Abstract
Purpose
Whether racial differences exist in the pattern of local disease progression among men treated with radical prostatectomy (RP) for localized prostate cancer (PCa) is currently unknown. In this study we evaluate the pattern of adverse pathologic features in an identical cohort of AA and Caucasian (CS) men with PCa.
Methods
The overall cohort consisted of 1,104 men (224 AA, and 880 CS) who underwent RP between 1990 and 2012. We compared preoperative factors and pathologic outcomes following RP across race groups. Multivariate analysis was used to identify factors predictive of adverse pathologic outcomes. The impact of race on adverse pathologic outcomes and biochemical control rate (BCR) was evaluated using multivariate regression model and Kaplan-Meier analysis.
Results
The 10-year BCR was 59 % vs. 82% in AA and CS men, respectively (p=0.003). There was no significant difference in extraprostatic spread (EPE; p = 0.14), positive surgical margin (SM; p = 0.81), lymph node involvement (LNI: p = 0.71) or adverse pathologic features (p = 0.16) across race groups. However, among patients with ≥1 adverse pathologic features, AA men had higher rate of seminal vesicle invasion (SVI) as compared with CS men (51% vs 30%; P = 0.01). Upon adjusting for known predictors of adverse pathologic features AA race remained a predictor of SVI.
Conclusions
AA men have increased risk of SVI following RP, particularly among men with Gleason ≤6 disease. This may represent racial differences in the biology of PCa disease progression contributing to poorer outcomes in AA men.
Keywords: African American race, disparities, biochemical failure, adverse pathologic features, seminal vesicle invasion
Introduction
African-American (AA) men are known to experience greater incidence of and mortality from prostate cancer (PCa) than their Caucasian (CS) counterparts1. AA men often present with higher Gleason score and clinical stage of disease at time of diagnosis2–4. A number of studies evaluating outcomes by race following radical prostatectomy have consistently reported a higher rate of adverse pathologic features among AA men2,3,5. These adverse features include higher pathologic Gleason grade, positive surgical margins (SM), seminal vesicle invasion (SVI), extraprostatic extension (EPE) and lymph node involvement (LNI). Other studies have reported worse biochemical control rate (BCR) in AA men despite favorable pathologic features following RP. A study from the SEARCH database showed that despite early clinical stage presentation and similar pathologic characteristics AA men were found to be at a slightly increased risk of biochemical disease recurrence6. A relatively large study on results from the Shared Equal Access Regional Cancer Hospital (SEARCH) and the Duke Prostate Center (DPC) databases also showed that despite favorable clinical or pathological staging and low risk disease at time of diagnosis, AA men were found to be at an increased risk of biochemical disease recurrence after RP7.
A recent study from the Johns Hopkins Hospital evaluated pathologic specimen of 200 prostatectomy samples (100 Caucasian men and 100 AA men) from men with very low-risk disease to determine whether there are systematic pathological differences by race. They demonstrated that AA men had a significantly higher prevalence of anterior cancer foci of higher grade and larger volume as compared to Caucasian men8. Whether differing patterns of local disease progression between race groups may account for worse outcomes in AA men following RP is currently unknown. To this end we evaluated the pattern of adverse pathologic features and biochemical outcomes among an identical cohort of AA and CS men treated with radical prostatectomy.
Materials and Methods
Patient selection
The present study is a retrospective analysis of a prospective cohort of 1104 men (224 AA, 880 CS) with PCa treated with RP at the University of Pennsylvania Health System (UPHS; Philadelphia, PA) recruited to the Study of Clinical Outcomes, Risk and Ethnicity (SCORE) between 1990 and 20129. The SCORE study includes information on patient age, race, height, weight, clinical stage, clinical Gleason on diagnostic biopsy, preoperative prostate-specific antigen (PSA) levels, surgical pathologic information (tumor grade, stage, surgical margins status, extraprostatic extension, or seminal vesicle involvement, lymph node status) and followup PSA levels for a mean and median of 51 and 29 months (IQR 10 to 79). Patients who received androgen depravation therapy (ADT) or adjuvant radiotherapy (RT) and/or ADT were included. Patients without adequate preclinical data including initial PSA, or biopsy GS at diagnosis were excluded from the analysis. Patients of non-CS and non-AA ethnicity were excluded. This study was approved by our Institutional Review Board.
Preoperative staging
Patients were evaluated at time of diagnosis by a thorough history and physical examination (including digital rectal examination [DRE]) followed by routine laboratory studies, including serum PSA levels, and GS determined by needle biopsy and reviewed at the UPHS. All patients were staged according to the 1992 American Joint Committee on Cancer staging system.10
Treatment
Surgical treatment consisted of a radical retropubic prostatectomy or robotic-assisted radical prostatectomy, and bilateral pelvic lymph node sampling. Adverse pathologic features consisting of extraprostatic extension (EPE), seminal vesicle invasion (SVI), surgical margin status (SM), and lymph node involvement (LNI) were noted and recorded. At the discretion of the treating physician, patients with adverse pathologic features including EPE, SVI, positive SM, or LNI were treated with adjuvant RT and/or ADT. ADT consisted of a gonadotropin-releasing hormone agonist (leuprolide acetate or goserelin acetate) with or without an antiandrogen (e.g. flutamide, bicalutamide).
Follow-Up and treatment endpoints
Patient information at each follow-up visit including DRE and serial PSA values were noted and recorded. PSA failure was defined as a single PSA≥0.2ng/ml or when two consecutive PSA values of 0.2ng/ml were obtained after an undetectable value. Start of the prospective follow-up (i.e., time zero) was defined at the date of surgery for all patients. If PSA was never undetectable postoperatively, then PSA failure was assigned at time zero. The median duration of follow up from time of surgery until last follow up PSA date was 27 months (range 1 to 207).
Statistical analysis
Clinical and pathologic variables were compared across the race groups using an analysis of variance model for continuous variables or contingency table χ2 test of homogeneity for categorical variables. Predictors of adverse pathologic features were examined using logistic regression models. Age, PSA, and year of surgery were examined as continuous variables. T-stage (T1a–c vs. T2), biopsy GS, and race were examined as categorical variables.
For multivariate analysis of factors predicting adverse pathologic features, a forward-stepwise logistic regression model was used with p<0.2 determining which variables were entered into the model at each step. The variable with the highest p value was successively deleted until only variables with p<0.2 remained. For survival analysis, the primary event of interest was PSA failure (biochemical disease recurrence). Individuals who did not experience PSA failure were censored at the time of last PSA measurement <0.2 ng/dl, or loss to follow-up. Time to PSA failure was used as a surrogate for biochemical control rate (BCR). The BCR was compared across the groups using a log-rank survivorship and Kaplan-Meier analyses. Analyses were conducted using STATA statistical software version 13.0 (STATA Corporation).
Results
The patient clinical and pathologic characteristics are listed in Table 1. Preoperative factors such as age, clinical T-stage, and year of RP were similar between groups. AA men had higher PSA at diagnosis (p<0.001), and biopsy Gleason score (p<0.001), and body mass index ≥30kg/m2 (p<0.001). Following RP, AA men were found to have higher pathologic Gleason score (p<0.001), as well as seminal vesicle invasion compared to CS men (5% vs. 10%; p=0.001). There was no significant difference in rate of Gleason score upgrade (31% vs 32%; p=0.93), extraprostatic spread (23% vs 27%; p = 0.14), positive surgical margin (17% vs 18%; p = 0.81), and lymph node involvement (0.7% vs 0.4%; p = 0.71) across race groups. The use of post-operative radiotherapy was higher in AA men compared with CS men (4% vs 1.5%, p = 0.016).
TABLE 1.
Pre-treatment characteristics and pathologic outcomes of a cohort of prostate cancer patients undergoing radical prostatectomy at University of Pennsylvania, 1990–2012.
| Caucasian Cohort; n=880 |
% | African-American Cohort; n=224 |
% | p- value | |
|---|---|---|---|---|---|
| Age, Y | 0.05 | ||||
| Median | 60 | 59 | |||
| mean | 59.3 | 58.3 | |||
| IQR | 55 – 64 | 53 to 63 | |||
|
| |||||
| iPSA ng/ml | <0.001 | ||||
| 0–4.0 | 231 | 27 | 54 | 25 | |
| 4.1–10 | 548 | 63 | 126 | 57 | |
| 10.1 – 20 | 78 | 9 | 24 | 11 | |
| > 20 | 12 | 1 | 15 | 7 | |
| median | 5 | 5.2 | |||
| mean | 5.7 | 6.67 | |||
| IQR | 4 –6.4 | 4.1 – 8.1 | |||
|
| |||||
| Clinical Stage | 0.25 | ||||
| T1A-C | 653 | 81 | 184 | 84 | |
| T2A | 121 | 15 | 27 | 12 | |
| T2B | 12 | 2 | 6 | 3 | |
| T2C | 18 | 2 | 2 | 1 | |
|
| |||||
| cGleason Score | <0.001 | ||||
| ≤6 | 683 | 82 | 133 | 61 | |
| 7 | 108 | 13 | 70 | 32 | |
| 8 to 10 | 39 | 5 | 15 | 7 | |
|
| |||||
| pGleason Score | <0.001 | ||||
| ≤6 | 472 | 54 | 87 | 39 | |
| 7 | 366 | 41 | 120 | 54 | |
| 8 to 10 | 42 | 5 | 17 | 7 | |
|
| |||||
| Gleason score upgrade | 260 | 31 | 69 | 32 | 0.93 |
|
| |||||
| Nodal Status | 0.7 | ||||
| pN0 | 858 | 99 | 212 | 100 | |
| pN1 | 6 | 0.7 | 1 | 0.4 | |
|
| |||||
| Seminal vesicle invasion | 41 | 5 | 23 | 10 | 0.001 |
|
| |||||
| Extracapsular spread | 198 | 23 | 61 | 27 | 0.14 |
|
| |||||
| Positive surgical margin | 148 | 17 | 39 | 18 | 0.81 |
|
| |||||
| Year of prostatectomy | 0.64 | ||||
| median | 2004 | 2005 | |||
| mean | 2004 | 2004.2 | |||
| IQR | 2000–2008 | 2001–2008 | |||
|
| |||||
| Radiotherapy | 13 | 1.5 | 9 | 4 | 0.016 |
|
| |||||
| ADT | 44 | 5 | 7 | 3 | 0.22 |
|
| |||||
| Body Mass Index (Kg/m2) | <0.001 | ||||
| <30 | 510 | 65 | 107 | 50 | |
| ≥30 | 279 | 35 | 107 | 50 | |
NOTE. Boldfaced values represent statistically significant differences between groups.
Abbreviations: iPSA- initial Prostate-specific antigen, IQR- interquartile range; ADT- Androgen depravation therapy
Among patients with low Gleason score disease defined as biopsy Gleason score ≤6, AA men were diagnosed at a slightly younger age (median age: 58 vs 59; p=0.04), and higher SVI (8% vs 2%; p<0.001) compared with CS men, (Table 2). There was no significant difference in any clinico-pathologic parameter including rate of SVI by race among patients with Gleason score of ≥7 (22% vs 26%; p=0.61; data not shown). Among patients with SVI, median PSA was two-fold higher in AA men compared to CS men (12.3 vs 6.5; p = 0.02). However, there was no significant difference in prostate size, percentage gland involvement, SVI laterality, and SVI with concurrent EPE or SM across race groups (Table 3).
TABLE 2.
Tumor characteristics and pathologic outcomes of prostate cancer patients with biopsy Gleason ≤6 disease following radical prostatectomy at University of Pennsylvania, 1990–2012.
| Caucasian Cohort n=760 |
% | African-American Cohort n=176 |
% | p- Value | |
|---|---|---|---|---|---|
| Age, Y | 0.04 | ||||
| Median | 59 | 58 | |||
| mean | 58.9 | 57.8 | |||
| IQR | 54–64 | 52–62 | |||
|
| |||||
| iPSA ng/ml | 0.09 | ||||
| 0–4.0 | 215 | 29 | 48 | 28 | |
| 4.1–10 | 468 | 62 | 101 | 58 | |
| 10.1 – 20 | 62 | 8 | 18 | 10 | |
| > 20 | 8 | 1 | 6 | 4 | |
| median | 5 | 5.2 | |||
| mean | 5.7 | 6.67 | |||
| IQR | 4 – 6.4 | 4.1 – 8.1 | |||
|
| |||||
| Clinical Stage | 0.2 | ||||
| T1A-C | 594 | 82 | 145 | 83 | |
| T2A | 112 | 15 | 23 | 15 | |
| T2B | 8 | 1 | 5 | 1 | |
| T2C | 12 | 2 | 1 | 1 | |
|
| |||||
| Adverse pathologic features‡ | 0.2 | ||||
| 0 | 575 | 76 | 122 | 69 | |
| 1 | 92 | 12 | 25 | 14 | |
| >2 | 93 | 12 | 29 | 17 | |
|
| |||||
| Seminal Vesicle invasion | 17 | 2 | 14 | 8 | <0.001 |
|
| |||||
| Extraprostatic spread | 150 | 20 | 44 | 25 | 0.12 |
|
| |||||
| Lymph Node Involvement | 2 | 0.3 | 0 | 0 | 0.5 |
|
| |||||
| Positive surgical margin | 119 | 16 | 29 | 17 | 0.76 |
NOTE. Boldfaced values represent statistically significant differences between groups.
Abbreviations: iPSA- initial Prostate-specific antigen, IQR- interquartile range;
Adverse pathologic features: extraprostatic extension, seminal vesicle invasion, positive surgical margin and/or lymph node involvement.
TABLE 3.
Pathologic characteristics of prostate cancer patients with seminal vesicle invasion following radical prostatectomy at University of Pennsylvania, 1990–2012.
| Caucasian Cohort n=41 |
% | African-American Cohort n=23 |
% | p- Value | |
|---|---|---|---|---|---|
| iPSA ng/ml | 0.02 | ||||
| 0–4.0 | 4 | 10 | 1 | 5 | |
| 4.1–10 | 22 | 56 | 8 | 36 | |
| 10.1 – 20 | 12 | 31 | 7 | 32 | |
| > 20 | 1 | 3 | 6 | 27 | |
| median | 6.5 | 12.3 | |||
| mean | 9.4 | 17 | |||
| IQR | 4.6 –11.6 | 7 –22 | |||
|
| |||||
| Prostate Size | 0.3 | ||||
| <50cc | 20 | 56 | 14 | 70 | |
| >50cc | 16 | 44 | 6 | 3 | |
| median | 47.7 | 45 | |||
| mean | 52 | 47 | |||
| IQR | 41 –56 | 34 – 56 | |||
|
| |||||
| Gland involvement (%) | 0.8 | ||||
| <10 | 10 | 27 | 4 | 18 | |
| 10 to 25 | 10 | 27 | 8 | 36 | |
| 25 to 50 | 10 | 27 | 7 | 32 | |
| > 50 | 7 | 19 | 3 | 14 | |
|
| |||||
| Seminal vesicle invasion | 0.3 | ||||
| Unilateral | 26 | 67 | 18 | 78 | |
| Bilateral | 13 | 33 | 5 | 22 | |
|
| |||||
| Extraprostatic spread | 0.4 | ||||
| Yes | 37 | 90 | 19 | 83 | |
| No | 4 | 10 | 4 | 17 | |
|
| |||||
| Positive Surgical Margins | 0.7 | ||||
| Yes | 21 | 51 | 10 | 45 | |
| No | 20 | 49 | 12 | 55 | |
NOTE. Boldfaced values represent statistically significant differences between groups.
Abbreviations: iPSA- initial Prostate-specific antigen, IQR- interquartile range.
Using the Kaplan-Meier survival analysis method, the impact of race on BCR was evaluated. The mean and median follow-up time from RP date until last follow-up PSA date was 45 months and 28.6 (range 1 to 207) months respectively. The median follow up for CS men was 27 months vs. 34.3 months for CS and AA men respectively. During this time period, 129 patients (11.6%) experienced biochemical recurrence. As shown in Figure 1, there was no difference in rate of adverse pathologic features between race groups (p=0.2; Fig 1A), however, AA men experienced worse 10-year biochemical control rates compared with CS men (59 % vs 82%, p = 0.003; Fig 1B). Using a Cox proportional hazard model, the predictors of biochemical control following RP were determined, (Table 4). In the multivariate model race (HR 0.63, 95%CI 0.39–0.99; p=0.028), T stage (HR 0.53, 95%CI 0.30–0.93; p=0.03), clinical Gleason score (HR 3.2, 95%CI 1.98–5.17; p<0.001), pathologic Gleason score (HR 2.11, 95%CI 1.47–3.02; p=0.001), EPE (HR 2.32, 95%CI 1.39–3.87; p=0.001), and SVI (HR 3.29, 95%CI 1.80–6.04; p<0.001) were predictors of biochemical recurrence.
FIG. 1.
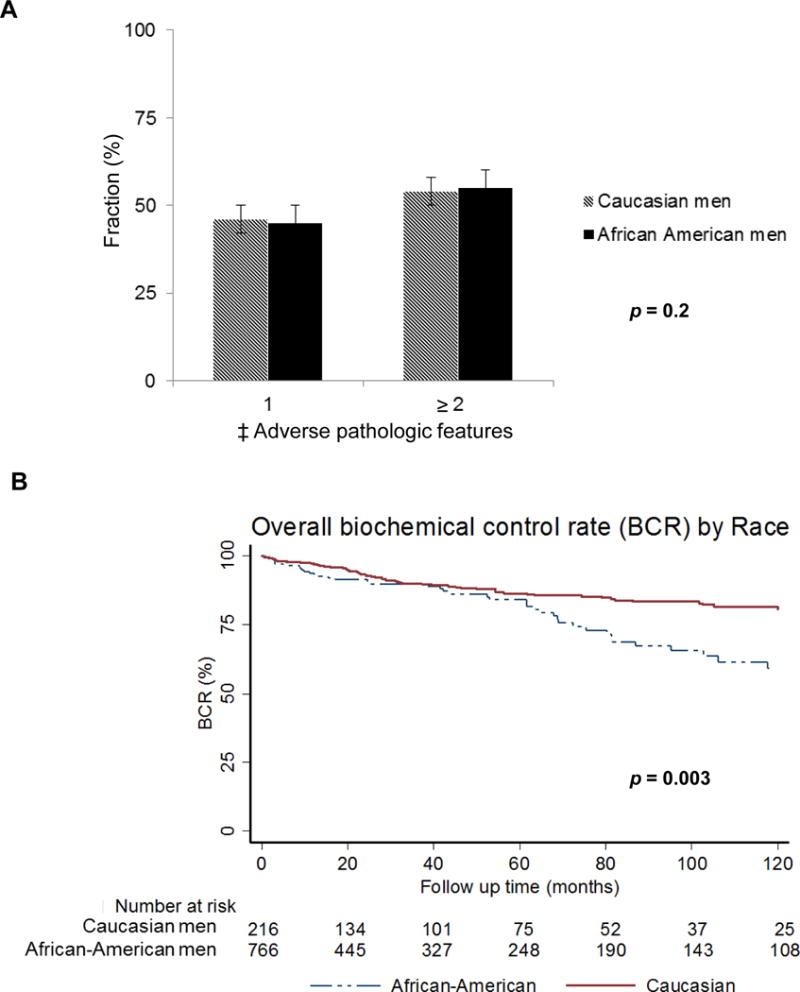
(A) Distribution of adverse pathologic features by race, and (B) Kaplan-Meier curves for biochemical control rate by race in prostate cancer patients treated with radical prostatectomy at University of Pennsylvania, 1990–2012.
Abbreviations: BCR- Biochemical control rate. ‡Adverse pathologic features: extraprostatic extension, seminal vesicle invasion, positive surgical margin and/or lymph node involvement.
TABLE 4.
Univariate and multivariate regression models of factors predicting biochemical recurrence in prostate cancer patients following radical prostatectomy at University of Pennsylvania, 1990–2012.
| Cox proportional regression analysis of factors predicting biochemical recurrence
| |||
|---|---|---|---|
| Univariate analysis | HR | 95% CI | p-Value |
| Age | 0.99 | 0.97 to 1.03 | 0.97 |
| Race | 1.73 | 1.20 to 2.51 | 0.003 |
| Serum PSA | 1.06 | 1.04 to 1.08 | <0.001 |
| Clinical stage | 1.29 | 0.84 to 1.99 | 0.24 |
| Clinical gleason score | 4.53 | 3.10 to 6.61 | <0.001 |
| Extraprostatic spread | 4.21 | 2.97 to 5.97 | <0.001 |
| Positive surgical margins | 4.09 | 2.88 to 5.82 | <0.001 |
| Seminal vesicle invasion | 5.07 | 3.34 to 7.69 | <0.001 |
| Pathologic gleason score | 3.28 | 2.49 to 4.31 | <0.001 |
| Body Mass Index | 1.07 | 1.02 to 1.11 | 0.002 |
| Adjuvant radiotherapy | 5.5 | 2.99 to 10.15 | <0.001 |
|
| |||
| Multivariate model of factors predicting biochemical recurrence | |||
| Age | 0.99 | 0.96 to 1.02 | 0.44 |
| Race | 0.63 | 0.39 to 0.99 | 0.028 |
| Serum PSA | 1.02 | 0.99 to 1.05 | 0.18 |
| Clinical stage | 0.53 | 0.30 to 0.93 | 0.03 |
| Clinical gleason score | 3.2 | 1.98 to 5.17 | <0.001 |
| Extraprostatic spread | 2.32 | 1.39 to 3.87 | 0.001 |
| Positive surgical margins | 1.63 | 1.39 to 3.87 | 0.055 |
| Seminal vesicle invasion | 3.29 | 1.80 to 6.04 | <0.001 |
| Pathologic gleason score | 2.11 | 1.47 to 3.02 | 0.001 |
| Body Mass Index | 1.01 | 0.96 to 1.06 | 0.71 |
| Adjuvant radiotherapy | 0.64 | 0.96 to 1.06 | 0.24 |
NOTE. Boldfaced values represent statistically significant differences between groups.
Abbreviations: PSA- Prostate-specific antigen, P values derived from a Cox proportional hazards model. Age, PSA, and body mas index were examined as continuous variables. T-stage (T1a–c vs. T2), clinical Gleason score, and race were examined as categorical variables.
Using the logistic regression model predictors of adverse pathologic features following RP were determined, (Table 5). In the initial univariate analysis, age, AA race, serum PSA, clinical T-stage, Gleason score, and body mass index were predictors of seminal vesicle invasion. However in the multivariate model, age (HR 1.06, 95%CI 1.01–1.12; p=0.02), serum PSA (HR 1.12, 95%CI 1.07–1.17; p<0.001), clinical Gleason score ≤6 (HR 5.18, 95%CI 2.67–10.1; p<0.001), and AA race (HR 2.00, 95%CI 1.01–3.94; p=0.046), were the only significant predictors of seminal vesicle invasion, (Table 5). Thus, upon adjusting for known predictors of adverse pathologic features AA race remained an independent predictor of seminal vesicle invasion. Using the multivariate model, AA race was not a significant predictor of EPE, positive SM, or LNI. However, known predictors of adverse pathologic features such as serum PSA, clinical T-stage and biopsy Gleason score remained significant. Of note, body mass index (BMI) was a predictor for EPE (HR 2.81, 95%CI 1.02–1.10; p=0.005), whereas year of prostatectomy predicted LNI (HR 3.31, 95%CI 1.34–8.17; p=0.01). Both BMI and year of prostatectomy were predictive for positive SM, (HR 1.05, 95%CI 1.00–1.09; p=0.025) and (HR 1.05, 95%CI 1.01–1.09; p=0.024), respectively.
TABLE 5.
Univariate and multivariate regression models of factors predicting adverse pathologic outcomes‡ in prostate cancer patients following radical prostatectomy at University of Pennsylvania, 1990–2012.
| Univariate analysis of factors predicting seminal vesicle invasion | |||
|---|---|---|---|
| Variables | HR | 95% CI | p value |
| Age | 1.06 | 1.02 to 1.10 | 0.003 |
| African-American Race | 2.34 | 1.37 to 3.99 | 0.002 |
| Serum PSA | 1.13 | 1.09 to 1.16 | <0.001 |
| Clinical stage | 1.6 | 1.14 to 2.27 | 0.007 |
| Clinical gleason score ≤ or > 6 | 9.1 | 5.21 to 16 | <0.001 |
| Year of prostatectomy | 1.02 | 0.97 to 1.07 | 0.51 |
| Body mass index, continuous | 1.06 | 1.00 to 1.12 | 0.07 |
|
| |||
|
Multivariate forward stepwise model of factors predicting seminal vesicle invasion
| |||
| Variables | HR | 95% CI | p value |
|
| |||
| Age | 1.06 | 1.01 to 1.12 | 0.02 |
| Serum PSA | 1.12 | 1.07 to 1.17 | <0.001 |
| Clinical stage | 1.86 | 0.91 to 3.80 | 0.089 |
| Clinical gleason score ≤ or > 6 | 5.18 | 2.67 to 10.1 | <0.001 |
| Year of prostatectomy | 1.07 | 1.01 to 1.16 | 0.055 |
| African-American Race | 2.00 | 1.01 to 3.94 | 0.046 |
|
| |||
|
Multivariate forward stepwise model of factors predicting extraprostatic extension
| |||
| Variables | HR | 95% CI | p value |
|
| |||
| Serum PSA | 1.12 | 1.08 to 1.16 | <0.001 |
| Clinical stage | 1.69 | 1.08 to 2.64 | 0.02 |
| Clinical gleason score ≤ or > 6 | 2.54 | 1.62 to 3.98 | <0.001 |
| Body mass index, continuous | 2.81 | 1.02 to 1.10 | 0.005 |
|
| |||
|
Multivariate forward stepwise model of factors predicting positive surgical margin
| |||
| Variables | HR | 95% CI | p value |
|
| |||
| Serum PSA | 1.08 | 1.04 to 1.11 | <0.001 |
| Clinical gleason score ≤ or > 6 | 1.67 | 1.02 to 2.73 | 0.04 |
| Year of prostatectomy | 1.05 | 1.01 to 1.09 | 0.024 |
| Body mass index, continuous | 1.05 | 1.00 to 1.09 | 0.025 |
|
| |||
|
Multivariate forward stepwise model of factors predicting Lymph node involvement
| |||
| Variables | HR | 95% CI | p value |
|
| |||
| Age | 0.89 | 0.78 to 1.03 | 0.13 |
| Serum PSA | 1.2 | 1.07 to 1.34 | 0.003 |
| Clinical gleason score ≤ or > 6 | 7.22 | 0.92 to 56.58 | 0.06 |
| Year of prostatectomy | 3.31 | 1.34 to 8.17 | 0.01 |
NOTE. Boldfaced values represent statistically significant differences between groups.
Abbreviations: PSA- Prostate-specific antigen, CI- confidence intervals,
Adverse pathologic features: seminal vesicle invasion, extraprostatic extension, positive surgical margin, and/or lymph node involvement. P values were derived from a forward-stepwise logistic regression model. Predictors of adverse pathologic features were examined using logistic regression models. Age, PSA, and year of surgery were examined as continuous variables. T-stage (T1a–c vs. T2), clinical Gleason score, and race were examined as categorical variables.
Discussion
In this report we show that AA men had worse BCR (Fig. 1) despite similar rate of Gleason score upgrading, and adverse pathologic features following RP. This remained true after accounting for other risk factors including body mass index, adverse pathologic features and use of adjuvant radiotherapy in a multivariate model (Table 4). Furthermore, among patients with ≥1 adverse pathologic features, AA men had higher rate of seminal vesicle invasion (Table 1). This trend held true for patients with low Gleason score ≤6 disease (Table 2) however, there was no significant difference in any clinico-pathologic parameter including rate of SVI by race among patients with Gleason score of ≥7. This might be due the relatively low patient numbers of patients with Gleason ≥7 disease to detect a difference by race. AA men with SVI were found to have significantly higher PSA compared to their CS counterparts (Table 3). In a multivariate model, AA race remained a significant predictor of seminal vesicle invasion (Table 5).
Patients with seminal vesicle invasion following RP have a poorer prognosis when compared with other adverse pathologic features such as EPE and positive SM11,12. SVI is found in approximately 5–15% of men with radical prostatectomy. Earlier studies evaluating the probability of biochemical recurrence by pathologic stage, Gleason score, and margin status showed that patients with SVI and LNI had similar 5- and 10- year BCR of 37% and 13%, respectively13. Long-term outcomes data on disease progression and survival rates following RP in 3,478 consecutive patients showed a 10-year disease BCR of only 26% in patient with SVI, even though some patients received adjuvant radiotherapy14. In a more recent study by Pierorazio et, al.15, patients with SVI had a 75% biochemical failure rate and 23% death rate by 12 years. In another study in which patients with LNI was excluded, patients with SVI only had a biochemical fail rate and mortality rate of 73% and 28% respectively, and increased to 88% and 34% in patients with concurrent EPE16. Numerous studies have shown poor outcomes in patients who have SVI, with concurrent Gleason score 8 to 10 disease, positive margins, and/or elevated initial PSA >10ng/ml17–19. These data suggest that SVI portends to a very high risk of failure and poorer prognosis.
D’Amico and colleagues initially proposed that patients with a 2-year PSA failure rate of >50% be offered adjuvant intervention due to the increased risk of subsequent metastatic disease. In that study almost all patient with SVI had a 2-year PSA failure rate of >50%20. Since then, adjuvant RT has been shown in randomized trials to improve PSA-relapse free survival,21–23 distant metastasis-free survival and overall survival,24 in patients with SVI or EPE and/or positive SM. A subset analysis of the South West Oncology Group 8794 trial evaluated the effect of SVI on outcomes after post-prostatectomy adjuvant RT. With a median follow up of 12.2 years the 10-year BCR and overall survival was only 22% and 61%25. However, patients with SVI who received adjuvant radiation showed an improvement in 10-year BCR from 12% to 36%, and overall survival from 51% to 71%. The authors conclude that although SVI is a negative prognostic factor, long-term control is possible especially if patients are given adjuvant radiation therapy25. In an attempt to determine the optimal treatment for patients with SVI after RP, Bastide and colleagues evaluated BCR according to different adjuvant treatments. They report that RP appears to be insufficient as monotherapy in patients with SVI, however ADT in combination with adjuvant RT provided substantial biochemical control benefit8.
The biologic mechanism for the increased SVI among AA men is not well understood. Nonetheless, our data showed that the difference in SVI by race was most significant in patients with biopsy Gleason score ≤6 disease (Table 2). This observation has major implications for treatment recommendations, which includes potentially undertreating biopsy Gleason score 6 disease due to the inability to adequately identify adverse risk features on biopsy. There is recent data to suggest that this could be a result of decreased sensitivity of transrectal ultrasound guided biopsies in AA men due to differences in tumor location by race. A study by Sundi et, al., showed that AA men with very low-risk disease have a significantly higher prevalence of anterior cancer foci that are of higher grade and larger volume26. In fact, this group also showed that in a cohort of patients who were eligible for active surveillance but underwent RP, AA men had a higher frequency of aggressive pathological findings compared to Caucasian men5.
It is imperative to accurately predict the risk of SVI prior to radical prostatectomy in patients with organ-confined PCa. Furthermore, other local therapies such as prostate seed implantation, external beam radiotherapy, and high-intensity focused ultrasound have gained popularity for the treatment organ-confined disease. These modalities although very effective do not always adequately treat cancer in the seminal vesicles. Therefore, better diagnostic techniques are needed to enhance the detection of SVI particularly in AA men prior to the initiation of treatment in order to provide appropriate treatment recommendations and improve outcomes in this patient population. Several studies have developed nomograms to improve the detection of SVI by utilizing the extent and location of cancer in systematic biopsies,27,28 or the status and percentage of cancer in prostate base29. More recently, the clinical utility of endorectal magnetic resonance imaging (MRI) in predicting SVI has gained some attention. A report by Wang et al. studied 573 patients to determine whether endorectal MRI findings provided additional value to the existing predictive nomogram for SVI. In this study, they showed that the addition of endorectal MRI findings significantly improved predictability of SVI above either nomogram alone or MRI alone30. Given the data from this study and others, AA men with low Gleason grade ≤6 disease and elevated PSA may require a more thorough staging work up to include endorectal MRI, with or without saturation biopsies as part of the initial assessment prior to recommending treatment.
A major limitation to this study is that it has a relatively small number of AA compared with CS men, and represents the experience from a single tertiary center. BCR outcomes were not adjusted for socioeconomic factors, diet, and comorbid conditions, and adherence to treatment recommendations. Information on tumor volume or percentage of cores positive for tumor were inconsistently reported, and hence we could not adequately evaluate the impact of these factors on the patterns of local PCa disease progression.
In conclusion, AA race predicts for increased SVI in a cohort of PCa patients with low Gleason disease following RP. This pattern of local disease progression may contribute to the observed worse BCR outcomes in AA men. This highlights the need for further studies to evaluate the biologic mechanisms that may contribute to disparities in patterns of local disease progression and aggressive phenotype in this patient population.
Acknowledgments
This work was funded in part by PHS grants R01-CA085074, P50-CA105641, P60-MD-006900 (to T.R.R.), and DOD grant PC-121189, and the Prostate Cancer Foundation Young Investigator Award (to K.Y.).
Role of Funding Source: provided funding for SCORE program, raw data acquisition as well as patient follow up data management infrastructure.
Footnotes
Disclaimer/Conflict of interest: none to disclose
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010 Aug;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Thompson I, Tangen C, Tolcher A, Crawford E, Eisenberger M, Moinpour C. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J Natl Cancer Inst. 2001 Feb 7;93(3):219–225. doi: 10.1093/jnci/93.3.219. [DOI] [PubMed] [Google Scholar]
- 3.Fowler JE, Jr, Terrell F. Survival in blacks and whites after treatment for localized prostate cancer. J Urol. 1996 Jul;156(1):133–136. [PubMed] [Google Scholar]
- 4.Roach M, 3rd, Krall J, Keller JW, et al. The prognostic significance of race and survival from prostate cancer based on patients irradiated on Radiation Therapy Oncology Group protocols (1976–1985) Int J Radiat Oncol Biol Phys. 1992;24(3):441–449. doi: 10.1016/0360-3016(92)91058-u. [DOI] [PubMed] [Google Scholar]
- 5.Sundi D, Ross AE, Humphreys EB, et al. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy: should active surveillance still be an option for them? J Clin Oncol. 2013 Aug 20;31(24):2991–2997. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamilton RJ, Aronson WJ, Presti JC, Jr, et al. Race, biochemical disease recurrence, and prostate-specific antigen doubling time after radical prostatectomy: results from the SEARCH database. Cancer. 2007 Nov 15;110(10):2202–2209. doi: 10.1002/cncr.23012. [DOI] [PubMed] [Google Scholar]
- 7.Moreira DM, Presti JC, Jr, Aronson WJ, et al. The effect of race on the discriminatory accuracy of models to predict biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. Prostate Cancer Prostatic Dis. Mar;13(1):87–93. doi: 10.1038/pcan.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastide C, Rossi D, Lechevallier E, et al. Seminal vesicle invasion: what is the best adjuvant treatment after radical prostatectomy? BJU international. 2012 Feb;109(4):525–530. doi: 10.1111/j.1464-410X.2011.10332.x. discussion 531–522. [DOI] [PubMed] [Google Scholar]
- 9.Zeigler-Johnson C, Friebel T, Walker AH, et al. CYP3A4, CYP3A5, and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer research. 2004 Nov 15;64(22):8461–8467. doi: 10.1158/0008-5472.CAN-04-1651. [DOI] [PubMed] [Google Scholar]
- 10.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010 Jun;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 11.Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. Journal of Cancer. 2010;2:1–19. [PMC free article] [PubMed] [Google Scholar]
- 12.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010 Feb;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 13.Khan MA, Partin AW, Mangold LA, Epstein JI, Walsh PC. Probability of biochemical recurrence by analysis of pathologic stage, Gleason score, and margin status for localized prostate cancer. Urology. 2003 Nov;62(5):866–871. doi: 10.1016/s0090-4295(03)00674-5. [DOI] [PubMed] [Google Scholar]
- 14.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004 Sep;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 15.Pierorazio PM, Epstein JI, Humphreys E, Han M, Walsh PC, Partin AW. The significance of a positive bladder neck margin after radical prostatectomy: the American Joint Committee on Cancer Pathological Stage T4 designation is not warranted. J Urol. 2010 Jan;183(1):151–157. doi: 10.1016/j.juro.2009.08.138. [DOI] [PubMed] [Google Scholar]
- 16.Swanson GP, Riggs M, Hermans M. Pathologic findings at radical prostatectomy: risk factors for failure and death. Urol Oncol. 2007 Mar-Apr;25(2):110–114. doi: 10.1016/j.urolonc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Mian BM, Troncoso P, Okihara K, et al. Outcome of patients with Gleason score 8 or higher prostate cancer following radical prostatectomy alone. J Urol. 2002 Apr;167(4):1675–1680. [PubMed] [Google Scholar]
- 18.Sofer M, Savoie M, Kim SS, Civantos F, Soloway MS. Biochemical and pathological predictors of the recurrence of prostatic adenocarcinoma with seminal vesicle invasion. J Urol. 2003 Jan;169(1):153–156. doi: 10.1016/S0022-5347(05)64057-8. [DOI] [PubMed] [Google Scholar]
- 19.Stephenson AJ, Wood DP, Kattan MW, et al. Location, extent and number of positive surgical margins do not improve accuracy of predicting prostate cancer recurrence after radical prostatectomy. J Urol. 2009 Oct;182(4):1357–1363. doi: 10.1016/j.juro.2009.06.046. [DOI] [PubMed] [Google Scholar]
- 20.D’Amico AV, Whittington R, Malkowicz SB, et al. The combination of preoperative prostate specific antigen and postoperative pathological findings to predict prostate specific antigen outcome in clinically localized prostate cancer. J Urol. 1998 Dec;160(6 Pt 1):2096–2101. doi: 10.1097/00005392-199812010-00041. [DOI] [PubMed] [Google Scholar]
- 21.Thompson IM, Jr, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathologically advanced prostate cancer: a randomized clinical trial. JAMA. 2006;296(19):2329–2335. doi: 10.1001/jama.296.19.2329. [DOI] [PubMed] [Google Scholar]
- 22.Wiegel T, Bottke D, Steiner U, et al. Phase III postoperative adjuvant radiotherapy after radical prostatectomy compared with radical prostatectomy alone in pT3 prostate cancer with postoperative undetectable prostate-specific antigen: ARO 96-02/AUO AP 09/95. J Clin Oncol. 2009 Jun 20;27(18):2924–2930. doi: 10.1200/JCO.2008.18.9563. [DOI] [PubMed] [Google Scholar]
- 23.Bolla M, van Poppel H, Collette L, van Cangh P, Vekemans K, Pozzo LD. Postoperative radiotherapy after radical prostatectomy: a randomised controlled trial (EORTC trial 22911) Lancet. 2005;366:572–578. doi: 10.1016/S0140-6736(05)67101-2. [DOI] [PubMed] [Google Scholar]
- 24.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: long-term followup of a randomized clinical trial. J Urol. 2009;181(3):956–962. doi: 10.1016/j.juro.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swanson GP, Goldman B, Tangen CM, et al. The prognostic impact of seminal vesicle involvement found at prostatectomy and the effects of adjuvant radiation: data from Southwest Oncology Group 8794. J Urol. 2008 Dec;180(6):2453–2457. doi: 10.1016/j.juro.2008.08.037. discussion 2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black american men. J Urol. 2014 Jan;191(1):60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001 Dec;58(6):843–848. doi: 10.1016/s0090-4295(01)01441-8. [DOI] [PubMed] [Google Scholar]
- 28.Koh H, Kattan MW, Scardino PT, et al. A nomogram to predict seminal vesicle invasion by the extent and location of cancer in systematic biopsy results. J Urol. 2003 Oct;170(4 Pt 1):1203–1208. doi: 10.1097/01.ju.0000085074.62960.7b. [DOI] [PubMed] [Google Scholar]
- 29.Ohori M, Kattan MW, Yu C, et al. Nomogram to predict seminal vesicle invasion using the status of cancer at the base of the prostate on systematic biopsy. International journal of urology : official journal of the Japanese Urological Association. 2010 Jun;17(6):534–540. doi: 10.1111/j.1442-2042.2010.02513.x. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Hricak H, Kattan MW, et al. Prediction of seminal vesicle invasion in prostate cancer: incremental value of adding endorectal MR imaging to the Kattan nomogram. Radiology. 2007 Jan;242(1):182–188. doi: 10.1148/radiol.2421051254. [DOI] [PubMed] [Google Scholar]


