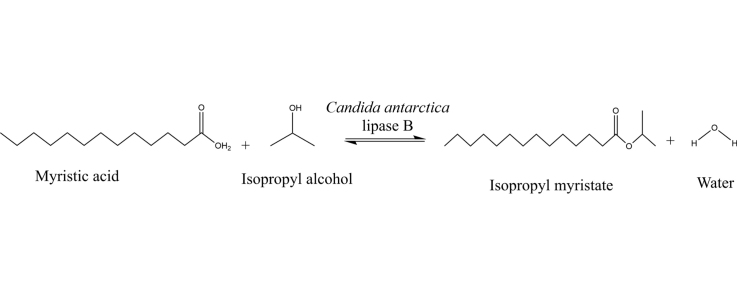
Graphical abstract
Keywords: Isopropyl myristate, Myristic acid, Isopropyl alcohol, Novozym 435, Esterification
Highlights
-
•
Green synthesis of isopropyl ester in homogenous phase reaction system.
-
•
Candida antarctica Lipase B (CAL-B) enzyme has efficiently catalyzed the esterification of myristic acid and isopropyl alcohol.
-
•
CAL-B activity depends on the polarity of an organic solvent.
-
•
Good operation stability of the enzyme was found in the single phase reaction system.
-
•
High purity of isopropyl myristate was obtained by the cold centrifugation technique.
Abstract
Isopropyl myristate finds many applications in food, cosmetic and pharmaceutical industries as an emollient, thickening agent, or lubricant. Using a homogeneous reaction phase, non-specific lipase derived from Candida antartica, marketed as Novozym 435, was determined to be most suitable for the enzymatic synthesis of isopropyl myristate. The high molar ratio of alcohol to acid creates novel single phase medium which overcomes mass transfer effects and facilitates downstream processing. The effect of various reaction parameters was optimized to obtain a high yield of isopropyl myristate. Effect of temperature, agitation speed, organic solvent, biocatalyst loading and batch operational stability of the enzyme was systematically studied. The conversion of 87.65% was obtained when the molar ratio of isopropyl alcohol to myristic acid (15:1) was used with 4% (w/w) catalyst loading and agitation speed of 150 rpm at 60 °C. The enzyme has also shown good batch operational stability under optimized conditions.
1. Introduction
The fatty acid esters have a broad range of applications in industries such as cosmetic industry, food industry, the pharmaceutical industry, the coating industry, lubricants, biodiesel etc. [1], [2]. The esters of fatty acids includes methyl esters, isopropyl esters, butyl esters, partial glycerides and wax esters (esters of fatty acids with long-chain fatty alcohols), and ester oils (esters of fatty acids with poly alcohols) [1], [2], [3]. The commonly used fatty acid ester, isopropyl myristate (IPM), is the main focus of present research.
IPM, the isopropyl ester of myristic acid, is used in cosmetics as a substitute for natural oils because it has excellent spreading properties and is absorbed easily into the skin. In many topical and transdermal preparations, IPM is also used as a co-solvent with skin penetration enhancement properties of active ingredients [4].
Conventional methods for IPM synthesis involve the use of chemical catalyst at high temperatures. But this result in undesirable changes in a final product with respect to colour, odour, and stability, hence there is need for post refining steps which increases overall unit processes [5]. Therefore, nowadays the use of an enzyme catalyzed reaction is in focus to replace the conventional methods. Recently, many lipase based catalysis for esters have been reported wherein the esterification reaction were enhanced by reaction/enzyme engineering [6], [7], [8], [9].
In accompanying papers the lipase-catalyzed esterification of myristic acid and isopropyl alcohol to produce isopropyl myristate ester was studied [10], [11]. They have used organic solvents like heptanes, petroleum ether to carry out esterification reaction. However, use of such organic solvents creates two phase system, hence has downstream processing problems. Further theses conditions affect an enzyme activity adversely. Hence, there is need for medium engineering in order to develop the improved esterification reaction system. Additionally, lower rate of reaction and yield in enzymatic route needed to be addressed for an efficient isopropyl myristate synthesis reaction.
In this paper, we have reported green synthesis of isopropyl myristate in a single phase medium. The novel approach for homogenization of two distinct substrates, i.e. myristic acid and isopropyl alcohol was established. Use of alcohol component in excess made the whole reaction system more realistic in terms of rate of synthesis and downstream processing. The various reaction parameters involved green synthesis of isopropyl myristate systematically studied. This paper promises a high performance synthesis of isopropyl myristate by enzymatic route using an immobilized lipase B from Candida antartica.
2. Experimental procedure
2.1. Materials
The commercial immobilized preparations like Novozym 435 (specific activity of 10,000 PLU/g), Lipozyme TLIM (specific activity of 250 IUN/g), Lipozyme RMIM (specific activity of 275 IUN/g) were supplied by Novo Nordisk Ltd. (Bagsvaerd, Denmark). HyLIP was indigenously immobilized 1, 3-regiospecific lipase provided by DBT-ICT-CEB (Mumbai, India). The myristic acid, isopropyl alcohol (0.2% water) and isopropyl myristate were purchased from SD fine chemicals (Mumbai, India). All the other chemicals and reagents were of analytical grade.
2.2. Esterification
Reactions were carried out in 100 ml stoppered conical flasks mixing isopropyl alcohol with myristic acid in a specific mole ratio. The reaction was initiated by adding catalyst, Novozym 435. All reactions were carried out by orbital shaking at a temperature of 60 °C. Samples were taken at different time period and analyzed for product formation.
2.3. Product analysis
Sample withdrawn from reaction mixture was suitably diluted in tert-butanol and filtered through 0.2 micron syringe filter. A 10 μl aliquot was injected into an Agilent Zorbax C-18 reverse phase HPLC column (4.6 mm I.D., 250 mm length and 5 μm particle size) at 30 °C. The mobile phase consisted of 80% acetonitrile (solvent A) and 20% of 30 mM phosphoric acid in water (solvent B) with a flow rate of 1 ml/min. The gradient started at 50% A followed by a linear increase to 90% A in 15 min. 100% A was then held for 10 min followed by returning to initial condition in next 10 min. Detection was carried out at 205 nm by UV detector. The concentration of the sample was determined from the standard curve for myristic acid and isopropyl myristate. The conversion (%) was calculated as follows
2.4. Recovery of an enzyme and operational stability studies
The immobilized enzyme after the completion of a reaction was removed by filtration and washed twice with isopropyl alcohol to remove traces of product left on the surface of an enzyme. The washed enzyme was then dried and reused for the next batch of esterification reactions to assess the operation stability.
3. Results and discussion
3.1. Screening of different lipases
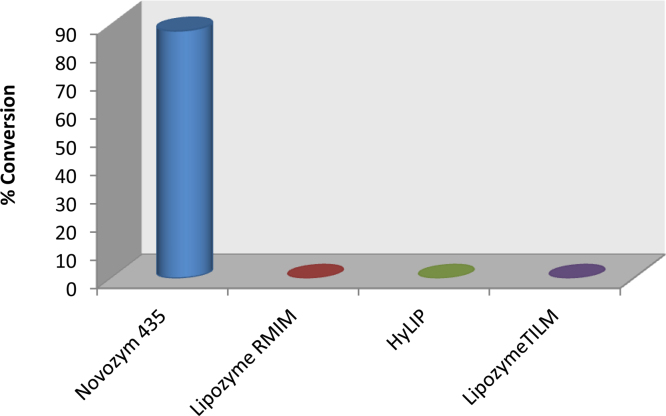
Immobilized enzymes have been used in continuous operations on a large scale due to various advantages like easy recovery, reusability, and stability. The commercially available lipases, Novozym 435, Lipozyme TLIM, Lipozyme RMIM, indigenously immobilized 1,3-specific Lipolase 100 L (HyLIP) was evaluated for synthesis of isopropyl myristate. In the case of Novozym 435, the conversion was 87.65%, while no conversion was obtained with other lipases (Fig. 1).
Fig. 1.
Screening of lipases for isopropyl myristate synthesis.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, time of reaction = 5 h, Enzyme sources: Novozym 435, Lipozyme RMIM and Lipozyme TLIM, Novo Nordisk, Denmark; HyLIP, Indigenous immobilized lipase preparation, DBT-ICT-CEB, India].
Novozym 435 has been found to be very effective in solvent-free reaction systems because of its versatility. Dimensional analysis based on the crystal structure of Candida antarctica lipase B (Novozym 435) clearly shows the presence of amino acid residue A281 as a part of an α-helix (α-10) at the top of the substrate-binding pocket in a highly hydrophobic environment [12], [13]. This micro-environment arrangement near the active site pocket favours proper interaction of myristic acid and isopropyl alcohol. While the flapping lid of Thermomyces lanuginosus (Lipozyme TLIM, Lipoalse 100 L) projects into the binding pocket of the enzyme, thereby creating steric hindrance in the binding of substrate at the active site [14]. Since Novozym 435 does not contain a flapping lid, there is a less steric hindrance as compared to other lipases and it shows greater activity. As Novozym 435 has given best conversion, it was used for further studies.
3.2. Effect of temperature
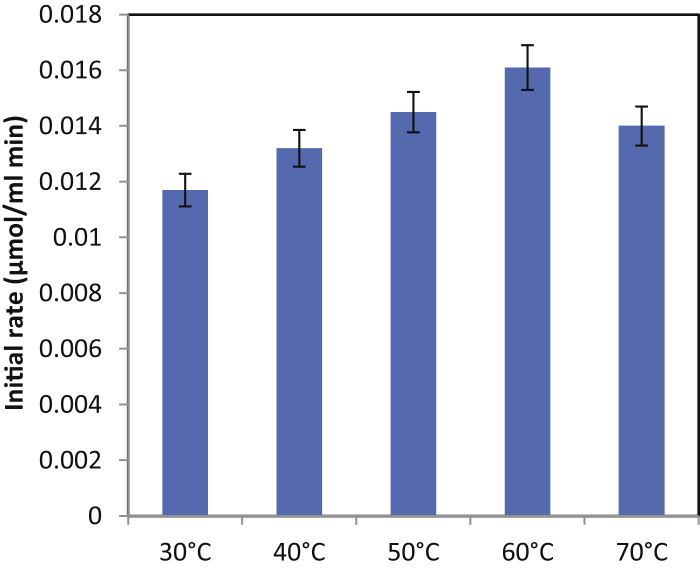
As the reaction temperature is a crucial parameter in enzyme catalysis [15], five different temperatures (30–70 °C) were selected for the synthesis of isopropyl myristate (Fig. 2). It was observed that the increase in temperature enhances the initial rate of synthesis. Higher temperatures increase the kinetic energy of the system and therefore collisions between enzyme and substrate molecules. An increase in temperature also improves solubility of the substrates and reduces viscosity, and mass transfer limitations [16]. Overall, there is acceleration of reaction rate. The thermal deactivation of lipase occurred at temperature of 70 °C which also being reported in literature [17].
Fig. 2.
Effect of temperature on the initial rate of isopropyl myristate synthesis.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, time of reaction = 5 h, enzyme—Novozym 435].
Table 1 shows value of thermodynamic and kinetic parameters calculated from Arrhenius and Van’t Hoff’s equation. The activation energy was estimated from Arrhenius equation which deals by means of a variation of the rate constant (k) with temperature (T). It is expected that a straight line will be obtained from the plot of ln k vs. 1/T, with the slope (Ea/R) giving an apparent activation energy Ea for the process. The activation energy for isopropyl myristate using the Arrhenius equation was found to be 37.13 kJ/mol. Kee et al. [18] reported the value of activation energy (43.67 kJ/mol) for synthesis of isopropyl palmitate. Slightly higher activation energy is attributed to high molecular weight of palmitic acid than myristic acid. The effect produced by the variation of temperature on the equilibrium constant is given by Van’t Hoff’s equation, which allows the estimation of enthalpy and entropy changes. A straight line was obtained when plot of ln keq is plotted against 1/T with slope of ΔH/R and intercept ΔS/R. The value of change in enthalpy (ΔH) was calculated and found to be −5.64 kJ/mol which is nearer to the reported value (−5.68 kJ/mol) for ethyl oleate synthesis by Trubiano et al. [19]. The negative value signifies that the reaction gives out energy to its surroundings and the energy needed for the reaction to occur is less than the total energy released.
Table 1.
Thermodynamic parameters, pre-exponential factor (k0) and activation energy determined for isopropyl myristate synthesis.
| ΔH (kJ/mol) | −5.641 |
|---|---|
| ΔS (kJ/mol × K) | 35.967 |
| k0 (l/mol/gcat/min) | 1.7691 × 103 |
| Ea (kJ/mol) | 37.13 |
3.3. Effect of agitation
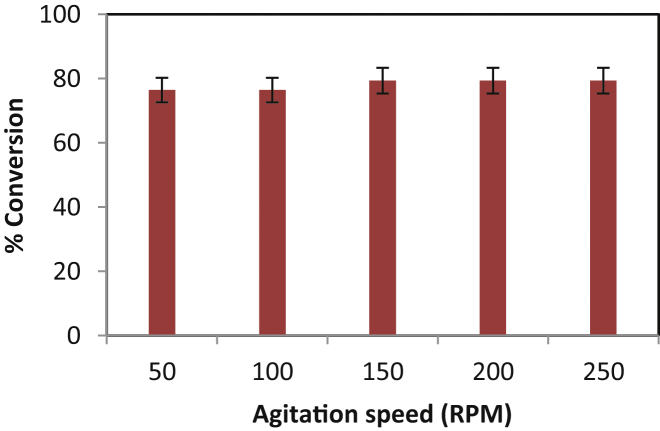
The present esterification reaction is a solid–liquid system having liquid phase consisting of myristic acid and isopropyl alcohol and the immobilized enzyme (Novozym 435) as a solid phase. The effect of agitation was studied in the range of agitation speed of 50–200 rpm (Fig. 3). The overall conversion remained constant at 75.65% within the time period of 60 min. The results indicated no external surface resistance of the reactants on catalyst particle. Similar results were obtained when isoamyl alcohol reacted with myristic acid in presence of Novozym 435 at a speed of 200–400 rpm [20].
Fig. 3.
Effect of agitation on isopropyl myristate.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, time of reaction = 5 h, enzyme—Novozym 435].
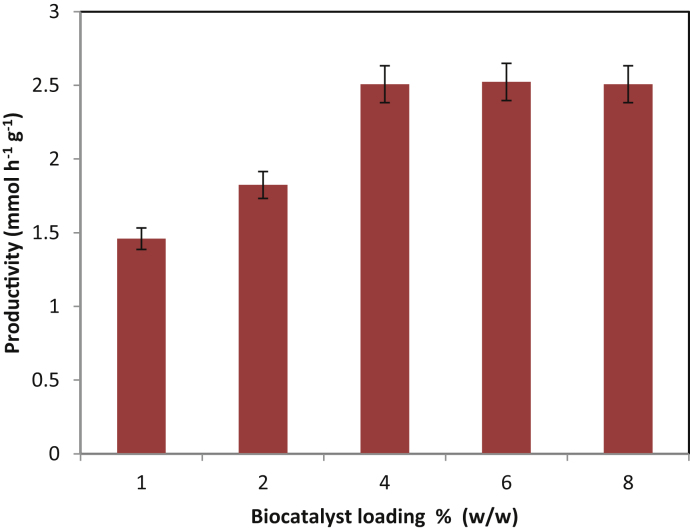
3.4. Effect of biocatalyst loading
For a successful application of a reaction, the substrate concentration needs to be higher so to obtain a higher degree of productivity. Simultaneously, the low amount of immobilized enzyme is desirable for economic feasibility of a reaction [21]. As it has influenced the process economics, the minimal amount necessary for achieving good productivity should be determined. The influence of varying amount of enzyme, 1–8% (w/w), on the productivity of isopropyl myristate is shown in Fig. 4. The productivity of ester was increased from 2.18 mmol of ester h−1 g of immobilized lipase−1 to 2.68 mmol of ester h−1 g of immobilized lipase−1 on increasing the biocatalyst leading from 1% to 4%. However, further increase of catalyst loading to 8% (w/w) increased productivity marginally. This would suggest that all available active sites of the enzyme are occupied by substrate molecules. Any further increase in enzyme loading will have no effect since no substrate molecules are available for binding [22]. As higher productivity with lower biocatalyst amount is desirable for process economics, 4% (w/w) of Novozym 435 was considered as optimum catalyst loading in further studies.
Fig. 4.
Effect of biocatalyst loading on synthesis of isopropyl myristate.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, time of reaction = 5 h, enzyme—Novozym 435]
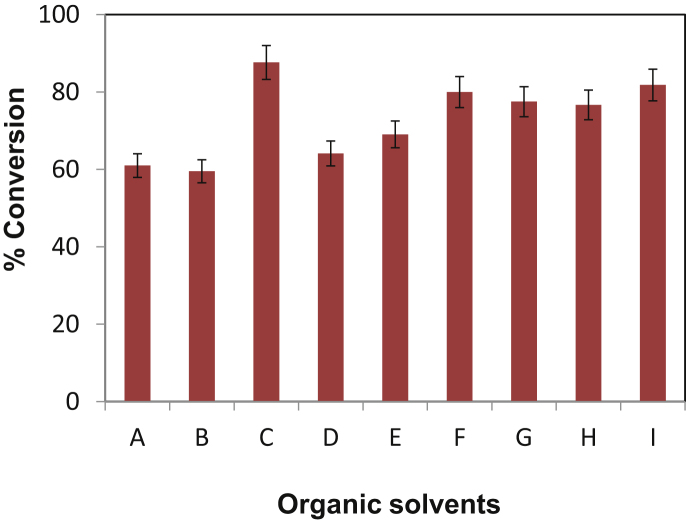
3.5. Effect of organic solvent
The selection of organic solvent that allows the dissolution of reactants without interacting with the enzyme or matrix/support is very important in achieving efficient esterification. The influence of organic solvents on the enzymatic reactions has been summarized in earlier research paper [23]. The enzyme activity is higher in non-polar solvent (log P > 4) and mid-polar solvents (2 < log P < 4), whereas the lowest activity is found in polar solvents (log P < 2). Solvents having log P > 4 do not strip off the essential water coat around the enzyme, thereby leaving the biocatalyst in an active state.
Fig. 5 shows the effects of log P value of organic solvent on isopropyl myristate conversion. Novozym 435 has shown the lower activity in polar solvents as occurred in acetone (log P = −0.24) and DMSO (log P = −1.37). The highest% conversion was obtained with hexane (log P = 4) and Toluene (log P = −2.73) with exception of isopropyl alcohol (log P = 0.05). There is optimum log P value after which the conversion is less than the maximum value. The organic solvent having higher log P values have indeed been recommended for optimal lipase activity and stability [24]. However, the highest conversion (87.65%) was obtained where isopropyl alcohol (log P value = 0.05) was used as a solvent which has created a single phase system. The esterification reaction, which is equilibrium in with respect to molar concentration to all reactants and products of the reaction, is favoured either by an excess of reactant or removal of by-product. Thus the excess of reactant (isopropyl alcohol, 15 M molar concentration) shifts the equilibrium towards the synthesis giving higher conversion. The use of single solvent also helps in the downstream processing, making a reaction industrially attractive. Additionally, isopropyl alcohol has not shown any water striping effect on immobilized enzyme, thus giving better conversion and high enzyme activity.
Fig. 5.
Effects of log P values of organic solvents on synthesis of isopropyl myristate.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, time of reaction = 5 h, enzyme—Novozym 435, Organic solvents (log P value)—A: DMSO (−1.37), B: acetone (0.24), C: isopropyl alcohol (0.05), D: tert-butyl alcohol (0.35), E: tetrahydrofuran (0.46), F: cyclohexanone (0.81), G: MIBK (1.31), H: toluene (2.73), I: hexane (4.0)].
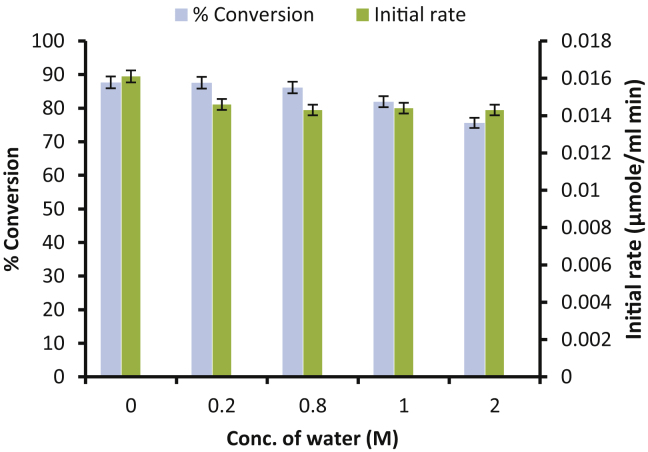
3.6. Effect of initial water activity
To determine the influence of the product (water) formed during the esterification reaction on enzyme activity, additional groups of experiments were carried out in a series operating in excess of water. The effect of the initial water percentage on the conversion is studied in the range of 0–2 M concentration of water in the reaction mixture (Fig. 6). Commonly, lipases are activated at interphase between oil and water [25], [26]. However, studies based on a three-dimensional structure [13], no interfacial activation have been reported for Novozym 435 (CALB). The fact that CALB does not require the presence of an oil/water interface to show higher activities is reported here for synthesis of isopropyl myristate. The presence of water has only an unfavourable effect on the equilibrium conversion obtained after 5 hrs. As the initial water percentage increases from 0 to 2 M, the equilibrium conversion decreases monotonically from 87.67% to 75.61%
Fig. 6.
Effects of initial addition of water on isopropyl myristate synthesis.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, time of reaction = 5 h, enzyme—Novozym 435].
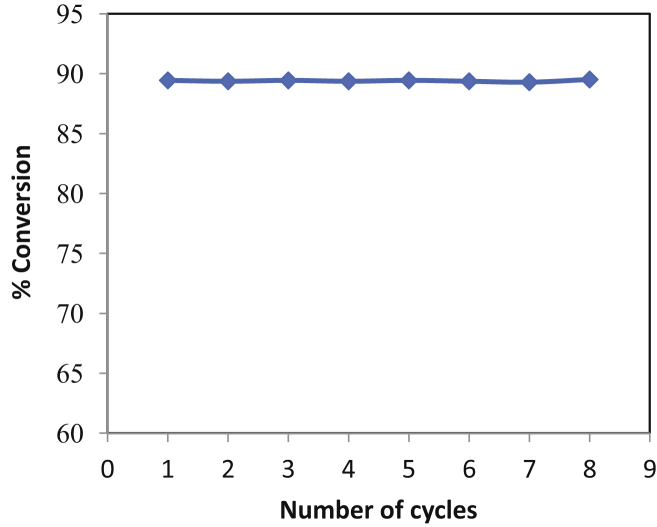
3.7. Batch operational stability of enzyme
The importance of economic and environmental aspects of immobilized enzyme lies in the possibility of their recovery and reusability. These were the main problems found with biocatalyst when performing the esterification reaction [27]. Therefore, it was necessary to ensure high operational stability of the Novozym 435. The results of the repeated batches tested for isopropyl myristate synthesis were presented in Fig. 7.
Fig. 7.
Batch operational stability of Novozym 435.
[Molar ratio of myristic acid to isopropyl alcohol = 1:15, temperature of reaction = 60 °C, time of each cycle = 18 h].
The results imply that there was no loss in the enzyme activity over a number of cycles studied. This indicates that there is no deactivation of the lipase enzyme occurring due to isopropyl alcohol, which is in excess to form homogenous reaction medium. The other reaction tested on same enzyme has indicated stability for over 4–5 months [28]. Earlier the operational stability of an enzyme has been reported for the synthesis of aliphatic esters [29], [30], [31], [32]. The majority of them stated that deactivation of an enzyme occurs as a number of cycles increased which is due to distortion of three dimensional structure by the solvent used. This is contradictory with our results where the solvent used (Isopropyl alcohol) do not show any adverse effects on enzyme activity and hence giving same conversion in numbers of cycles. These results demonstrate the possible use of Novozym 435 for large-scale enzymatic synthesis of isopropyl myristate.
3.8. Recovery of residual substrate and final product
The purity of isopropyl myristate is important for its industrial acceptance. At the end of the esterification reaction, the excess of isopropyl alcohol was distilled and recovered for use after dehydration. The unreacted myristic acid was recovered by employing the cold centrifugation wherein, myristic acid (melting point = 54.4 °C) at low temperature (0 °C) can be isolated as solid. The liquid isopropyl myristate is further separated from the solid mass by cold centrifugation to achieve a final purity of 97%.
4. Conclusions
Isopropyl myristate was successfully synthesized by novel single phase reaction system using an immobilized commercial lipase, Novozym 435. The esterification reaction was highly dependent on the polarity of organic solvent used and isopropyl alcohol was chosen as it met requirements of solvent properties and was also one of the substrate molecules. Optimization in terms of the operating molar ratio of the fatty acid to alcohol (1:15) and catalyst loading (4% by weight of substrate) results in >87% conversion in 5 hrs of processing time. The recovery of isopropyl myristate (97%) by distillation and cold centrifugation ensures the recyclability of both the substrates. The present work therefore clearly demonstrates the advantages of use of a homogenous single phase reaction medium for immobilized lipase mediated esterification of fatty acids and the possibility of operating the system on a continuous mode to achieve high productivity for process intensification.
Acknowledgements
This study was supported by the Department of Biotechnology, Ministry of India and Institute of Chemical Technology, Mumbai. We wish to express our gratitude to the DBT-ICT-Centre for Energy Biosciences for providing facilities for the experimental work.
Footnotes
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.btre.2015.10.006.
Contributor Information
Rajeshkumar N. Vadgama, Email: rajesh.vdgm@gmail.com.
Annamma A. Odaneth, Email: a.annamma@ictmumbai.edu.in, a.dbtceb@gmail.com.
Arvind M. Lali, Email: arvindmlali@gmail.com.
Appendix A. Supplementary data
The following are Supplementary data to this article:
References
- 1.Brockmann R., Demmering G., Kreutzer U., Lindemann M., Plachenka Steinberner J.U. Ullmann’s Encyclopedia of Industrial Chemistry. John Wiley & Sons Inc.; Weinheim: 2005. Fatty acids; pp. 1–34. Electronic ed. [Google Scholar]
- 2.Kalish J., Pattison E.S. Marcel Dekker Inc.; New York: 1968. Fatty Acids and Their Industrial Applications; pp. 221–232. [Google Scholar]
- 3.Gervajio G.C., Shahidi F. Bailey’s Industrial Oil and Fat Products. fourth ed. Wiley-Interscience; 2005. pp. 1–56. vol. 6. [Google Scholar]
- 4.Klaffenbach P., Kronenfeld D. J. Chrom. A. 1997;767:330–334. [Google Scholar]
- 5.L.H. Staal, To Esters via Biotechnology, Proceedings World Conference on Oleochemicals Into the 21st Century (Eds.) (1990).
- 6.Alves J.S., Garcia-Galan C., Danelli D., Paludo N., Barbosa O., Rodrigues R.C., Fernandez-Lafuente R. Catal. Today. 2015;255:27–32. [Google Scholar]
- 7.Fallavena L.P., Antunes F.H.F., Alves J.S., Paludo N., Ayub M.A.Z., Fernandez-Lafuente R., Rodrigues R.C. RSC Adv. 2014;4(17):8675–8681. [Google Scholar]
- 8.Martins A.B., Da Silva A.M., Schein M.F., Garcia-Galan C., Záchia Ayub M.A., Fernandez-Lafuente R., Rodrigues R.C. J. Mol. Catal. B: Enzyme. 2014;105:18–25. [Google Scholar]
- 9.Alves J.S., Garcia-Galan C., Schein M.F., Silva A.M., Barbosa O., Ayub M.A.Z., Fernandez-Lafuente R., Rodrigues R.C. Molecules. 2014;19(7):9562–9576. doi: 10.3390/molecules19079562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Verma M.L., Chauhan G.S., Kanwar S.S. Acta micro. et immune. hung. 2008;55(3):327–342. doi: 10.1556/AMicr.55.2008.3.4. [DOI] [PubMed] [Google Scholar]
- 11.Chun-hua Y., Ming G., Jiang-fan L. China Surfactant Deterg. Cosmet. 2009:06–09. [Google Scholar]
- 12.Valério A., Krüger R.L., Ninow J.L., Corazza F.C., Oliveira D., Oliveira J.V., Corazza M.L. J. Agric. Food Chem. 2009;57:8350–8356. doi: 10.1021/jf901771m. [DOI] [PubMed] [Google Scholar]
- 13.Uppenberg J., Patkar S., Bergfors T., Jones T.A. J. Mol. Biol. 1994;235:790–792. doi: 10.1006/jmbi.1994.1035. [DOI] [PubMed] [Google Scholar]
- 14.Jaeger K.E., Eggert T. Curr. Opin. Biotechnol. 2002;13:390–397. doi: 10.1016/s0958-1669(02)00341-5. [DOI] [PubMed] [Google Scholar]
- 15.Lidstrom P., Tierney J., Wathey B., Westman J. Tetrahedron. 2001;57:9225–9283. [Google Scholar]
- 16.Soo E.L., Salleh A.B., Basri M., Rahman R.N.Z.A., Kamaruddin K. Process Biochem. 2004;39(11):1511–1518. [Google Scholar]
- 17.S. Šabeder, M. Habulin, Ž. Knez, 10th European Meeting on Supercritical Fluids: Reactions, Materials and Natural Products Processing, Proceedings of the meeting in Colmar (France), 2005.
- 18.Kee C.Y., Hassan M., Ramachandran K.B. Artif. Cells Blood Substit. Immobil. Biotechnol. 1999;27(5–6):393–398. doi: 10.3109/10731199909117709. [DOI] [PubMed] [Google Scholar]
- 19.Trubiano G., Borio D., Errazu A. Enzyme Microb. Technol. 2007;40:716–722. [Google Scholar]
- 20.Yadav G.D., Pooja A.T. J. Mol. Catal. B: Enzyme. 2012;83:16–22. [Google Scholar]
- 21.Lozano P., de Diego Teresa, Carrie Daniel, Vaultier Michel, Iborra Jose. J. Mol. Catal. B. Enzyme. 2003;21(1–2):9–13. [Google Scholar]
- 22.Yadav G.D., Sajgure A.D. J. Chem. Technol. Biotechnol. 2007;82:964–970. [Google Scholar]
- 23.Lanne S., Vos B.K., Veeger C. Biotechnol. Bioeng. 1987;30:81–87. doi: 10.1002/bit.260300112. [DOI] [PubMed] [Google Scholar]
- 24.Peters G.H., van Aalten D.M.F., Svendsen A., Bywater R. Prot. Eng. 1997;10:149–158. doi: 10.1093/protein/10.2.149. [DOI] [PubMed] [Google Scholar]
- 25.Monot F., Borzeix F., Bardin M., Vandecasteele J.P. Appl. Microbiol. Biotechnol. 1991;35:759–765. [Google Scholar]
- 26.Wehtje E., Aldercreutz P. Water Biotechnol. Bioeng. 1997 796-806;55(5) doi: 10.1002/(SICI)1097-0290(19970905)55:5<798::AID-BIT10>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Martins A.B., Graebin N.G., Lorenzoni A.S.G., Fernandez-Lafuente R., Ayub M.A.Z., Rodrigues R.C. Process Biochem. 2011;46:2311–2316. [Google Scholar]
- 28.Vadgama R.N. (2014), Ph.D. Thesis—Designing lipases for Hydrolysis and Synthesis—Submitted to Institute of Chemical Technology, Mumbai, India.
- 29.Yadav G.D., Lathi P.S. J. Mol. Catal. B. Enzyme. 2004;27:109–115. [Google Scholar]
- 30.Harikrishna S., Divakar S., Prapull S.G., Karanth N.G. J. Biotechnol. 2001;87:193–201. doi: 10.1016/s0168-1656(00)00432-6. [DOI] [PubMed] [Google Scholar]
- 31.Yadav G.D., Lathi P.S. Biochem. Eng. J. 2003;16:245–252. [Google Scholar]
- 32.Shimada Y., Watanable Y., Sugihara A., Bata T., Ooguri T., Moriyama S., Terai T., Tominaga Y. J. Biosci. Bioeng. 2001;92:19–23. doi: 10.1263/jbb.92.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.