Abstract
Traditionally, bio-butanol is produced with the ABE (Acetone Butanol Ethanol) process using Clostridium species to ferment sugars from biomass. However, the route is associated with some disadvantages such as low butanol yield and by-product formation (acetone and ethanol). On the other hand, butanol can be directly produced from ethanol through aldol condensation over metal oxides/ hydroxyapatite catalysts. This paper suggests that the chemical conversion route is more preferable than the ABE process, because the reaction proceeds more quickly compared to the fermentation route and fewer steps are required to get to the product.
Keywords: Bio-butanol, ABE fermentation, Chemical synthesis
1. Introduction
Butanol (ethyl alcohol) is a four-carbon alcohol which has been mainly used as a solvent, chemical intermediate, and extractant in cosmetics and pharmaceutical industries and also for the production of butyl acrylate and methacrylate [1], [2], [3]. This alcohol class is sometimes known as bio-butanol when produced biologically from fermentation of starchy and sugar feedstock. Butanol is mainly comprised of four isomeric structures, i.e. n-butanol (n-C4H9OH), sec-butanol (sec-C4H9OH), iso-butanol (iso-C4H9OH) and tert-butanol (tert-C4H9OH) [4].
In recent years, n-butanol has caught the attention of researchers as an alternative biofuel to bioethanol. Although most researchers and industries previously focused on ethanol as a fuel than butanol [60], [61], butanol could be a better direct option The straight chain alcohol (n-butanol) is considered as the next generation biofuel due to many advantages over ethanol (see Table 1), such as higher energy content, lower volatility, and does not readily adsorb moisture [1], [3], [5]. The other added advantage is carbon atoms of butanol double that of ethanol. It is known that the higher the number of carbon atoms, the more energy is contained (approximately 30% more energy) in butanol compared to ethanol [1], [62]. The high boiling point of butanol than that of ethanol causes butanol to take much longer to be burned in the motor engine than ethanol. It is also less corrosive and more suitable for distribution through existing petrol pipelines. The Reid vapor pressure of n-butanol is 7.5 times lower than that of ethanol, thus making it less evaporative/explosive [6]. If use in a blend, n-butanol can be mixed in higher ratios than ethanol with petrol for use in existing cars without the need for modification as the air-fuel ratio and energy content are closer to that of petrol [7], [8]. In addition, high octane rating makes the alcohol more suitable to be used in internal combustion engines. A fuel with lower octane rating is more prone to knocking (extremely rapid and spontaneous combustion by compression) and will lower efficiency. Knocking can also cause engine damage [9]. Unlike other alcohols, the Environmental Energy Company (US) confirmed that n-butanol can be used as a total replacement for petrol without any modifications to car engines [10].
Table 1.
Characteristics of butanol compared to ethanol [72].
| Characteristic | Ethanol | Butanol |
|---|---|---|
| Formula | C2H5OH | C4H9OH |
| Boiling point (°C) | 78 | 118 |
| Energy density (MJ Kg−1) | 26.9 | 33.1 |
| Air fuel ratio | 9.0 | 11.2 |
| Research octane number | 129 | 96 |
| Motor octane number | 102 | 78 |
| Heat of vaporization (MJ Kg−1) | 0.92 | 0.43 |
Despite all benefits associated with the use of the alcohol as a fuel, there are glitches associated with the bio-production of butanol such as low production yield and high substrate cost. Many efforts are being directed toward utilization of lower-costs substrate such as maize stover [11], agricultural waste [12] and rice straw [13], barley straw [14] and switchgrass [15]. First generation bio-butanol causes problem ranging from a negative impact on food security, increased food prices and net energy losses [16].
Other studies have focused on the utilisation of crude glycerol that is obtained as a byproduct during biodiesel production to lower the impact of biofuels production on food security [11], [17].
Search for cheaper second generation substrates, technology and other carbon sources will be required if renewable biofuels are to make more significant advantages into the world's energy portfolio. Lignocellulose is potentially the best feedstock for bio-butanol production and therefore more efficient bioconversion of cellulose and hemicellulose to be sought for economic success of the industrial production of bio-butanol [18], [19]. Research into more effective conversion of biomass material using economically feasible processes is thus of principal importance. This article is a detailed review of the current production options for n-butanol from biomass using biochemical and chemical strategies. In addition, the review discusses the history of butanol, the various synthesis mechanisms, their advantages and drawbacks and also the future in the current bio-butanol industry on the global scenario.
2. History of bio-butanol production from biomass
The acetone butanol ethanol (ABE) fermentation was discovered by a French Microbiologist known as Louis Pasteur in 1861. Restoring of research in butanol became alive again as large quantities of acetone were demanded in World War I when [20] filed a patent for the process which was tested in the United Kingdom (UK). However, there was a need for an organism that could increase acetone production. Strange and Chaim Weizmann succeeded in isolating Clostridium acetobutylicumin from a garden soil for ABE fermentation that could produce large amounts of acetone [21].
During World War I, it was important to produce high quantities of acetone yield since in Britain it was used as an essential chemical for the production of cordite that was used as an alternative to gunpowder during World War I. With the increasing demand for butanol after the war, the first large-scale industrial plants were created in Canada and USA. After 1936 a number of ABE production industries were established in Soviet Union, Japan, China, South Africa, and Egypt. By 1945, during the start of the Second World War, Japan commenced the production of butanol from sugar plants mainly as fuel for airplanes [22].
In the 1950s, a petrochemical route for n-butanol production emerged. The process was mostly based on aldol condensation of acetaldehydes, followed later by dehydration and then hydrogenation of crotonaldehyde. With this fast-growing industrial discovery also known as the Oxo synthesis, the fermentation processes were abandoned [23]. For example, by the 1960s, most of the industrial ABE fermentation facilities were closed due to cheap oil prices that favored the chemical production route. The last factory was closed in 1986 in South Africa. The chemical route did not last for a long time until rise in crude oil prices when industrial ABE fermentation facilities started to emerge again in China and Brazil. To date, many plants have been established in several locations globally including the USA, Slovakia, France and UK where bio-butanol is produced for use a fuel.
The n-butanol (as biofuel) application was demonstrated by running an old Buick on pure n-butanol in the year 2005. The reported fuel consumption increase was 9% higher than traditional petrol [24]. Regardless of the increase, emissions of carbon monoxide (CO), hydrocarbons, and nitrogen oxides (NOx) were substantially reduced; due to the positive characteristic effects that butanol has on the environment. This trend is regarded as a positive impact towards the environment. This resulted the Fuel Butyl Company to increase the capacity to produce 10 L of n-butanol per 25 kg of maize. A year after 2005, two major global players, BP and DuPont, also announced plans for manufacturing plants to produce n-butanol through fermentation process. The first new commercial plant was built by BP in Saltend (UK) with a capacity to produce 420 million L of n-butanol through fermentation [24], [62].
3. Bio-chemical production route
The most attractive routes for production of bio-butanol are through fermentation of sugar [25] glycerol [26] or lignocellulose [27] feedstock in the presence of different micro-organisms from the Clostridiaceae family. Acetone–butanol–ethanol (ABE) fermentation has many benefits since it largely depends on the availability of inexpensive and abundant raw materials. Bio-butanol fermentation from biodiesel derived glycerol is also considered as an alternative route, since it uses waste products from biodiesel production. The renewed interest of butanol production has not only highlighted its use as a chemical, but also as an alternative biofuel, due to a rise in oil prices [28], [3].
3.1. Metabolic pathway-ABE fermentation
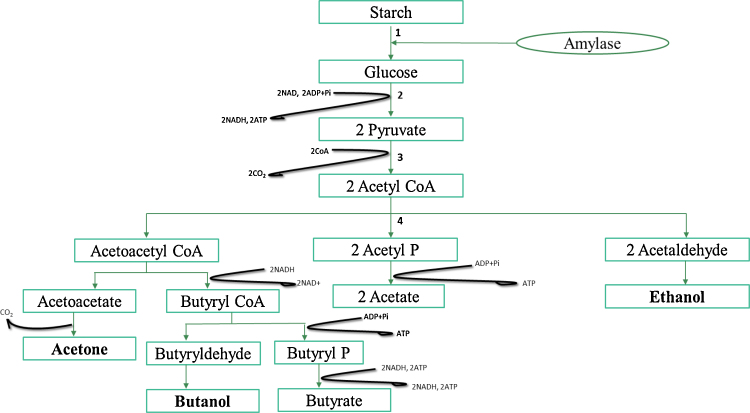
The metabolic pathway utilizes glucose derived from the hydrolysis of carbohydrates (Fig. 1, step 1) that is broken down by amylase enzyme to form fatty acids, and solvents by C. acetobutylicum via anaerobic fermentation [22], [29]. The mechanism of ABE has been elucidated, carbon from carbohydrates in form of pentose and hexose sugars (mono-, di- tri-, and polysaccharides) are metabolized via the Embden–Meyerhof pathway (Step 2) to pyruvate. The degradation of 1 mole of sugar leads up to 2 moles pyruvate with a net formation of 2 moles of adenosine triphosphate (ATP) and 2 moles of nicotinamide adenine dinucleotide (NADH).
Figure 1.
Biochemical pathway of ABE fermentation [24].
Pyruvate is further converted to acetyl-CoA and Carbon dioxide (CO2) (Fig. 1, step 3). Thereafter, step 4 shows acetyl CoA being converted to other intermediates (acetaldehyde, butyraldehyde) which ultimately lead to oxidized products (acetone, acetate), and/ reduced products such as butanol and ethanol. The first occurrence of intermediates and acid formation is known as acidogenesis, and this occurs under specific growth conditions, such as pH values >5 and iron limitation. Therefore, ATP is continuously generated during this process [5].
During the accumulations of organic acids (Acidogenesis), the culture pH reduces, due to the metabolic switch of C. acetobutylicum from acidogenesis to solvontogenesis [1], [24]. Organic acids are utilized for the formation of solvents (Solvontogenesis). The second phase of ABE fermentation is solventogenesis, a stage during which acids are re-assimilated to produce acetone, butanol, ethanol, acetic acid, butyric acid, hydrogen, and carbon dioxide as the main products. This stage of fermentation occurs frequently in various bacterial strains of Clostridia species utilizing a wide range of carbon sources such as starch. An abundant and cheaper feedstock investigated and identified as alternative substrates for butanol production via ABE fermentation is lignocellulosic biomass, which includes agricultural residues namely maize fiber, wheat straw and switchgrass [30], [73], [74].
Acetate and butyrate are assimilated to their corresponding CoA derivatives catalyzed by the acetoacetyl-CoA:acyl-CoA transferase, with acetoacetyl-CoA as the CoA donor. The utilization of acetate and butyrate occurs via the acetoacetyl-CoA:acetate/butyrate:CoA transferase (CoAT) pathway with the formation of acetone. Butyryl-CoA converts to butaraldehydes, and finally to ethanol and butanol. Meanwhile, acotoacetyl-CoA converts to acetone and acetaldehydes to ethanol [31], [32], [74].
3.2. Typical feedstock for butanol production
Bio-butanol is usually produced from food crop biomass which are first generation feedstock,nevertheless second generation feedstock which are not considered for human consumption are preferred. Second generation feedstock are desired in ABE fermentation due to the fact that they are non-food and do not compete for land use. Therefore, these new methods that can make use of a greater proportion of plant material are considered to have long-term economic advantages. Research is also currently in progress for third generation feedstock, but not much literature has been found on the third generation feedstock.
Bio-butanol can be produced by a variety of different micro-organisms from the Clostridiaceae bacterial family. Clostridia are well-known to produce several products which cannot be achieved by use of chemical synthesis [33]. Clostridia are rod-shaped, spore-forming gram-positive bacteria and very strict anaerobes. The yield of butanol varies, depending on the type of biomass and bacteria used, and different parameters are relevant. The forthcoming section shows a summary and understanding of literature that has been previously found on butanol production by different clostridium species and biomass, thereof.
3.2.1. First generation bio-butanol
First-generation bio-butanol requires a relatively simple process to be produced mostly afforded by fermentation of mostly hexose sugars. These sugars are derived through hydrolysis of starch-rich crops such as maize, wheat, rice and cassava. Prior to use, the raw materials (grains) are usually hydrolysed into dextrose, which can be subsequently bio-converted into glucose using glucoamylase enzyme. Several researchers have demonstrated that first generation butanol production can be achieved at significantly high yields. Table 2 shows the literature associated with different types of feedstock for first generation bio-butanol, the various micro-organisms used and their product yields.
Table 2.
Examples of first generation biomass used in butanol fermentation.
| First generation feedstock | Fermentation conditions (T °C and pH) | Clostridium species | Yields |
Productivity (g L−1 h−1) | Product distribution | Reference | |
|---|---|---|---|---|---|---|---|
| g L−1 | g g−1 | ||||||
| Cassava starch | 37 and 5 |
C.beijerincki tyrobutyricum |
6.66 | 0.18 | 0.96 | Butanol | [34] |
| Glucose | 37 and over 4 | C.acetobutylicum CICC 8012 | 0.13 | Butanol | [25] | ||
| Cassava flour | 37 and pH controlled | C.acetobutylicum DP 217 | 574.3 | 0.76 | ABE | [35] | |
| Oil palm sap | 37 and 6 | C.acetobutylicum DSM 1731 | 14.4 | 0.35 | Butanol | [36] | |
| Maize meal | 37 and 6 | C. beijerinckii, BA101 | 26 | Acetone and Butanol | [33] | ||
Li et al. [34], investigated co-cultures of Clostridium beijerinckii and Clostridium tyrobutyricum to enhance butanol production yield. The experiments were conducted using glucose, cassava starch, or cane molasses. Fibrous-bed bioreactor (FBB) was used for immobilized fermentation. According to the findings of the study, two-strain co-culture for butanol production yields and volumetric productivity that increased compared to using a single culture. The reported butanol production was 6.66 g L−1, yield and productivity 0.18 g g−1 and 0.96 g L−1 h−1, respectively, when using cassava as a starting material. Acetone–butanol–ethanol (ABE) yield of 0.36 g g−1 was produced. The study confirms that co-culture fermentation could enhance butanol productivity.
According to [25], glucose as a starting material produced 0.14 and 0.13 g L−1 h−1 butanol using Clostridium acetobutylicum strain CICC 8012. Fermentation was monitored under slight pressure on a continuous and closed-circulating fermentation (CCCF) system. Fermentation conditions were 37 °C, and the pH value was adjusted to over 4. As fermentation proceeded, glucose concentration was maintained at about of 30 g L−1 by adding it every 2 h of fermentation. The study indicated that pressures have no effect on Clostridium acetobutylicum strain CICC 8012 performance during fermentation.
Production of acetone–butanol–ethanol (ABE) from cassava was investigated by [35]. Fermentation was done using pervaporation (PV) coupled process. ABE products were in situ removed from fermentation broth to alleviate the toxicity of solvent to the Clostridium acetobutylicum DP217. Compared to the batch fermentation without PV, glucose consumption rate and solvent productivity increased by 15% and 21%, respectively, in batch fermentation–PV coupled process, while in continuous fermentation–PV coupled process running for 304 h, the substrate consumption rate, solvent productivity and yield increased by 58%, 81% and 15%, reaching 2.02 g L−1 h−1, 0.76 g L−1 h−1, and 0.38 g g−1, respectively. After phase separation, a final product containing 574.3 g L−1 ABE with 501.1 g/L butanol was obtained. Therefore, the results from the study proved that fermentation–PV coupled process has the potential to decrease the cost in ABE production.
Komonkiat et al. [36], conducted a study using a sap of palm oil to produce biobutanol. The findings gave an indication that palm oil could be used as a feedstock as it was effective for high butanol yield without any nutrient supplementation. An amount of 14.4 g L−1 butanol was obtained from the sap which contained sugar concentration of 50 g L−1 with butanol yield of 0.35 g g−1.
Ezeji et al. [33], tested liquefied maize meal for acetone–butanol production. Fermentation was done using hyper amylolytic C. beijerinckii BA101. C. beijerinckii has the ability to utilize starch and accumulate higher butanol concentrations of 17–21 g L−1 in the medium. Batch fermentation was used, and solvent (acetone and butanol) recovery of 26 g L−1 was obtained. The results show that maize meal is an effected starch biomass to yield butanol using a batch scale in the presence of C. beijerinckii BA101.
3.2.2. Second generation bio-butanol
Biofuels from different agricultural residues and part of the plant biomass are often termed second generation because the fuels are derived from feedstock that are non-edible residues of food crop production or non-edible plant biomass (e.g. grasses, trees and energy crops). The main advantage of the production of second-generation biofuels is that there is no competition with food-feed chain and their availability is diverse. Lignocellulosic materials are associated with low cost, sufficiently abundance and usually generate low net greenhouse emissions, thus must be ideal precursors to produce biofuels. Second generation liquid biofuels are generally produced by two fundamentally different approaches i.e. biological or thermochemical processing, due to their structural complexity. Table 3 reveals literature on some of different second generation biomass that is currently used to produce butanol, and the corresponding clostridium bacteria strains.
Table 3.
Examples of second generation biomass used in butanol fermentation.
| Fermentation conditions (T°C and pH) | Clostridium species | Yields |
Productivity (g L−1 h−1) | Product distribution | References | ||
|---|---|---|---|---|---|---|---|
| g L−1 | g g−1 | ||||||
| Barley liquor silage | 37 and 6.5 | C. acetobutylicum DSM 1731 | 9.0 | ABE | [27] | ||
| Glycerol | 37 and over 6.5 | C. acetobutylicum KF 158795 | 13.57 | Butanol | [26] | ||
| Rice straw | 37 and 6.7 | C. sporogenes BE01 | 5.52 | Butanol | [37] | ||
| Oil palm trunk fiber | 37 and 6 | C. beijerinckii TISTR 1461 | 10 | 0.41 | Butanol | [36] | |
| Crude cellulose | 37 and 6.7 | C. acetobutylicum DP 217 | 0.33 | Butanol | [38] | ||
| Spoilage palm fruits | 30 and 6 | C. acetobutylicum ATCC 824 and Bacillus subtilis DSM 4451 | 21.56 | 0.42 | 0.30 | ABE | [39] |
Several research attempts have been developed to utilize second generation biomass with appropriate fermenting bacteria to yield higher butanol. Studies developed a C. acetobutylicum strain that can directly utilize cellulose as feedstock [3], [32]. There is evidence that C. acetobutylicum ATCC 824 contains a cellulosome, that is, a cellulose-degrading multi-enzyme complex consisting of several catalytic components surrounding a scaffold protein. In an effort to make C. acetobutylicum utilize cellulose directly, the cellulase gene from C. cellulovorans or the gene encoding the scaffold protein from C. cellulolyticum and C. thermocellum were introduced into C. acetobutylicum [32].
Although the gene has been identified, more studies still need to be done for the characterization of the existing cellulase gene cluster in C. acetobutylicum before further metabolic engineering. As a result this will further increase feasibility of lignocellulosic material usage compared to first generation biofuels. The production of second-generation biofuel requires most sophisticated processing production equipment, more investment per unit of production and larger-scale facilities. To achieve the potential energy and economic outcome of second-generation biofuels, further research, development and application are required on feedstock production and conversion technologies. The future production of ethanol is expected to include both the use of traditional grain/sugar crops and lignocellulosic biomass feedstock [26].
Various studies have been conducted using different second generation feedstock. Batch fermentations of barley silage liquor which were supplemented with gelatinised barley grain, the results produced good fermentability butanol yields of 0.20, 0.17 and ABE yields of 0.28, 0.26 g g−1 monosaccharide were obtained [27]. The study showed that starch in silage could be a possible replacement of media components that provide the nutrients for butanol fermentation.
Trunk fiber was utilised as one of the second generation feedstock types [36]. The feedstock was firstly, hydrolysed to fermentable sugars prior to fermentation. A number of clostridia strains were screened, and Clostridium beijerinckii TISTR was the most suitable organism for utilizing sugars from hydrolysed trunk fiber with highest amount of 10 g L−1 butanol concentration and butanol yield of 0.41 g g−1. The results presented herein suggest that oil palm trunk is a promising renewable substrate for bio-butanol. A study conducted by [40] showed that sweet sorghum bagasse (SSB) hydrolysate, produced 12.3 ± 0.1 g L−1 butanol. The results were only obtained after pervaporation membrane was used for sweet sorghum bagasse detoxification.
Gottumukkala et al. [37] used an acid hydrolysed rice straw for bio-butanol production. Clostridium sporogenes BE01 was used a fermenting bacterium. This strain gave a butanol yield of 3.43 g L−1 and a total solvent yield of 5.32 g L−1. After the conditions of ABE fermentation enhanced, biobutanol reached 5.52 g/L. The study also showed biomass potential on bio-butanol production.
Yadav et al. [26] used glycerol as feedstock for bio-butanol production. Twenty anaerobic bacteria were screed for biomass conversion. Among the twenty, Clostridium acetobutylicum KF158795 was found to be the most effective bacterium in producing bio-butanol. Results showed that the strain was able to utilize glycerol as a sole carbon source to produce 1.4 g/L of butanol. After optimization, production increased to 13.57 g L−1 of butanol. [38] also conducted research on crude cellulose as a starting material for bio-butanol production using Clostridium acetobutylicum. The findings were 0.33 g g−1 butanol yield. Various researchers have shown the use of Clostridium acetobutylicum as a suitable strain for bio-butanol production, and the study also supports the literature.
Spoilage date palm fruits were used as substrate for ABE production by mixed culture of Clostridium acetobutylicum ATCC 824 and Bacillus subtilis DSM 4451. B. The total ABE production of 21.56 g L−1 was achieved from the date fruits. The productivity and yield obtained was 0.30 g.L−1 h−1 0.42 g g−1, respectively [39].
Marchal et al. [41] used maize cobs to produce an ABE yield of 0.96 g L−1 h−1 in a 48 m3 reactor. Fed-batch and continuous fermentation techniques have been developed in addition to conventional batch fermentation to utilize concentrated substrates and eliminate downtime, thereby reducing the reactor size and capital cost with enhanced reactor productivity. Cell immobilization with bone char, brick, and cotton towels as supporting materials has also been applied in ABE fermentation to achieve high cell density and reactor productivity
Khanna et al. [17b] used biodiesel derived crude glycerol as a feedstock to produce n-butanol with a maximum yield of 5 g L−1 h−1 butanol via an anaerobic fermentation pathway, using immobilized Clostridium pasteurianum cells. This was the first report to apply immobilized cells to convert glycerol to butanol. A mutant strain of C. pasteurianum which can tolerate high concentrations of crude glycerol was developed and reported a maximum glycerol utilization rate of 7.59 g L−1 h−1 and a butanol productivity of 1.80 g L−1 h−1.
With the detailed literature reports on first and second generation bio-butanol and stringent food regulations, there are still drawbacks associated with the feedstock. This is ascribed to demand of large space for cultivation, processing and storing of second generation during harvest season can be a challenge. However, the disadvantages can be overcome by third generation biomass as an alternative resource for bio-butanol which can produce large quantity of biomass on much smaller areas.
3.2.3. Third generation bio-butanol
Algae has increasingly became one of the promising feedstock, arising from its vast availability. It is catagorised as a third generation feedstock. A few reports on bio-butanol production from algae. Reason for this, most species have high oil content of approximately 50% [63], and that makes it suitable for biodiesel production. The remaining green waste which left after oil extraction can be further used for the production of bio-butanol. The two distinct types of algae is microalgae and macroalgae. Microalgae is made of unicellular organisms which are classified as microscopic. The latter, contains multiple cells with a structure like a plant with roots, stems and leaves. They are categorized into i.e. red, green and brown, depending on their pigmentation. The specific characteristics of macroalgae are; lower protein and lipid content, but higher carbohydrates content compared to microalgae [64]. Most research reports [65], [66], [67], [68] have mainly focused on microalgae over macro-algae, e.g. seaweed [63], [69].
Castro et al. [65], first optimized acid hydrolysis of mixed microalgae for sugar release. The sugars were subsequently fermented to produce acetone, butanol, and ethanol (ABE) by Clostridium saccharoperbutylacetonicum N1-4. The findings provided an optimal sugar yield of 166.1 g kg of dry algae, with concentrations of 3.74 g/L butanol. [70], also conducted acetone, butanol, and ethanol (ABE) fermentation by C. saccharoperbutylacetonicum N1-4 using wastewater algae. The higher ABE yield of 0.311 g g−1 and volumetric productivity of 0.102 g L−1 h−1 were obtained when enzyme supplementation on the biomass was done. It was concluded that the use of wastewater algae to produce industrial solvents which are of high value could have substantial implications in terms of economic use. The study showed that the use of organic waste is an alternative method of utilizing almost 100% of the biomass. The additional [66] conducted research on the use of microalgae biodiesel residues using C. acetobutylicum produced 3.86 g/L of butanol with the yield of 0.13 g/g-carbohydrate during ABE fermentation. This observation confirmed that bio-butanol production from microalgae biodiesel residues is attainable. However, advance research on fermentation approaches are required to improve bio-butanol yield.
Van der Wal et al. [67], conducted research on the application of potential green seaweed Ulva lactuca for the production of acetone, ethanol and ethanol. Two clostridium species were used i.e. C. acetobutylicum and C. beijerinckii. According to the findings, C. beijerinckii was the organism capable of producing ABE of 0.35 g g−1 while C. acetobutylicum produced mainly organic acids (acetic and butyric acids). These results demonstrate the great potential use of U. lactuca as feedstock for fermentation. On the other hand, [69], reported potential feedstocks to produce bio-butanol from a macroalgae. They found that it can produce approximately 4 g L−1 butanol by C. beijerinckii and C. saccharoperbutylacetonicum, with C. beijerinckii being the highest butanol producing organism.
The topic of algal conversion to bio-butanol is not new, thorough research has not been done on the different algal feedstock. Table 4, ascertains the different feedstock (micro algae and macroalgae) for bio-butanol production, conditions used, and the amounts produced thereof.
Table 4.
Examples of third generation biomass used in butanol fermentation.
| Fermentation conditions (T °C and pH) | Clostridium species | Yields |
Productivity (g L−1 h−1) | Product distribution | References | ||
|---|---|---|---|---|---|---|---|
| g L−1 | g g−1 | ||||||
| Mixed microalgae | 35 and 6.5 | C. Saccharoperbutylacetonicum N1-4 | 3.74 | Butanol | [65] | ||
| Wastewater algae | 35 and over 6.5 | C. Saccharoperbutylacetonicum N1-4 | 9.74 | 0.311 | 0.102 | ABE | [70] |
| Micro algae biodiesel residues | 37 and 6 | C. acetobutylicum ATCC 824 | 0.13 | Butanol | [66] | ||
| Green seaweed (Ulva lactuca) | 37 and 6.0–6.4 | C. beijerinckii NCIMB 8052 | 0.35 | ABE | [67] | ||
| Macroalgae | 37 and 6 | C. beijerinckii ATCC 35702 | 4 | Butanol | [69] | ||
This section reveals a number of studies which have been conducted on different biomass and Clostridium species for bio-butanol production. The results reported are showing different bio-butanol yields. Therefore, diversity on bio-butanol production has been shown. However, there are still setbacks on technology discoveries, and inhibitions on optimization of the existing bio-butanol production process (fermentation).
3.3. Inhibitions and other problems associated with ABE fermentation
Although a number of feedstock and microorganisms have successfully been utilized to produce bio-butanol, there are numerous drawbacks associated with ABE fermentation which hinders it to compete economically with petrochemical synthesis. Some of the identified limitations of the ABE based on the process aspect are as follows:
-
1.
Low final butanol concentration (<20 g L−1) caused by inhibition during fermentation,
-
2.
Low yield of butanol due to hetero-fermentation (0.28–0.33 g g−1),
-
3.
High cost of butanol recovery from low-concentration yields [31].
Not only is inhibition a major challenge for the ABE fermentation, research has also shown that lack of feedstock biomass alternatives is widespread. Current ABE fermentation depends on very expensive feedstock and efforts must be directed towards decreasing substrate costs and several other drawbacks. Rational approaches and advanced knowledge necessary for the effective strategies to limit the inhibitors whilst improving the yields of the products of ABE fermentation are needed.
4. Chemical synthesis of n-butanol from ethanol
As the interests in bio-butanol keeps on growing, there is also a rising attention on the use of chemical routes to produce n-butanol. Since the use of fermentation still faces significant technical and economic challenges such as finding efficient microorganisms to convert fermentable sugars to bio-butanol, therefore it is still mandatory to find other alternate routes. On the other hand, the chemical route usually involves simple step in the presence of catalysts which are employed to achieve suitable ethanol conversion into n-butanol at relatively higher yield and conversions. The primary advantage of chemical route is that only one-step is required for producing n-butanol from ethanol, whereas bio-chemical route may involve several steps. Chemical processes for n-butanol are wide-spread and have been well documented [42], [23].
4.1. Reaction mechanism of n-butanol formation from ethanol
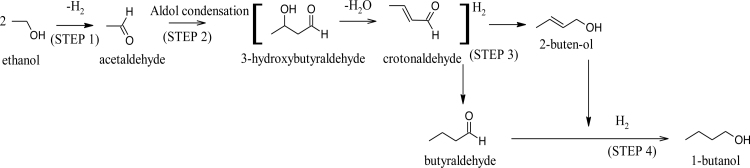
There are three consecutive reactions associated with the conversion of n-butanol from ethanol. The first liquid phase reaction is the dehydrogenation of ethanol to form acetaldehydes [43], followed by aldol condensation of acetaldehyde [44] and then hydrogenation to n-butanol (see Fig. 2). Ndou et al. [45] and Ueda et al. [46] proposed a mechanism for the gas phase reaction of ethanol over a zeolite catalyst to synthesize butanol. According to [23] gas phase reaction does not involve the formation of intermediates such as acetaldehydes and crotonaldehydes. To date, few reports are made on the gas phase reaction formation of n-butanol and the reaction mechanism. Ethanol to n-butanol conversion is an important industrial process which has been used to increase the carbon number of alcohols by coupling two molecules. Aldol condensation was first studied by Marcel Guerbet in the 1890s, and it is widely known as Guebert reaction [23]. Although this method is rarely utilized nowadays, it may again become significant in the future [71].
Figure 2.
The reaction mechanism for production of n-butanol from ethanol in a liquid phase [42].
4.1.1. Dehydrogenation
Dehydrogenation of ethanol allows the removal of hydrogen in a chemical reaction. In this instance, ethanol is mostly used to synthesize ethyl acetate and acetaldehydes. [43] demonstrated that dehydrogenation is of outmost importance in industrial process to produce fine chemicals, petrochemicals, and oleochemicals. In Scheme 1, the first step (labelled as step 1) presents dehydrogenation of a molecule and followed by Adol condensation in the presence heterogeneous catalysts to produce a desired product [47].
4.1.2. Aldol condensation
Juben et al. [44] described aldol condensation as well-known phenomenon used to produce a β-hydroxyaldehyde or β-hydroxyketone. This occurs in an organic reaction containing enol or an enolate ion that reacts with a carbonyl compound and followed by dehydration to give a desired product. Aldol condensation is one of the important organic synthesis method with advantage to form carbon–carbon bonds. Guerbet reaction is one of aldol condensation types, whereby an in-situ aldehyde is formed from an alcohol and then self-condenses to the dimerized alcohol [48].
4.1.3. Hydrogenation
The subsequent step after aldol-condensation is hydrogenation of aldol-adducts to increase their solubility in the aqueous phase. In addition, selective hydrogenation of the furan ring in HMF and furfural can lead to additional carbonyl-containing compounds that can undergo aldol self-condensation to form heavier alkanes. Thermodynamic considerations favor hydrogenation of the C C bond over the C O bond for hydrogenation reactions involving unsaturated aldehydes Reaction kinetics considerations also favour hydrogenation of the C C bond over the C O bond for small molecules. Whereas steric constraints for larger molecules decrease the rates for hydrogenation of C C bonds. Accordingly, the C C bonds of furfural are less reactive than the C O bond, probably due to steric effects, making the production of tetrahydrofurfural (THF2A) by hydrogenation of furfural difficult [44].
The chemical approach is improving by the ongoing research to develop appropriate catalyst, or pair of catalysts, for the hydroformylation of alkenes to form aldehydes and the subsequent hydrogenation of the aldehydes to produce alcohols [49].
4.2. Catalysts used for n-butanol synthesis
Several researchers, to name few [50], [51], [52] have devoted their work on a quest to discovering suitable catalysts which would be able to produce high yields of n-butanol (see Table 5). These new technologies are compared to that of existing biological technologies in order to understand the two approaches. Table 5 presents several catalysts which have been previously used to produce n-butanol with ethanol as a feedstock.
Table 5.
Examples of few catalysts and conditions studied in the literature.
| Catalyst loading |
Catalytic test temperature (°C) | n-Butanol selectivity (%) | References | ||
|---|---|---|---|---|---|
| mg | wt% | ||||
| Mg and Al mixed oxides | 300 | 500 | >20 | [50] | |
| ZrO2-supported Cu | 500 | 300 | ≥10 | [52] | |
| Ni/Al2O3 | 10–50 | 250 | 80 | [53] | |
| Hydroxyapatite | 350–450 | 50 | [54] | ||
| Na to ZrO2 | 1 | 340–400 | – | [55] | |
| Ni/C-alumina | 3000–3500 | 250 | 62 | [56] | |
| Co2+ + / Ca2+ hydroxyapatite |
1.35 | 120–240 | – | [57] | |
| MgO | 500 | 450 | – | [44] | |
Researches have been conducted on the direct conversion of ethanol to n-butanol. Different findings have been observed with different suitable conditions of the catalytic reaction. [50] studied Mg and Al mixed oxides for the conversion of ethanol to n-butanol. The results showed butanol selectivity over 20%. The results proved that Mg and Al can be used as a suitable combination for butanol production. However the findings were not as promising as the other studies conducted by other researchers. [52] used ZrO2-supported Cu catalysts with different Cu content (5–30 wt%). The final observations of the study provided the highest selectivity with Cu content ≥10%. [53] studied direct catalytic one-pot valorization of bio-ethanol to 1-butanol over different alumina supported catalyst. n-butanol selectivity was found to be 80%.
Scalbert et al. [54] investigated a commercial hydroxyapatite catalyst, and 50% selectivity of butanol was formed. Kozlowski et al. [55] studied the influence of adding sodium to zirconia on the acid-base properties of the surface and on the catalytic conversion of ethanol. According to the study, an overall selectivity of ethanol to butanol was improved when using 1.0 wt% Na/ZrO2. [56] performed a continuous process of ethano to butanol conversion. Approximately 62% -butanol selectivity was found. [57] used a series of exchanged cobalt/calcium (Co2+ + /Ca2+). According to [45], modified MgO was able to convert ethanol into 1-butanol.
Hydroxapatite based catalysts have been proven to show high selectivity towards n-butanol, but suffer from deactivation by high molecular weight by-products that form during the reaction. Metal oxide based catalysts deactivates slower, but suffer from low selectivity. [58] showed that magnesium (Mg) and aluminum (Al) mixed oxides catalysts with a high sensitivity of strong basic sites are the most selective to n-butanol. The same authors also observed a very high selectivity to ethylene, which is associated with the presence of acid sites on the surface of these mixed oxides. [59] also verified that the selectivity to n-butanol increases when the density of strong basic sites increases as was observed when Cu–Mg–Al-based catalysts were used. It was concluded that the formation of n-butanol requires both basic and acid sites. According to some researchers [50], a specific surface atomic arrangement composed of two acid sites and one basic site is needed in order to adsorb the acetaldehyde molecules during the aldol condensation step.The use of various heterogeneous catalysts for ethanol conversion to n-butanol has been extensively researched by the aforementioned studies. However, the studies are limited to the evaluation of catalyst performance, and they give less information on the use of these catalysts at a commercial scale. In developing countries such as South Africa, butanol production has not been well documented. It is necessary to evaluate all the possible technologies which can aid in fuel production to boost the country's economy. This will also allow the comparison of different processes and their impacts on the entire production process, which would be much harder to achieve in an experimental scale.
5. Concluding remarks
5.1. Bio-chemical derived butanol
Bio-butanol has the potential to provide a new generation energy that can offer many attractive features for transportation fuel. However, the acetone butanol ethanol (ABE) fermentation process has a number of limitations. This makes it easier for other processes such aldol condensation which has been discussed in the current literature to take the lead as one of the promising technologies. Currently researchers are undertaking various studies on the development of butanol fermentation, such as Clostridium genetic manipulation and feedstock investigation which can lower fermentation constraints. Lignocellulose feedstock is one of the cheaper biomass that is being currently studied, and has been reported as a sugar source for fermentation of ABE. In the current paper, a promising approach after ABE fermentation for butanol production has been discussed.
5.2. Chemical derived butanol
Chemical butanol production research mainly from ethanol as a substrate has been conducted. The process has been known for over a century, but it was not studied extensively. However, due to renewed interest of biofuels, many researchers are investigating potential catalysts which can lead to higher butanol selectivity. The process only involves the addition of the catalyst to hydroformulation to form a high carbon atom alcohol (butanol). Many reports regard the process more advantageous than ABE fermentation as it takes lesser time than the ABE process.
Overall, butanol offers a great promise as petrol substitute. The primary reason is that butanol is a pure alcohol with longer hydrocarbon chain, making it nonpolar, with an energy content similar to that of petrol and, hence, can be directly used as fuel in existing internal combustion engines by either blending or using it in pure form. Therefore, with all the literature key points stressed in this paper, showing the sustainability of considering bio-butanol as a fuel, it is vital to use this type of fuel in the near future
Acknowledgements
Authors are thankful to the South African Research Chairs Initiative of the Department of Science and Technology and National Research Foundation (NRF) of South Africa.
References
- 1.Durre P. Biobutanol: an attractive biofuel. Biotechnol. J. 2007;2:1525–1534. doi: 10.1002/biot.200700168. [DOI] [PubMed] [Google Scholar]
- 2.García V., Päkkiläa J., Ojamo H., Muurinena E., Keiski R.L. Challenges in biobutanol production: how to improve the efficiency? Renew. Sustain. Energy Rev. 2011;15:964–980. [Google Scholar]
- 3.Lee S.Y., Park J.H., Jang S.H., Nielsen L.K., Kim J., Jung K.S. Fermentative butanol production by Clostridia. Biotechnol. Bioeng. 2008;101:209–228. doi: 10.1002/bit.22003. [DOI] [PubMed] [Google Scholar]
- 4.Grana R., Frassoldati A., Faravelli T., Niemann U., Ranzi E., Seiser R., Cattolica R., Seshadri K. An experimental and kinetic modeling study of combustion of isomers of butanol. Combust. Flame. 2010;157:2137–2154. [Google Scholar]
- 5.Nigam P.S., Singh A. Production of liquid biofuels from renewable resources. Prog. Energy Combust. Sci. 2011;37:52–68. [Google Scholar]
- 6.Morone A., Pandey R.A. Lignocellulosic biobutanol production: gridlocks and potential remedies. Renew. Sustain. Energy Rev. 2014;37:21–35. [Google Scholar]
- 7.Sarathy S., Vranckx S., Yasunanga K., Mehl M., Osswald P., Metcalfe W.K., Westbrook C.K., Pitz W.J., Kohse-Hoingaus K., Fernandes R.X., Curran H.J. A comprehensive chemical kinetic combustion model for the four butanol isomers. Combust. Flame. 2012;159:2028–2055. [Google Scholar]
- 8.Campos-Fernandez J., Arnal J.M., Gomez J., Porado M.P. A comparison of higher alcohols/diesel fuel blends in a diesel engine. Appl. Energy. 2012;95:267–275. [Google Scholar]
- 9.Rudloff J., Zaccardi J.M., Richard S., Anderlohr J.M. Analysis of pre-ignition in highly charged SI engines: emphasis on the auto-ignition mode. Proceed. Combust. Inst. 2013;34:2959–2967. [Google Scholar]
- 10.Brekke K. Butanol: an energy alternative? Ethan. Today. 2007:36–39. [Google Scholar]
- 11.He Q., Chen H. Improved efficiency of butanol production by absorbed lignocellulose. Ferm. J. Biosci. Bioeng. 2013;115:298–302. doi: 10.1016/j.jbiosc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 12.(a) Cheng C., Che P., Chen B., Lee W., Lin C.Y., Chang J. Biobutanol production from agricultural waste by an acclimated mixed bacterial microflora. Appl. Energy. 2012;100:3–9. [Google Scholar]; (b) Cheng C.L., Che P.Y., Chen B.Y., Lee W.J., Chien L.J., Chang J.S. High yield biobutanol production by solvent-producing bacterial microflora. Bioresour. Technol. 2012;113:58–64. doi: 10.1016/j.biortech.2011.12.133. [DOI] [PubMed] [Google Scholar]
- 13.Ranjan A., Mayank R., Moholkar V.S. Process optimization for butanol production from developed rice straw hydrolysate using Clostridium acetobutylicum MTCC481 strain. Biomass Conv. Bioref. 2013;3:143–155. [Google Scholar]
- 14.Qureshi N., Liu S., Ezeji T.C. Cellulosic butanol production from agricultural biomass and residues: recent advances in technology. Adv. Biofuels Bioprod. 2013:247–265. [Google Scholar]
- 15.Jain A., Hammonds R.E., kerrigan J.L., Henson J.M. Characterization of trichoderma atroviride strain isolated from switchgrass bales and its use to saccharify ammonia-pretreated switchgrass for biobutanol production. Biomass Bioenergy. 2014;64:299–308. [Google Scholar]
- 16.Martin A. First generation biofuels compete. New biotechnol. 2010;27:577–607. doi: 10.1016/j.nbt.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 17.(a) Khanna S., Goyal A., Moholkar V.S. Bioconversion of biodiesel derived crude glycerol by immobilized Clostridium pasteurianum: effect of temperature. Int. J. Chem. Biol. Eng. 2013;6:301–304. [Google Scholar]; (b) Khanna S., Goyal A., Moholkar V.S. Production of n-butanol from biodiesel derived crude glycerol using Clostridium pasteurianum immobilized on Amberlite. Fuel. 2013;112:557–561. [Google Scholar]
- 18.Howard R.L., Abotsi E.L., Van Rensburg J., Howard S. Lignocellulosic biotechnology: issues of bioconversion and enzyme production. Afr. J. Biotechnol. 2003;2:602–619. [Google Scholar]
- 19.Kumar P., Barrett D.M., Delwiche M.J., Stroeve P. Methods for pretreatment of lignocellulosic biomass for efficient hydrolysis and biofuel production. Indust. Eng. Chem. Res. 2009;48:3713–3729. [Google Scholar]
- 20.Fernbach A., Strange H. Sugars; and Other Carbohdrate Material: 1912. Fermentation Process for the Production of Acetone and Higher Alcohols from Starch. [Google Scholar]
- 21.C. Weismann, L. Alliston, Production of Secondary Butly Alcohol, 1922.
- 22.Ezeji T., Milne C., Price N.D., Blaschek H.P. Achievements and perspectives to overcome the poor solvent resistance in acetone and butanol-producing microorganisms. Appl. Microbiol. Biotechnol. 2010;85:1697–1712. doi: 10.1007/s00253-009-2390-0. [DOI] [PubMed] [Google Scholar]
- 23.Uyttebroek M., Vam Hecke W., Vanbroekhoven K. Sustainability metrics of 1-butanol. Cat. Today. 2015;239:7–10. [Google Scholar]
- 24.Durre P. Fermentative butanol production—bulk chemical and biofuel. Ann. N. Y Acad Sci. 2008;1125:353–362. doi: 10.1196/annals.1419.009. [DOI] [PubMed] [Google Scholar]
- 25.Chen C., wang L., Xiao G., Liu Y., Xiao Z., Deng Q., Yao P. Continuous acetone–butanol–ethanol (ABE) fermentation and gas production under slight pressure in a membrane bioreactor. Bioresour. Technol. 2014;163:6–11. doi: 10.1016/j.biortech.2014.04.004. [DOI] [PubMed] [Google Scholar]
- 26.Yadav S., Rawat G., Tripathi P., Saxena R.K. A novel approach for biobutanol production by Clostridium acetobutylicum using glycerol: a low cost substrate. Renew. Energy. 2014;71:37–42. [Google Scholar]
- 27.Yang M.K., Keinänen S., Vepsäläinen M., Romar J., Tynjälä H., Lassi P.U., Pappinen A. The use of (green field) biomass pretreatment liquor for fermentative butanol production and the catalytic oxidation of biobutanol. Chem. Eng. Res. Des. 2014;92:1531–1538. [Google Scholar]
- 28.Kumar M., Gayen K. Developments in biobutanol production: new insights. Appl. Energy. 2011;88:1999–2012. [Google Scholar]
- 29.Huang H., Liu H., Gan Y. Genetic modification of critical enzymes and involved genes in butanol biosynthesis from biomass. Biotechnol. Adv. 2010;28:651–657. doi: 10.1016/j.biotechadv.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Qureshi N., Saha B.C., Dien B., Hector R.E., Cotta M.A. Production of butanol (a biofuel) from agricultural residues: part I-use of barley straw hydrolysate. Biomass Bioenergy. 2010;34:559–565. [Google Scholar]
- 31.Jones D.T., Woods D.R. Acetone-butanol fermentation revisited. Microbiol. Rev. 1986;4:484–524. doi: 10.1128/mr.50.4.484-524.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin C., Yao M., Liu H., Chia-fon F., Lee E., Ji J. Progress in the production and application of n-butanol as a biofuel. Renew. Sustain. Energy Rev. 2011;15:4080–4106. [Google Scholar]
- 33.Ezeji T., Qureshi N., Blaschek H.P. Production of acetone–butanol–ethanol (ABE) in a continuous flow bioreactor using degermed corn and Clostridium beijerinckii. Process Biochem. 2004;42:34–39. [Google Scholar]
- 34.Li L., Ai H., Zhang S., Li S., Liang Z., Wua Z., Yang S., Wang J. Enhanced butanol production by coculture of Clostridium beijerinckii and Clostridium tyrobutyricum. Bioresour. Technol. 2013;143:397–404. doi: 10.1016/j.biortech.2013.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Li J., Chen X., Qi B., Luo J., Zhang Y., Sub Y., Wan Y. Efficient production of acetone–butanol–ethanol (ABE) from cassava by a fermentation–pervaporation coupled process. Bioresour. Technol. 2014;169:251–257. doi: 10.1016/j.biortech.2014.06.102. [DOI] [PubMed] [Google Scholar]
- 36.Komonkiat I., Cheirsilp B. Felled oil palm trunk as a renewable source for biobutanol production by Clostridium spp. Bioresour. Technol. 2013;146:200–207. doi: 10.1016/j.biortech.2013.07.067. [DOI] [PubMed] [Google Scholar]
- 37.Gottumukkala L.D., Parameswaran B., Valappil S.K., Mathiyazhakan K., Pandey A., Sukumaran R.K. Biobutanol production from rice straw by a non acetone producing Clostridium sporogenes BE01. Bioresour. Technol. 2013;145:182–187. doi: 10.1016/j.biortech.2013.01.046. [DOI] [PubMed] [Google Scholar]
- 38.Tippkötter N., Duwe A.M., Wiesen S., Sieker T., Ulber R. Enzymatic hydrolysis of beech wood lignocellulose at high solid contents and its utilization as substrate for the production of biobutanol and dicarboxylic acids. Bioresour. Technol. 2014;167:447–455. doi: 10.1016/j.biortech.2014.06.052. [DOI] [PubMed] [Google Scholar]
- 39.Abd-Alla M.H., El-Enany A.E. Production of acetone-butanol-ethanol from spoilage date palm (Phoenix dactylifera L.) fruits by mixed culture of Clostridium acetobutylicum and Bacillus subtilis. Biomass Bioenergy. 2012;2:172–178. [Google Scholar]
- 40.Cai D., Zhang T., Zheng J., Chang Z., Wang Z., Qin P., Tan T. Biobutanol from sweet sorghum bagasse hydrolysate by a hybrid pervaporation process. Bioresour. Technol. 2013;145:97–102. doi: 10.1016/j.biortech.2013.02.094. [DOI] [PubMed] [Google Scholar]
- 41.Marchal R., Ropars M., Pourquie J., Fayolle F., Vandecasteele J.P. Large-scale enzymatic hydrolysis of agricultural lignocellulosic biomass. Part 2: conversion into acetone butanol. Bioresour. Technol. 1992;42:205–217. [Google Scholar]
- 42.Ogo S., Onda A., Yanagisawa K. Selective synthesis of 1-butanol from ethanol over strontium phosphate hydroxyapatite catalysts. Appl. Catal. 2011;402:188–195. [Google Scholar]
- 43.Santacesaria E., Carotenuto G., Tesser R., Di Serio M. Ethanol dehydrogenation to ethyl acetate by using copper and copper chromite catalysts. Chem. Eng. J. 2012;179:209–220. [Google Scholar]
- 44.Juben N., James C., Dumesi A. An overview of dehydration, aldol-condensation and hydrogenation processes for production of liquid alkanes from biomass-derived carbohydrates. Catal. Today. 2007;123:59–70. [Google Scholar]
- 45.Ndou A.S., Plint N., Coville N.J. Dimerisation of ethanol to butanol over solid base catalysts. Appl. Catal. 2003;251:337–345. [Google Scholar]
- 46.Ueda W., Ohshida T., Kuwabara T., Morikawa Y. Condensation of alcohol over solid-base catalyst to form higher alcohols. Catal. Lett. 1992;12:97–104. [Google Scholar]
- 47.Fijita S.I., Iwasa N., Tani H., Nomura W., Arai M., Takezawa N. Dehydrogenation of ethanol over Cu/ZnO catalysts prepared from various coprecipitated precursors. React. Kinet. Catal. Lett. 2001;2:367–372. [Google Scholar]
- 48.Casale M.T., Richman A.R., Elroda M.J., Garland R.M., Beaver M.R., Tolbert M.A. Kinetics of acid-catalyzed aldol condensation reactions of aliphatic aldehydes. Atmos. Environ. 2007;41:6212–6224. [Google Scholar]
- 49.Zakzeski J., Lee H.R., Leung Y.L., Bell A.T. One-pot synthesis of alcohols from olefins catalyzed by rhodium and ruthenium complexes. Appl. Catal. 2010;374:201–212. [Google Scholar]
- 50.Carvalho D.L., de Avillez R.R., Michelly B., Rodriguesc T., Luiz E.P., Lucia G. Mg and Al mixed oxides and the synthesis of n-butanol from ethanol. Appl.Catal. 2012;416:96–100. [Google Scholar]
- 51.Dowson G.R.M., Haddow M.F., Lee J., Wingad R.L., Wass D.F. Catalytic conversion of eEthanol into an advanced biofuel: unprecedented selectivity for n-butanol. Angew. Chem. 2013;125:9175–9178. doi: 10.1002/anie.201303723. [DOI] [PubMed] [Google Scholar]
- 52.Freitas I.C., Damyanova S., Oliveira D.C., Marques C.M.P., Bueno J.M.C. Effect of Cu content on the surface and catalytic properties of Cu/ZrO2 catalyst for ethanol dehydrogenation. J. Mol. Catal. Chem. 2014;381:26–37. [Google Scholar]
- 53.Riittonen T., Toukoniitty E., Madnani D.K., Leino R., Kordas K., Szabo M., Sapi A., Arve K., Wärnå J., Mikkola J.P. One-pot liquid-phase catalytic conversion of ethanol to 1-butanol over aluminium oxide—the effect of the active metal on the selectivity. Catalysts. 2012;2:68–84. [Google Scholar]
- 54.Scalbert J., Thibault-Starzyk F., Jacquot R., Morvan D., Meunier F. Ethanol condensation to butanol at high temperatures over a basic heterogeneous catalyst: how relevant is acetaldehyde self-aldolization? J. Catal. 2014;311:28–32. [Google Scholar]
- 55.Kozlowski J.T., Davis R.J. Sodium modification of zirconia catalysts for ethanol coupling to 1-butanol. J. Energy Chem. 2013;22:58–64. [Google Scholar]
- 56.Ghaziaskar H.S., Xu C.C. One-step continuous process for the production of 1-butanol and 1-hexanol by catalytic conversion of bioethanol at its sub-/supercritical state. RSC Adv. 2013;3:4271–4280. [Google Scholar]
- 57.Elkabouss K., Kacimi M., Ziyad M., Ammar S., Bozon-Verduraz F. Cobalt-exchanged hydroxyapatite catalysts: magnetic studies, spectroscopic investigations, performance in 2-butanol and ethane oxidative dehydrogenations, 226 16 -24. J. Catal. 2004:226. [Google Scholar]
- 58.León M., Diaz E., Ordó̃nez S. Ethanol catalytic condensation over Mg–Al mixed oxides derived from hydrotalcites. Catal. Today. 2011;164:436–442. [Google Scholar]
- 59.Marcu I.C., Tichit D., Fajula F., Tanchoux N. Catalytic valorization of bioethanol over Cu–Mg–Al mixed oxide catalysts. Catal. Today. 2009;147:231–238. [Google Scholar]
- 60.Hansen A.C., Zhang Q., Lyne P.W.L. Ethanol diesel fuel blends—a review. Bioresour. Technol. 2005;96:277–285. doi: 10.1016/j.biortech.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 61.Niven R.K. Ethanol in gasoline: environmental impacts and sustainability review article. Renew. Sustainable Energy Rev. 2005;9:535–555. [Google Scholar]
- 62.Wackett L.P. Biomass to fuels via microbial transformations. Curr. Opin. Chem. Biol. 2008;12:187–193. doi: 10.1016/j.cbpa.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 63.Ullah K., Ahmad M., Sharma V.K., Lu P., Harvey A., Zafar M., Sultana S. Assessing the potential of algal biomass opportunities for bioenergy industry: a review. Fuel. 2015;143:414–423. [Google Scholar]
- 64.Monlau F., Sambusiti C., Barakat A., Quéméneur M., Trably E., Steyer J.P., Carrère H. Do furanic and phenolic compounds of lignocellulosic and algae biomass hydrolyzate inhibit anaerobic mixed cultures? A comprehensive review. Biotechnol. Adv. 2014;32:934–951. doi: 10.1016/j.biotechadv.2014.04.007. [DOI] [PubMed] [Google Scholar]
- 65.Castro Y.A., Ellis J.T., Miller C.D., Sims R.C. Optimization of wastewater microalgae saccharification using dilute acid hydrolysis for acetone, butanol, and ethanol fermentation. Appl. Energy. 2015;140:14–19. [Google Scholar]
- 66.Cheng H.H., Whang L.M., Chan K.C., Chung M.C., Wua S.H., Liu C.P., Tien S.Y., Chen S.Y., Chang J.S., Lee W.J. Biological butanol production from microalgae-based biodiesel residues by Clostridium acetobutylicum. Bioresour. Technol. 2015;184:379–385. doi: 10.1016/j.biortech.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 67.Van der Wal H., Sperber B.L.H.M., Houweling-Tan B., Bakker R.R.C., Brandenburg W., López-Contreras A.M. Production of acetone, butanol, and ethanol from biomass of the green seaweed Ulva lactuca. Bioresour. Technol. 2013;128:431–437. doi: 10.1016/j.biortech.2012.10.094. [DOI] [PubMed] [Google Scholar]
- 68.Maity J.P., Bundschuh J., Chen C.Y., Bhattacharya P. Microalgae for third generation biofuel production, mitigation of greenhouse gas emissions and wastewater treatment: present and future perspectives— a mini review. Energy. 2014;xxx:1–10. [Google Scholar]
- 69.Potts T., Du J., Paul M., May P., Beitle R., Hestekin J. The production of butanol from Jamaica bay macro algae. Environ. Prog. Sustain. Energy. 2012;31:29–36. doi: 10.1002/ep.10606. [DOI] [Google Scholar]
- 70.Ellis J.T., Hengge N.N., Sims R.C., Miller C.D. Acetone, butanol, and ethanol production from wastewater algae. Bioresour. Technol. 2012;111:491–495. doi: 10.1016/j.biortech.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 71.Swodenk W. Ethanol als Rohstoff für die chemische Industrie. Chem. Ing. Tech. 1983;55:683–688. [Google Scholar]
- 72.L. Gholizadeh, Enhanced Butanol Production by Free and Immobilized Clostridium sp. Cells Using Butyric Acid as Co-Substrate, Master Thesis, University College of Borås. 2009.
- 73.Ezeji T.C., Qureshi N., Blaschek H.P. Bioproduction of butanol from biomass: from genes to bioreactors. Curr. Opin. Biotechnol. 2007;18:220–227. doi: 10.1016/j.copbio.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 74.Huang H., Liu H., Gan Y.R. Genetic modification of critical enzymes and involved genes in butanol biosynthesis from biomass. Biotechnol. Adv. 2010;28:651–657. doi: 10.1016/j.biotechadv.2010.05.015. [DOI] [PubMed] [Google Scholar]