Abstract
PURPOSE
To explore whether disparities in outcomes exist between African-American (AA) and Caucasian (CS) men with low-grade prostate cancer (PCa) and similar Cancer of the Prostate Risk Assessment post-Surgery (CAPRA-S) features following prostatectomy (RP)
METHODS
The overall cohort consisted of 1,265 men (234 AA, and 1,031 CS) who met National comprehensive cancer network (NCCN) criteria for low-intermediate risk PCa and underwent RP between 1990 and 2012. We first evaluated whether clinical factors were associated with adverse pathologic outcomes and freedom from biochemical failure (FFbF) using the entire cohort. Next, we studied a subset of 705 men (112 AA, and 593 CS) who had pathologic Gleason score ≤6 (low-grade disease). Using this cohort, we determined whether race impacted FFbF in men with prostatectomy-proven low-grade disease and similar CAPRA-S score.
RESULTS
With a median follow up time of 27 months, the overall 7-year FFbF rate was 86% vs. 79% in CS and AA men, respectively (p=0.035). There was no significant difference in ≥1 adverse pathologic features between CS vs. AA men (27% vs. 31%; P =0.35) or CAPRA-S score (p=0.28). In the subset analysis of patients with low-grade disease, AA race was associated with worse FFbF outcomes (p=0.002). Furthermore, AA race was a significant predictor of FFbF in men with low-grade disease (HR 2.01, 95%CI 1.08–3.72; p=0.029).
CONCLUSIONS
AA race is a predictor of worse FFbF outcomes in men with low-grade disease after RP. These results suggest that a subset of AA men with low-grade disease may benefit from more aggressive treatment.
Keywords: African American race, disparities, biochemical failure, adverse pathologic features
Introduction
Men of African descent are known to experience greater incidence of and mortality from PCa than men of other races[1]. AA men have been shown to experience PCa at an earlier age as compared to CS men. Furthermore, AA men often present with higher grade and stage of disease at time of diagnosis[2]. This observation has been partly attributed to socio-economic factors and inadequate access to healthcare[3]. However, there is recent evidence suggesting that differences in genetic susceptibility play a major role in this disparity[4, 5].
Due to the relatively indolent nature of most PCa diagnosed in the US, the decision-making process for determining whether to pursue active surveillance, or alternative management options, is complicated by the balance between life expectancy, comorbidities, clinical benefits, as well as the side effects of treatment[6]. The ability to predict clinical outcomes is critical in recommending appropriate treatment options for PCa patients. Current NCCN guidelines recommend active surveillance as the preferred option for very low-risk PCa in men, defined as PSA <10 ng/ml, clinical stage ≤T1c. Gleason score (GS) ≤6, positive cores ≤2, and cancer involvement of ≤50% per core. The goal of these recommendations is to prevent overtreatment of indolent cancers while identifying patients who develop disease progression and offering treatment with curative intent. However, most predictive tools currently used to risk-stratify PCa patients for treatment recommendations have not been developed or validated in AA men[7]. Furthermore, randomized clinical trials reporting on low-risk prostate treatment outcomes have been unable to effectively address whether interventions depend on race due to inadequate numbers of AA participants[8].
Whether AA race acts as a prognostic factor for freedom from biochemical failure (FFbF) in patients with pathologic GS ≤6 disease (referred to here as low-grade disease) and minimal adverse pathologic features after prostatectomy (RP) is poorly understood. The goal of this study is to determine whether disparities in adverse pathologic features and FFbF outcomes exist among an identical cohort of AA and CS men using a prospective cohort of PCa patients treated with RP.
Patients and Methods
Patient selection
The present study is a retrospective analysis of a prospective cohort of 2,012 men (298 AA, 1,673 CS and 41 Other race) with PCa treated with RP at the University of Pennsylvania Health System (UPHS; Philadelphia, PA) recruited to the Study of Clinical Outcomes, Risk and Ethnicity (SCORE) between 1990 and 2012[9]. Patients without adequate preclinical data including initial PSA, or biopsy GS at diagnosis were excluded from the analysis (N=457). Patients of non-CS and non-AA ethnicity were excluded (N=41). Patients with >T3 tumors, or a GS between 7(4+3) and 10, or a PSA level ≥20 ng/ml, or patients found to have regional lymph node metastasis on imaging or following bilateral pelvic lymph node dissection were excluded from the study (N=249). The remaining 1,265 patients comprising the overall cohort who met NCCN criteria for low-intermediate risk PCa with biopsy GS ≤ 7 (3+4), T-stage ≤ T2c, PSA ≤ 20ng/ml and underwent RP were selected for this study[10]. Of the 1,265 patients, a subset of 705 men (112 AA, and 593 CS) with pathologic GS ≤6 (low-grade disease determined post-RP), and were further analyzed in this study. We selected low-intermediate risk patients in the overall cohort in order to capture patients with biopsy GS 7 (3+4) who were downgraded to pathologic GS 6 (3+3) following RP.
Preoperative staging
Patients were evaluated at time of diagnosis by a thorough history and physical examination (including digital rectal examination [DRE]) followed by routine laboratory studies, including serum PSA levels, and GS determined by needle biopsy and reviewed at the UPHS. All patients were staged according to the 1992 American Joint Committee on Cancer staging system[11].
Treatment
Surgical treatment consisted of a radical retropubic prostatectomy or robotic-assisted radical prostatectomy, and bilateral pelvic lymph node sampling. All pathology slides were prepared as per standard institutional protocol. Prostatectomy specimen was initially coated with india ink and fixed in formalin. The whole gland was step sectioned at 3 mm intervals and the resulting sections were fixed into tissue cassettes. Tissue sections were embedded in paraffin blocks, from which sections were prepared and stained with hematoxylin and eosin for routine histological analysis by a dedicated GU pathologist. Adverse pathologic features consisting of extraprostatic extension (EPE), seminal vesicle invasion (SVI), and surgical margin status (SM) were noted and recorded. At the discretion of the treating physician, patients with adverse pathologic features including EPE, SVI or positive surgical margins were treated with adjuvant radiation therapy (RT) and/or androgen depravation therapy (ADT). ADT consisted of a gonadotropin-releasing hormone agonist (leuprolide acetate or goserelin acetate) with or without an antiandrogen (e.g. flutamide, bicalutamide).
Follow-Up and treatment endpoints
Patient information at each follow-up visit including DRE and serial PSA values were noted and recorded. PSA failure was defined as a single PSA≥0.2ng/ml along with documentation of failure by a physician or when two consecutive PSA values of 0.2ng/ml were obtained after an undetectable value. Start of the prospective follow-up (i.e., time zero) was defined at the date of surgery for all patients. If PSA was never undetectable postoperatively, then PSA failure was assigned at time zero. Patients with no follow-up PSA measurements (N=190, 14.5%) were included for the evaluation of differences in preoperative and pathologic characteristics, but not for analysis on FFbF outcomes.
Statistical analysis
Clinical and pathologic variables were compared across the race groups using an analysis of variance model for continuous variables or contingency table χ2 test of homogeneity for categorical variables. Predictors of adverse pathologic features were examined using logistic regression models. Age, PSA, and year of surgery were examined as continuous variables. T-stage (T1a-c vs. T2), biopsy GS, and race were examined as categorical variables. Based on pathologic findings following surgery, patients were further stratified using the Cancer of the Prostate Risk Assessment post-Surgery (CAPRA-S), a validated post-surgical score that predicts risk of a cancer recurrence following RP[12]. Variables for determining CAPRA-S score included preoperative PSA, pathologic GS, SM, EPE, and SVI. Patients were categorized into low (CAPRA-S <3), intermediate (CAPRA-S 3–5) and high (CAPRA-S >5) risk of recurrence.
For survival analysis, the primary event of interest was PSA failure (biochemical disease recurrence). Individuals who did not experience PSA failure were censored at the time of last PSA measurement <0.2 ng/dl, or loss to follow-up. Time to PSA failure was used as a surrogate for freedom from biochemical failure (FFbF). The FFbF rates were compared across the groups using a log-rank survivorship and Kaplan-Meier analyses. For multivariate analysis, a forward-stepwise Cox proportional hazards model was used with p<0.2 determining which variables were entered into the model at each step. The variable with the highest p value was successively deleted until only variables with p<0.2 remained. Analyses were conducted using STATA statistical software version 13.0 (STATA Corporation). This study was approved by our Institutional Review Board.
Results
Baseline clinical and pathologic characteristics of overall cohort are listed in Table 1. Preoperative factors such as age at RP, PSA at diagnosis, and clinical T-stage were similar between groups. Compared with CS men, AA men had higher biopsy GS (p<0.001). There was no difference in ≥1 adverse pathologic features among race groups (28% vs. 31%; p=0.41). However, a greater number of AA men had pathologic GS of ≥7 (52% vs. 43%; p=0.01), as well as SVI (6% vs. 3%; p=0.02). There was no difference in use of radiotherapy or ADT between groups.
Table 1.
Pre- and post- treatment characteristics and pathologic outcomes of NCCN low–& intermediate- risk men undergoing radical prostatectomy at University of Pennsylvania, 1990–2012 (Overall Cohort).
| Caucasian Cohort n=1031 | % | African-American Cohort n=234 | % | p-Value | |
|---|---|---|---|---|---|
| Age, Y | 0.48† | ||||
| Median | 60 | 58 | |||
| mean | 59.1 | 57.8 | |||
| IQR | 54–64 | 52–62 | |||
|
| |||||
| iPSA ng/ml | 0.89† | ||||
| 0–4.0 | 271 | 26 | 59 | 25 | |
| 4.01 - 10 | 659 | 64 | 150 | 65 | |
| 10.01- 20 | 101 | 10 | 25 | 11 | |
| median | 5.1 | 5.6 | |||
| mean | 5.8 | 6.2 | |||
| IQR | 4.1 -- 6.7 | 4.1 -- 7.8 | |||
|
| |||||
| Biopsy Gleason | <0.001* | ||||
| ≤6 | 948 | 90 | 162 | 67 | |
| 7(3+4) | 103 | 10 | 54 | 23 | |
|
| |||||
| Clinical Stage | 0.63* | ||||
| T1A-C | 583 | 81 | 149 | 85 | |
| T2A | 111 | 16 | 22 | 12 | |
| T2B | 8 | 1 | 4 | 2 | |
| T2C | 12 | 2 | 1 | 1 | |
| Year of prostatectomy | 0.006† | ||||
| median | 2003 | 2004 | |||
| mean | 2002.7 | 2003.7 | |||
| IQR | 1999–2007 | 2000–2008 | |||
|
| |||||
| Pathologic Stage | 0.07* | ||||
| pT2N0 | 802 | 77 | 175 | 74 | |
| pT3aN0 | 202 | 20 | 44 | 19 | |
| pT3bN0 | 23 | 2 | 13 | 6 | |
| pT4aN0 | 4 | 1 | 2 | 1 | |
|
| |||||
| Pathologic Gleason | <0.001* | ||||
| ≤6 | 596 | 57 | 113 | 46 | |
| 7(3+4) | 229 | 22 | 81 | 36 | |
| 7(4+3) | 35 | 4 | 14 | 7 | |
| 7(unspecified) | 145 | 14 | 22 | 9 | |
| 8 to 10 | 26 | 3 | 4 | 2 | |
|
| |||||
| Gleason upgrading | 0.25* | ||||
| 6/7 to 7/(8–10) | 369 | 35 | 72 | 30 | |
|
| |||||
| Adverse pathologic features‡ | 0.35* | ||||
| 0 | 757 | 73 | 164 | 69 | |
| 1 | 147 | 15 | 33 | 15 | |
| ≥2 | 127 | 12 | 37 | 16 | |
|
| |||||
| Extraprostatic spread | 223 | 22 | 58 | 25 | 0.32* |
|
| |||||
| Seminal Vesicle invasion | 27 | 3 | 13 | 6 | 0.02* |
|
| |||||
| Positive surgical margin | 162 | 16 | 39 | 17 | 0.71* |
|
| |||||
| Radiotherapy | 11 | 1 | 3 | 1 | 0.78* |
|
| |||||
| ADT | 35 | 3 | 8 | 3 | 0.5* |
NOTE. Boldfaced values represent statistically signi cant differences between groups.
Abbreviations: iPSA- initial Prostate-specific antigen, IQR- interquartile range;
NCCN- National Comprehensive Cancer Network; ADT- Androgen depravation therapy
P value derived from Person’s chi-square test.
P value derived from analysis of variance model.
Adverse pathologic features: extraprostatic extension, seminal vesicle invasion, and/or positive surgical margin.
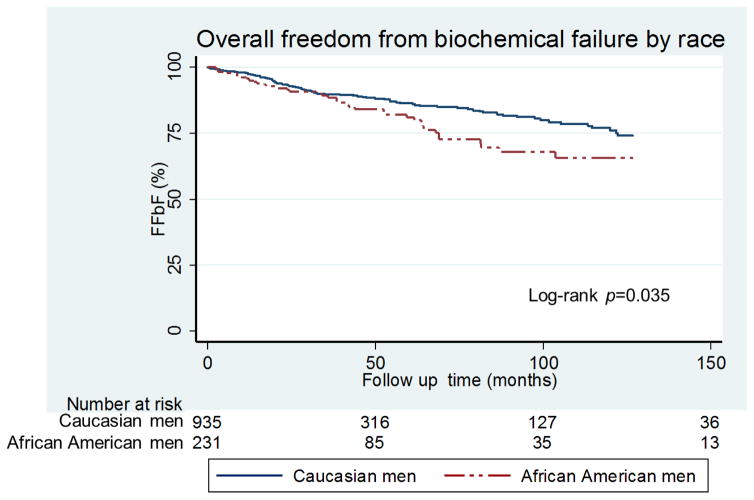
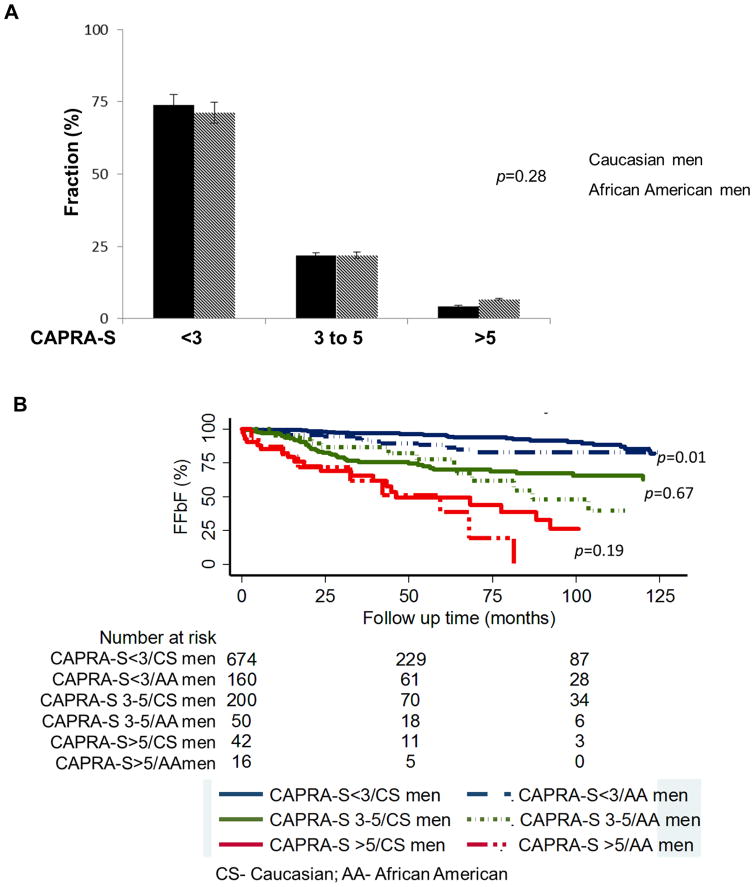
Using the Kaplan-Meier survival analysis method, the impact of race on FFbF was evaluated in the overall cohort. The mean and median follow-up time from RP date until last follow-up PSA date was 45 months and 27 (range 1 to 207) months respectively. During this time period, 144 patients (11.5%) experienced biochemical failure. The 7-year FFbF rate between CS men and AA men was 86% versus 79%, respectively (Fig. 1; p=0.035). There was no difference in adverse pathologic features using the validated CAPRA-S score for risk of recurrence, (Fig. 2A; p=0.28). However, the corresponding Kaplan-Meier estimates of FFbF showed worse outcomes among AA men in the CAPRA-S <3 group, Fig. 2B (p=0.01). There was no statistically significant difference in the CAPRA-S 3–5 and >5 risk groups likely due to small numbers in both groups (Fig. 2B; p=0.67 and p=0.19), respectively.
FIGURE 1.
Kaplan-Meier curves for FFbF outcomes by race in NCCN Low–& intermediate- risk men undergoing radical prostatectomy at University of Pennsylvania, 1990–2012 (Overall Cohort).
Abbreviations: FFbF- Freedom From biochemical Failure, NCCN- National Comprehensive Cancer Network P values derived from the Mantel-Cox log-rank test.
FIG. 2.
(A). Distribution of CAPRA-S score grouping by race and (B) Kaplan-Meier curves for FFbF outcomes by race stratified by CAPRA-S score group in NCCN low- & intermediate- risk men undergoing radical prostatectomy at University of Pennsylvania, 1990–2012 (overall cohort).
Abbreviations: FFbF- Freedom From biochemical Failure, NCCN- National Comprehensive Cancer Network, CAPRA-S- Cancer of the Prostate Risk Assessment Post-Surgical scoring system
Using a Cox proportional hazard model, the predictors of FFbF following RP were determined, (Table 2). In the multivariate model of overall cohort, T stage (HR 2.92, 95%CI 1.17–7.32; p=0.02) serum PSA (HR 1.14, 95%CI 1.09–1.20; p<0.001), clinical GS (HR 1.51, 95%CI 1.01–2.27; p=0.045), pathologic GS (HR 1.59, 95%CI 1.18–2.15; p=0.002), EPE (HR 2.01, 95%CI 1.33–3.04; p=0.001), SVI (HR 2.47, 95%CI 1.48–4.12; p=0.001), and SM (HR 1.7, 95%CI 1.13–2.56; p=0.01) were predictors of FFbF.
Table 2.
Univariate and multivariate regression models of factors predicting FFbF in NCCN low–& intermediate- risk men undergoing radical prostatectomy at University of Pennsylvania, 1990–2012 (Overall Cohort).
| Univariate analysis | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | 0.99 | 0.96 to 1.01 | 0.48 |
| Race | 1.43 | 0.99 to 2.05 | 0.05 |
| Serum PSA | 1.16 | 1.11 to 1.21 | <0.001 |
| T-stage | 3.79 | 1.55 to 9.26 | 0.003 |
| Clinical gleason score | 2.63 | 1.80 to 3.83 | <0.001 |
| Year of prostatectomy | 1.04 | 0.99 to 1.08 | 0.09 |
| Extraprostatic spread | 3.89 | 2.81 to 5.38 | <0.001 |
| Positive surgical margins | 3.72 | 2.67 to 5.19 | <0.001 |
| Seminal vesicle invasion | 5.9 | 3.71 to 9.38 | <0.001 |
| Pathologic gleason score | 2.63 | 2.01 to 3.44 | <0.001 |
|
| |||
| Multivariate analysis | |||
| Age | 0.99 | 0.96 to 1.02 | 0.50 |
| Race | 1.38 | 0.92 to 2.07 | 0.12 |
| Serum PSA | 1.13 | 1.08 to 1.19 | <0.001 |
| T-stage | 2.92 | 1.17 to 7.32 | 0.02 |
| Prostate specific antigen | 1.14 | 1.09 to 1.20 | <0.001 |
| Extraprostatic spread | 2.01 | 1.33 to 3.04 | 0.001 |
| Seminal vesicle invasion | 2.47 | 1.48 to 4.12 | 0.001 |
| Positive surgical margins | 1.7 | 1.13 to 2.56 | 0.01 |
| Clinical gleason score | 1.11 | 0.69 to 1.79 | 0.67 |
| Pathologic gleason score | 1.59 | 1.18 to 2.15 | 0.009 |
NOTE. Boldfaced values represent statistically signi cant differences between groups.
Abbreviations: PSA- Prostate-specific antigen, NCCN- National Comprehensive Cancer Network, FFbF- Freedom From biochemical Failure
P values derived from a Cox proportional hazards model.
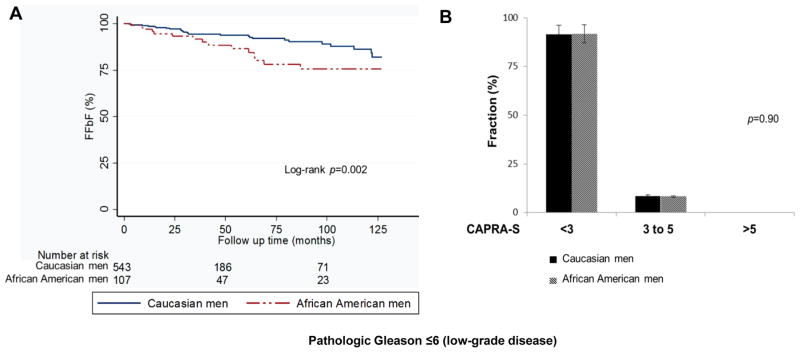
In order to study outcomes in men with prostatectomy-proven low-grade PCa, we analyzed the characteristics of 705 men (112 AA, and 593 CS) who had pathologic GS ≤6 (i.e. low-grade disease) following RP, using similar analytic methods employed in the overall cohort. For this analysis patients who initially had biopsy Gleason <7 and then upon RP were upgraded to pathologic Gleason grade ≥7 were excluded. This represents a true cohort of patients with low-grade disease. In this cohort, there was no difference in any pre- and post-treatment characteristics between race groups among patients with low-grade disease (Table 3). To determine the effect of race on FFbF we analyzed this cohort with low-grade disease with similar CAPRA-S score. This group received prostatectomy as monotherapy with <5% needing any additional therapy, (Table 3). Among patients with low-grade disease, AA men demonstrated worse 7-year FFbF (Fig. 3A; p=0.002), despite similar CAPRA-S score compared with CS men (Fig. 3B; p=0.90).
Table 3.
Pre- and post- treatment characteristics and pathologic outcomes of men with pathologic Gleason ≤6 (low-grade disease) following radical prostatectomy at University of Pennsylvania, 1990–2012.
| Caucasian Cohort n=593 | % | African-American Cohort n=112 | % | p- Value | |
|---|---|---|---|---|---|
| Age, Y | 0.39† | ||||
| Median | 59 | 58 | |||
| mean | 58.4 | 57.8 | |||
| IQR | 54–63 | 52–62 | |||
|
| |||||
| iPSA ng/ml | 0.05† | ||||
| 0–4.0 | 179 | 30 | 29 | 26 | |
| 4.1–10 | 357 | 60 | 79 | 70 | |
| 10.1 - 20 | 57 | 10 | 4 | 4 | |
| median | 5 | 5.4 | |||
| mean | 5.6 | 5.6 | |||
| IQR | 3.7 -- 6.5 | 4.1 -- 7.0 | |||
|
| |||||
| Clinical Stage | 0.17* | ||||
| T1A-C | 357 | 86 | 73 | 91 | |
| T2A | 61 | 14 | 7 | 9 | |
|
| |||||
| Pathologic Stage | 0.45* | ||||
| pT2N0 | 515 | 87 | 96 | 86 | |
| pT3aN0 | 72 | 12 | 13 | 12 | |
| pT3bN0 | 4 | 1 | 2 | 2 | |
| pT4aN0 | 2 | 0 | 0 | 0 | |
|
| |||||
| Adverse pathologic features‡ | 0.85* | ||||
| 0 | 497 | 84 | 92 | 82 | |
| 1 | 56 | 9 | 11 | 10 | |
| ≥2 | 40 | 7 | 9 | 8 | |
|
| |||||
| Extraprostatic spread | 76 | 13 | 16 | 14 | 0.64* |
|
| |||||
| Seminal Vesicle invasion | 6 | 1 | 2 | 2 | 0.47* |
|
| |||||
| Positive surgical margin | 54 | 9 | 11 | 10 | 0.81* |
|
| |||||
| Radiotherapy | 3 | 0.5 | 1 | 1 | 0.62* |
|
| |||||
| ADT | 28 | 5 | 5 | 5 | 0.94* |
NOTE. Abbreviations: iPSA- initial Prostate-specific antigen, IQR- interquartile range;
ADT- Androgen depravation therapy
P value derived from Person’s chi-square test.
P value derived from analysis of variance model.
Adverse pathologic features: extraprostatic extension, seminal vesicle invasion, and/or positive surgical margin.
FIG. 3.
(A, B) Kaplan-Meier curves for FFbF outcomes and CAPRA-S score grouping by race in men with pathologic Gleason ≤6 following radical prostatectomy at University of Pennsylvania, 1990–2012.
Abbreviations: FFbF- Freedom From biochemical Failure, CAPRA-S- Cancer of the Prostate Risk Assessment Post-Surgical scoring system
Using a multivariate model, the significant predictors of risk for FFbF following RP were determined for patients with low-grade disease, (Table 4). Serum PSA (HR 1.24, 95%CI 1.15–1.34; p<0.001), EPE (HR 3.77, 95%CI 1.79–7.95; p<0.001), and AA race (HR 2.01, 95%CI 1.08–3.72; p=0.029) remained predictors of FFbF.
Table 4.
Univariate and multivariate regression models of factors predicting FFbF in men with pathologic Gleason ≤6 (low-grade disease) following radical prostatectomy at University of Pennsylvania, 1990–2012.
| Univariate analysis | HR | 95% CI | p-Value |
|---|---|---|---|
| Age | 1.01 | 0.96 to 1.05 | 0.63 |
| African-American Race | 2.02 | 1.09 to 3.74 | 0.025 |
| Serum PSA | 1.22 | 1.06 to 1.41 | 0.005 |
| T-stage | 1.37 | 0.87 to 2.14 | 0.17 |
| Clinical Gleason score | 2.48 | 0.76 to 8.19 | 0.13 |
| Year of prostatectomy | 0.99 | 0.91 to 1.06 | 0.61 |
| Extraprostatic spread | 4.05 | 2.27 to 7,23 | <0.001 |
| Positive surgical margins | 3.71 | 1.94 to 7.04 | <0.001 |
| Seminal vesicle invasion | 8.1 | 2.87 to 22.8 | <0.001 |
|
| |||
| Multivariate analysis | |||
| Age | 1.02 | 0.97 to 1.06 | 0.44 |
| Year of prostatectomy | 0.99 | 0.92 to 1.07 | 0.81 |
| Clinical Gleason score | 1.23 | 0.35 to 4.41 | 0.74 |
| Serum PSA | 1.24 | 1.15 to 1.34 | <0.001 |
| Extraprostatic spread | 3.77 | 1.79 to 7.95 | <0.001 |
| African-American Race | 2.01 | 1.08 to 3.72 | 0.029 |
| Seminal vesicle invasion | 2.71 | 0.89 to 8.57 | 0.089 |
| Positive surgical margins | 1.83 | 0.81 to 4.12 | 0.15 |
NOTE. Boldfaced values represent statistically signi cant differences between groups.
Abbreviations: PSA- Prostate-specific antigen, NCCN- National Comprehensive Cancer Network, FFbF- Freedom From biochemical Failure
P values derived from a Cox proportional hazards model.
Discussion
In this report, we show that AA men with low-grade disease have worse FFbF as compared with their CS counterparts (Fig. 3A). This observation is not likely due to treatment differences since patient groups had similar adverse pathologic features as demonstrated by comparable CAPRA-S scores between AA and CS men (Fig. 3B) and there were no differences by race in the utilization of adjuvant radiotherapy or ADT. Additionally, there was no difference in extent of positive margin status by race to suggest sub-optimal surgical technique in AA patients (Table 3). Less than 5% of entire cohort had documented treatment with additional RT or ADT. This data may reflect the low physician referral patterns for adjuvant treatment for eligible patients [13, 14]. However, these results should be interpreted with caution, since a number of patients may have undergone RP at UPHS and then received RT at another institution.
Overtreatment of GS ≤6 PCa diagnosed on biopsies triggered by elevated PSA remains an ongoing controversy[15]. In fact, a few recent studies have suggested that removing the label “cancer” from biopsy GS ≤6 disease could potentially reduce overtreatment of low-grade disease[16, 17]. However, our results suggest caution in applying this to some men, and particularly AA men. Biopsy GS alone usually underestimates both grade and extent of disease, thus relabeling of biopsy GS ≤6 disease as non-cancer could result in a missed opportunity of curative treatment in some individuals. Consistent with our study (Table 1), the rate of upgrading from biopsy GS ≤6 to pathologic GS ≥7 at prostatectomy is estimated at 25% to 35%[18]. A number of studies have shown a suboptimal correlation between biopsy Gleason scoring and radical prostatectomy, despite the migration from sextant biopsies to 12-core sampling. Cookson et, al. showed that biopsy GS was identical to specimen core in 31% of cases, while discrepant by >2 GS in 26%[19]. In more contemporary series utilizing 12 or more biopsy cores, the upgrade rate is approximately 30%[20]. Furthermore, there is evidence to suggest that the zonal distribution of cancer foci within the prostate may differ between AA and CS men, thus influencing the result of core biopsies[21]. Therefore, the current practice of recommending no active treatment for patients by relying heavily on parameters such as biopsy grade, number of biopsy positive cores, and initial PSA may need to be validated in AA men.
As per the NCCN guidelines, active surveillance (AS) is the preferred treatment option for men with very low-risk PCa and life expectancy of ≤20 years or those with low-risk disease and life expectancy of less than 10 years[22]. The advantage of AS is to prevent overtreatment of indolent disease while actively monitoring the course of the disease and intervene only when progression occurs in patients with more aggressive disease[23]. However, evidence for the benefit of AS was based on studies conducted in primarily CS cohort[24, 25]. In studies where race was reported 5% to 10% of patients enrolled in AS program were African-American[20, 26]. One retrospective study evaluated the effect of race on discontinuation of AS for patients with low-risk PCa. Their results showed that AA men had more aggressive disease and were more likely to progress on AS, and proceed to treatment faster than CS men[27]. A large study on pathologic and FFbF outcomes in very low-risk AA men who qualify for AS but underwent immediate RP showed that AA men had significantly higher rates of upgrading, positive surgical margins, and CAPRA-S score than do CS men[28]. Data from our study however, showed worse FFbF even in AA patients despite similar CAPRA-S score and low-grade disease when compared to their CS counterparts (Fig. 2,3). Potential reasons for the discrepancy in pathologic outcomes between our low-grade study and the prior study is likely due to the fact that, unlike the prior study that evaluated low-risk patients as determined by biopsy Gleason grade, we analyzed a cohort of patients with truly low-grade (pathologic Gleason grade ≤6) disease. Nonetheless, these emerging data suggests that further study is needed to determine whether some AA men with low-grade disease and CAPRA-S score of >2 may derive benefit from additional/adjuvant therapy such as radiation or ADT. In light of these findings, AA men found to have biopsy GS ≤6 with clinically low-risk disease who choose AS should undergo more careful monitoring due to possibility of increased oncologic risk.
Of note, several studies have been conducted regarding the effect of race on FFbF after definitive PCa treatment with radical prostatectomy (RP), or radiotherapy. However, results from these studies have proven inconclusive[28–30]. These inconsistencies may in part be due to differences in selection criteria and imbalances in comparison groups.
The strength of our study is that it provides a stringent analysis of AA and CS men with similar adverse pathologic features. Therefore, known socio-economic factors such as inaccessibility to healthcare, late diagnosis, and sub-optimal treatment are less likely account for outcomes disparity in this cohort. Our data has major clinical implications for treatment recommendations, which includes potentially undertreating low-grade disease in AA men. Furthermore, AA men with low-grade disease need to be enrolled on clinical trials evaluating biomarker driven risk-adapted treatment options to improve outcomes.
A major limitation to this study is that it has a relatively small number of AA compared with CS men, and represents the experience from a single tertiary center. Though the men in this study had identical adverse pathologic risk features, a randomized controlled trial is required to adequately answer the question of race and FFbF outcomes in men with low-grade disease. Outcomes were not adjusted for socioeconomic factors, diet, obesity, comorbid conditions, and adherence to treatment recommendations. Information on tumor volume or percentage of cores positive for tumor were inconsistently reported, and hence we could not adequately investigate outcomes in very low-risk patients who might have been eligible for active surveillance.
In conclusion, AA race is a predictor of worse FFbF in patients with pathologic GS ≤6 or low-grade disease and favorable pathologic features. This highlights the need for clinically useful biomarkers that will enable us to identify AA men appropriate for active surveillance vs. those harboring aggressive disease that may ultimately benefit from exploration of additional/adjuvant therapy such as radiation or ADT.
Acknowledgments
This work was funded in part by PHS grants R01-CA085074, P50-CA105641, P60-MD-006900 (to T.R.R.), and DOD grant PC-121189, and the Prostate Cancer Foundation Young Investigator Award (to K.Y.).
Footnotes
Disclaimer/Conflict of interest: none to disclose
References
- 1.Jemal A, Center MM, DeSantis C, Ward EM. Global patterns of cancer incidence and mortality rates and trends. Cancer Epidemiol Biomarkers Prev. 2010;19(8):1893–1907. doi: 10.1158/1055-9965.EPI-10-0437. [DOI] [PubMed] [Google Scholar]
- 2.Thompson I, Tangen C, Tolcher A, Crawford E, Eisenberger M, Moinpour C. Association of African-American ethnic background with survival in men with metastatic prostate cancer. J Natl Cancer Inst. 2001;93(3):219–225. doi: 10.1093/jnci/93.3.219. [DOI] [PubMed] [Google Scholar]
- 3.Albain KS, Unger JM, Crowley JJ, Coltman CA, Jr, Hershman DL. Racial disparities in cancer survival among randomized clinical trials patients of the Southwest Oncology Group. J Natl Cancer Inst. 2009;101(14):984–992. doi: 10.1093/jnci/djp175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haiman CA, Chen GK, Blot WJ, Strom SS, Berndt SI, Kittles RA, Rybicki BA, Isaacs WB, Ingles SA, Stanford JL, et al. Characterizing genetic risk at known prostate cancer susceptibility loci in African Americans. PLoS Genet. 2011;7(5):e1001387. doi: 10.1371/journal.pgen.1001387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kerns SL, Ostrer H, Stock R, Li W, Moore J, Pearlman A, Campbell C, Shao Y, Stone N, Kusnetz L, et al. Genome-wide association study to identify single nucleotide polymorphisms (SNPs) associated with the development of erectile dysfunction in African-American men after radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2010;78(5):1292–1300. doi: 10.1016/j.ijrobp.2010.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shariat SF, Kattan MW, Vickers AJ, Karakiewicz PI, Scardino PT. Critical review of prostate cancer predictive tools. Future Oncol. 2009;5(10):1555–1584. doi: 10.2217/fon.09.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eastham JA, May R, Robertson JL, Sartor O, Kattan MW. Development of a nomogram that predicts the probability of a positive prostate biopsy in men with an abnormal digital rectal examination and a prostate-specific antigen between 0 and 4 ng/mL. Urology. 1999;54(4):709–713. doi: 10.1016/s0090-4295(99)00213-7. [DOI] [PubMed] [Google Scholar]
- 8.Roach M, 3rd, Lu J, Pilepich MV, Asbell SO, Mohiuddin M, Grignon D. Race and survival of men treated for prostate cancer on radiation therapy oncology group phase III randomized trials. J Urol. 2003;169(1):245–250. doi: 10.1016/S0022-5347(05)64078-5. [DOI] [PubMed] [Google Scholar]
- 9.Zeigler-Johnson C, Friebel T, Walker AH, Wang Y, Spangler E, Panossian S, Patacsil M, Aplenc R, Wein AJ, Malkowicz SB, et al. CYP3A4, CYP3A5, and CYP3A43 genotypes and haplotypes in the etiology and severity of prostate cancer. Cancer research. 2004;64(22):8461–8467. doi: 10.1158/0008-5472.CAN-04-1651. [DOI] [PubMed] [Google Scholar]
- 10.Mohler JL. The 2010 NCCN clinical practice guidelines in oncology on prostate cancer. J Natl Compr Canc Netw. 2010;8(2):145. doi: 10.6004/jnccn.2010.0010. [DOI] [PubMed] [Google Scholar]
- 11.Edge SB, Compton CC. The American Joint Committee on Cancer the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 12.Cooperberg MR, Hilton JF, Carroll PR. The CAPRA-S score. A straightforward tool for improved prediction of outcomes after radical prostatectomy. Cancer. 2011;117(22):5039–5046. doi: 10.1002/cncr.26169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghia AJ, Shrieve DC, Tward JD. Adjuvant radiotherapy use and patterns of care analysis for margin-positive prostate adenocarcinoma with extracapsular extension. Postprostatectomy adjuvant radiotherapy. A SEER analysis. Urology. 2010;76(5):1169–1174. doi: 10.1016/j.urology.2010.04.047. [DOI] [PubMed] [Google Scholar]
- 14.Swanson GP, Basler JW. Prognostic factors for failure after prostatectomy. Journal of Cancer. 2010;2:1–19. [PMC free article] [PubMed] [Google Scholar]
- 15.Ganz PA, Barry JM, Burke W, Col NF, Corso PS, Dodson E, Hammond ME, Kogan BA, Lynch CF, Newcomer L, et al. National Institutes of Health State-of-the-Science Conference role of active surveillance in the management of men with localized prostate cancer. Annals of internal medicine. 2012;156(8):591–595. doi: 10.7326/0003-4819-156-8-201204170-00401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nickel JC, Speakman M. Should we really consider Gleason 6 prostate cancer? BJU international. 2012;109(5):645–646. doi: 10.1111/j.1464-410X.2011.10854.x. [DOI] [PubMed] [Google Scholar]
- 17.Chabner BA, Smith M. Call it cancer. The oncologist. 2012;17(2):149–150. doi: 10.1634/theoncologist.2012-0004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Epstein JI, Feng Z, Trock BJ, Pierorazio PM. Upgrading and downgrading of prostate cancer from biopsy to radical prostatectomy incidence and predictive factors using the modified Gleason grading system and factoring in tertiary grades. Eur Urol. 2012;61(5):1019–1024. doi: 10.1016/j.eururo.2012.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cookson MS, Fleshner NE, Soloway SM, Fair WR. Correlation between Gleason score of needle biopsy and radical prostatectomy specimen accuracy and clinical implications. J Urol. 1997;157(2):559–562. [PubMed] [Google Scholar]
- 20.Sundi D, Ross AE, Humphreys EB, Han M, Partin AW, Carter HB, Schaeffer EM. African American men with very low-risk prostate cancer exhibit adverse oncologic outcomes after radical prostatectomy. should active surveillance still be an option for them? J Clin Oncol. 2013;31(24):2991–2997. doi: 10.1200/JCO.2012.47.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sundi D, Kryvenko ON, Carter HB, Ross AE, Epstein JI, Schaeffer EM. Pathological examination of radical prostatectomy specimens in men with very low risk disease at biopsy reveals distinct zonal distribution of cancer in black american men. J Urol. 2014;191(1):60–67. doi: 10.1016/j.juro.2013.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mohler J, Bahnson RR, Boston B, Busby JE, D'Amico A, Eastham JA, Enke CA, George D, Horwitz EM, Huben RP, et al. NCCN clinical practice guidelines in oncology prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 23.Dall'Era MA, Albertsen PC, Bangma C, Carroll PR, Carter HB, Cooperberg MR, Freedland SJ, Klotz LH, Parker C, Soloway MS. Active surveillance for prostate cancer a systematic review of the literature. Eur Urol. 2012;62(6):976–983. doi: 10.1016/j.eururo.2012.05.072. [DOI] [PubMed] [Google Scholar]
- 24.Klotz L, Zhang L, Lam A, Nam R, Mamedov A, Loblaw A. Clinical results of long-term follow-up of a large, active surveillance cohort with localized prostate cancer. J Clin Oncol. 2010;28(1):126–131. doi: 10.1200/JCO.2009.24.2180. [DOI] [PubMed] [Google Scholar]
- 25.Dall'Era MA, Konety BR, Cowan JE, Shinohara K, Stauf F, Cooperberg MR, Meng MV, Kane CJ, Perez N, Master VA, et al. Active surveillance for the management of prostate cancer in a contemporary cohort. Cancer. 2008;112(12):2664–2670. doi: 10.1002/cncr.23502. [DOI] [PubMed] [Google Scholar]
- 26.Iremashvili V, Soloway MS, Rosenberg DL, Manoharan M. Clinical and demographic characteristics associated with prostate cancer progression in patients on active surveillance. J Urol. 2012;187(5):1594–1599. doi: 10.1016/j.juro.2011.12.082. [DOI] [PubMed] [Google Scholar]
- 27.Abern MR, Bassett MR, Tsivian M, Banez LL, Polascik TJ, Ferrandino MN, Robertson CN, Freedland SJ, Moul JW. Race is associated with discontinuation of active surveillance of low-risk prostate cancer results from the Duke Prostate Center. Prostate cancer and prostatic diseases. 2013;16(1):85–90. doi: 10.1038/pcan.2012.38. [DOI] [PubMed] [Google Scholar]
- 28.Resnick MJ, Canter DJ, Guzzo TJ, Brucker BM, Bergey M, Sonnad SS, Wein AJ, Malkowicz SB. Does race affect postoperative outcomes in patients with low-risk prostate cancer who undergo radical prostatectomy? Urology. 2009;73(3):620–623. doi: 10.1016/j.urology.2008.09.035. [DOI] [PubMed] [Google Scholar]
- 29.Yamoah K, Stone N, Stock R. Impact of race on biochemical disease recurrence after prostate brachytherapy. Cancer. 2011;117(24):5589–5600. doi: 10.1002/cncr.26183. [DOI] [PubMed] [Google Scholar]
- 30.Moreira DM, Presti JC, Jr, Aronson WJ, Terris MK, Kane CJ, Amling CL, Sun LL, Moul JW, Freedland SJ. The effect of race on the discriminatory accuracy of models to predict biochemical recurrence after radical prostatectomy. results from the Shared Equal Access Regional Cancer Hospital and Duke Prostate Center databases. Prostate Cancer Prostatic Dis. 13(1):87–93. doi: 10.1038/pcan.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]