WHAT TRANSPIRED? THE FALL OF THE CURRENT CLINICALLY AVAILABLE β-LACTAM-β-LACTAMASE INHIBITOR COMBINATIONS
In gram-negative pathogens, the production of β-lactamases, which hydrolyze β-lactam antibiotics, is a foremost threat in modern medicine.1–3 β-Lactam-β-lactamase inhibitor combinations (ie, amoxillin-clavulanic acid, ampicillin-sulbactam, cefoperazone-sulbactam, piperacillin-tazobactam, and ticarcillin-clavulanic acid) were first introduced into the clinic in the 1980s and 1990s (Fig. 1). The premise was simple: the β-lactamase inhibitor targeted the β-lactamase inactivating it, so that the partner β-lactam could inactivate the penicillin binding protein (PBP) target, eventually resulting in bacterial cell death. When introduced, these compounds were highly effective because they mimicked the β-lactam core. However, resistance to these inhibitors (ie, clavulanic acid, tazobactam, and sulbactam) is highly prevalent in the clinic because of 3 mechanisms. First, from the beginning, clavulanic acid, sulbactam, and tazobactam targeted only class A serine β-lactamases, thus 3 structurally and functionally distinct groups of β-lactamases: metallo-β-lactamases (MBLs) of class B, AmpCs serine β-lactamases belonging to class C, and OXAs serine β-lactamases of class D, were resistant to inhibition. Second, variants of previously susceptible class A β-lactamases (eg, TEM-1 and SHV-1) evolved single amino acid substitutions (eg, S130G, K234R) that resulted in these inhibitors failing to inactivate these enzymes.3 These variant β-lactamases were considered inhibitor-resistant and are classically more resistant to clavulanic acid than the sulfone inhibitors, sulbactam and tazobactam. Last, new class A β-lactamases, such as KPC-2, evolved (circa 1996) with the ability to hydrolyze clavulanic acid, sulbactam, and tazobactam.4 Thus, these first-generation β-lactam-β-lactamase inhibitor combinations are unable to inactivate most β-lactamases expressed by multi-drug-resistant (MDR) clinical isolates today.
Fig. 1.
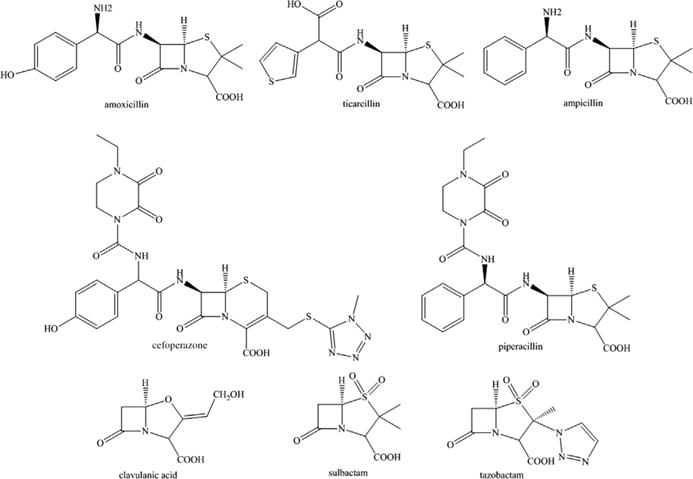
β-Lactamase inhibitors of the past and their β-lactam partners.
MAJOR OBSTACLES IN β-LACTAMASE INHIBITOR DEVELOPMENT
The development of novel inhibitors is an arduous task because the mechanisms by which β-lactamases are resistant to clavulanic acid, tazobactam and/or sulbactam, are different even within the same class of β-lactamase. The mechanistic characterization of the greater than 1600 β-lactamases identified to date is critical to understanding how to evade their action (http://www.lahey.org/Studies/). In addition, most MDR Gram-negatives possess more than one of these β-lactamases. A large gap exists that needs to be filled by identifying novel β-lactamase inhibitors or modified β-lactams that can inhibit this ever-growing population of diverse enzymes. MBLs and OXA β-lactamases pose the most difficult challenge. MBLs possess a Zn2+-mediated noncovalent mechanism, whereas the OXA class is extremely heterogeneous with over 500 different variants at the time this article was written. In addition, the β-lactam hydrolytic mechanism of OXAs is fundamentally different and not like the other serine-based mechanisms. As a result, a single “magic bullet” β-lactam-β-lactamase inhibitor combination that targets all clinically important β-lactamases (eg, KPC-2, OXA-24/40, AmpC, and NDM-1) seems unlikely. This debated question is addressed and the novel β-lactam-β-lactamase inhibitor combinations are discussed in this article.
CHANGING THE β-LACTAM PARTNER: CEFTOLOZANE-TAZOBACTAM
One technique to combat strains that carry multiple different classes of β-lactamases is to switch the β-lactam partner of a clinically available inhibitor. Cubist (now owned by Merck) used this approach with tazobactam when they paired tazobactam with the novel cephalosporin, ceftolozane (Fig. 2 and Table 1). Ceftolozane-tazobactam was approved in December 2014 by the US Food and Drug Administration (FDA) for the treatment of complicated intra-abdominal infections (cIAIs) and complicated urinary tract infections (cUTIs). Ceftolozane-tazobactam will also be tested in clinical trials for ventilator-associated pneumonia, cystic fibrosis patients, and for diabetic lower limb infections (Table 1). What makes this combination successful? Ceftolozane is more stable against the AmpC β-lactamase than the predecessor β-lactam partners of tazobactam. AmpC possesses a low catalytic efficiency (kcat/Km) for ceftolozane.5 Thus, ceftolozane inhibits PBPs and inhibitor-resistant TEMs and SHVs as well as AmpC (unlike tazobactam), allowing tazobactam to target class A serine β-lactamases (eg, TEM-1) and extended-spectrum beta-lactamases (ESBLs) (eg, CTX-M-15). In addition, ceftolozane works against some class D oxacillinases (eg, OXA-1).6 Thus, the ceftolozane-tazobactam combination is able to target class A, C, and some class D β-lactamases; the major exception is carbapenemases. Ceftolozane or ceftolozane-tazobactam was demonstrated to be similarly effective or superior to other β-lactams in a variety (eg, lung, urinary tract, burn wound, sepsis, and thigh) of animal infection models using Pseudomonas aeruginosa, Escherichia coli, or Klebsiella pneumoniae6; therefore, its utility in the clinic may be expanded for other infections. In addition, clinical trials are recruiting pediatric patients to test ceftolozane-tazobactam (Table 1).
Fig. 2.
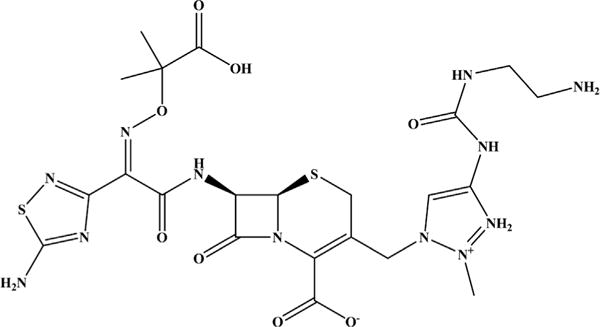
Chemical structure of ceftolozane.
Table 1.
New β-lactam-β-lactamase inhibitor combinations in the clinic or in development
| Combination | Company | Type of β-Lactamase Inhibitor | Development Phase | US Clinical Trial Numbers (Status) |
|---|---|---|---|---|
| Ceftolozane-tazobactam | Merck/Cubist Pharmaceuticals | Sulfone | FDA approved (2014) | NCT01147640 (completed); NCT01853982 (terminated); NCT02266706, NCT02070757, and NCT02387372 (recruiting); NCT02508753 (completed); NCT02421120 (recruiting); NCT02620774 (not open yet) |
| Ceftazidime-avibactam | AstraZeneca Pharmaceuticals, Forest-Cerexa, Actavis-Allergan | DBO | FDA approved (2014) | NCT01395420, NCT01430910, NCT01290900, NCT01644643, NCT01291602, NCT00752219, NCT00690378, NCT01599806, NCT01595438, NCT01893346, NCT01499290, NCT01500239, NCT01920399, NCT01534247, and NCT01789528 (completed); NCT01726023 (completed); and NCT01808092 (completed); NCT02475733 and NCT02497781 (recruiting) |
| Ceftaroline-avibactam | AstraZeneca Pharmaceuticals, Forest-Cerexa, Actavis-Allergan | DBO | Phase 2 | NCT01624246, NCT01281462, NCT01290900, and NCT01789528 (completed) |
| Aztreonam-avibactam | AstraZeneca Pharmaceuticals, Forest-Cerexa, Actavis-Allergan | DBO | Phase 1 | NCT01689207 (completed); NCT02655419 (not open yet) |
| Imipenem-relebactam | Merck Sharp & Dohme Corporation | DBO | Phase 2 | NCT01275170 and NCT01506271 (completed); and NCT01505634 (completed); NCT02452047 and NCT02493764 (recruiting) |
| RG6080 (formerly OP0595) | Meiji Seika Pharma Co, Ltd, Roche, and Fedora | DBO | Phase 1 | NCT02134834 (completed) |
| Meropenem-RPX7009 | Rempex Pharmaceuticals (The Medicines Company) | Boronate | Phase 3 | NCT01897779, NCT02020434, and NCT02073812 (completed); and NTC02168946 and NCT02166476 (recruiting); NCT01751269 (completed); NCT02687906 (not open yet) |
| Biapenem-RPX7009 | Rempex Pharmaceuticals (The Medicines Company) | Boronate | Phase 1 | NCT01772836 (completed) |
| S-649266 | Shionogi | Cephalosporin | Phase 2 | NCT02321800 (recruiting); NCT02714595 (not open yet) |
Laboratory-mediated selection of resistance (minimum inhibitory concentration [MIC] range of 4–8 mg/L) to ceftolozane-tazobactam was slower to occur than that with ceftazidime, meropenem, and ciprofloxacin in P aeruginosa PAO1; resistance was attributed to global pleiotrophic changes.7 Higher levels of ceftolozane-tazobactam resistance (MIC range of 32–128 mg/L) were obtained only with a ΔmutS strain of PAO1; in these resistant strains, mutations were identified in blaampC and the blaampC regulatory pathway. Since the writing of this article, additional amino acid substitutions in the AmpC from Pseudomonas were found to confer resistance to ceftolozane-tazobactam.8
DIAZABICYCLOOCTANONES, THE “FUTURE” OF β-LACTAMASE INHIBITOR MEDICINAL CHEMISTRY
DBOs are synthetic non-β-lactam-based β-lactamase inhibitors that were discovered in the early 2000s. Over a period of 15 years, this class of compounds has expanded almost exponentially with most modifications occurring at the C2 side chain (Fig. 3). Most studies published to date indicate that DBOs possess class A and class C activity with minor class D activity. More recently, the DBOs, FPI-1465 and RG6080 (formerly, OP0595), showcased at the 2013 and 2014 Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) meetings, and WCK 5153 and WO2013/030735, claim activity against PBPs as well. Many pharmaceutical companies (eg, Actavis-Allergan [formerly Forest-Cerexa], AstraZeneca, Fedora Pharmaceuticals, Meiji Seika Pharmaceuticals, Merck [formerly Cubist], Naeja Pharmaceuticals, Roche, and Wockhardt) are developing DBO derivatives (see Fig. 3).
Fig. 3.
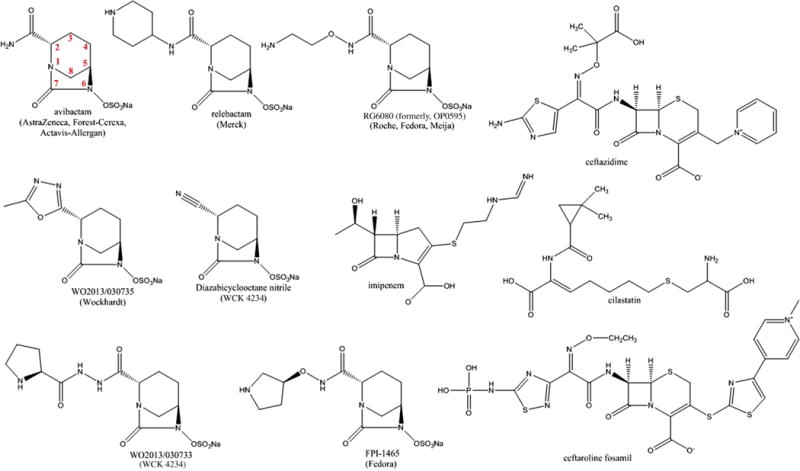
DBOs and DBO β-lactam partners.
AVIBACTAM, THE “PIONEER” DIAZABICYCLOOCTANONE IN THE CLINIC
In December 2014, avibactam was the first DBO to be approved by the FDA in combination with ceftazidime for the treatment of cUTIs and cIAIs; currently the combination is being tested in the pediatric population (see Fig. 3 and Table 1). Thus, the microbiological, pharmacologic, and biochemical characteristics of avibactam are the most known and are discussed in more detail than the other DBOs. Depending on the partner β-lactam (eg, ceftazidime, ceftaroline, aztreonam, cefepime, or imipenem), β-lactam-avibactam combinations have the potential to be highly effective against many MDR gram-negative pathogens, including Enterobacteriaceae and P aeruginosa, producing class A, B, C, and some D β-lactamases.1 Avibactam has been studied primarily with 2 partner cephalosporins, ceftazidime and ceftaroline; these combinations target Gram-negatives expressing class A, C, and some D β-lactamases (see Fig. 3 and Table 1). However, partnership with aztreonam would expand the spectrum of activity to include class B MBLs, because aztreonam is not hydrolyzed by MBLs (see Fig. 5 and Table 1). Imipenem-avibactam and cefepime-avibactam or aztreonam-avibactam and ceftaroline-avibactam are effective against E coli and K pneumoniae carrying blaOXA-48, respectively, but not Acinetobacter baumannii producing blaOXAs.9–12 Avibactam is restores susceptibility to ceftazidime when tested against clinical isolates of Enterobacteriaceae possessing porin and outer membrane permeability defects.13
Fig. 5.
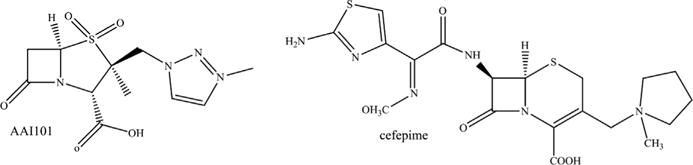
Chemical structures of AAI101 and cefepime.
Avibactam forms a stable carbamyl-adduct with serine β-lactamases that is reversible through recyclization of the 5-membered urea ring.14 Thus far, decarbamylation-hydrolysis of avibactam was only observed after 24 hours with class A β-lactamase, KPC-2.15 Formation of the carbamyl-enzyme complex, represented by the kinetic value, k2/K and recyclization-decarbamylation to reform active avibactam, denoted by a koff value differs for the various classes of serine β-lactamases. These kinetic parameters translate directly to efficacy within bacterial cells; thus, an ideal DBO is one that possesses a high k2/K value and low koff value. The tested class A and C β-lactamases (with the exception of the class A β-lactamase BlaC from Mycobacterium tuberculosis) acylate rapidly with high k2/K values (range: 104 to 106 M−1s−1) and recyclize slowly with low koff values (range: 10−3 to 10−4 s−1).15,16 Conversely, BlaC and class D β-lactamases are slow to acylate with low k2/K values in the range of 101 to 103 M−1s−1, but once acylated, recyclization is very slow with koff values of 10−5 to 10−6 s−1. X-ray crystallography as well as molecular modeling revealed that avibactam adopts very similar active site conformations in class A, C, and D β-lactamases.16–20
Recent crystal structures and biochemical analysis with avibactam and OXA-24/40 and OXA-48 were conducted and provided some insights into why select class D β-lactamases are inhibited, whereas others are not.17,19 OXA-24/40’s k2/K value is much lower than OXA-48’s, whereas the koff values are very similar. The crystal structures revealed that the binding pocket for avibactam in class D β-lactamases is more hydrophobic with fewer polar residues present, thus potentially affecting binding and acylation. In addition, OXA-24/40 possesses additional hydrophobic moieties (eg, hydrophobic bridge between Y112 and M223), whereas OXA-48 possesses polar residues (eg, T213, R214, and D101) that could aid in Michaelis-complex formation with avibactam. Slow recyclization was suggested to occur because of a decarboxylated Lys84/73.17,19
RESISTANCE TO β-LACTAM-AVIBACTAM COMBINATIONS
Selection via passaging of E coli producing blaCTX-M-15 and E cloacae with de-repressed blaAmpC on ceftaroline-avibactam identified strains with elevated ceftaroline-avibactam MICs; however, most of the mutations were unstable.21 E coli producing CTX-M-15 Lys237Gln variant conferred resistance with the cost of ESBL activity. Selected-resistant E cloacae possessed Ω loop deletions within AmpC as well as porin loss. A similar approach was conducted using P aeruginosa with de-repressed blaAmpC with ceftazidime-avibactam.22 Ceftazidime-avibactam–resistant variants possessed MICs from 64 to 256 mg/L as a result of deletions in the Ω loop of the AmpC. Mechanistic analyses revealed that the AmpC variants were less susceptible to avibactam inactivation and possessed improved ceftazidime kinetics.
Resistance to ceftazidime-avibactam was observed in a panel of clinical isolates of P aeruginosa.23 The mechanism of resistance was dissected by sequencing blaAmpC, the genes in the blaAmpC regulon, pbps, and oprD, measuring efflux pump expression, and combination antibiotic therapy with ceftazidime-avibactam. Using these methods, membrane permeability and drug efflux were found to be the most important factor influencing ceftazidime-avibactam resistance in these isolates. Resistance to ceftazidime-avibactam was overcome with ceftazidime-avibactam/fosfomycin; this combination targets PBPs, β-lactamases, and MurA, and UDP-N-acetylglucosamine-3-enolpyruvyltransferase, which is involved in peptidoglycan synthesis.
In a panel of clinical isolates of E coli producing blaNDM-1, resistance to aztreonam-avibactam was detected.24 Aztreonam was still able to inhibit the β-lactamases; however, a 4-amino-acid insertion was identified in PBP3.
Variant β-lactamases with single amino acid substitutions in residues that typically result in inhibitor resistance (ie, 69, 130, 234, 220/244, and 276) and ceftazidime-resistance (ie, 164, 167, 169, and 179) in clinical isolates were tested in SHV-1 and KPC-2 β-lactamase isogenic E coli strain backgrounds with β-lactam-avibactam combinations.25–27 The S130G, K234R, and R220M (KPC-2)/R244S (SHV-1) substitutions in the SHV-1 and KPC-2 backgrounds resulted in elevated MICs to ampicillin-avibactam when expressed in E coli.25,27 The S130G variants of SHV-1 and KPC-2 were found to have severely compromised k2/K values (~1 M−1s−1), thus avibactam failed to inactivate these variants. S130 is an important residue for avibactam acylation. The resistance mechanisms of the K234R and R220M/R244S variants remain to be defined. The R164A, R164P, D179A, D179Q, and D179N substitutions in KPC-2 resulted in increased ceftazidime-avibactam MICs.26 Loss of susceptibility to ceftazidime-avibactam is thought to be due to enhanced ceftazidime kinetics of the variants because avibactam was still able to inactivate the R164A and D179N variants. In another study, selection of Enterobacteriaceae producing blaKPC for resistance to ceftazidime-avibactam resulted in the identification of KPC variants with D179Y amino acid substitutions.28 Resistance to a β-lactam-β-lactamase inhibitor combination due to resistance to the partner β-lactam is a very intriguing observation. The choice of a β-lactam partner is critical, as described above with ceftolozane-tazobactam.
During the writing of this manuscript, the first clinical observation of ceftazidime-avibactam resistance was reported.29 The resistance was observed in Klebsiella pneumoniae expressing blaKPC-3 and mechanism of resistance is unclear.
DIAZABICYCLOOCTANONES, RELEBACTAM AND OP0595, ON THE HORIZON
Relebactam partnered with imipenem-cilastatin demonstrates a similar spectrum of activity as avibactam, thus lacking activity against MBLs and most OXAs (see Fig. 3 and Table 1).30–32 RG6080 (formerly OP0595) not only is an inhibitor of class A and C β-lactamases but also inhibits PBP-2 of Enterobacteriaceae (see Fig. 3 and Table 1).33,34 Thus, RG6080 is unique compared with avibactam and relebactam, because it does not need a β-lactam partner for antimicrobial activity. In addition, there is evidence that RG6080 acts to enhance the activity of β-lactams.35
DIAZABICYCLOOCTANONES IN PRECLINICAL DEVELOPMENT
FPI-1465 when combined with aztreonam and ceftazidime possesses activity against Enterobacteriaceae containing ESBLs and class A, B, and D carbapenemases (see Fig. 3; Table 2).36,37 In addition, FPI-1465 is also active against PBPs (ie, PBP2) from E coli and P aeruginosa.38
Table 2.
Promising new β-lactams or β-lactamase inhibitors in preclinical development
| β-Lactamase Inhibitor Name | Partner β-Lactam | Company | Type of β-Lactamase Inhibitor |
|---|---|---|---|
| FPI-1465 | Aztreonam or ceftazidime | Fedora | DBO (also inhibits PBP activity) |
| WCK 4234 | Meropenem | Wockhardt, Ltd | DBO |
| WO2013/030735 | Not necessary? | Wockhardt, Ltd | DBO (also inhibits PBP activity) |
| WCK 5153 | Not necessary? | Wockhardt, Ltd | DBO (also inhibits PBP activity) |
| Benzo(b)thiophene-2-boronic acid | Ceftazidime | Therabor and Regents of the University of California | Boronate |
| Sulfonamide boronates (CR161, compound 4, and compound 9) | Ceftazidime or cefotaxime | Therabor and Regents of the University of California | Boronate |
| S02030 | Cefepime | Case Western Reserve University and Università degli Studi di Modena e Reggio Emilia | Boronate |
| 3,4-dihydro-2H-benzo[e][1,2] oxaborinine-8-carboxylic acids | Ceftazidime or meropenem | VenatoRx Pharmaceuticals | Boronate |
| α-Aminoboronic acids | Ceftazidime | VenatoRx Pharmaceuticals | Boronate |
| 3,4-Dihydro-2H-benzo[e][1,2] oxaborinine-8-carboxylic acids | Carbapenem | Rempex Pharmaceuticals (The Medicines Company) | Boronate |
| AA101 | Cefepime | Allecra Therapeutics | Sulfone |
| Sulfone derivatives | Meropenem or imipenem | Orchid Pharmaceuticals | Sulfone |
| Sulfone derivatives | Meropenem or imipenem | Dr John D. Buynak (Southern Methodist University) | Sulfone |
| Clavam derivatives | Ceftazidime | Nabriva Therapeutics | Clavam |
| MG96077 | Imipenem | Mirati Therapeutics | Phosphonate |
| BAL30072 | Meropenem or no β-lactam required | Basilea Pharmaceuticals | Siderophore monobactam |
| BAL30376 (BAL19764, BAL29880, & clavulanic acid) | No β-lactam required | Basilea Pharmaceuticals | Siderophore monobactam, bridged monobactam, and a clavam |
| MK-8712 | Imipenem | Merck Sharp & Dohme Corporation | Bridged monobactam |
| Siderophore monobactams | Aztreonam or meropenem | Pfizer | Siderophore monobactam |
| Syn2190 | Ceftazidime | Taiho Pharmaceuticals Co | Siderophore monobactam |
| 3′-Thiobenzoyl cephalosporins | Meropenem | University of Waterloo, Wilfrid Laurier University | β-Lactam |
| FSI-1686 and FSI-1671 | No β-lactam required | FOB Synthesis Inc | β-Lactam |
| BTZs | Imipenem | Universidad de la República, Montevideo, Uruguay | Bisthiazolidine |
| ME1071 | Ceftazidime or biapenem | Meiji Seika Kaisha Ltd | Maleic acid derivative |
Wockhardt, Ltd has 3 DBOs in the pipeline: WCK 4234, WO2013/030735, and WCK 5153 (see Fig. 3 and Table 2).39 The WCK 4234 combined with meropenem demonstrates activity against oxacillinase-producing strains of A baumannii.39 WO2013/030735 and WCK 5153 possess antibacterial activity against P aeruginosa and E coli.
During the publication process of this article, Merck (formerly Cubist) published work with another DBO, CB-618.40 CB-618 tested in combination with meropenem displays activity against clinical isolates of Enterobacteriaceae expressing the KPC-2, KPC-3, FOX-5, OXA-48, SHV-11, SHV-27, and/or TEM-1 beta-lactamases.
BORONIC ACID β-LACTAMASE INHIBITORS ARE MAKING GREAT STRIDES
In the late 1970s, boronic acids were recognized as inhibitors of serine β-lactamases in vitro.41 Boron forms a reversible bond with the β-lactamase.42 Boronic acids serve as competitive inhibitors and were not shown to be hydrolyzed by any β-lactamase to date. Historically, despite good affinities for many class A and C serine β-lactamases, boronic acids failed to make it into clinical development, but the tides are changing.
A novel cyclic boronic acid–based β-lactamase inhibitor, RPX7009, is in phase 3 clinical trials in combination with meropenem (RPX2014) under the name Carbavance; in addition, clinical trials in pediatric patients are in the works (Fig. 4; see Table 1).43 Initially, development began with biapenem (RPX2003) (see Fig. 4 and Table 1). Biapenem-RPX7009 and meropenem-RPX7009 are most effective against Enterobacteriaceae producing class A carbapenemases and demonstrated against class A ESBLs and AmpC; impermeability had a negative impact on the activity of carbapenem-RPX7009 combinations.44,45 In addition, biapenem-RPX7009 was not effective against Enterobacteriaceae expressing MBLs or OXA-48.44
Fig. 4.
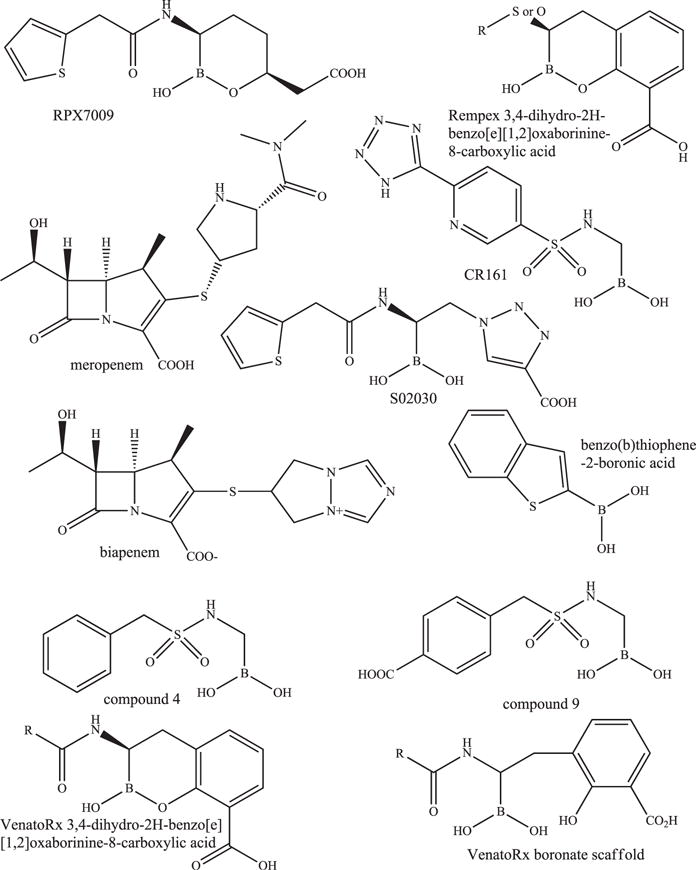
Chemical structures of the carbavance (meropenem-RPX7009) combination, biapenem, and other boronates.
RPX7009 did not potentiate the activity of carbapenems against nonfermenters P aeruginosa and A baumannii.45 RPX7009 also did not increase the activity of biapenem against anaerobes.46 A neutropenic lung model of infection in mice with K pneumoniae producing blaKPC-2 revealed that RPX-7009 reduced colony forming units (CFUs) by 2 logs in combination with biapenem and meropenem compared with the carbapenem alone.43
BORONIC ACIDS IN PRECLINICAL DEVELOPMENT
In addition, several boronic acid inhibitors in preclinical development showed promise because they reduced MICs or were successful in animal models of infection. Benzo(b)thiophene-2-boronic acids combined with ceftazidime are effective against Enterobacteriaceae and P aeruginosa (see Fig. 4 and Table 2).47 S02030, a novel boronic acid possessing thiophene and triazole carboxylate side chains demonstrates activity against Enterobacteriaceae carrying blaKPCs with a k2/K value (1.2 ± 0.2 × 10(4) M(−1) s(−1)) comparable to avibactam (Fig. 4 and Table 2).48 TheraBor Pharmaceuticals and the Regents of the University of California developed and patented (patent WO2013/056079) several sulfonamide boronates (eg, CR161) that were shown to reduce ceftazidime MICs against Enterobacteriaceae and P aeruginosa (see Fig. 4 and Table 2).16,49 Moreover, when mice were infected intraperitoneally with E coli overexpressing AmpC, after 120 hours, the mice treated with CR161 combined with cefotaxime possessed a 65% survival compared with cefotaxime alone at 15%. VenatoRx Pharmaceuticals also patented a series of cyclic boronic acids, 3,4-dihydro-2H-benzo[e][1,2]oxaborinine-8-carboxylic acids (US 8,912,169 B2), and a set of novel a-aminoboronic acids (US 20100120715 A1) (see Fig. 4 and Table 2). Select cyclic boronates combined with either ceftazidime or meropenem demonstrated activity against E cloacae, K pneumoniae with blaKPC-3, P aeruginosa with blaVIM-2, and K pneumoniae with blaKPC-2 and blaVIM-4 and inhibited SHV-5, KPC-2, VIM-2, AmpC, and OXA-1 with concentration of inhibitor at which 50% inhibition of substrate hydrolysis is observed (IC50) values <1 μM. Select α-aminoboronic acids combined with ceftazidime possessed activity against E coli with blaSHV-5, K pneumoniae with blaCTX-M-15, E cloacae with blaP99, and K pneumoniae with blaKPC-2 and inhibited SHV-5, CTX-M-15, P99, and KPC-2, with IC50 values <0.1 μM. Rempex, a subsidiary of The Medicines Company, also patented another group of cyclic boronic acids, 3,4-dihydro-2H-benzo[e][1,2]oxaborinine-8-carboxylic acids (see Fig. 4 and Table 2) (WO2014/107536 A1). Select compounds in this class have Ki values of less than 1 μM against SHV-12, TEM-10, CTX-M-14, KPC-2, P99, CMY-2, OXA-48, VIM-1, and NDM-1, and when combined with carbapenems, demonstrated MICs less than 1 mg/L for Enterobacteriaceae producing blaNDM-1, blaVIM-1, and blaKPC-2 and even A baumannii expressing blaNDM-1.
NOVEL SULFONES AND CLAVAMS IN PRECLINICAL DEVELOPMENT
Allecra Therapeutics is developing a novel sulfone, AAI101, in combination with cefepime; cefepime-AAI101 demonstrated activity against some Enterobacteriaceae with ESBLs or carbapenemases (Fig. 5; see Table 2).50 In a neutropenic thigh mouse infection model, the cefepime-AAI101 combination reduced bacterial CFUs by more than 0.5 log CFU for 12 of the 20 strains tested; cefepime alone only worked in 3 of 20 strains. Orchid Pharmaceuticals synthesized a series of sulfone derivatives with no R1 side chain and different R2 side chains, and some lowered meropenem and imipenem MICs from 32 to 64 mg/L to 1 to 2 mg/L against K pneumoniae with blaKPC-2 (WO/2012/070071) when tested in combination (see Table 2). These sulfones were also tested in combination with imipenem or meropenem against other Enterobacteriaceae with blaKPC-2 or blaKPC-3, and MICs decreased from 2 to 4 mg/L to 0.25 to 0.5 mg/L for some compounds. Another set of novel sulfones was developed by Dr John D. Buynak, and one of these compounds lowered meropenem and imipenem MICs for carbapenem-susceptible A baumannii producing blaOXA-24/40 from 32 mg/L to 1 mg/L when tested in combination (see Table 2).51 Nabriva Therapeutics created clavam spinoffs that possessed activity against K pneumoniae and Citrobacter freundii with MICs decreasing for ceftazidime from 26 mg/L to 0.2 mg/L and 3.2 mg/L, respectively, with a clavam derivative (see Table 2).
PHOSPHONATES IN PRECLINICAL DEVELOPMENT
Like with the boronates, literature shows that phosphonates are good inhibitors of class A and C and even some B and D β-lactamases kinetically.52–54 Mirati Therapeutics conducted preclinical studies on a phosphonate, MG96077, which is a novel broad-spectrum, non-β-lactam β-lactamase inhibitor (Fig. 6; see Table 2).39,55 Imipenem combined with MG96077 decreased greater than 90% of the MICs for imipenem-resistant P aeruginosa and K pneumoniae to 4 mg/L. In a mouse spleen infection model with imipenem-resistant P aeruginosa, imipenem-MG96077 caused a 4 to 6 log reduction in CFUs and increased mouse survival.
Fig. 6.
Phosphonate, MG96077.
MONOBACTAMS ARE PROMISING β-LACTAMASE INHIBITORS OR EVADE β-LACTAMASE ACTIVITY
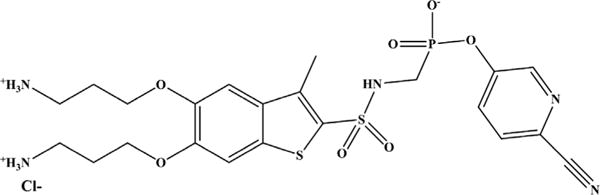
Already available in the clinic, the monobactam, aztreonam, is a β-lactam that can also circumvent certain β-lactamases (Fig. 7). Aztreonam inhibits certain AmpC β-lactamases and binds poorly to MBLs.56–58
Fig. 7.
Chemical structures of monobactams and bridged monobactams.
MONOBACTAMS AND DERIVATIVES IN PRECLINICAL DEVELOPMENT
The monobactam scaffold is encouraging for future MBL inhibitors as well as inhibitors of AmpCs, and Basilea Pharmaceuticals, Merck, Pfizer, and Taiho Pharmaceutical Co are working on these agents.
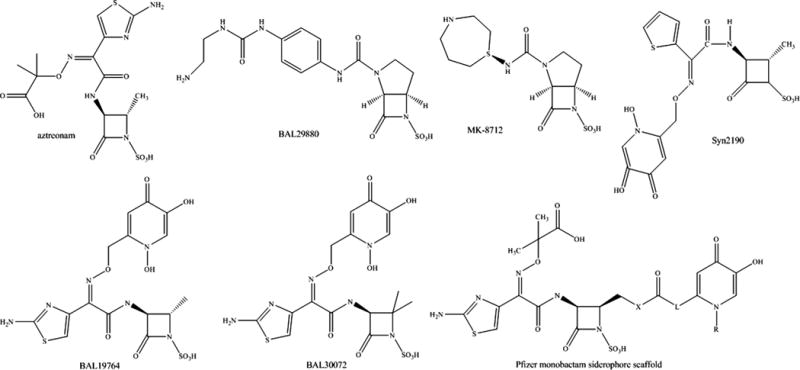
For Basilea, expansion of the monobactam class has resulted in several new monobactams (eg, BAL30072 and BAL19764) and a novel class of bridged monobactams (eg, BAL29880) that serve as β-lactamase inhibitors. BAL30072 contains a 1,5-dihydroxy-4-pyridone group that is a siderophore moiety that allows for transport of the compound into the bacterial cell via the TonB iron transport system (see Fig. 7 and Table 2). BAL30072 possessed bactericidal activity against Acinetobacter spp, which include those with some blaOXAs, P aeruginosa, Burkholderia spp, Enterobacteriaceae with class A carbapenemases, and against strains that produced blaMBLs.59–62 BAL30072 demonstrated efficacy in rat soft tissue infection by A baumanniii as well as a mouse septicemia model when combined with meropenem against Serratia marcescens producing blaSME-1 (a class A carbapenemase), P aeruginosa, and A baumannii.61,63
BAL30376 combines BAL19764, another siderophore monobactam, with the bridged monobactam BAL29880 and clavulanic acid (see Fig. 7 and Table 2).64,65 BAL30376 was effective against most clinical Enterobacteriaceae isolates producing blaAmpCs and blaESBLs, P aeruginosa, A baumannii producing blaOXA-23, or the southeast region clonal lineage and some Stenotrophomonas maltophilia. BAL30376 was not effective against Enterobacteriaceae expressing blaMBLs, blaKPC, or blaOXA-48, or those with impermeability, P aeruginosa or B cepacia complex isolated from cystic fibrosis patients, P aeruginosa expressing blaMBLs, and other A baumannii. In addition, in a mouse septicemia model of infection, BAL30376 demonstrated efficacy against selected isolates of A baumannii, E cloacae, and P aeruginosa.65
Merck is conducting preclinical testing with a bridged monobactam, MK-8712 (see Fig. 7 and Table 2).66 MK-8712 combined with imipenem reduced MICs against P aeruginosa strain CL5701from 32 mg/L to 4 mg/L. MK-8712 was also a potent inhibitor of AmpC of P aeruginosa with an IC50 value of 1 μM. MK-8712 combined with imipenem-cilastatin resulted in a 4.6 log fold reduction in CFU in a mouse spleen model of infection compared with imipenem-cilastatin alone.67
Pfizer developed a series of siderophore monobactams, similar to BAL30072; however, the siderophore moiety is on the R2 side chain (see Fig. 7 and Table 2).68 Several of these compounds worked in combination with aztreonam or meropenem to lower MIC values to the susceptible range against P aeruginosa and also possessed efficacy in a mouse pneumonia model of infection. The siderophore monobactams were modified to increase the length of the R2 side chain with linkers resulting in expansion of activity against K pneumoniae, E coli, and A baumannii, including P aeruginosa carrying a blaMBL; these compounds target the PBPs and evade MBL activity.69
Similar to Merck, Taiho Pharmaceutical Co is working on a siderophore monobactam, Syn2190, that inhibited AmpC β-lactamases and reduced ceftazidime MICs to the susceptible range against Enterobacteriaceae and P aeruginosa expressing blaAmpC (see Fig. 7 and Table 2).70 In addition, Syn2190 combined with ceftazidime or cefpirome demonstrated activity in mouse systemic and urinary tract infection models using P aeruginosa. However, Syn2190 induced expression of blaAmpC.
A NEW SIDEROPHORE CEPHALOSPORIN IN CLINICAL DEVELOPMENT
S-649266, a novel catechol-substituted siderophore cephalosporin, is in phase II clinical trials (see Table 1 and Fig. 8).71 This compound demonstrated potent in vitro activity against a diverse panel of gram-negative bacteria39 S-649266 was not hydrolyzed by KPC-2, P99, or OXA-23; minimal hydrolysis with kcat/Km values in the 103–104 M−1s−1 for MBLs, IMP-1, VIM-2, L1, and CTX-M-15 was observed.72 S-649266 demonstrated activity against P aeruginosa, S maltophilia, K pneumoniae, and A baumannii with MIC90 values <2 mg/L.73 Furthermore, against MDR strains of Enterobacteriaceae, P aeruginosa, and A baumannii, the MIC90 values were less than 4 mg/L.73 In a rat lung infection model, S-649266 was shown to have efficacy against P aeruginosa and A baumannii, including MDR strains.74 In addition, several different mouse models of infections (ie, systemic, lung, urinary tract, and subcutaneous infection) using various Gram-negatives were used to assess the efficacy of S-649266; the compound was effective against K pneumoniae producing blaKPC-2.75
Fig. 8.
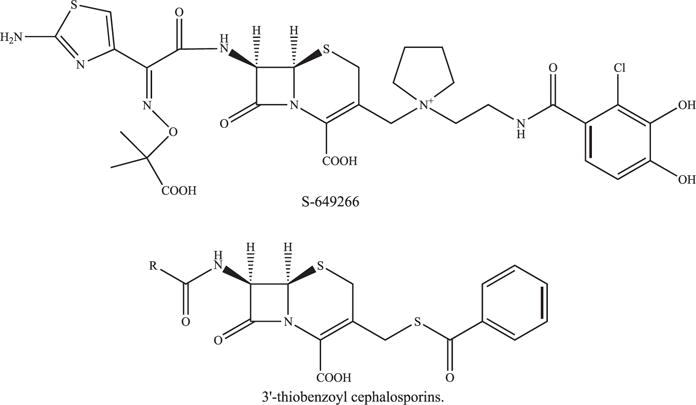
Chemical structure of 3′-thiobenzoyl cephalosporins.
NOVEL 3′-THIOBENZOYL CEPHALOSPORINS IN PRECLINICAL DEVELOPMENT
3′-Thiobenzoyl cephalosporins demonstrated inhibitory activity against class A, B, C, and D β-lactamases with IC50 values in the range of 1.4 μM to 140 μM (Fig. 8 and see Table 2) (US 20120329770 A1). In addition, when combined with meropenem, selected compounds possessed activity against P aeruginosa, S maltophilia, and Chryseobacterium meningosepticum.
NOVEL CARBAPENEMS IN PRECLINICAL DEVELOPMENT: FSI-1671 AND FSI-1686
Two novel carbapenems, FSI-1671 and FSI-1686, were shown to possess activity against MDR A baumannii, Enterobacteriaceae, and some P aeruginosa (Fig. 9; see Table 2).76 Mice were intraperitoneally infected by carbapenem-resistant A baumannii, K pneumoniae, and P aeruginosa and treated with FSI-1671, FSI-1686, meropenem, doripenem, colistin, or tigecycline.77 Both novel carbapenems demonstrated good potency (lower ED50 [50% effective dose] values) against carbapenem-resistant A baumannii, K pneumoniae, and P aeruginosa compared with the other carbapenems tested. The β-lactamase inhibitory properties of these compounds were not assessed, but all other clinically available carbapenems are dual agents inhibiting PBPs and some β-lactamases.
Fig. 9.
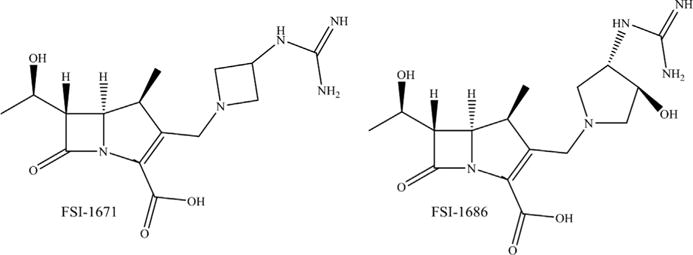
Chemical structures of FSI-1671 and FSI-1686.
METALLO-β-LACTAMASE-SPECIFIC INHIBITORS IN PRECLINICAL DEVELOPMENT: BISTHIAZOLIDINES AND ME1071
Four bisthiazolidine (BTZ) inhibitors were tested against VIM-2 and VIM-24 and possessed Ki values between 3.7 and 14 μM (Fig. 10; see Table 2).78 Most importantly, the BTZs restored imipenem susceptibility of clinical isolates, P aeruginosa producing blaVIM-2 and K pneumoniae carrying blaVIM-24. In addition, BTZs demonstrate activity against NDM-1 with Ki values from 7 to 19 μM and are effective against A. baumannii, K. pneumoniae, and P. rettgeri expressing blaNDM-1 when combined with imipenem (ACS Infect. Dis., 2015, 1 (11), pp 544–54).
Fig. 10.
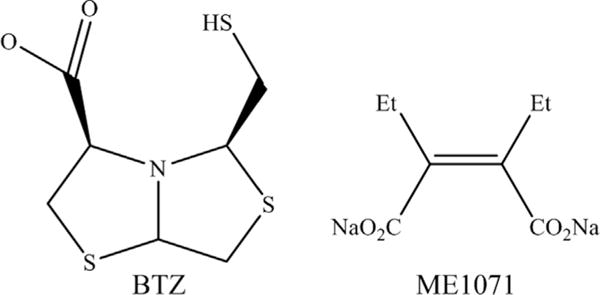
Chemical structure of MBL-specific inhibitors the BTZ, (3R,5R,7aS)-5-(sulfanylmethyl) tetrahydro[1,3] thiazol[4,3-b][1,3]thiazole-3-carboxylic acid and ME1071.
ME1071 is a maleic acid derivative, and when combined with ceftazidime, increased susceptibility to ceftazidime against P aeruginosa expressing blaIMP and blaVIM, although never for 100% of the isolates tested (see Fig. 10 and Table 2).79 Biapenem-ME1071 combination decreased MICs for Enterobacteriaceae with blaIMP and blaVIM, but not blaNDM.80 ME1071 in combination with biapenem was effective in a mouse model for ventilator-associated pneumonia by P aeruginosa producing blaMBL.81
SUMMARY
When one thinks about designing drugs to treat infections caused by MDR bacteria, there are 2 approaches to keep in mind. One strategy involves designing “niche” drugs to target specific bacteria with certain resistance mechanisms. These “niche” agents would be highly useful especially for uncommon or hard-to-treat infections (eg, S maltophilia or MDR A baumannii or Gram-negatives producing blaMBLs). However, using drugs that only target a specific pathogen will force clinicians to rely heavily on accurate and rapid molecular diagnostics. The second tactic is designing drugs that possess a very broad spectrum and can be given as empirical therapy. With this second scenario, “time is no longer the enemy,” and molecular diagnostics are not nearly as important. However, the risk of the bacteria evolving resistance to the novel agents is higher the more these agents are used in the clinic. If other older agents would work equally well, why accelerate the decline in utility of a novel agent?
Taking these ideas into consideration and looking back at this article, how do these approaches fit with the novel β-lactam-β-lactamase inhibitor combinations described? Clavulanic acid and the sulfones seem to be classified between a “niche” and a broad-spectrum agent category because their spectrum is somewhat limited given the MDR organisms observed today. Moreover, because they were first introduced more than 30 years ago, further derivatization of these compounds has not resulted in any novel inhibitors reaching clinical development. There were slight gains such as increased penetration and the ability to target other classes of β-lactamase. However, based on this history, the novel inhibitors may be a better route.
The DBOs in the preclinical development target PBPs. Is this PBP activity enough to propel this class forward as a broad-spectrum agent? With that in mind, could a novel DBO be a potential “magic bullet” to target all Gram-negatives producing β-lactamases? Will β-lactamases ever evolve to hydrolyze DBOs? Probably, as KPC-2 hydrolyzes avibactam albeit at a very slow rate.
Boronates have historically possessed activity against class A and C β-lactamases. Now, boronates were expanded to inhibit class A and B carbapenemases and some class D β-lactamases with 3,4-dihydro-2H-benzo[e][1,2]oxaborinine-8-carboxylic acids showing the most promise. Can they be expanded to target OXA carbapenemases? Could a novel carbapenem-3,4-dihydro-2H-benzo[e][1,2]oxaborinine-8-carboxylic acid combination be a potential “magic bullet” to inhibit all Gram-negatives producing β-lactamases? Will β-lactamases evolve to hydrolyze them? Hydrolysis seems less likely because, to date, hydrolysis of boronic acids has not been documented. However, β-lactamases could still evolve to resist inhibition by boronates.
The other compounds discussed here possess more limited spectra compared with the DBOs and boronates that are in preclinical development. Some inhibitors would be categorized as “niche” agents, such as the BTZs and ME1071. Where does this leave us now, and what is the best path forward? Clinical agents need to be developed that treat the resistant pathogens that exist now. It should be kept in mind that other resistance mechanisms (eg, loss of porins, expression of efflux pumps, and other permeability barriers) are still going to be a challenge. Also, the evolution of resistance in both PBPs and β-lactamases to these novel β-lactams and β-lactamase inhibitor should be studied to understand their mechanisms of action in order to come up with strategies to circumvent resistance, when it eventually appears. The war against resistant pathogens will most likely not have a definitive end. However, luckily, several new agents are in the arsenal that momentarily will keep pace with the challenging pathogens in the clinic today.
KEY POINTS.
Obstacles in the development of beta-lactamase inhibitors.
Diazabicyclooctanones, an ever expanding novel beta-lactamase inhibitor class.
Boronic acids, non-hydrolyzable beta-lactamase inhibitors.
Beta-lactams as beta-lactamase inhibitors.
Acknowledgments
Funding: Research reported in this publication was supported in part by funds and/or facilities provided by the Cleveland Department of Veterans Affairs to K.M. Papp-Wallace and R.A. Bonomo, the Veterans Affairs Career Development Program to K.M. Papp-Wallace, the Veterans Affairs Merit Review Program Award 1I01BX002872 to K.M. Papp-Wallace and the Veterans Affairs Merit Review Program Award 1I01BX001974 to R.A. Bonomo and the Geriatric Research Education and Clinical Center VISN 10 to R.A. Bonomo. R.A. Bonomo is also supported by the Harrington Foundation, and The National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Numbers R01 AI100560 and R01 AI063517 to R.A. Bonomo. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Drawz SM, Papp-Wallace KM, Bonomo RA. New β-lactamase inhibitors: a therapeutic renaissance in an MDR world. Antimicrob Agents Chemother. 2014;58:1835–46. doi: 10.1128/AAC.00826-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. Twenty-fifth Informational supplement M100-S25. Wayne (PA): CLSI; 2015. Performance standards for antimicrobial susceptibility testing. [Google Scholar]
- 3.Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201. doi: 10.1128/CMR.00037-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papp-Wallace KM, Bethel CR, Distler AM, et al. Inhibitor resistance in the KPC-2 β-lactamase, a preeminent property of this class A β-lactamase. Antimicrob Agents Chemother. 2010;54:890–7. doi: 10.1128/AAC.00693-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takeda S, Ishii Y, Hatano K, et al. Stability of FR264205 against AmpC β-lactamase of Pseudomonas aeruginosa. Int J Antimicrob Agents. 2007;30:443–5. doi: 10.1016/j.ijantimicag.2007.05.019. [DOI] [PubMed] [Google Scholar]
- 6.Zhanel GG, Chung P, Adam H, et al. Ceftolozane/tazobactam: a novel cephalosporin/β-lactamase inhibitor combination with activity against multidrug-resistant gram-negative bacilli. Drugs. 2014;74:31–51. doi: 10.1007/s40265-013-0168-2. [DOI] [PubMed] [Google Scholar]
- 7.Cabot G, Bruchmann S, Mulet X, et al. Pseudomonas aeruginosa ceftolozane-tazobactam resistance development requires multiple mutations leading to overexpression and structural modification of AmpC. Antimicrob Agents Chemother. 2014;58:3091–9. doi: 10.1128/AAC.02462-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berrazeg M, Jeannot K, Ntsogo Enguéné VY, et al. Mutations in β-Lactamase AmpC Increase Resistance of Pseudomonas aeruginosa Isolates to Antipseudomonal Cephalosporins. Antimicrob Agents Chemother. 2015;59(10):6248–55. doi: 10.1128/AAC.00825-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aktas Z, Kayacan C, Oncul O. In vitro activity of avibactam (NXL104) in combination with β-lactams against Gram-negative bacteria, including OXA-48 β-lactamase-producing Klebsiella pneumoniae. Int J Antimicrob Agents. 2012;39:86–9. doi: 10.1016/j.ijantimicag.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Livermore DM, Mushtaq S, Warner M, et al. Activities of NXL104 combinations with ceftazidime and aztreonam against carbapenemase-producing Enterobacteriaceae. Antimicrob Agents Chemother. 2011;55:390–4. doi: 10.1128/AAC.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mushtaq S, Warner M, Livermore DM. In vitro activity of ceftazidime1NXL104 against Pseudomonas aeruginosa and other non-fermenters. J Antimicrob Chemother. 2010;65:2376–81. doi: 10.1093/jac/dkq306. [DOI] [PubMed] [Google Scholar]
- 12.Mushtaq S, Warner M, Williams G, et al. Activity of chequerboard combinations of ceftaroline and NXL104 versus β-lactamase-producing Enterobacteriaceae. J Antimicrob Chemother. 2010;65:1428–32. doi: 10.1093/jac/dkq161. [DOI] [PubMed] [Google Scholar]
- 13.Pagès JM, Peslier S, Keating TA, et al. Role of the Outer Membrane and Porins in Susceptibility of β-Lactamase-Producing Enterobacteriaceae to Ceftazidime-Avibactam. Antimicrob Agents Chemother. 2015;60(3):1349–59. doi: 10.1128/AAC.01585-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ehmann DE, Jahic H, Ross PL, et al. Avibactam is a covalent, reversible, non-β-lactam β-lactamase inhibitor. Proc Natl Acad Sci U S A. 2012;109:11663–8. doi: 10.1073/pnas.1205073109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ehmann DE, Jahic H, Ross PL, et al. Kinetics of avibactam inhibition against class A, C, and D β-lactamases. J Biol Chem. 2013;288:27960–71. doi: 10.1074/jbc.M113.485979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu H, Hazra S, Blanchard JS. NXL104 irreversibly inhibits the β-lactamase from Mycobacterium tuberculosis. Biochemistry. 2012;51:4551–7. doi: 10.1021/bi300508r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lahiri SD, Mangani S, Jahic H, et al. Molecular basis of selective inhibition and slow reversibility of avibactam against class D carbapenemases: a structure-guided study of OXA-24 and OXA-48. ACS Chem Biol. 2015;10:591–600. doi: 10.1021/cb500703p. [DOI] [PubMed] [Google Scholar]
- 18.Lahiri SD, Mangani S, Durand-Reville T, et al. Structural insight into potent broad-spectrum inhibition with reversible recyclization mechanism: avibactam in complex with CTX-M-15 and Pseudomonas aeruginosa AmpC β-lactamases. Antimicrob Agents Chemother. 2013;57:2496–505. doi: 10.1128/AAC.02247-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.King DT, King AM, Lal SM, et al. Molecular mechanism of avibactam-mediated β-lactamase inhibition. ACS Infect Dis. 2015;1:175–84. doi: 10.1021/acsinfecdis.5b00007. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan NP, Nguyen NQ, Papp-Wallace KM, et al. Inhibition of Klebsiella β-Lactamases (SHV-1 and KPC-2) by Avibactam: A Structural Study. PLoS One. 2015;10(9):e0136813. doi: 10.1371/journal.pone.0136813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Livermore DM, Mushtaq S, Barker K, et al. Characterization of β-lactamase and porin mutants of Enterobacteriaceae selected with ceftaroline + avibactam (NXL104) J Antimicrob Chemother. 2012;67:1354–8. doi: 10.1093/jac/dks079. [DOI] [PubMed] [Google Scholar]
- 22.Lahiri SD, Walkup GK, Whiteaker JD, et al. Selection and molecular characterization of ceftazidime/avibactam-resistant mutants in Pseudomonas aeruginosa strains containing derepressed AmpC. J Antimicrob Chemother. 2015;70:1650–8. doi: 10.1093/jac/dkv004. [DOI] [PubMed] [Google Scholar]
- 23.Winkler ML, Papp-Wallace KM, Hujer AM, et al. Unexpected challenges in treating multidrug-resistant Gram-negative bacteria: resistance to ceftazidime-avibactam in archived isolates of Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;59:1020–9. doi: 10.1128/AAC.04238-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alm RA, Johnstone MR, Lahiri SD. Characterization of Escherichia coli NDM isolates with decreased susceptibility to aztreonam/avibactam: role of a novel insertion in PBP3. J Antimicrob Chemother. 2015;70:1420–8. doi: 10.1093/jac/dku568. [DOI] [PubMed] [Google Scholar]
- 25.Papp-Wallace KM, Winkler ML, Taracila MA, et al. Variants of the KPC-2 β-lactamase which are resistant to inhibition by avibactam. Antimicrob Agents Chemother. 2015;59(7):3710–7. doi: 10.1128/AAC.04406-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winkler ML, Papp-Wallace KM, Bonomo RA. Activity of ceftazidime/avibactam against isogenic strains of Escherichia coli containing KPC and SHV β-lactamases with single amino acid substitutions in the Ω-loop. J Antimicrob Chemother. 2015;70(8):2279–86. doi: 10.1093/jac/dkv094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Winkler ML, Papp-Wallace KM, Taracila MA, et al. Avibactam and inhibitor resistant SHV β-lactamases. Antimicrob Agents Chemother. 2015;59(7):3700–9. doi: 10.1128/AAC.04405-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livermore DM, Warner M, Jamrozy D, et al. In vitro selection of ceftazidime-avibactam resistance in Enterobacteriaceae with KPC-3 carbapenemase. Antimicrob Agents Chemother. 2015;59(9):5324–30. doi: 10.1128/AAC.00678-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Humphries RM, Yang S, Hemarajata P, et al. First Report of Ceftazidime-Avibactam Resistance in a KPC-3-Expressing Klebsiella pneumoniae Isolate. Antimicrob Agents Chemother. 2015;59(10):6605–7. doi: 10.1128/AAC.01165-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hirsch EB, Ledesma KR, Chang KT, et al. In vitro activity of MK-7655, a novel β-lactamase inhibitor, in combination with imipenem against carbapenem-resistant Gram-negative bacteria. Antimicrob Agents Chemother. 2012;56:3753–7. doi: 10.1128/AAC.05927-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livermore DM, Warner M, Mushtaq S. Activity of MK-7655 combined with imipenem against Enterobacteriaceae and Pseudomonas aeruginosa. J Antimicrob Chemother. 2013;68(10):2286–90. doi: 10.1093/jac/dkt178. [DOI] [PubMed] [Google Scholar]
- 32.Young K, Hackel M, Lascols C, et al. Response to imipenem plus MK-7655, a novel β-lactamase inhibitor, among 212 recent clinical isolates of P aeruginosa, Abstr 1620, abstr IDSA Week 2012. San Diego (CA): Oct 17–21, 2012. [Google Scholar]
- 33.Morinaka A, Tsutsumi Y, Yamada M, et al. F-946: OP0595, a novel serine-β-lactamase inhibitor: mode of action as β-lactamase inhibitor, antibiotic agent and β-lactam enhancer. Abstr 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 34.Ishii Y, Tsutsumi Y, Yoshizumi A, et al. F-953: OP0595, a novel serine-β-lactamase inhibitor: enzymatic studies of OP0595 against serine-β-lactamases. Abstr 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 35.Morinaka A, Tsutsumi Y, Yamada M, et al. OP0595, a new diazabicyclooctane: mode of action as a serine β-lactamase inhibitor, antibiotic and β-lactam ‘enhancer’. J Antimicrob Chemother. 2015;70(10):2779–86. doi: 10.1093/jac/dkv166. [DOI] [PubMed] [Google Scholar]
- 36.Mendes RE, Rhomberg P, Becker H, et al. F-1188: Activity of β-lactam agents tested in combination with novel β-lactamase inhibitor compounds against Enterobacteriaceae producing extended-spectrum β-lactamases. Abstr 53rd International Interscience Conference on Antimicrobial Agents Chemotherapy; Denver (CO). Sep 10–13, 2013. [Google Scholar]
- 37.Mendes RE, Rhomberg PR, Becker HK, et al. F-1189: β-lactam activity tested in combination with β-lactamase inhibitor candidates against Enterobacteriaceae producing class A, B and D carbapenemases. Abstr 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Denver (CO). Sep 10–13, 2013. [Google Scholar]
- 38.Salama SM, Brouillette E, Malouin F, et al. F-1191: mechanistic studies of FPI-1465 a novel β-lactamase inhibitor. Abstr 54th Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 39.Qin W, Panunzio M, Biondi S. β-Lactam antibiotics renaissance. Antibiotics. 2014;3:193–215. doi: 10.3390/antibiotics3020193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.VanScoy BD, Trang M, McCauley J, et al. Pharmacokinetics-Pharmacodynamics of a Novel Beta-Lactamase Inhibitor, CB-618, in Combination with Meropenem in an. In Vitro Infection Model. Antimicrob Agents Chemother. 2016 doi: 10.1128/AAC.02943-15. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kiener PA, Waley SG. Reversible inhibitors of penicillinases. Biochem J. 1978;169:197–204. doi: 10.1042/bj1690197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Beesley T, Gascoyne N, Knott-Hunziker V, et al. The inhibition of class C β-lactamases by boronic acids. Biochem J. 1983;209:229–33. doi: 10.1042/bj2090229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hecker SJ, Reddy KR, Totrov M, et al. Discovery of a cyclic boronic acid β-lactamase inhibitor (RPX7009) with utility vs class A serine carbapenemases. J Med Chem. 2015;58:3682–92. doi: 10.1021/acs.jmedchem.5b00127. [DOI] [PubMed] [Google Scholar]
- 44.Livermore DM, Mushtaq S. Activity of biapenem (RPX2003) combined with the boronate β-lactamase inhibitor RPX7009 against carbapenem-resistant enterobacteriaceae. J Antimicrob Chemother. 2013;68:1825–31. doi: 10.1093/jac/dkt118. [DOI] [PubMed] [Google Scholar]
- 45.Lapuebla A, Abdallah M, Olafisoye O, et al. Activity of meropenem combined with RPX7009, a novel β-lactamase inhibitor, against Gram-negative clinical isolates in New York City. Antimicrob Agents Chemother. 2015;59(8):4856–60. doi: 10.1128/AAC.00843-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldstein EJ, Citron DM, Tyrrell KL, et al. In vitro activity of biapenem plus RPX7009, a carbapenem combined with a serine β-lactamase inhibitor, against anaerobic bacteria. Antimicrob Agents Chemother. 2013;57:2620–30. doi: 10.1128/AAC.02418-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Powers RA, Blazquez J, Weston GS, et al. The complexed structure and antimicrobial activity of a non-β-lactam inhibitor of AmpC β-lactamase. Protein Sci. 1999;8:2330–7. doi: 10.1110/ps.8.11.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rojas LJ, Taracila MA, Papp-Wallace KM, et al. Boronic Acid Transition State Inhibitors Active against KPC and Other Class A β-Lactamases: Structure-Activity Relationships as a Guide to Inhibitor Design. Antimicrob Agents Chemother. 2016;60(3):1751–9. doi: 10.1128/AAC.02641-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Eidam O, Romagnoli C, Caselli E, et al. Design, synthesis, crystal structures, and antimicrobial activity of sulfonamide boronic acids as β-lactamase inhibitors. J Med Chem. 2010;53:7852–63. doi: 10.1021/jm101015z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Crandon JL, Nicolau DP. In vivo activities of simulated human doses of cefepime and cefepime-AAI101 against multidrug-resistant Gram-negative Enterobacteriaceae. Antimicrob Agents Chemother. 2015;59:2688–94. doi: 10.1128/AAC.00033-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bou G, Santillana E, Sheri A, et al. Design, synthesis, and crystal structures of 6-alkylidene-2′-substituted penicillanic acid sulfones as potent inhibitors of Acinetobacter baumannii OXA-24 carbapenemase. J Am Chem Soc. 2010;132:13320–31. doi: 10.1021/ja104092z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Majumdar S, Pratt RF. Inhibition of class A and C β-lactamases by diaroyl phosphates. Biochemistry. 2009;48:8285–92. doi: 10.1021/bi900807e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Adediran SA, Nukaga M, Baurin S, et al. Inhibition of class D β-lactamases by acyl phosphates and phosphonates. Antimicrob Agents Chemother. 2005;49:4410–2. doi: 10.1128/AAC.49.10.4410-4412.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lassaux P, Hamel M, Gulea M, et al. Mercaptophosphonate compounds as broad-spectrum inhibitors of the metallo-β-lactamases. J Med Chem. 2010;53:4862–76. doi: 10.1021/jm100213c. [DOI] [PubMed] [Google Scholar]
- 55.Martell LA, Rahil G, Vaisburg A, et al. C1–1373: novel β-lactamase inhibitor potentiates and extends the antibacterial activity of imipenem against β-lactam-resistant P aeruginosa and K pneumoniae. Abstr 49th Interscience Conference on Antimicrobial Agents and Chemotherapy; San Francisco (CA). Sep 12–15, 2009. [Google Scholar]
- 56.Poeylaut-Palena AA, Tomatis PE, Karsisiotis AI, et al. A minimalistic approach to identify substrate binding features in B1 metallo-β-lactamases. Bioorg Med Chem Lett. 2007;17:5171–4. doi: 10.1016/j.bmcl.2007.06.089. [DOI] [PubMed] [Google Scholar]
- 57.Sakurai Y, Yoshida Y, Saitoh K, et al. Characteristics of aztreonam as a substrate, inhibitor and inducer for β-lactamases. J Antibiot (Tokyo) 1990;43:403–10. doi: 10.7164/antibiotics.43.403. [DOI] [PubMed] [Google Scholar]
- 58.Papp-Wallace KM, Mallo S, Bethel CR, et al. A kinetic analysis of the inhibition of FOX-4 β-lactamase, a plasmid-mediated AmpC cephalosporinase, by monocyclic β-lactams and carbapenems. J Antimicrob Chemother. 2014;69:682–90. doi: 10.1093/jac/dkt434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Page MG, Dantier C, Desarbre E. In vitro properties of BAL30072, a novel siderophore sulfactam with activity against multiresistant Gram-negative bacilli. Antimicrob Agents Chemother. 2010;54:2291–302. doi: 10.1128/AAC.01525-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mushtaq S, Warner M, Livermore D. Activity of the siderophore monobactam BAL30072 against multiresistant non-fermenters. J Antimicrob Chemother. 2010;65:266–70. doi: 10.1093/jac/dkp425. [DOI] [PubMed] [Google Scholar]
- 61.Russo TA, Page MG, Beanan JM, et al. In vivo and in vitro activity of the siderophore monosulfactam BAL30072 against Acinetobacter baumannii. J Antimicrob Chemother. 2011;66:867–73. doi: 10.1093/jac/dkr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mima T, Kvitko BH, Rholl DA, et al. In vitro activity of BAL30072 against Burkholderia pseudomallei. Int J Antimicrob Agents. 2011;38:157–9. doi: 10.1016/j.ijantimicag.2011.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hofer B, Dantier C, Gebhardt K, et al. Combined effects of the siderophore monosulfactam BAL30072 and carbapenems on multidrug-resistant Gram-negative bacilli. J Antimicrob Chemother. 2013;68:1120–9. doi: 10.1093/jac/dks527. [DOI] [PubMed] [Google Scholar]
- 64.Livermore DM, Mushtaq S, Warner M. Activity of BAL30376 (monobactam BAL19764 + BAL29880 + clavulanate) versus Gram-negative bacteria with characterized resistance mechanisms. J Antimicrob Chemother. 2010;65:2382–95. doi: 10.1093/jac/dkq310. [DOI] [PubMed] [Google Scholar]
- 65.Page MG, Dantier C, Desarbre E, et al. In vitro and in vivo properties of BAL30376, a β-lactam and dual β-lactamase inhibitor combination with enhanced activity against Gram-negative bacilli that express multiple β-lactamases. Antimicrob Agents Chemother. 2011;55:1510–9. doi: 10.1128/AAC.01370-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H, Blizzard TA, Kim S, et al. Side chain SAR of bicyclic β-lactamase inhibitors (BLIs). 2. N-Alkylated and open chain analogs of MK-8712. Bioorg Med Chem Lett. 2011;21:4267–70. doi: 10.1016/j.bmcl.2011.05.065. [DOI] [PubMed] [Google Scholar]
- 67.Blizzard TA, Chen H, Kim S, et al. Side chain SAR of bicyclic β-lactamase inhibitors (BLIs). 1. Discovery of a class C BLI for combination with imipinem. Bioorg Med Chem Lett. 2010;20:918–21. doi: 10.1016/j.bmcl.2009.12.069. [DOI] [PubMed] [Google Scholar]
- 68.Mitton-Fry MJ, Arcari JT, Brown MF, et al. Novel monobactams utilizing a siderophore uptake mechanism for the treatment of Gram-negative infections. Bioorg Med Chem Lett. 2012;22:5989–94. doi: 10.1016/j.bmcl.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 69.Brown MF, Mitton-Fry MJ, Arcari JT, et al. Pyridone-conjugated monobactam antibiotics with Gram-negative activity. J Med Chem. 2013;56:5541–52. doi: 10.1021/jm400560z. [DOI] [PubMed] [Google Scholar]
- 70.Nishida K, Kunugita C, Uji T, et al. In vitro and in vivo activities of Syn2190, a novel β-lactamase inhibitor. Antimicrob Agents Chemother. 1999;43:1895–900. doi: 10.1128/aac.43.8.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ito A, Kohira N, Bouchillon SK, et al. In vitro antimicrobial activity of S-649266, a catechol-substituted siderophore cephalosporin, when tested against non-fermenting Gram-negative bacteria. J Antimicrob Chemother. 2016;71(3):670–7. doi: 10.1093/jac/dkv402. [DOI] [PubMed] [Google Scholar]
- 72.Ishii Y, Horiyama T, Nakamura R, et al. F-1557: S-649266, a novel siderophore cephalosporin: III. Stability against clinically relevant β-lactamases. Abstr 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 73.Ito A, Yoshizawa H, Nakamura R, et al. F-1562: S-649266, a novel siderophore cephalosporin: I In vitro activity against Gram-negative bacteria including multidrug-resistant strains. Abstr 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 74.Horiyama T, Singley CM, Nakamura R, et al. F-1556: S-649266, a novel siderophore cephalosporin: VIII Efficacy against Pseudomonas aeruginosa and Acinetobacter baumannii in rat lung infection model with humanized exposure profile of 2 gram dose with 1 hour and 3 hours infusion. Abstr 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 75.Nakamura R, Toba S, Tsuji M, et al. F-1558: S-649266, a novel siderophore cephalosporin: IV In vivo efficacy in various murine infection models. Abstr 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Washington, DC. Sep 5–9, 2014. [Google Scholar]
- 76.Joo HY, Kim DI, Kowalik E, et al. F1-1202: FSI–1671, a novel anti-Acinetobacter carbapenem: in vivo efficacy against carbapenem-resistance Gram-negative bacterial infection. Abstr 53rd Interscience Conference on Antimicrobial Agents and Chemotherapy; Denver (CO). Sep 10–13, 2013. [Google Scholar]
- 77.Joo HY, Kim DI, Kowalik E, et al. F1–143: efficacy of FSI-1686 in animal model of carbapenem-resistance Gram-negative bacterial infection. Abstr 51st Interscience Conference on Antimicrobial Agents and Chemotherapy; Chicago (IL). Sep 17–20, 2011. [Google Scholar]
- 78.Mojica MF, Mahler SG, Bethel CR, et al. Exploring the role of residue 228 in substrate and inhibitor recognition by VIM metallo-β-lactamases. Biochemistry. 2015;54:3183–96. doi: 10.1021/acs.biochem.5b00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ishii Y, Eto M, Mano Y, et al. In vitro potentiation of carbapenems with ME1071, a novel metallo-β-lactamase inhibitor, against metallo-β-lactamase- producing Pseudomonas aeruginosa clinical isolates. Antimicrob Agents Chemother. 2010;54:3625–9. doi: 10.1128/AAC.01397-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Livermore DM, Mushtaq S, Morinaka A, et al. Activity of carbapenems with ME1071 (disodium 2,3-diethylmaleate) against Enterobacteriaceae and Acinetobacter spp. with carbapenemases, including NDM enzymes. J Antimicrob Chemother. 2013;68:153–8. doi: 10.1093/jac/dks350. [DOI] [PubMed] [Google Scholar]
- 81.Yamada K, Yanagihara K, Kaku N, et al. In vivo efficacy of biapenem with ME1071, a novel metallo-β-lactamase (MBL) inhibitor, in a murine model mimicking ventilator-associated pneumonia caused by MBL-producing Pseudomonas aeruginosa. Int J Antimicrob Agents. 2013;42:238–43. doi: 10.1016/j.ijantimicag.2013.05.016. [DOI] [PubMed] [Google Scholar]


