Abstract
Regenerative medicine therapies, underpinned by the core principles of rejuvenation, regeneration and replacement, are shifting the paradigm in healthcare from symptomatic treatment in the 20th century to curative treatment in the 21st century. By addressing the reasons behind the rapid expansion of regenerative medicine research and presenting an overview of current clinical trials, we explore the potential of regenerative medicine to reshape modern healthcare.
Keywords: Regenerative medicine, Cell therapy, Tissue engineering, Stem cells, Clinical translation
Background
The current dilemmas for modern day healthcare, such as an aging population and the increasing prevalence of chronic diseases, require solutions that limit organ dysfunction and tissue degeneration and which potentially offer replacement. This was first addressed through transplantation, a field that advanced rapidly in the 1950s through a combination of surgical innovations and fundamental scientific breakthroughs in immunosuppression [1]. In contrast to the allogenic replacement of transplantation, regenerative medicine seeks to apply stem cell research with developmental biology principles to regenerate cells, tissues and organs de novo [2].
The regenerative medicine research field resulted from the convergence of multiple scientific avenues, such as successful culture of cells in the laboratory [3], identification, characterization and differentiation of stem cells [4–7], and an improved understanding of developmental and molecular biology [8], to conceivably allow control of the intracellular and extracellular environment to promote tissue and organ formation in the laboratory (Fig. 1).
Fig. 1.
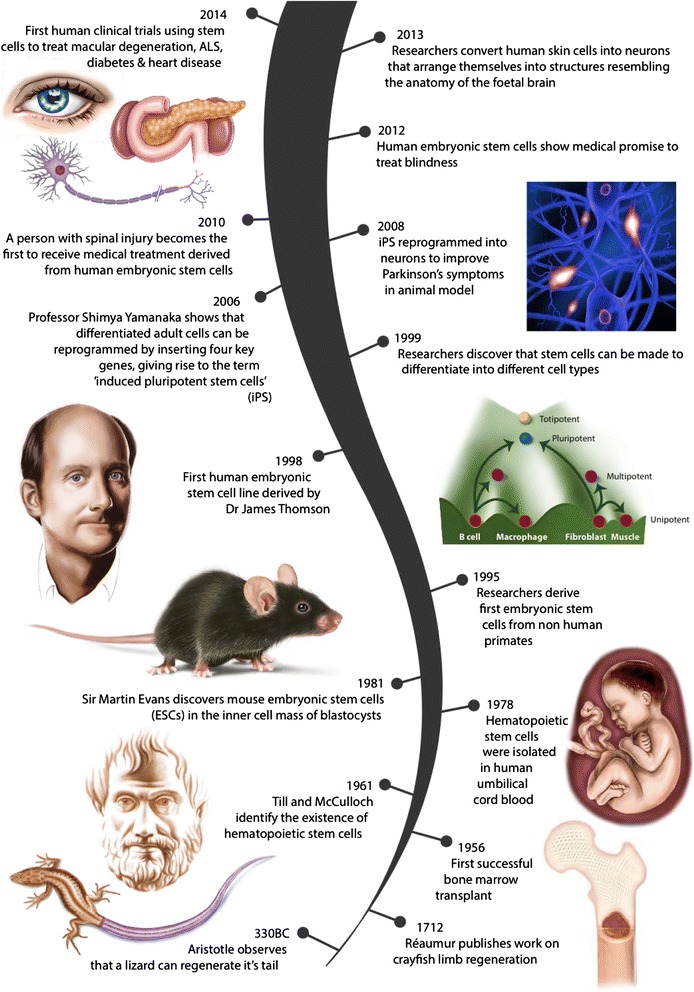
Regenerative medicine origins
Regenerative medicine has been recognized worldwide as a developing research field that offers the potential to revolutionize patient care in the 21st century [9]. The prospect of addressing massive healthcare markets, such as cardiovascular disease, neurological conditions or chronic metabolic diseases (e.g. end-stage renal disease or diabetes), means that there has been sustained scientific, public and commercial interest despite early setbacks and slow progress.
Expansion and potential impact of regenerative medicine
Demand for regenerative medicine products has been driven by an increase in degenerative and chronic diseases which place cost pressures on healthcare providers, combined with advances in new technologies such as nanotechnology, bioengineering and stem cell therapy [10]. Long-term cell, tissue and organ replacement will not only provide an alternative to transplantation [11], but will also provide therapeutic options for degenerative conditions (e.g. neurodegenerative conditions (Parkinson’s), stroke and heart failure), which are currently only managed through palliation [12, 13].
According to the World Regenerative Medicines Market forecast for 2013–2020 [14], the global regenerative medicines market for small molecules and biologics, gene therapy and cell therapy is expected to reach $67.5 billion by 2020, which is an increase of $51.1 billion from 2013, thus reflecting its commercial potential. Governments across Europe and the US, as well as their medical research councils, have identified tissue engineering and regenerative medicine at the top of their research priorities [9]. Removal of previous restrictions in embryonic stem cell research in 2009 by the Obama organization is predicted to contribute to further considerable growth within the field as well as improved potential for clinical translation [15].
Clinical trials in regenerative medicine
The expansion of regenerative medicine as a scientific discipline, with its core principles of rejuvenation, regeneration and replacement (the 3R’s), is shifting the paradigm in healthcare from symptomatic treatment in the 20th century to curative treatment in the 21st century [13]. This is evidenced by the rapid increase in regenerative medicine clinical trials in each specialty [16, 17], which can be broadly classified as using either cell- or tissue-based products (Table 1). The Food and Drug Administration in the US and the European Medicines Agency have more complex classification systems of regenerative medicine products, including cellular therapy, gene therapy, stimulators of endogenous repair, biologic-device combination products, and human tissue and xenotransplantation [18]. Broadly, the regulatory requirements can be based on the pillars of sterility, stability and potency, and these need to be addressed prior to successful clinical translation in the future (Table 2).
Table 1.
Applications of regenerative medicine therapies in different medical specialties
| Medical Speciality | Pathology | Cell/Tissue Therapy | Clinical Trial Phase | Patient numbers | Clinical Trial Study |
|---|---|---|---|---|---|
| Pubmed Clinical Trial database | |||||
| Neurology | Parkinson’s | Fetal porcine cells Transplantation of embryonic dopamine neurons |
I, II | 34 | Fink 2001; Freed 2001 |
| Paraplegia, Spinal cord injuries | MSCs transplanted directly into injured spinal cord. Bone marrow nucleated cells injected intrathecally and intravenously coupled with MSC infusion by lumbar puncture |
I, II | 80 | Park 2012; Jarocha 2015 | |
| Multiple Sclerosis | IV infusion of MSCs Haemopoietic stem cell transplants |
I, II | 30 | Connick 2012 | |
| Cardiology | Ischaemic cardiomyopathy, heart failure | Transendocardial injection of MSC derived from BM or adipose tissue Intracoronary injection of cardiac stem cells IV infusion of MSC |
I, II | 104 | Heldman 2014; Hare 2014; Chugh 2012; Perin 2014 |
| Respiratory | Idiopathic pulmonary fibrosis | IV infusion of placental- Chambers derived MSC | I | 8 | Chambers 2014 |
| Chronic lung disease | IV infusion of HLA-matched allogeneic MSCs derived from BM/umbilical cord | I,II | 62 | Weiss 2013 | |
| Rheumatology | Osteoarthritis | Intra-articular injection of autologous or allogeneic MSC | I, II, III | 104 | Orozco 2013; Jo 2014 |
| Osteogenesis Imperfecta | Allogeneic bone marrow derived MSC Haemopoietic stem cell transplant plus MSC infusion |
I | 8 | Horwitz 2002; Horwitz 2002 | |
| Orthopaedics | Fracture healing; Joint resurfacing; Osteoporosis |
MSC combined with/without calcium sulphate Allogenic bone graft containing stem cells G-CSF-mobilised Haemopoietic stem cells with collagen scaffold for non-union fracture healing |
I,II | 96 | Kuoroda 2014; Jones 2015; Bajada 2007 |
| Haematology | Hematopoietic stem cell transplant (HSCT); Graft versus Host Disease (GvHD) | Prochymal (MSC) for severe refractory acute GvHD MSC infused with or following hematopoietic stem cell transplant |
I, II, III | 240 | Prasad 2011; Ringden 2006; Perez-Simon 2011 |
| Opthalmology | Macular degeneration | ESC-derived retinal pigment epithelium | I | 2 | Schwartz 2012 |
| Gastroenterology | Liver cirrhosis; Decompensated liver disease | MSC injected into peripheral or portal vein Autologous bone marrow mononuclear cells infused IV for liver cirrhosis UC-MSC IV in fusion in decompensated liver disease |
I,II | 45 | Kharaziha 2009; terai 2006; Zhang 2012 |
| Crohn’s disease | Autologous hematopoietic)stem cell transplantation for refractory Crohn’s | I, II, III | 98 | Oyama 2005 | |
| Endocrinology | Diabetes (type I & 2) | Stem cell educator therapy with cord blood derived stem cells for insulin resistant type II diabetes Hematopoietic stem cell transplantation for new onset type I diabetes |
I, II | 65 | Zhao 2013, D’Addio 2014 |
| Nephrology/Urology | Kidney transplant rejection | MSC based therapy to prevent rejection in living-related kidney transplants | I, II | 159 | Tan 2012 |
MSC mesenchymal stem cells, BM Bone marrow, ESC Embryonic Stem cells, iPSC induced Pluripotent stem cells, IV intravenous, G-CSF granulocyte-colony stimulating factor, 3D 3-dimensional, UC umbilical cord
Table 2.
Overview of testing of regenerative medicine products to validate sterility, stability and potency
| Pillar | Obstacles | Method | Reference |
|---|---|---|---|
| Sterility | Sterility testing | Direct inoculation test in aerobic and anaerobic media | [32] |
| Stability | Chromosomal stability | Karyotyping | [33] |
| Cell metabolism | Mitochondrial bioenergetics | [34] | |
| Safety | Animal testing to investigate interactions between native tissue and product | [35] | |
| Potency | Cell identity | Flow cytometry and immunohistochemical analysis | [36] |
| Reproducibility | Purity and viability of cell population | [37] | |
| Cell tracking | Fluorescent/superparamagnetic iron oxide cell labeling prior to animal implantation | [35] |
Cell-based therapies work either via stimulation of endogenous repair through extracellular factors or differentiation and functional replacement of endogenous cell types [17]; they include stem cell implantation or infusion to treat hematopoietic diseases, cardiac conditions and Parkinson’s disease. Most of the pioneering work has been performed using haematopoietic stem cells due to the early bone marrow transplant work, making them the most well-studied stem cell type [19]. In particular, adult mesenchymal stem cells have gained interest as they avoid the ethical concerns of using embryonic stem cells, can be rapidly expanded in vitro and avoid immunogenicity. Studies have shown contradictory results on the efficacy of the transplanted cells, with patient variability with regards to response (Table 1); further work is needed to elucidate cell identity and health to ensure patient safety (Table 2).
The tissue engineering strand of regenerative medicine incorporates cells with biodegradable scaffolds to engineer replacement tissues like dermis or cartilage [20] and whole organs such as trachea and bladder [21, 22]. Limitations of synthetic polymer scaffolds, such as infection, extrusion and degradation product toxicity, have encouraged interest in decellularised matrices as well biologics for use as scaffolds as one of the more effective ways of replicating native tissue anisotropy [21, 22]. Decellularised matrices provide durability, enhanced integration and biocompatibility whilst avoiding allosensitization [21]. This may explain why many of the significant breakthroughs and first-in-man studies have utilized this technique combined with autologous cell-seeding with some success [21–23], and even showed promise in vitro for more complex structures such as pulmonary and aortic valves as well as whole organs such as heart and liver [24, 25]. However, despite early interest and investment in tissue engineering research, with annual R&D spending estimated at US$580 million, initial clinically applicable product release has been slow but steady [26].
Controversies in the field
The regenerative medicine field has been shrouded in controversy. Significant potential gains have led to several high profile allegations of research misconduct [27, 28]. There is also a growing stem cell tourism industry based on unproven treatments that aims to capitalize on stem cell hype [29, 30]. Desperate patients would rather approach private clinics offering experimental stem cell treatments, with unproven safety and efficacy profiles, than wait for outcomes of clinical trials [30]. Media coverage and direct advertising of stem cell therapies as well as the political, ethical and religious controversies surrounding human embryonic stem cells, can contribute not only to increased public awareness but also inflated expectations of regenerative medicine products, and there continues to be a significant gap between the perceived and realistic benefits [31]. A concerted effort from the scientific community as well as robust outcome data from clinical trials will be needed to temper unrealistic claims [16, 17].
Conclusion
Medical breakthroughs often require the convergence of multiple scientific advances for which interdisciplinary collaboration is fundamental. Similar to transplant medicine, regenerative medicine requires the convergence of a number of scientific disciplines, including stem cell biology, developmental and molecular biology, engineering and biomaterials. Despite media hype, scientific overclaim and unrealistic expectations, which have been previously witnessed for a number of healthcare technologies, regenerative medicine continues to make steady progress reflected by the increasing number of clinical trials [16, 17]. Significant potential has been demonstrated in the cell therapy field to treat haematological, neurological and rheumatological conditions. The tissue engineering field, although holding great promise, still has some way to develop before the excitement surrounding novel biofabrication strategies, such as 3D bioprinting, is translated to patient care. The fast moving and versatile field of regenerative medicine is at the cutting edge of translational research and could shift the paradigm in healthcare from symptomatic to curative treatment. BMC Medicine is very interested in breakthroughs in regenerative medicine/stem cell therapy and submission of such relevant articles is encouraged.
Acknowledgements
Mr. Steve Atherton RMIP MIMI, Medical Illustrator, ABMU Health Board for Fig. 1.
Authors’ contributions
ZMJ, AA and WF undertook a literature review, collated data and wrote the final manuscript. ISW conceived the manuscript, contributed content and provided a critical overview. All authors read and approved the final manuscript.
Authors’ information
Ms Zita M. Jessop is a Medical Research Council (MRC) Clinical Research Training Fellow in the Reconstructive Surgery & Regenerative Medicine Research Group, at Swansea University Medical School and a Clinical Lecturer on the Welsh Clinical Academic Track Fellowship Scheme.
Dr Ayesha Al-Sabah is a Postdoctoral Scientist in the Reconstructive Surgery & Regenerative Medicine Research Group, at Swansea University Medical School.
Dr Wendy R. Francis is a Postdoctoral Scientist at the Centre for NanoHealth at Swansea University.
Professor Iain S Whitaker is the Chair of Plastic & Reconstructive Surgery and Director of the Reconstructive Surgery & Regenerative Medicine (ReconRegen) Research Group at Swansea University Medical School and Honorary Consultant Plastic Surgeon at The Welsh Centre for Burns & Plastic Surgery.
Competing interests
There are no sources of financial or other support, or any financial or professional relationships that might pose a competing interest for any of the authors.
References
- 1.Calne R. The history and development of organ transplantation: biology and rejection. Baillieres Clin Gastroenterol. 1994;8:389–97. doi: 10.1016/0950-3528(94)90026-4. [DOI] [PubMed] [Google Scholar]
- 2.Haseltine W. Regenerative medicine 2003: an overview. J Regener Med. 2003;4:15–8. [Google Scholar]
- 3.Landecker H. Culturing Life. Cambridge, MA: Harvard University Press; 2009. [Google Scholar]
- 4.Stevens LC. The development of transplantable teratocarcinomas from intratesticular grafts of pre- and postimplantation mouse embryos. Developmen Biol. 1970;21:364–82. doi: 10.1016/0012-1606(70)90130-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans MJ, Kaufman MH. Establishment in culture of pluripotential cells from mouse embryos. Nature. 1981;292:154–6. doi: 10.1038/292154a0. [DOI] [PubMed] [Google Scholar]
- 6.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 7.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–76. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Sunderland ME. Studying Development: The Value of Diversity, Theory, and Synthesis. PhD Thesis. Tempe, AZ: Arizona State University; 2008. [Google Scholar]
- 9.O’Dowd A. Peers call for UK to harness “enormous” potential of regenerative medicine. BMJ. 2013;347:f4248. doi: 10.1136/bmj.f4248. [DOI] [PubMed] [Google Scholar]
- 10.Mason C, Dunnill P. The strong financial case for regenerative medicine and the regen industry. Regen Med. 2008;3(3):351–63. doi: 10.2217/17460751.3.3.351. [DOI] [PubMed] [Google Scholar]
- 11.Orlando G, Wood KJ, Stratta RJ, Yoo JJ, Atala A, Soker S. Regenerative medicine and organ transplantation: past, present, and future. Transplantation. 2011;91(12):1310–7. doi: 10.1097/TP.0b013e318219ebb5. [DOI] [PubMed] [Google Scholar]
- 12.Tsukamoto A, Abbot SE, Kadyk LC, DeWitt ND, Schaffer DV, Wertheim JA, Whittlesey KJ, Werner MJ. Challenging regeneration to transform medicine. Stem Cells Transl Med. 2006;5(1):1–7. doi: 10.5966/sctm.2015-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nelson TJ, Behfar A, Terzic A. Strategies for therapeutic repair: the “R3” regenerative medicine paradigm. Clin Transl Sci. 2008;1:168–71. doi: 10.1111/j.1752-8062.2008.00039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.World Regenerative Medicines Market - Opportunities and Forecasts, 2013–2020. Allied Market Research Report. 2014. https://www.alliedmarketresearch.com/regenerative-medicines-market. Accessed 6 July 2016.
- 15.Remarks of President Barack Obama. The White House. 2009. https://www.whitehouse.gov/the-press-office/remarks-president-prepared-delivery-signing-stem-cell-executive-order-and-scientifi. Accessed 6 July 2016.
- 16.Trounson A. New perspectives in human stem cell therapeutic research. BMC Med. 2009;7:29. doi: 10.1186/1741-7015-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Trounson A, Thakar RG, Lomax G, Gibbons D. Clinical trials for stem cell therapies. BMC Med. 2011;9:52. doi: 10.1186/1741-7015-9-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee MH, Arcidiacono JA, Bilek AM, Wille JJ, Hamill CA, Wonnacott KM, Wells MA, Oh SS. Considerations for tissue-engineered and regenerative medicine product development prior to clinical trials in the United States. Tissue Eng Part B Rev. 2010;16(1):41–54. doi: 10.1089/ten.teb.2009.0449. [DOI] [PubMed] [Google Scholar]
- 19.Ford CE, Hamerton JL, Barnes DW, Loutit JF. Cytological identification of radiation-chimaeras. Nature. 1956;177:452–4. doi: 10.1038/177452a0. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Vacanti JP, Paige KT, Upton J, Vacanti CA. Transplantation of chondrocytes utilizing a polymer-cell construct to produce tissue-engineered cartilage in the shape of a human ear. Plast Reconstr Surg. 1997;100:297–304. doi: 10.1097/00006534-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 21.Macchiarini P, Jungebluth P, Go T, Asnaghi MA, Rees LE, Cogan TA, Dodson A, Martorell J, Bellini S, Parnigotto PP, Dickinson SC, Hollander AP, Mantero S, Conconi MT, Birchall MA. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–30. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 22.Atala A, Bauer SB, Soker S, Yoo JJ, Retic AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 23.Gonfiotti A, Jaus MO, Barale D, Baiguera S, Comin C, Lavorini F, Fontana G, Sibila O, Rombolà G, Jungebluth P, Macchiarini P. The first tissue-engineered airway transplantation: 5-year follow-up results. Lancet. 2014;383(9913):238–44. doi: 10.1016/S0140-6736(13)62033-4. [DOI] [PubMed] [Google Scholar]
- 24.Ott HC, Matthiesen TS, Goh SK, Black LD, Kren SM, Netoff TI. Perfusion-decellularized matrix: using nature’s platform to engineer a bioartificial heart. Nat Med. 2008;14(2):213–21. doi: 10.1038/nm1684. [DOI] [PubMed] [Google Scholar]
- 25.Soto-Gutierrez A, Zhang L, Medberry C, Fukumitsu K, Faulk D, Jiang H. A whole-organ regenerative medicine approach for liver replacement. Tissue Eng Part C Methods. 2011;17(6):677–86. doi: 10.1089/ten.tec.2010.0698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kemp P. History of regenerative medicine: looking backwards to move forwards. Regen Med. 2006;1(5):653–69. doi: 10.2217/17460751.1.5.653. [DOI] [PubMed] [Google Scholar]
- 27.Cyranoski D. Acid bath offers easy path to stem cells. Nature News. 2014. http://www.nature.com/news/acid-bath-offers-easy-path-to-stem-cells-1.14600. Accessed 6 July 2016 [DOI] [PubMed]
- 28.Vogel G. Regenerative medicine. Report finds misconduct by surgeon. Science. 2015;348(6238):954–5. doi: 10.1126/science.348.6238.954-b. [DOI] [PubMed] [Google Scholar]
- 29.Alta CR. On the road (to a cure?) — Stem-cell tourism and lessons for gene editing. N Engl J Med. 2016;374:901–3. doi: 10.1056/NEJMp1600891. [DOI] [PubMed] [Google Scholar]
- 30.Matthews KR, Iltis AS. Unproven stem cell-based interventions and achieving a compromise policy among the multiple stakeholders. BMC Med Ethics. 2015;16:75. doi: 10.1186/s12910-015-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bubela T, Li MD, Hafez M, Bieber M, Atkins H. Is belief larger than fact: expectations, optimism and reality for translational stem cell research. BMC Med. 2012;10:133. doi: 10.1186/1741-7015-10-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galbraith DN. Regulatory and microbiological safety issues surrounding cell and tissue-engineering products. Biotechnol Appl Biochem. 2004;40:35–9. doi: 10.1042/BA20030163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Carpenter MK, Frey-Vasconcells J, Rao MS. Developing safe therapies from human pluripotent stem cells. Nat Biotechnol. 2009;27:606–13. doi: 10.1038/nbt0709-606. [DOI] [PubMed] [Google Scholar]
- 34.Chacko BK, Kramer PA, Ravi S, Benavides GA, Mitchell T, Dranka BP, Ferrick D, Singal AK, Ballinger SW, Bailey SM, Hardy RW, Zhang J, Zhi D, Darley-Usmar VM. The Bioenergetic Health Index: a new concept in mitochondrial translational research. Clin Sci (Lond) 2014;127:367–73. doi: 10.1042/CS20140101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Progatzky F, Dallman MJ. Lo Celso. From seeing to believing: labelling strategies for in vivo cell-tracking experiments. Interface. Focus. 2013;3(3):20130001. doi: 10.1098/rsfs.2013.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Christensen JL, Weissman IL. Flk-2 is a marker in hematopoietic stem cell differentiation: A simple method to isolate long-term stem cells. Proc Natl Acad Sci. 2001;98:14541–14546. doi: 10.1073/pnas.261562798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ram-Liebig G, Bednarz J, Stuerzebecher B, Fahlenkamp D, Barbagli G, Romano G, Balsmeyer U, Spiegeler ME, Liebig S, Knispel H. Regulatory challenges for autologous tissue engineered products on their way from bench to bedside in Europe. Adv Drug Deliv Rev. 2015;82-83:181–91. doi: 10.1016/j.addr.2014.11.009. [DOI] [PubMed] [Google Scholar]


