4% PFA for 5 and 60 minutes, respectively. The monolayer cultures and whole mounts were permeabilized and blocked in 0.1% Triton X-100/1% horse serum in PBS for 15 and 60 minutes, respectively. Tissue sections were blocked in 1% horse serum in PBS for 30 minutes. For immunolabeling, primary antibodies recognized NC cell markers (p75, HNK1 [human natural killer-1], SOX10), glial markers (GFAP [glial fibrillary acidic protein], S100β, SOX10), the neuronal marker (Hu), neuronal nitric oxide synthase (nNOS), nerve fibers (TUJ1, neuron-specific class III β-tubulin), and the muscle marker SMA. Secondary antibodies appropriate for these were coupled to AMCA (methyl N-(4′-(9-acridinylamino)-phenyl) carbamate hydrochloride), Alexa 647, 594, 488, and Texas Red. A complete list of these antibodies is in Table 3. For monolayer culture and tissue sections, antibodies were incubated for 2 hours. For whole mount, antibodies were incubated overnight. Cell nuclei were counterstained with 5 μg/mL Hoechst 33342 (Invitrogen/Life Technologies, Carlsbad, CA), which labels all cell nuclei. Staining was visualized using a Leica SP2 confocal microscope (Leica Microsystems) or LSM 780 confocal microscope (Carl Zeiss Light Microscopy, Göttingen, Germany). Postacquisition image analysis was performed by LAS AF light software (Leica Microsystems) or ZEN Blue software (Carl Zeiss Light Microscopy).
Table 3.
Antibodies and Probes Used to Label Enteric Nervous System and Muscle Cells
| Cell Target | Identity | Type | Source | Product No. | Research Resource Identifiers (RRID) |
|---|---|---|---|---|---|
| NC cell surface | p75 | Rabbit IgG | Promega | G3231 | AB_430853 |
| NC cell surface/FACS | p75: Alexa 647, clone c40–1457 | Mouse IgG | BD Pharmingen | 560326 | AB_2033986 |
| NC cell surface | HNK1 | Mouse IgM | MCRI | NA | NA |
| NC/glial cell nucleus | SOX10 | Goat IgG | R&D Systems | AF2864 | AB_442208 |
| Glial cytoplasm | GFAP | Rabbit IgG | Dako, Denmark | Z0334 | AB_2314535 |
| Glial cytoplasm | S100β, clone SH-B1 | Mouse IgG | Sigma-Aldrich, Australia | S2532 | AB_477499 |
| Neuron nucleus/cytoplasm | Hu | Human IgG | Vanda Lennon, Rochester, MN | NA | NA |
| Nitrergic neuron | nNOS | Sheep IgG | EMSON | H212 | AB_2314957 |
| Nerve fiber | Neuronal class III β-tubulin clone (TUJ1) | Mouse IgG | Biolegend/Covance | MMS-435P | AB_2315514 |
| Smooth muscle actin | SMA, clone SH-B1 | Mouse IgG | Sigma-Aldrich, Australia | A2547 | AB_476701 |
| DNA | Hoechst 33342 | NA | Life Technologies | H3570 | NA |
| DNA/dead cells | Propidium iodide | NA | Sigma-Aldrich Australia | P4170 | NA |
| Mitochondria | MitoTracker CMXRos | NA | Life Technologies | M7512 | NA |
| Secondary antibodies and probes | |||||
| Human IgG | Texas Red | Donkey IgG | Jackson ImmunoResearch | 709 075 098 | AB_2532064 |
| Mouse IgM | Texas Red | Donkey IgG | Jackson ImmunoResearch | 715 065 140 | AB_2340783 |
| Mouse IgG | Alexa 594 | Donkey IgG | Life Technologies | A21203 | AB_10563558 |
| Mouse IgG | Alexa 647 | Goat IgG | Life Technologies | A21235 | AB_10562370 |
| Goat IgG | Alexa 488 | Donkey IgG | Life Technologies | A11015 | AB_10561557 |
| Goat IgG | Alexa 594 | Donkey IgG | Life Technologies | A11058 | AB_142540 |
| Goat IgG | Biotin | Donkey IgG | Jackson ImmunoResearch | 705 065 147 | AB_2340397 |
| Biotin | AMCA | Streptavidin | Jackson ImmunoResearch | 016 150 084 | AB_2337243 |
AMCA, methyl N-(4′-(9-acridinylamino)-phenyl) carbamate hydrochloride; GFAP, glial fibrillary acidic protein; HNK1, human natural killer-1; NA, not applicable; NC, neural crest; nNOS, neuronal nitric oxide synthase; SMA, smooth muscle actin; SOX10, sex-determining region Y–box 10.
Quantification of Cell Types in Aggregates
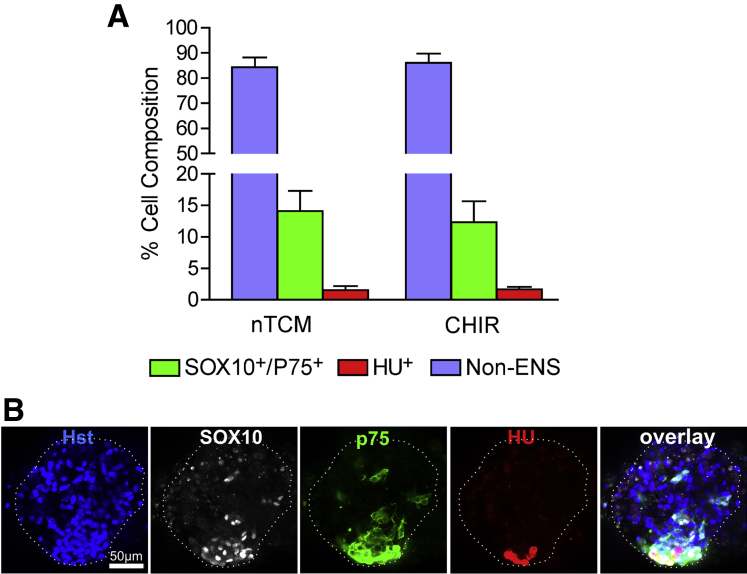
Cell types present in spontaneously forming aggregates was detected by immunolabeling aggregates for SOX10, p75, and Hu and then counterstaining with Hoechst 33342. For each labeled sphere, a Z-stack confocal microscopy series was generated with optical sections taken every 10 μm. Three optical sections for each sphere were assessed and for cells staining positive for SOX10+/p75+, Hu+, or Hoechst 33342 were counted. Ten spheres were counted for each treatment group (nTCM/CHIR-99021, n = 2 patients). Data were represented as a percentage of SOX10+/p75+ or Hu+ cells of all cells present (Hoechst 33342+).
Statistical Analysis
Data were analyzed with GraphPad Prism version 5.1 software (GraphPad Software, San Diego, CA). Data were expressed as mean ± standard error of the mean (SEM). Statistical analysis was performed by one-way analysis of variance or Kruskal-Wallis test and the appropriate parametric (Student t test) or nonparametric (Mann-Whitney) post test. P < .05 was considered statistically significant.
Results
Enteric Nervous System Cells Can Be Identified in the Hirschsprung Disease Patient Colon and Persist in Organ Culture
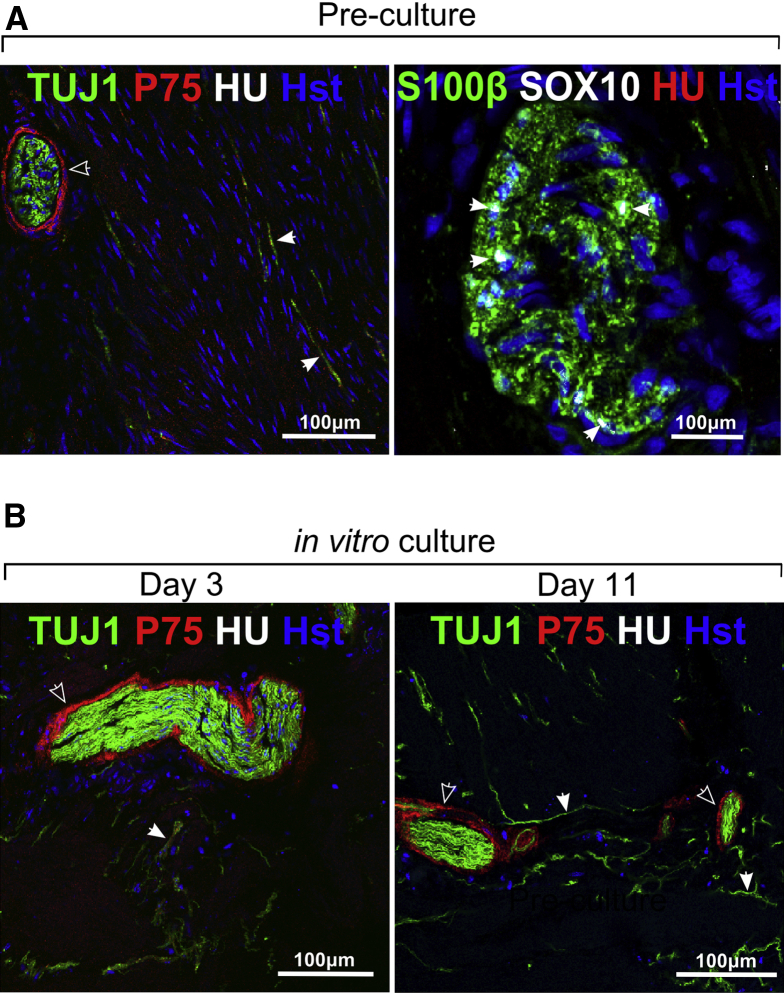
Serial sections of the distal aneuronal segment of HSCR colon from four HSCR patients were examined. These contained no Hu+ neurons, but p75+, SOX10+, and S100β+ cells were plentiful. The neural elements of the aneuronal segment consisted of large bundles of TUJ1+ fibers surrounded by p75+ cells as well as single TUJ1+ fibers (Figure 2A). This confirmed that the HSCR patient distal colon was aneuronal but not aneural. Aneuronal colon muscle maintained for up to 11 days in organ culture maintained expression of these markers, including the persistence of TUJ1+ nerve fibers (see Figure 2B). In sections of proximal neuronal colon from four HSCR patients, ganglia within the myenteric plexus could be identified containing the NC markers p75, SOX10 and HNK1 (not shown), neuronal (HU) and neurite (TUJ1; not shown) markers and glia markers (SOX10, GFAP; not shown S100β) (Figure 3A and B). Closely associated with ganglia were putative ENS progenitor cells, which were SOX10+ but lacked neuronal and glial markers, including GFAP (see Figure 3B).
Figure 2.
Characterization of human aneuronal tissue. (A) Aneuronal tissue taken directly from the distal end of the resected colon (on the day of Hirschsprung disease [HSCR] surgery) showed (left) hypertrophic neuron-specific class III β-tubulin positive (TUJ1+) nerve bundles and p75+ cells mainly at the perimeter of the bundles (open arrowheads). TUJ1+ fibers also were present throughout the muscle (closed arrowheads). In this aneuronal colon tissue (right) no Hu+ cell bodies were detected, consistent with the extrinsic origin of these fibers, but SOX10+/S100β+ (closed arrowheads) cells were detected. (B) Cultured aneuronal explants at 3 and 11 days in vitro showed the persistence of hypertrophic nerve bundles and fibers immunoreactive for TUJ1 (closed arrowhead), and p75+ cells (open arrowhead). Hu+ cell bodies were not detected after culture. Images acquired using confocal microscopy.
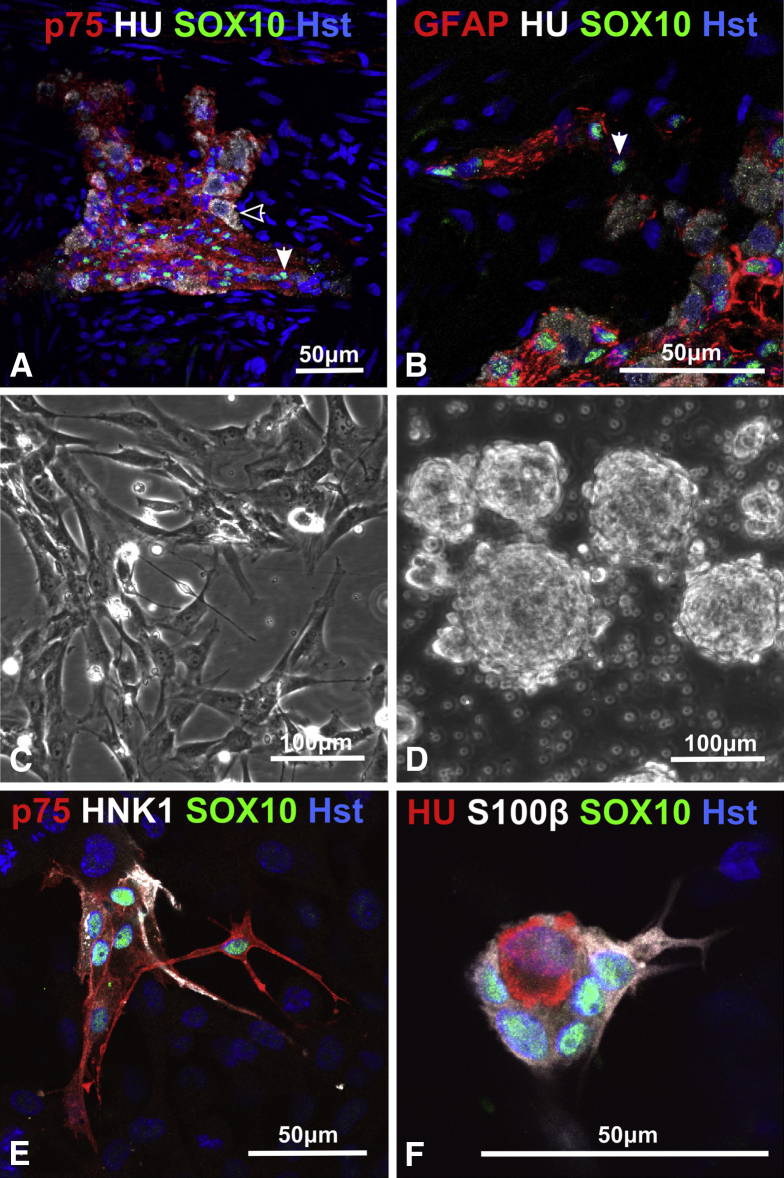
Figure 3.
Characterization of human enteric nervous system (ENS) in vivo and in vitro. (A) The proximal resected Hirschsprung disease (HSCR) colon contained myenteric ganglia, positive for p75 and the neuron marker Hu (open arrowhead) and glia/progenitor marker SOX10 (closed arrowhead). (B) Near the enteric ganglia were putative progenitor cells, identified by SOX10 and absence of the glia marker GFAP (glial fibrillary acidic protein) (closed arrowhead). (C) Dissociated colon cells on fibronectin grew initially as a monolayer. (D) Dissociated colon cells in vitro without fibronectin mainly formed spheres. (E) Immunolabeling of NC cell-surface antigens p75 and HNK1 (human natural killer-1) and transcription factor SOX10 reveal ENS cells within colon monolayer cultures. (F) Glia cells (SOX10+/S100β+) and neurons (SOX10−/HU+) occur in monolayer cells. Hst, Hoechst nuclear stain. Images acquired using confocal microscopy. Data derived from six HSCR patient samples.
Human Enteric Nervous System and Mesoderm Cells Can Be Propagated in Vitro
Cells dissociated from proximal colon (21 HSCR patients) produced monolayers on fibronectin; these showed multiple morphologically distinct cell types (see Figure 3C). Neurosphere-like bodies (see Figure 3D) also appeared in cell cultures, and omission or reduction of substrate fibronectin promoted this.
Within the monolayer cultures, ENS cells could be identified by staining for NC surface antigens p75 and HNK1 (see Figure 3E) and by the pan-neuronal marker Hu and glia/progenitor cell markers SOX10 and S100β (see Figure 3E and F). The ENS-derived cells formed clusters of 5–30 cells in a carpet of fibroblast-like cells, many of which stained strongly for SMA (not shown) and were presumed to have originated from smooth muscle cells. Fluorescence-activated cell sorting analysis (see below) indicated that ENS cells formed <5% of the total cells present in these cultures.
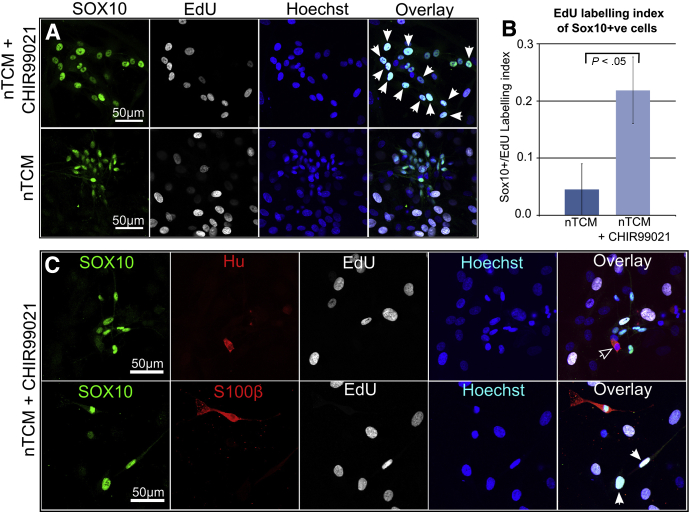
To test whether the proliferation rate of SOX10+ cells could be increased in monolayer cultures, we added the GSK3 inhibitor CHIR-99021 to the nTCM culture medium. This molecule was chosen because inhibition of the GSK3 pathway to mimic canonical Wnt signaling promotes proliferation of central nervous system progenitors in vitro.29, 30 Additionally, Wnt in combination with bone morphogenetic proteins (BMP) signaling promotes the survival and proliferation of mouse NC progenitor cells.31 Proliferation of SOX10+ cells in monolayer cultures with nTCM alone was low but could be increased more than 4-fold by the addition of CHIR-99021 (Figure 4A and B). The fibroblast-like cells did not require CHIR-99021 for proliferation. Proliferating ENS cells in the presence of CHIR-99021 were either glia (SOX10+/S100β+) or putative progenitor (SOX10+/S100β-ve) cell types (Figure 4C). Neurons (SOX10-ve/Hu+) could not be identified as differentiating in culture from a proliferating (ie, EdU+) precursor labeled during the prior EdU exposure period (see Figure 4C). Primary colon monolayer cells were routinely passaged and cryopreserved without loss of the ENS cell moiety (not shown).
Figure 4.
Human enteric nervous system (ENS)–derived cells proliferate in vitro. (A) Incorporation of ethynyl-2′-deoxyuridine (EdU) (white arrowheads) into SOX10+ ENS cells with and without CHIR-99021. (B) Proliferation of SOX10+ cells is significantly increased by CHIR-99021, expressed as a ratio of EdU+/SOX+ cells to total SOX10+ cells. (C) Proliferation occurs in putative progenitor cells (EdU+/SOX10+/S100β-ve; white arrowheads), but was not detected in neurons (HU+/SOX10-ve; open arrowhead) during the culture period. Images acquired using confocal microscopy. Data derived from two Hirschsprung disease (HSCR) patient samples.
Neurosphere-like bodies were immunolabeled with SOX10, p75, and Hu (Figure 5) to determine the percentage of ENS and non-ENS cells present. From confocal sections, on average the neurosphere-like bodies were 14.11% (SEM = 3.20%) p75+/SOX10+ cells when grown in nTCM (total 4781 cells sampled) and 12.31% (SEM = 3.34%) when grown with the addition of CHIR-99021 (total 4126 cells sampled). Less than 1% of cells were Hu+ neurons in these neurosphere-like bodies after culture with or without CHIR-99021 (see Figure 5). Although there was slight enrichment for ENS cells in neurosphere-like bodies compared with primary cultures of monolayered colon cells, the major constituents (over 85%) were not ENS cells.
Figure 5.
Cell types present in neurosphere-like bodies. (A) Bar graph displaying the percentage of either SOX10+/p75+, HU+ or non–enteric nervous system (ENS) cells present in neurosphere-like bodies grown in nTCM with and without CHIR-99021 (no statistical difference was found between these groups). (B) A Z-stack optical section shows the characteristic staining of a SOX10+ and p75+, which were always found coexpressed in cells. Hu+ cells were often found in association with SOX10+/p75+ cells. However, the majority of cells did not stain for SOX10, p75, or Hu and were non-ENS population derived from the intestinal muscle.
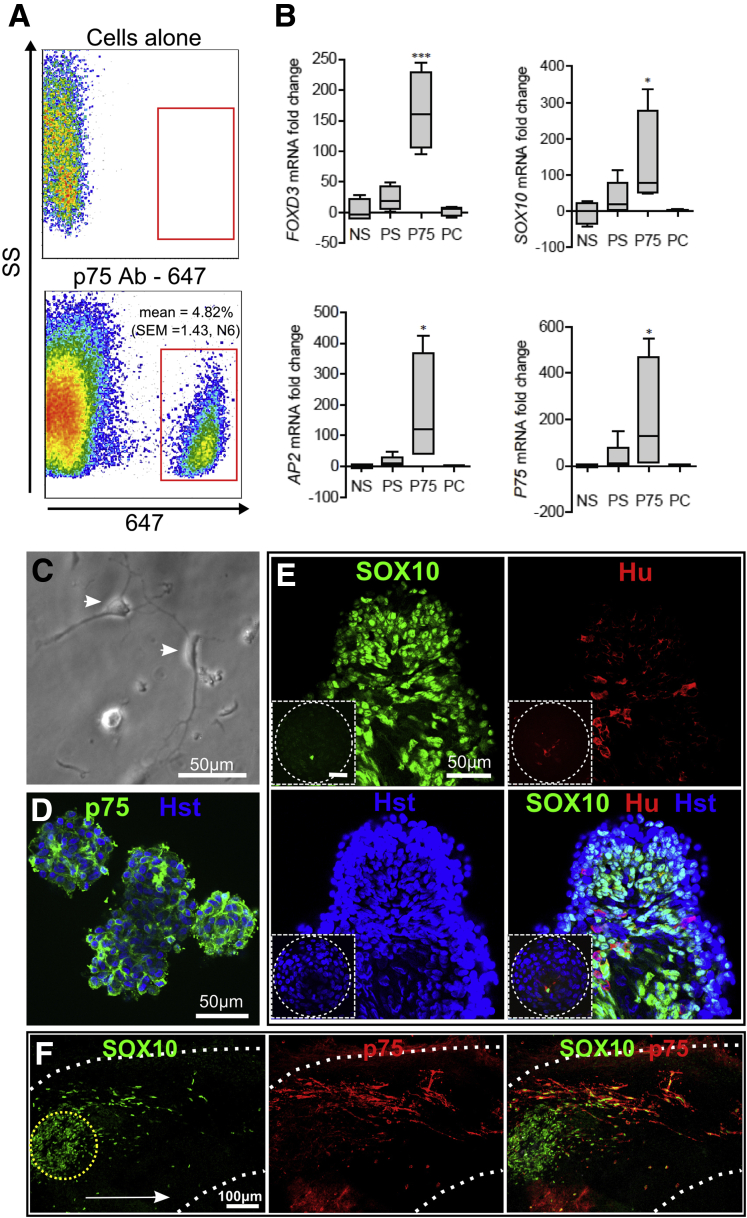
Enrichment of Human Enteric Nervous System Cells by Flow Cytometry Cell Sorting
We did not find sorting for p75+ ENS cells effective using cells directly dissociated from the proximal colon. Instead, proximal colon cells were cultured for 1–7 days in monolayer, and then ENS lineage cells were isolated by flow cytometric sorting of p75+ cells (see Figure 1). The average percentage of p75+ cells in colon cultures was 4.82% (SEM = 1.43, from six patients) (Figure 6A). Analysis by qRT-PCR (see Figure 6B) showed an increase of NC gene expression in p75-sorted cells. These were SOX10 (mean 407-fold enrichment, SEM = 205), FOXD3 (mean 165-fold, SEM = 32), AP2 (mean 176-fold, SEM = 90), and p75 (mean 204-fold, SEM = 126) when compared with cells negatively sorted for p75 (negative sort) (n = 4 patients). The p75+ve sorted cells showed enrichment of ENS markers when compared with unsorted cells on the day of tissue dissociation (precultured), or cultured cells on the day of flow cytometry (presort) (see Figure 6B). A comparison of precultured and presort groups revealed that no significant loss of ENS markers occurred during the cell culture period. The p75-sorted ENS cells on replating showed neuronal or multipolar morphologies (see Figure 6C). These cells formed aggregates after centrifugation into low-attachment plates (see Figure 6D). Immunologic staining of these p75+ spin-aggregates confirmed that almost all cells expressed either p75∖SOX10 or Hu (see Figure 6D and E).
Figure 6.
Enrichment of enteric nervous system (ENS) cells from human colon cell cultures, and behavior in embryonic intestine explants. (A) p75+ cells could be isolated from colon monolayer cultures and comprised less than 5% of the population sorted by flow cytometry. (B) Neural crest (NC) gene expression levels after quantitative reverse transcription and polymerase chain reaction (qRT-PCR) of FOXD3, SOX10, AP2, and p75 mRNA levels. Groups compared were unsorted colon cells analyzed prior to cell culture (PC), cells that were cultured and analyzed prior to sorting (PS), and cells that were sorted for p75+ (p75) and p75 negatively sorted (NS). Box plots show the first quartile to interquartile range, whiskers show minimum and maximum range, and the median is represented by horizontal line. *P < .05; ***P < .01. (C) p75-sorted cells displayed neuronal (white arrowhead) and multipolar morphologies in vitro. (D) p75-sorted cells formed aggregates after centrifugation. (E) p75+ aggregates stained for SOX10 and Hu. Inset image is a cell aggregate of unsorted cells (PS) that underwent spin aggregation. In this case, ENS cells are a minor proportion of the total cell population. Data derived from six HSCR patients for flow cytometry study, four Hirschsprung disease (HSCR) patients for qRT-PCR analysis, and three HSCR patients for immunohistochemical study. (F) Human p75+ cells migrate distally in aneural quail embryo gut (gut border indicated by dotted white line) (four explants) from the initial placement of the p75+ cell aggregate (dotted yellow line). The migrating p75+ cells coexpressed SOX10. The direction of migration proximal to distal is indicated by the white arrow. Images acquired by confocal microscopy.
Embryonic Avian Aneural Gut Supports Human Enteric Nervous System Cell Colonization
The avian embryonic aneural gut favors ENC cell colonization,24 so we used this to test the colonization ability of the postnatal human p75+ ENS-derived cells. When p75+ cell aggregates were combined in explants with aneural quail guts, cells migrated as chains into the gut and expressed SOX10 (n = 4 quail intestines, ENS cells from two HSCR patients) (see Figure 6F). This was similar to migrating avian embryonic ENC cells, but the colonization distance was less; approximately 600 μm in 4 days, compared with >1500 μm for avian ENC cells in similar culture conditions.24
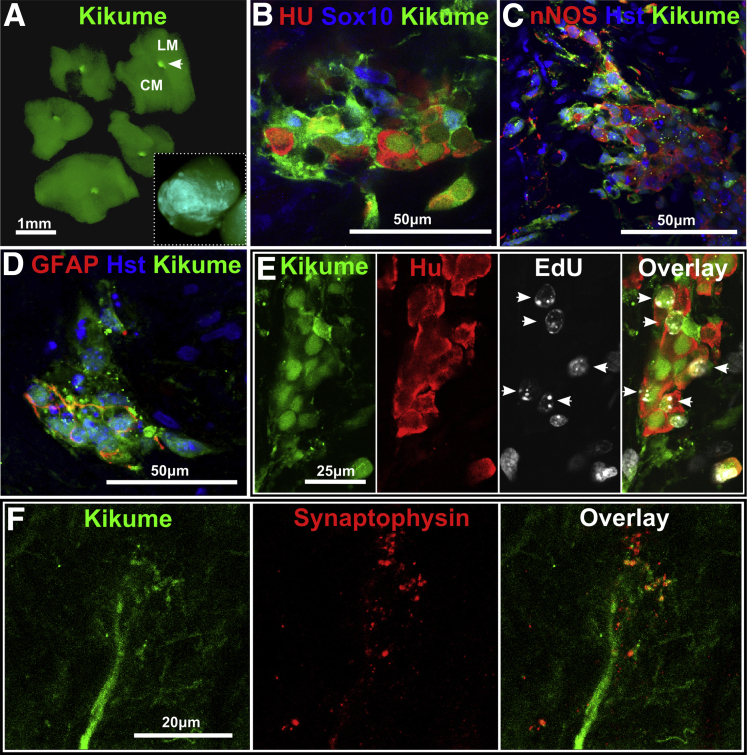
Postnatal Human Aneuronal Hirschsprung Disease Colonic Muscle Supports Mouse Enteric Neural Crest Cell Colonization
To test whether postnatal human HSCR colon tissue could support colonization by ENC cells, we employed cells with demonstrated colonization potential: embryonic ENC cells from EdnrbKik mice.23 Aggregates of these cells were combined with human HSCR aneuronal colonic muscle in vitro (n = 2 patients, eight explants). The diameter of the mouse ENS-derived spheres were approximately 150 μm at the time of combination (Figure 7A); however, after 1 week the ENC cells had migrated greater than 1 mm radially over and into the surrounding human HSCR colon tissue (see Figure 7A, inset). Mouse ENS cells formed small ganglion-like clusters of neuronal (Hu) and glial (SOX10, GFAP, and S100β) cells, and some neurons showed immunoreactivity for nNOS (see Figure 7B–D). EdU was detected in the nuclei of Hu+ neurons in the cocultures (see Figure 7E), demonstrating de novo differentiation of neurons from proliferating mouse ENS progenitors in human HSCR tissue. Immunoreactivity for the synaptic protein synaptophysin was closely associated with mouse Kikume+ nerve fibers (see Figure 7F).
Figure 7.
Mouse enteric nervous system (ENS) cells colonize Hirschsprung disease (HSCR) aneuronal colonic muscle. (A) EdnrbKik+ mouse ENS cell spheres (white arrowhead) were combined with human HSCR muscle tissue (day 0) by positioning the sphere between the longitudinal muscle (LM) and circular muscle (CM) layers. The inset image shows that after 7 days of culture in vitro, Kikume cells have spread throughout the explant. (B) EdnrbKik+ ENC cells formed ganglion-like clusters with neurons (Hu+) and glial or progenitor cells (SOX10+). These ganglion-like clusters contained nitric oxide synthase positive (NOS+) neural subtypes (C) and also glia (GFAP+, glial fibrillary acidic protein positive) (D). (E) Proliferating EdnrbKik+ ENC cells differentiated into neurons (EdU+/Hu+) (white arrowheads) within human HSCR muscle. (F) Combination cultures of EdnrbKik cells with HSCR colon tissue for 5 days shows synaptophysin immunoreactivity closely associated with Kikume graft–derived nerve fibers. Results were from 8 explants from two HSCR patients. Images were acquired by confocal microscopy.
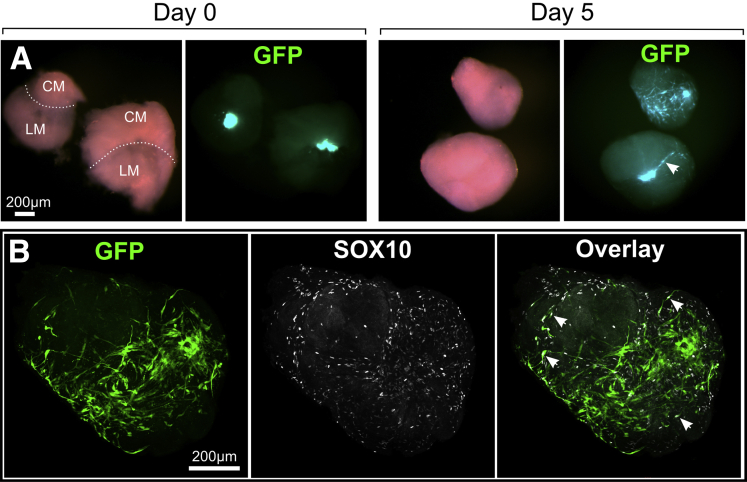
Postnatal Human Aneuronal Hirschsprung Disease Colon Muscle Supports Autologous Human Enteric Nervous System Cell Colonization
HSCR patient ENS-derived cell aggregates were cocultured with aneuronal colon muscle from the same patient. To investigate the degree of ENS cell spreading, eGFP+/p75+ ENS-derived cells were monitored in HSCR muscle from one patient, in two explants (Figure 8A). SOX10+/eGFP+ cells extended with surprising speed, and by 4–5 days were spread, principally over the circular muscle layer of the explanted aneuronal muscle tissue (see Figure 8B). ENS aggregate cells (from six patients, 30 explants) were also tracked by MTR vital stain (Figure 9A) and by prior EdU-labeling.
Figure 8.
Autologous transplant of human p75+/eGFP enteric nervous system (ENS)-derived cells in Hirschsprung disease (HSCR) muscle. (A) p75+/eGFP ENS aggregate combined with HSCR muscle (day 0) and after the culture period (day 5). During this period enhanced green fluorescent protein (eGFP) cells have spread throughout the muscle explant. Most eGFP+ cells spread on the circular muscle (CM) and at the interface (dotted line) with the longitudinal muscle (LM); this is shown (white arrowhead) in the side view of the lower explant at day 5. (B) Confocal whole-mount immunolabeling revealed that the majority of eGFP cells maintained expression of SOX10 and these cells were found extensively over muscle tissue (white arrowheads). SOX10+ cells not expressing eGFP were also detected and are likely of glial origin, which reside in the aneuronal tissue (see Fig. 2).
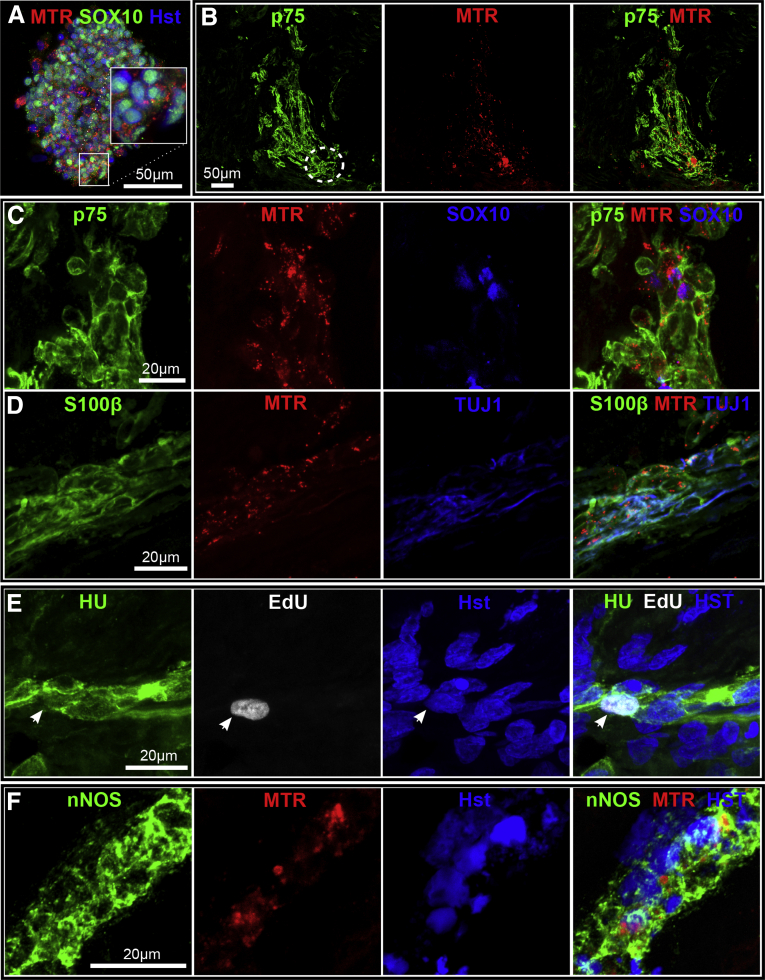
Figure 9.
Autologous transplant of human p75+/MTR (MitoTracker Red) enteric nervous system (ENS)–derived cells in Hirschsprung disease (HSCR) muscle. (A) p75+ ENS aggregate prestained with MTR before implantation into HSCR muscle. The insert shows MTR labeling within the cytoplasm of SOX10+ cells. (B) Implantation of aggregate with HSCR muscle shows distribution of MTR+/p75+ cells from initial graft site (indicated by white dotted circle) after 5 days. (C, D) Ganglion-like structures originating from MTR+ aggregates contain SOX10+ and p75+ cells, glia markers (S100β+) and nerve fibers (TUJ1+). (E) Proliferating human p75+ ENS cells differentiate into neurons (EdU+/Hu+) within HSCR muscle (white arrowhead). (F) Neuronal nitric oxide synthase (nNOS) immunoreactivity in MTR-labeled cells indicates neuronal differentiation appropriate of an ENS neuron type from human p75+ ENS cells. Images acquired using confocal microscopy.
In all explants from all patients, within a week MTR-labeled cells spread from the initial graft, and were detected as p75+ cells in fixed specimens using confocal microscopy (see Figure 9B). Ganglion-like clusters of MTR+/p75+ cells within HSCR muscle stained for neuronal (HU), axonal (TUJ1) and glial (SOX10, S100β) markers (see Figure 9C and D). Neuronal differentiation from previously cycling ENS precursor cells was shown by the presence of some EdU+/HU+ double-positive cells (see Figure 9E). Some neurons showed the characteristic enteric neuron type marker nNOS (see Figure 9F). These p75+ ENS-derived neurons were often at and close to the implant site, but MTR+/p75+/SOX10+ cells occurred more distally from the implant site. In no cases were neurons detected in aneuronal explant tissue (six patients, 21 explants) without the prior engraftment of p75+ ENS cells.
Discussion
Delivery of neural progenitor cells to the postnatal aneuronal (aganglionic) colon is a promising therapeutic strategy for HSCR.23 Avoidance of immunologic rejection of such cells is obviously of prime importance, hence autologous cells would be preferred. Here we have shown that HSCR patients’ ENS-derived cells can form ENS in aneuronal bowel muscle tissue of the same HSCR patient. Our findings have fulfilled three key requirements: 1) human postnatal ENS-derived cells can be harvested from the colon of HSCR patients; 2) postnatal aneuronal human colonic tissue from HSCR patients is still permissive and supportive of de novo ENS formation, despite derivation from a much later developmental stage than is typical for this process; and 3) postnatal ENS-derived cells from a HSCR patient colon are still competent to colonize not only embryonic gut (when colonization occurs normally) but are also able colonize and differentiate in postnatal colon tissue of the same patient.
A number of sources of autologous cells for ENS reconstitution for the HSCR distal colon can be considered, but potentially favorable sources are NC-derived cells already associated with the ENS. The aneuronal HSCR distal colon has numerous sacral and trunk NC-derived cells associated mostly with nerve trunks, as we show here. These can be isolated and can form neurons in culture and in rodent embryonic aneural colon in organ coculture.32 In addition, normal enteric neurogenesis has recently been reported in postnatal mice, the source being trunk and/or sacral NC-derived Schwann cell precursors.16 However, animal model experiments suggest that neither of these has adequate ENS-forming ability in terms of cell numbers and neuron types and cannot replace the absent vagal NC-derived ENS source.13, 14, 15 The most favorable cell population for ENS reconstitution may be that derived from NC with vagal positional identity13 that has experienced prior enteric inductive influences33—that is, cells from the ENS of the upstream proximal ganglionated colon of HSCR patients. An alternative route to autologous ENS-competent cells is via an iPS cell route. This would solve problems of ENS progenitor cell number, but would require the iPS cells to be converted to NC identity,34 and preferably to vagal and ENC identity. Techniques to achieve these higher-order specification steps are as yet not known.
It is important to use optimal methods for harvesting and maintaining ENS-competent cells. Spontaneous formation of neurosphere-like bodies, requiring one or more weeks in vitro, has been used for isolating ENS cells from dissociated gut.35, 36, 37 However, we found that spontaneously forming spheres consisted mostly of mesodermal cells, reflecting the low proportion of ENS cells in the intestinal wall. This means that spherogenesis is a slow and imperfect method for isolating ENS cells, as concluded by others.38 We obtained human ENS-derived cells rapidly by flow cytometry of dissociated and replated colon cells using the NC cell-surface marker antibody p75. This method produced NC–derived cells, shown by enrichment for NC markers detected by qRT-PCR (p75, FOXD3, SOX10, and AP2) and by immunolabeling (p75, SOX10, HNK1, GFAP, S100β, HU, TUJ1). NC markers were expressed similarly in cells isolated from freshly resected colon and in cells cultured for 1 week, suggesting that the in vitro environment was not, at least in the short term, detrimental to the maintenance of ENS cell types.
Proliferation of SOX10+ ENS cells in the mixed monolayer cell cultures was greatly increased by the GSK3 inhibitor CHIR-99021. Inhibition of the GSK3 pathway to mimic canonical Wnt signaling promotes proliferation of central nervous system progenitors in vitro.29, 30 Activation of the Wnt pathway in part mimics the signals necessary for NC cell proliferation,39 which drives colonization of the gastrointestinal tract.24 Nevertheless, more work is required to optimize the culture conditions for human ENS cells.
It is assumed that the most effective NC-derived cell for ENS reconstitution would be a progenitor cell, combining proliferative ability and multilineage differentiation potential. However, it is difficult to unequivocally assign progenitor status to p75+ sorted cells described here due to the lack of markers for bona fide ENS progenitors. For instance SOX10, present in ENS progenitor cells, remains expressed in differentiated glial cells.40 However, we were able to identify potential progenitors as SOX10+ cells that were negative for glial markers. Nevertheless, glial cells have been reported to be capable of enteric neurogenesis after wounding at least in rodents.41 Moreover, recent analyses in mice of development of the related NC-derived parasympathetic ganglia suggest that some neurons are normally derived from cells that had expressed glial markers,42, 43 while some late-originating calretinin+ ENS neurons in mice are of Schwann cell origin.16
Experiments with embryonic mice on colonization of gut by ENS progenitors show that the stage of the ENC cells and of the recipient colon are influential, and a temporal mismatch between ENC cells and colon restricts innervation.44, 45 For HSCR patients, temporal variation options are limited: only postnatal cells and tissues are available. Therefore, the study of enteric neurogenesis abilities used postnatal human ENS-derived cells and recipient colon from HSCR patients. Comparison with age-matched cells from healthy neonatal donor colons is difficult because the latter are so rarely obtainable, but it can be compared with that of equivalent postnatal ENS cells of normal rodent models.23 We investigated whether postnatal human HSCR patient ENS-derived cells could migrate into receptive gut and whether postnatal human HSCR colon tissue was permissive for ENS formation, given that these processes are normally confined to embryonic development. To address the first question, we used the embryonic quail aneural intestine. In this model, the human ENS-derived cells adopted the morphology of motile ENC cell chains, which are typical of NC migration in the embryonic gut,46 and expressed NC cell markers, but the rate of colonization of these human HSCR-derived ENS cells was slower than that of avian embryonic ENC cells. This may be because the rate of occupation is driven by proliferation,13 which may be expected to be lower in cells of older and/or human origin. However, this confirmed that p75-sorted human HSCR ENS cells are capable of enteric colonization in a permissive embryonic tissue. To answer the second question, we challenged human HSCR colon tissue with mouse embryonic ENC progenitors of known colonization ability.23 We conclude that postnatal human aneuronal HSCR colon muscle tissue is permissive of mouse embryonic ENC cell migration, proliferation, ganglion-like aggregation, ENS neuronal and glial differentiation, and neurite extension.
Previously, neonatal and adult human bowel has been dissociated and cultured in vitro to form spontaneously neurosphere-like bodies for transplantation into aneural embryonic mouse hindgut in organ culture or into postnatal mouse colon in vivo.35, 37, 47, 48 Neurons (Hu, PGP9.5) and glia (S100β, GFAP) were presumed to have arisen from the implanted human cells.35, 37, 47, 48 Human bowel-derived neurosphere-like bodies have also been cocultured with postnatal aneuronal human gut, although analysis was limited to detection of TUJ1+ fibers.36 We have confirmed32 that the aneuronal tissue contains many single TUJ1+ nerve fibers and large nerve fiber bundles that persist after in vitro culture. We also observed TUJ1+/p75+ cells that were SOX10+ and lacked neuronal morphology in aneuronal (aganglionic) HSCR colon both in vivo and in explants. We concur that this antibody does not discriminate between neurons/axons and non-neuronal cells in human ENS as clearly as it does in mouse ENS.49 Therefore, it is difficult based on TUJ1 labeling alone to unequivocally identify whether neurosphere-derived donor cells had colonized and differentiated in the human gut tissue.
We prelabeled HSCR patient ENS-derived cells with eGFP by viral transfection and by vital staining with MTR to allow detection in HSCR muscle. Because of the rapidity with which we could obtain these cells, we could implant them into aneuronal muscle explants from the same individual. We conclude that postnatal human ENS-derived cells from HSCR colon are capable of migration, proliferation, ganglion-like aggregation, and neuronal and glial differentiation in autologous aneuronal colon muscle tissues.
HSCR is genetically complex, with numerous mutations in many gene(s) expressed in ENS cells,50 most frequently the gene for the tyrosine kinase receptor RET that mediates signaling from mesoderm-derived GDNF (glial-derived neurotrophic factor). It has been argued that, without gene correction, autologous cells may therefore be poor candidates for ENS replacement therapy. Yet in our studies all patient donor cells were able to colonize autologous smooth muscle explants. In the majority of HSCR patients a large portion of the ENS is present and is functional, so an alternative argument is that in HSCR ENC cells could form ENS if they could be delivered to the region lacking ENS.
In summary, we provide strong evidence that ENS-derived cells from HSCR patients can establish ENS in aneuronal (aganglionic) bowel of the same patients. Critical to this is our demonstration that postnatal human colon tissue remains permissive for colonization by a competent ENS source. Nevertheless, we caution against overinterpretation of our conclusions as there are still quantitative and distributional hurdles to surmount before cell therapy can become practicable in HSCR, or achieve the lesser goal of improving the outcome of current surgery by reinnervating the remaining aneuronal muscular cuff.51 Generation of sufficient numbers of progenitor cells to be effective in the large postnatal aneuronal area is challenging. Even with adequate cell numbers, how to surgically transplant them to achieve coverage of the large area of HSCR colon in vivo is a problem. It is likely that the refinement of cell growth conditions combined with the use of elastic polymer substrates permissive for cell proliferation and cell delivery will assist in achieving this therapeutic goal.52
Acknowledgments
Surgery was performed by Michael Nightingale, Elizabeth McLeod, Russell Taylor, Joe Crameri and Tom Clarnette, and the authors are grateful for their interest and participation. The authors thank Duncan MacGregor, C.W. Chow, and Jessica Ng for directing the anatomic pathology analysis of specimens; Matthew Burton for assisting with flow cytometry and confocal microscopy; and Dr Vanda Lennon (Mayo Clinic) for kindly supplying the human HU antibody. The EdnrbKik mouse was generated by Hideki Enomoto and maintained by Prof. Heather Young. The authors also thank the Victorian Government for the Operational Infrastructure Support Program’s support of MCRI. Above all the authors thank the parents who allowed their children’s tissue to be used in this study.
Footnotes
Conflicts of interest The authors disclose no conflicts.
Funding This study was funded by NHMRC grant 607380 (to D.F.N. and J.M.H.), MCRI-Cell Biology Theme grants (to L.S. and B.N.R.), and generous private bequests by Graham Burke and Yvonne Spencer, and by the Federation of Chinese Associations (VIC) Inc. L.S. was sponsored by Fonds du Service de chirurgie pédiatrique et de Perfectionnement du CHUV, the SICPA Fondation, and the Société Académique Vaudoise, Lausanne, Switzerland. S.K.K. is generously supported by a Career Development Award from the Murdoch Children’s Research Institute.
References
- 1.Furness J.B. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 2.Newgreen D., Young H.M. Enteric nervous system: development and developmental disturbances—part 1. Pediatr Dev Pathol. 2002;5:224–247. doi: 10.1007/s10024-001-0142-y. [DOI] [PubMed] [Google Scholar]
- 3.Engum S.A., Grosfeld J.L. Long-term results of treatment of Hirschsprung’s disease. Semin Pediatr Surg. 2004;13:273–285. doi: 10.1053/j.sempedsurg.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 4.Furness J.B. The enteric nervous system: normal functions and enteric neuropathies. Neurogastroenterol Motil. 2008;20(Suppl 1):32–38. doi: 10.1111/j.1365-2982.2008.01094.x. [DOI] [PubMed] [Google Scholar]
- 5.Burns A.J., Pasricha P.J., Young H.M. Enteric neural crest-derived cells and neural stem cells: biology and therapeutic potential. Neurogastroenterol Motil. 2004;16(Suppl 1):3–7. doi: 10.1111/j.1743-3150.2004.00466.x. [DOI] [PubMed] [Google Scholar]
- 6.Heanue T.A., Pachnis V. Enteric nervous system development and Hirschsprung’s disease: advances in genetic and stem cell studies. Nat Rev Neurosci. 2007;8:466–479. doi: 10.1038/nrn2137. [DOI] [PubMed] [Google Scholar]
- 7.Newgreen D., Young H.M. Enteric nervous system: development and developmental disturbances—part 2. Pediatr Dev Pathol. 2002;5:329–349. doi: 10.1007/s10024-002-0002-4. [DOI] [PubMed] [Google Scholar]
- 8.Wallace A.S., Burns A.J. Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res. 2005;319:367–382. doi: 10.1007/s00441-004-1023-2. [DOI] [PubMed] [Google Scholar]
- 9.Fu M., Tam P.K., Sham M.H. Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol (Berl) 2004;208:33–41. doi: 10.1007/s00429-003-0371-0. [DOI] [PubMed] [Google Scholar]
- 10.Fu M., Chi Hang Lui V., Har Sham M. HOXB5 expression is spatially and temporarily regulated in human embryonic gut during neural crest cell colonization and differentiation of enteric neuroblasts. Dev Dyn. 2003;228:1–10. doi: 10.1002/dvdy.10350. [DOI] [PubMed] [Google Scholar]
- 11.Burns A.J., Le Douarin N.M. The sacral neural crest contributes neurons and glia to the post- umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development. 1998;125:4335–4347. doi: 10.1242/dev.125.21.4335. [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Chan A.K., Sham M.H. Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology. 2011;141:992–1002. doi: 10.1053/j.gastro.2011.06.002. e1–6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang D., Brinas I.M., Binder B.J. Neural crest regionalisation for enteric nervous system formation: implications for Hirschsprung’s disease and stem cell therapy. Dev Biol. 2010;339:280–294. doi: 10.1016/j.ydbio.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 14.Hearn C., Newgreen D. Lumbo-sacral neural crest contributes to the avian enteric nervous system independently of vagal neural crest. Dev Dyn. 2000;218:525–530. doi: 10.1002/1097-0177(200007)218:3<525::AID-DVDY1003>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 15.Burns A.J., Champeval D., Le Douarin N.M. Sacral neural crest cells colonise aganglionic hindgut in vivo but fail to compensate for lack of enteric ganglia. Dev Biol. 2000;219:30–43. doi: 10.1006/dbio.1999.9592. [DOI] [PubMed] [Google Scholar]
- 16.Uesaka T., Nagashimada M., Enomoto H. Neuronal differentiation in Schwann cell lineage underlies postnatal neurogenesis in the enteric nervous system. J Neurosci. 2015;35:9879–9888. doi: 10.1523/JNEUROSCI.1239-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Natarajan D., Grigoriou M., Marcos-Gutierrez C.V. Multipotential progenitors of the mammalian enteric nervous system capable of colonising aganglionic bowel in organ culture. Development. 1999;126:157–168. doi: 10.1242/dev.126.1.157. [DOI] [PubMed] [Google Scholar]
- 18.Bondurand N., Natarajan D., Thapar N. Neuron and glia generating progenitors of the mammalian enteric nervous system isolated from foetal and postnatal gut cultures. Development. 2003;130:6387–6400. doi: 10.1242/dev.00857. [DOI] [PubMed] [Google Scholar]
- 19.Young H.M., Hearn C.J., Farlie P.G. GDNF is a chemoattractant for enteric neural cells. Dev Biol. 2001;229:503–516. doi: 10.1006/dbio.2000.0100. [DOI] [PubMed] [Google Scholar]
- 20.Druckenbrod N.R., Epstein M.L. The pattern of neural crest advance in the cecum and colon. Dev Biol. 2005;287:125–133. doi: 10.1016/j.ydbio.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 21.Martucciello G., Brizzolara A., Favre A. Neural crest neuroblasts can colonise aganglionic and ganglionic gut in vivo. Eur J Pediatr Surg. 2007;17:34–40. doi: 10.1055/s-2007-964952. [DOI] [PubMed] [Google Scholar]
- 22.Tsai Y.H., Murakami N., Gariepy C.E. Postnatal intestinal engraftment of prospectively selected enteric neural crest stem cells in a rat model of Hirschsprung disease. Neurogastroenterol Motil. 2011;23:362–369. doi: 10.1111/j.1365-2982.2010.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotta R., Stamp L.A., Foong J.P. Transplanted progenitors generate functional enteric neurons in the postnatal colon. J Clin Invest. 2013;123:1182–1191. doi: 10.1172/JCI65963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson M.J., Zhang D.C., Mariani M. Cell proliferation drives neural crest cell invasion of the intestine. Dev Biol. 2007;302:553–568. doi: 10.1016/j.ydbio.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Bennett C.N., Ross S.E., Longo K.A. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 26.Nishiyama C., Uesaka T., Manabe T. Trans-mesenteric neural crest cells are the principal source of the colonic enteric nervous system. Nat Neurosci. 2012;15:1211–1218. doi: 10.1038/nn.3184. [DOI] [PubMed] [Google Scholar]
- 27.Allan I.J., Newgreen D.F. The origin and differentiation of enteric neurons of the intestine of the fowl embryo. Am J Anat. 1980;157:137–154. doi: 10.1002/aja.1001570203. [DOI] [PubMed] [Google Scholar]
- 28.Peterson A.J., Menheniott T.R., O’Connor L. Helicobacter pylori infection promotes methylation and silencing of trefoil factor 2, leading to gastric tumor development in mice and humans. Gastroenterology. 2010;139:2005–2017. doi: 10.1053/j.gastro.2010.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morales-Garcia J.A., Luna-Medina R., Alonso-Gil S. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci. 2012;3:963–971. doi: 10.1021/cn300110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Adachi K., Mirzadeh Z., Sakaguchi M. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25:2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 31.Kleber M., Lee H.Y., Wurdak H. Neural crest stem cell maintenance by combinatorial Wnt and BMP signaling. J Cell Biol. 2005;169:309–320. doi: 10.1083/jcb.200411095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkinson D.J., Bethell G.S., Shukla R. Isolation of enteric nervous system progenitor cells from the aganglionic gut of patients with Hirschsprung’s disease. PLoS One. 2015;10:e0125724. doi: 10.1371/journal.pone.0125724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Simkin J.E., Zhang D., Rollo B.N. Retinoic Acid upregulates ret and induces chain migration and population expansion in vagal neural crest cells to colonise the embryonic gut. PLoS One. 2013;8:e64077. doi: 10.1371/journal.pone.0064077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Denham M., Hasegawa K., Menheniott T. Multipotent caudal neural progenitors derived from human pluripotent stem cells that give rise to lineages of the central and peripheral nervous system. Stem Cells. 2015;33:1759–1770. doi: 10.1002/stem.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Metzger M., Bareiss P.M., Danker T. Expansion and differentiation of neural progenitors derived from the human adult enteric nervous system. Gastroenterology. 2009;137:2063–2073.e4. doi: 10.1053/j.gastro.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 36.Metzger M., Caldwell C., Barlow A.J. Enteric nervous system stem cells derived from human gut mucosa for the treatment of aganglionic gut disorders. Gastroenterology. 2009;136:2214–2225. doi: 10.1053/j.gastro.2009.02.048. e1–3. [DOI] [PubMed] [Google Scholar]
- 37.Almond S., Lindley R.M., Kenny S.E. Characterisation and transplantation of enteric nervous system progenitor cells. Gut. 2007;56:489–496. doi: 10.1136/gut.2006.094565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Binder E., Natarajan D., Cooper J. Enteric neurospheres are not specific to neural crest cultures: implications for neural stem cell therapies. PLoS One. 2015;10:e0119467. doi: 10.1371/journal.pone.0119467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barembaum M., Bronner-Fraser M. Early steps in neural crest specification. Semin Cell Dev Biol. 2005;16:642–646. doi: 10.1016/j.semcdb.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 40.Schreiner S., Cossais F., Fischer K. Hypomorphic Sox10 alleles reveal novel protein functions and unravel developmental differences in glial lineages. Development. 2007;134:3271–3281. doi: 10.1242/dev.003350. [DOI] [PubMed] [Google Scholar]
- 41.Laranjeira C., Sandgren K., Kessaris N. Glial cells in the mouse enteric nervous system can undergo neurogenesis in response to injury. J Clin Invest. 2011;121:3412–3424. doi: 10.1172/JCI58200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Espinosa-Medina I., Outin E., Picard C.A. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science. 2014;345:87–90. doi: 10.1126/science.1253286. [DOI] [PubMed] [Google Scholar]
- 43.Dyachuk V., Furlan A., Shahidi M.K. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science. 2014;345:82–87. doi: 10.1126/science.1253281. [DOI] [PubMed] [Google Scholar]
- 44.Druckenbrod N.R., Epstein M.L. Age-dependent changes in the gut environment restrict the invasion of the hindgut by enteric neural progenitors. Development. 2009;136:3195–3203. doi: 10.1242/dev.031302. [DOI] [PubMed] [Google Scholar]
- 45.Hotta R., Anderson R.B., Kobayashi K. Effects of tissue age, presence of neurones and endothelin-3 on the ability of enteric neurone precursors to colonize recipient gut: implications for cell-based therapies. Neurogastroenterol Motil. 2010;22 doi: 10.1111/j.1365-2982.2009.01411.x. 331–e86. [DOI] [PubMed] [Google Scholar]
- 46.Druckenbrod N.R., Epstein M.L. Behavior of enteric neural crest-derived cells varies with respect to the migratory wavefront. Dev Dyn. 2007;236:84–92. doi: 10.1002/dvdy.20974. [DOI] [PubMed] [Google Scholar]
- 47.Hetz S., Acikgoez A., Voss U. In vivo transplantation of neurosphere-like bodies derived from the human postnatal and adult enteric nervous system: a pilot study. PLoS One. 2014;9:e93605. doi: 10.1371/journal.pone.0093605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lindley R.M., Hawcutt D.B., Connell M.G. Human and mouse enteric nervous system neurosphere transplants regulate the function of aganglionic embryonic distal colon. Gastroenterology. 2008;135:205–216. doi: 10.1053/j.gastro.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 49.Badizadegan K., Thomas A.R., Nagy N. Presence of intramucosal neuroglial cells in normal and aganglionic human colon. Am J Physiol Gastrointest Liver Physiol. 2014;307:G1002–1012. doi: 10.1152/ajpgi.00164.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amiel J., Sproat-Emison E., Garcia-Barcelo M. Hirschsprung disease, associated syndromes and genetics: a review. J Med Genet. 2008;45:1–14. doi: 10.1136/jmg.2007.053959. [DOI] [PubMed] [Google Scholar]
- 51.Dickie B.H., Webb K.M., Eradi B. The problematic Soave cuff in Hirschsprung disease: Manifestations and treatment. J Pediatr Surg. 2014;49:77–81. doi: 10.1016/j.jpedsurg.2013.09.034. [DOI] [PubMed] [Google Scholar]
- 52.Xu B., Rollo B., Stamp L.A. Non-linear elasticity of core/shell spun PGS/PLLA fibers and their effect on cell proliferation. Biomaterials. 2013;34:6306–6317. doi: 10.1016/j.biomaterials.2013.05.009. [DOI] [PubMed] [Google Scholar]