Abstract
Two closely related series of novel β-carboline derivatives, electronically similar to tadalafil (CAS 171596-29-5), were synthesized and evaluated for their inhibitory effects upon phosphodiesterase 5 (PDE5) and phosphodiesterase 11 (PDE11) and their in vitro tumor cell growth inhibitory activity versus HT29 colorectal carcinoma cell line. Interestingly, some of the synthesized compounds showed growth inhibitory properties that appear to be associated with their ability to inhibit PDE5. Moreover, the PDE5 inhibition seems relevant to the stereochemical aspects of the compounds.
Keywords: Antitumor agents, β-Carboline derivatives, anticancer activity, phosphodiesterase 5 inhibition, CAS 171596-29-5, Tadalafil
1. Introduction
To date, the phosphodiesterase (PDE) superfamily has been classified into 11 different isozyme families (PDE1-11) according to their primary protein and cDNA sequences, kinetic properties, substrate specificity, mechanisms of regulation, cellular/subcellular localization, and sensitivities to various pharmacological agents. PDEs 5, 6, 9 are relatively cGMP-specific; PDEs 1, 2, 3, 10, 11 are of cGMP/cAMP dual specificity, while PDEs 4, 7, 8 are cAMP specific [1]. Hence, PDE5, 6 and 9 might be considered appropriate targets for drugs with capacity to selectively affect biological systems relying on cGMP as an important messenger. The extent to which such drugs can affect one isozyme, and not an-other, along with the expression of the isozyme in the target tissue may influence their effectiveness, severity of side effects, and therapeutic uses.
The physiological significance of PDE5 inhibition is exemplified by the commercial and clinical success of sildenafil, vardenafil and tadalafil as drugs for the treatment of the male erectile dysfunction [2]. These drugs display high potency to inhibit PDE5, although they show variable potencies to inhibit PDE11. The latter shares a high degree of homology with PDE5, but its physiological function is unknown [3]. Recently, it has been found that PDE5 and PDE11 are overexpressed in various tumor types, including colon carcinomas [4–6]. In addition, certain non-selective cGMP-PDEs inhibitors, particularly exisulinid (sulindac sulfone, Formula 1), the related CP248, CP461 and MY5445 were able to induce apoptosis and suppress the growth of numerous tumor cell lines. This effect appears to be mediated via sustained increase in cGMP levels, activation of the cGMP-dependent protein kinase G (PKG), and proteosomal degradation of β-catenin as a result of its phosphorylation by PKG. Apparently, it is important to maintain cross reactivity among the specific and dual cGMP-PDE isozymes, because highly selective PDE5 inhibitors were unable to affect cGMP levels or induce apoptosis in tumor cells [7–9].
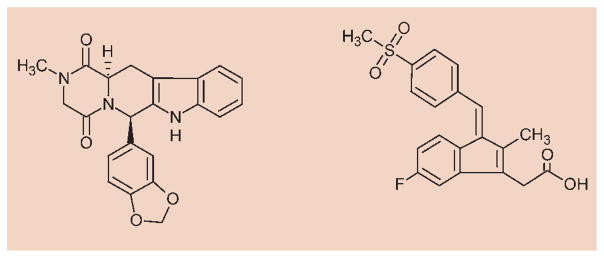
Formula 1.
Chemical structure of the reference compounds: tadalafil (left) and exisulind (right).
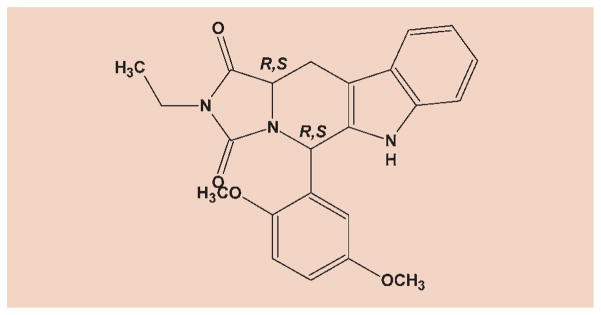
The above findings encourage further synthesis of novel tadalafil analogues as cGMP-PDEs inhibitors and study their isozyme selectivity and anticancer activity (Formula 2).
Formula 2.
(R, R) isomer: IC50 vs HT29 cancer cell line = 2.5 μm, IC50 vs PDE5 = 5.0 μm.
2. Materials and methods
2.1 Chemistry
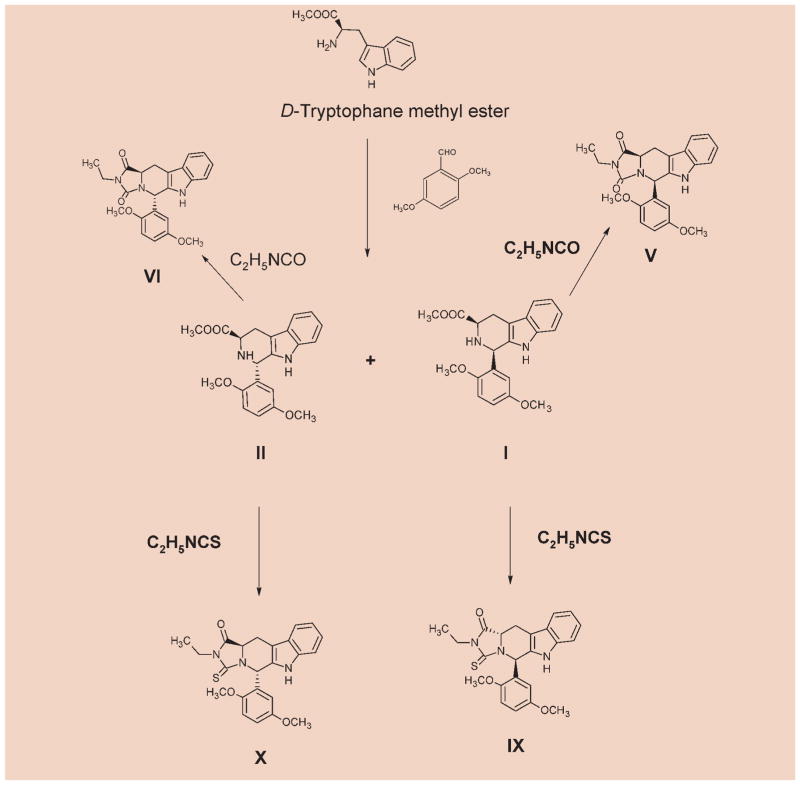
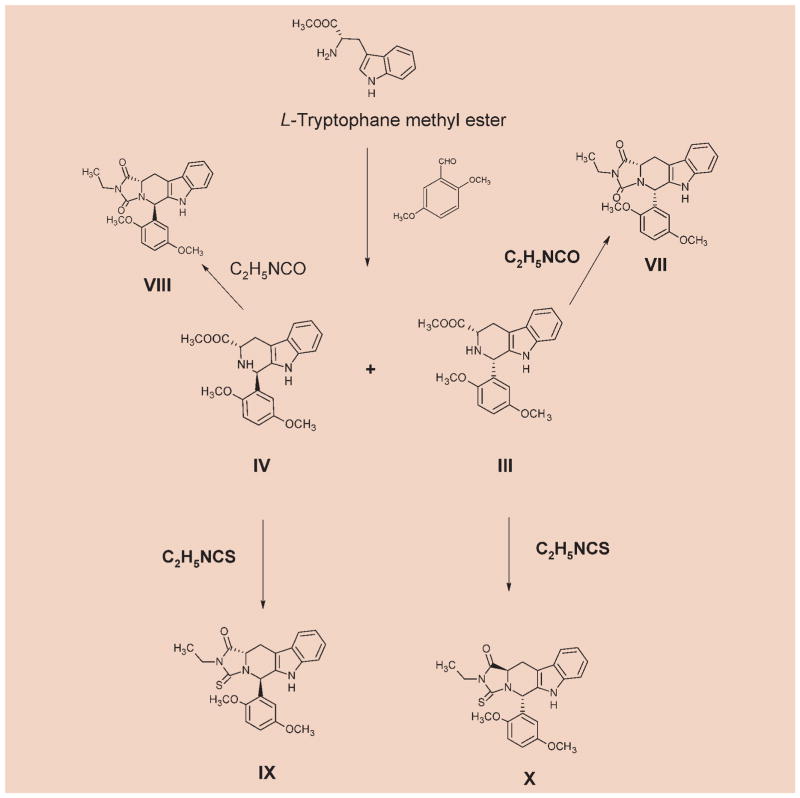
The synthetic pathways are depicted in Schemes 1 and 2. In brief, we started from the commercially available L- or D-tryptophan, depending upon the desired stereochemistry of the final product. Methyl esters were synthesized by a general procedure for the synthesis of esters of amino acids [10]. The appropriate tryptophan methyl ester and 2,5-dimethoxybenzaldehyde were subjected to Pictet-Spengler reaction. Since we initially desired access to both the cis- and trans-isomers, the reaction was carried out under non-stereospecific conditions. This leads to 4 isomers (I–IV), with I and III, II and IV being enantiomeric, while I relative to II and III relative to IV are diastereomeric. The diastereomeric cis- and trans-1,3-disubstituted tetrahydro-β-carbolines were separated by column chromatography and reacted with the commercially available ethyl isocyanates to afford the desired cis- and trans-hydantoins. The reaction with ethyl isothiocyanates exclusively yielded only the trans-thiohydantoins. This is confirmed by the matching physicochemical properties of the isomers obtained from I, II, III or IV indicating their enantiomeric nature. Such has been re-ported previously [11, 12].The assignment of cis/trans stereochemistry for tetrahydro-β-carbolines was based on a detailed study of 1H and 13C NMR spectroscopy and by comparison with the literature data [13, 14].
Scheme 1.
Scheme 2.
Thus, the signals for C-1 and C-3 in the trans-isomer appeared at higher field in the carbon spectrum than the analogous carbons of the corresponding cis-isomer, probably due to the 1,3-interactions present in the trans-isomer.
Moreover, a correlation exists between Rf value on TLC and the stereochemistry of the 1,3-disubstituted tetrahydro-β-carbolines as described previously in the literature [14]. The cis-isomers I, III are systematically less polar than the trans-isomers II, IV as indicated by the order of elution during the purification on silica gel (dichloromethane as eluent). However, in the hydantoin series, the polarity is reversed, the cis-isomer becomes more polar than the trans-isomer.
2.2 General considerations
All reactions were performed with commercially available re-agents and were used without further purification. Solvents were dried by standard methods and stored over molecular sieves. All reactions were carried out under inert gas (nitrogen). TLC performed on precoated silica gel plates Alugram SIL G/UV254, (Macherey-Nagel, Düren, Germany), monitored reaction progress and short UV light were used for the detection of the components. Column chromatography was performed using silica gel 70 –230 mesh (Merck, Darmstadt, Germany). Melting points were determined on Büchi Melting Point apparatus and are uncorrected (Büchi Labortechnik, Flawil, Switzerland). FTIR spectra were recorded on Nicolet Avatar 380 spectrometer (Thermo Scientific, Waltham, MA, USA). 1H-NMR spectra were recorded on Varian Mercury VX-300 MHz spectrometer (Varian, Palo Alto, CA, USA) using CDCl3 as a solvent; 13C-NMR spectra were recorded at 75 MHz, chemical shifts (δ) were reported in parts per million (ppm) downfield from TMS; multiplicities are abbreviated as: s: singlet; d: doublet; q: quartet; m: multiplet; dd: doublet of doublet; brs: broad. Mass spectra were made on Hewlett Packard GC-MS, model 5890, series II at an ionization potential of 70 eV. Elemental analyses values were within ±0.4 % of the theoretical ones unless otherwise indicated (Elementar Analysensysteme, Hanau, Germany).
The following fine chemicals were purchased and used with-out further purification: L-tryptophan (98 %, Sigma-Aldrich, St. Louis, MO, USA); D-tryptophan (Sigma, St. Louis, MO, USA); acetyl chloride (99 %, Fluka); 2,5-dimethoxybenzaldehyde (99 %, Aldrich); ethyl isocyanate (99 %, Aldrich); ethyl isothiocyanate (97 %, Aldrich).
2.3 Synthesis
General procedures for the Pictet-Spengler reaction
The appropriate tryptophan methyl ester (3.42 g, 15.7 mmol) and 2,5-dimethoxybenzaldehyde (2.86 g, 17.25 mmol) were dissolved in CH2Cl2 (10 mL) and cooled to 0 °C in an ice bath. To this solution was added dropwise trifluoroacetic acid (1 mL), and the mixture was stirred at room temperature for 4 days under N2 atmosphere. The reaction mixture was then made alkaline with dilute NH4OH solution and extracted with CH2Cl2 (3 × 10 mL). The organic layer was washed with water, brine, dried over Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified and the isomers were separated by column chromatography on silica gel eluting with CH2Cl2, to give first the appropriate cis-isomer followed by the trans-isomer.
2.3.1 Methyl (1R,3R)-1-(2,5-dimethoxyphenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (I)
Yield: 25 %; buff powder; mp 102–105 °C; Rf: 0.39 (CH2Cl2/MeOH 98 : 2); 1H-NM: 7.79 (brs, 1H, NH),7.53–7.50 (d, 1H, Ar), 7.25–6.82 (m, 6H, Ar), 5.71(s, 1H, CHPh), 4.02 – 3.97 (m, 1H, CHCOOCH3), 3.87 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.711(s, 3H, OCH3), 3.37–3.17 (m, 1H, CHaHb), 3.05 – 3.00 (m, 1H, CHaHb).13C-NMR: 173.1, 154.1, 151.3, 135.9, 134.5, 127.1, 121.6, 119.4, 117.9, 114.7, 114.1, 112.2, 110.9, 108.2, 56.9, 56.4, 55.8 (C1), 52.2 (C3), 52.0, 25.4. MS m/z (%): M+ 366 (100), 307(45), 248 (70). Anal (C21H22N2O4) C, H, N.
2.3.2 Methyl (1S,3R)-1-(2,5-dimethoxyphenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (II)
Yield: 45 %; yellow powder; mp 139–142 °C; Rf 0.18 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 7.93 (s, 1H, NH), 7.56–7.53 (d, 1H, Ar), 7.25–6.62 (m, 6H, Ar), 5.81 (s, 1H, CHPh), 3.97–3.95 (m, 1H, CHCOOCH3), 3.93 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.28 – 3.21 (dd, 1H, CHaHb), 3.10–3.02 (m, 1H, CHaHb). 13C-NMR: 173.8, 153.5, 151, 136.3, 132.4, 127.8, 126.9, 125.8, 121.8, 119.2, 118, 115.8, 112.6, 111.5, 108.5, 56.0, 55.7, 52.4, 52.2 (C1), 49.4 (C3), 29.7. MS m/z (%): M+ 366 (100), 307 (39), 248 (43). Anal. (C21 H22 N2 O4) C, H, N.
2.3.3 Methyl (1S,3S)-1-(2,5-dimethoxyphenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (III)
Yield: 25 %; yellow powder; mp 100–102 °C; Rf 0.38 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 7.78 (s, 1H, NH), 7.53–7.51 (d, 1H, Ar), 7.27–6.82 (m, 6H, Ar), 5.69 (s, 1H, CHPh), 4.02 – 3.97 (dd, 1H, CHCOOCH3), 3.88 (s, 3H, OCH3), 3.83 (s, 3H, OCH3), 3.71 (s, 3H, OCH3), 3.25–3.18 (m,1H, CHaHb), 2.95–3.05 (m, 1H, CHaHb).13C-NMR: 173.1, 154.2, 151.3, 135.9, 134.4, 127, 121.6, 119.4, 117.9, 114.7, 114.1, 112.3, 110.8, 108.1, 56.9, 56.4, 55.8 (C1), 52.2 (C3), 52, 25.5. MS m/z (%): M+ 366 (100), 307 (48), 248 (86). Anal. (C21H22N2O4) C, H, N.
2.3.4 Methyl (1R,3S)-1-(2,5-dimethoxyphenyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (IV)
Yield: 45 %; yellow powder; mp 138–141 °C; Rf 0.17 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 7.96 (brs, 1H, NH), 7.56–7.53 (d, 1H, Ar), 7.18 – 6.61 (m, 6H, Ar), 5.82 (s, 1H, CHPh), 3.98–3.96 (m, 1H, CHCOOCH3), 3.920 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.66 (s, 3H, OCH3), 3.29 – 3.22 (m, 1H, CHaHb), 3.04–3.12 (m, 1H, CHaHb). 13C-NMR: 173.9, 153.5, 151.1, 136.1, 132.8, 131.5, 126.9, 121.7, 119.2, 118, 115.6, 112.5, 111.6, 110.9, 108.7, 56.1, 55.7, 52.5, 52.0 (C1), 49.1 (C3), 24.9. MS m/z (%): M+ 366(100), 307 (42), 248 (46). Anal. (C21H22N2O4) C, H, N.
General procedures for the synthesis of the hydantoin tetrahydro-β-carbolines
Ethyl isocyanate (135 μl, 1.6 mmol) was added to a stirred solution of the appropriate β-carboline I, II, III, IV (0.36 g, 1 mmol) in methyl ethyl ketone (10 ml) and the mixture was stirred at reflux for 16 h. The solvent was evaporated under reduced pressure; the residue was purified by column chromatography using CH2Cl2 as an eluent.
2.3.5 (5R,11aR)-2-Ethyl-5-(2,5-dimethoxyphenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido [3,4-b]indole-1,3(2H)-dione (V)
Yield: 45 %, white powder; mp 256–258 °C; Rf 0.57 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 8.5 (brs, 1H, NH), 7.56–7.53 (d, 1H, Ar), 7.23–6.68 (m, 6H, Ar), 6.42 (s, 1H, CHPh), 4.41–4.35 (dd, 1H, CHC(O)N), 3.97 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.63 – 3.56 (m, 2H, NCH2), 3.48–3.46 (m, 1H, CHaCHb), 2.88–2.86 (m, 1H, CHaHb), 1.27 – 1.22 (t, 3H, CH3). MS m/z (%): M+ 405 (11), 374 (31), 57 (100). Anal. (C23H23N3O4) C, H, N.
2.3.6 (5S,11aR)-2-Ethyl-5-(2,5-dimethoxyphenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido [3,4-b]indole-1,3(2H)-dione (VI)
Yield: 54 %, yellow powder; mp 240–242 °C; Rf 0.53 (CH2Cl2/MeOH 98:2); 1H-NMR: 8.52 (brs, 1H, NH), 7.52–7.50 (d, 1H, Ar), 7.30 – 6.68 (m, 6H, Ar), 6.58 (brs, 1H, CHPh), 4.59–4.65 (dd, 1H, CHC(O)N), 3.98 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.58–3.61 (q, 2H, NCH2), 3.47 – 3.54 (dd, 1H, CHaCHb), 2.92 – 2.83 (m, 1H, CHaHb), 1.28–1.26 (t, 3H, CH3). MS m/z (%): M+ 405 (45), 374 (100). Anal. (C23H23N3O4) C, H, N.
2.3.7 (5S,11aS)-2-Ethyl-5-(2,5-dimethoxyphenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido [3,4-b]indole-1,3(2H)-dione (VII)
Yield: 51 %; yellow powder; mp 255–258 °C; Rf 0.58 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 8.53 (brs, 1H, NH), 7.55–6.68 (m, 7H, Ar), 6.39 (brs, 1H, CHPh), 4.41–4.36 (dd, 1H, CHC(O)N), 3.992 (s, 3H, OCH3), 3.73 (s, 3H, OCH3), 3.61 – 3.58 (q, 2H, NCH2), 3.07 – 2.86 (m, 1H, CHaHb), 2.92 – 2.82 (m, 1H, CHaCHb), 1.27 – 1. 22 (t, 3H, CH3). MS m/z (%): M+ 405 (30), 374 (64), 57 (100). Anal. (C23H23N3O4) C, H, N.
2.3.8 (5R,11aS)-2-Ethyl-5-(2,5-dimethoxyphenyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6]pyrido [3,4-b]indole-1,3(2H)-dione (VIII)
Yield: 54 %; yellow powder; mp 241 – 43 °C; Rf 0.53 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 8.522 (brs, 1H, NH), 7.521–7.496 (d, 1H, Ar), 7.292–6.767 (m, 6H, Ar), 6.578 (brs, 1H, CHPh), 4. 647–4.593 (dd, 1H, CHC(O)N), 3.984 (s, 3H, OCH3), 3.730 (s, 3H, OCH3), 3.702 – 3.630 (q, 2H, NCH2), 3.542 – 3.474 (dd, 1H, CHaCHb), 2.914–2.816 (m, 1H, CHaHb), 1.283 – 1.26 (t, 3H, CH3). MS m/z (%):M+ 405 (21), 374 (64), 57 (100). Anal. (C23H23N3O4) C, H, N.
General procedures for the synthesis of the thiohydantoin tetrahydro-β-carbolines
Ethyl isothiocyanate (135 μl, 1.6 mmol) was added to a stirred solution of the appropriate β-carboline (0.36 g, 1 mmol) in methyl ethyl ketone (10 ml) and the mixture was stirred at reflux for 16 h under a nitrogen atmosphere. The solvent was evaporated under reduced pressure; the residue was purified by column chromatography using CH2Cl2 as an eluent.
2.3.9 (5R,11aS)-2-ethyl-1-oxo-5-(2,5-dimethoxyphe-nyl)-5,6,11,11a-tetrahydro-1H-imidazo[1′,5′:1,6] pyrido[3,4-b]indole-3 (2H)-thione (IX)
Yield: 53 %; yellow powder; mp 144–146 °C; Rf 0.93 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 8.56 (brs, 1H, NH), 7.50 – 7.48 (d, 1H, Ar), 7.30 – 6.68 (m, 6H, Ar) 6.66–6.65 (m, 1H, CHPh), 4.79–4.73 (m, 1H, CHC(O)N), 4.01–4.00 (q, 2H, NCH2), 3.98 (s, 3H, OCH3), 3.75 (s, 3H, OCH3), 3.55 – 3.47 (m, 1H, CHaHb), 2.90 – 2.77 (brs, 1H, CHaCHb),1.34–1.29 (t, 3H, CH3). MS m/z (%): M+ 421 (12), 368 (8), 55 (100). Anal. (C23H23N3O3S) C, H, N.
2.3.10 (5S,11aR)-2-ethyl-1-oxo-5-(2,5-dimethoxy-phenyl)-5,6,11,11a-tetrahydro-1H-imidazo [1′,5′:1,6]pyrido[3,4-b]indole-3 (2H)-thione (X)
Yield: 48 %; white powder; mp 142–144 °C; Rf 0.92 (CH2Cl2/MeOH 98 : 2); 1H-NMR: 8.56 (brs, 1H, NH), 7.50–7.48 (d, 1H, Ar), 7.27 – 6.84 (m, 6H, Ar), 6.66–6.65 (m, 1H, CHph), 4.80–4. 75 (dd, 1H, CHC(O)N), 3.980–4.02 (q, 2H, CH2), 3.973 (s, 3H, OCH3), 3.748 (s, 3H, OCH3), 3.57 – 3.56 (m, 1H, CHaHb), 2.97 – 2.89 (m, 1H, CHaCHb), 1.34–1.29 (t, 3H, CH3). MS m/z (%): M+ 421 (32), 368 (7.5), 55 (100). Anal. (C23H23N3O3S) C, H, N.
2.4 Biological testing [15, 16]
2.4.1 Cell culture
Human HT29 colon tumor cells were obtained from American Type Culture Collection (Rockville, MD, USA) and grown in RPMI 1640 media supplemented with 5 % fetal bovine serum, 2 mM glutamine, 100 units/ml penicillin, 100 units/ml streptomycin, and 0.25 μg/ml amphotericin. Cells were harvested at 70 to 90 % confluence with either trypsin or EDTA and used immediately. All compounds were solubilized in 100 % DMSO and diluted with media to obtain a final DMSO concentration of 0.1 %.
2.4.2 Cell growth inhibition [16]
Cell growth inhibition was determined by plating cells at 1 000–2 000 cells/well in 96-well plates. All test compounds were solubilized in DMSO and diluted with media to obtain a final concentration of 0.1 %. Cells were dosed after 18 h and incubated for 3 days. The number of viable cells was measured based on ATP content using the CellTiter Glo luminescence assay (Promega, Madison, WI USA) as per the manufacturer’s specifications. Luminescence was measured by a Perkin Elmer Victor® multi-label plate reader (Vienna, VA, USA).
2.4.3 Phosphodiesterase inhibitory activity [15]
PDE activity was measured using an adaptation of the IMAP® fluorescence polarization phosphodiesterase assay (Molecular Devices, Sunnyvale, CA, USA). PDE hydrolysis of the fluorescent-labeled substrate allows it to bind the IMAP® reagent, which increases fluorescence polarization (FP). The assay used fluorescein (Fl)-cAMP and tetramethylrhodamine (TAMRA)-cGMP as substrates. The different excitation and emission spectra of the substrates (485–530 nm for Fl and 530–590 nm for TAMRA) allowed for simultaneous measurement of cAMP and cGMP hydrolysis in the same well. The assays were performed in 96-well microtiter plates using a reaction buffer containing 10 mM Tris-HCl (pH 7.2) 10 mM MgCl2, 0.05 % NaN3 and 0.1 % phosphate-free bovine serum albumin as the carrier. Each well contained 20 μl of recombinant enzyme (BPS Biosciences, San Diego, CA, USA) and 10 μl inhibitor. The reaction was initiated by the addition of 10 μl of a substrate solution containing 50 nM Fl-cAMP and/or TAMRA-cGMP. After incubating at room temperature for 60 min. the reaction was terminated by adding 120 μl of binding solution. FP was measured with a BioTek Synergy 4 (BioTek Instruments, Winooski, VM, USA).
3. Results and discussion
All the synthesized compounds were evaluated for their ability to inhibit recombinant PDE5 and PDE11 using a fluorescence polarization based enzyme assay [15]. Furthermore, we tested all the compounds for their ability to inhibit growth using the human HT29 colon tumor cell lines known to overexpress PDE5. For all assays, tadalafil (PDE5/PDE11 inhibitor) and exisulind (non-specific cGMP-PDE inhibitor) were used as positive controls. The results are summarized in Table 1.
Table 1.
IC50 values of the test compounds for ability to inhibit PDE5, PDE11 and the growth of human HT29 colon tumor cells. The results are the average of at least 3 experiments each made in triplicate.
| Cpd. | IC50 HT29 growth (μM) | IC50 PDE5, cGMP (μM) | IC50 PDE11, cGMP (μM) | IC50 PDE11, cAMP (μM) |
|---|---|---|---|---|
| I | 15.5 ± 1.03 | 3.9 ± 1.09 | > 100 | > 100 |
| II | > 20 | > 100 | > 100 | > 100 |
| III | > 20 | 14.3 ± 1.09 | > 100 | > 100 |
| IV | > 20 | 17.4 ± 1.09 | > 100 | > 100 |
| V | 2.5 ± 1.02 | 5.0 ± 1.14 | > 100 | > 100 |
| VI | 11 ± 1.03 | > 100 | > 100 | > 100 |
| VII | 17 ± 1.02 | > 100 | > 100 | > 100 |
| VIII | > 20 | 87.4 ± 1.10 | > 100 | > 100 |
| IX | > 20 | 78.6 ± 1.14 | > 100 | > 100 |
| X | > 20 | > 100 | > 100 | > 100 |
| Tadalafil | > 50 | 0.004 ± 0.001 | 0.782 ± 0.001 | > 2 |
| Exisulind | 364.8 ± 1.04 | 295 ± 1.11 | > 300 | > 300 |
Thus, among the tetrahydro-β-carbolines I, II, III, and IV, compound I showed the most potent PDE5 and HT29 growth inhibitory activity with comparable IC50 values. This indicates the relevance of spatial orientation of the substituent at C1 and C3 (R, R) upon both activities and the potential correlation between the two biological activities.
For the hydantoins V, VI, VII, VIII, compound V, which is derived from compound I, was the most active both for HT29 growth inhibition and for PDE5 inhibition. These results support the relevance of the (R, R) spatial orientation at C5 and C11a for both activities. As the IC50 of V versus HT29 is lower than that of I, the hydantoin moiety may be a more favorable group for growth inhibitory activity.
In addition, the IC50 values of the hydantoins VI and VII were also lower than their precursors II, III, which provides further support for the importance of the hydantoin moiety for growth inhibitory activity. It is worthy to mention that tadalafil is also a diastereomeric compound of the R, R spatial orientation.
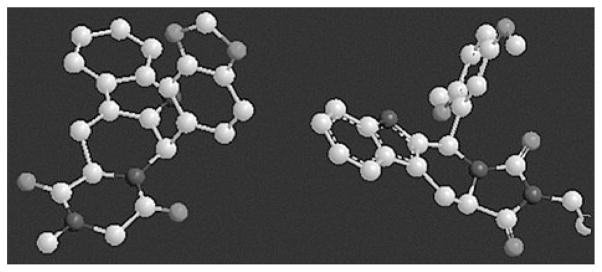
To further study the similarity in conformational aspects of both tadalafil and compound V, the crystal structure of tadalafil was extracted from its complex with PDE5 (PDB 1UDU) and used to measure the torsional angle of the pendant 1,3-benzodioxole relative to the tetracycle. Compound V was subjected to minimization by MM+ followed by conformational search and measuring the torsional angle of the pendant 2,5-dimethoxyphenyl ring relative to the tetracycle. Interestingly, both pendant aryls showed non-coplanar orientation relative to the tetracycle (Fig. 1).
Fig. 1.
Torsional angle of the pendant aryl relative to the tetracyclic part for tadalafil (left) and compound V (right).
To analyze the electronic similarity of tadalafil and compound V, both compounds were subjected to MM+ minimization, followed by computing its heat of formation, gradient norm, dipole, charges, cosmo solvation in water, electrostatic potential, molecular surface, spin density and hyperfine coupling using AM1 procedure in the MOPAC package of ChemOffice 9.0. Presentation of the entire electrostatic potential on the molecular surface in a single picture allows the perception of similarity of substrates that bind to the same receptor. Interestingly, both compounds showed highly similar electrostatic surfaces both in the tetracyclic and the pendant aryl areas which indicates that both may be able to bind the same receptor in similar fashion.
The thiohydantoin derivatives were almost inactive in all assays, which may be due to the nature of the sulphur or the spatial arrangement of the molecule as we were only able to synthesize the trans-isomer.
None of the compounds inhibited PDE11, regardless of whether cGMP or cAMP was used as substrates. By contrast, tadalafil inhibited PDE5 and PDE 11 with IC50 values of 0.004 and 0.782 μmol, respectively.
These results suggest that inhibition of the cGMP selective PDE5 rather than the dual substrate PDE11 may be required for growth inhibition.
Since compounds I and V maintained similar rank orders of potency for growth inhibition and PDE5 inhibition, we suggest that the growth inhibitory activity of these compounds may be mediated by PDE5 inhibition and cGMP elevation. However, we cannot rule out the possible involvement of other PDE isozymes, since the highly potent PDE5 inhibitor, tadalafil did not inhibit tumor cell growth. In support of this possibility, the non-selective PDE inhibitor, exisulind inhibited tumor cell growth at concentrations that inhibit PDE5, albeit with low potency.
Acknowledgments
The authors are grateful to the authority of the Faculty of Graduate Studies, the German University in Cairo, for financial support. Thanks are also due to the Alexander von Humboldt foundation, Germany for donating some of the instruments used in this research.
Literature
- 1.Manallack DT, Hughes RA, Thompson PE. The next generation of phosphodiesterase inhibitors: structural clues to ligand and substrate selectivity of phosphodiesterases. J Med Chem. 2005;48:3449–62. doi: 10.1021/jm040217u. [DOI] [PubMed] [Google Scholar]
- 2.Mulhall JP, Montorsi F. Evaluating preference trials of oral phosphodiesterase 5 inhibitors for erectile dysfunction. Eur Urol. 2006;49:30–7. doi: 10.1016/j.eururo.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Weeks JL, Zoraghi R, Beasley A, Sekhar KR, Francis SH, Corbin JD. High biochemical selectivity of tadalafil, sildenafil and vardenafil for human phosphodiesterase 5A1 (PDE5) over PDE11A4 suggests the absence of PDE11A4 cross-reaction in patients. Int J Impot Res. 2005;17:5–9. doi: 10.1038/sj.ijir.3901283. [DOI] [PubMed] [Google Scholar]
- 4.Piazza GA, Thompson WJ, Pamukcu R, Alila HW, White-head CM, Liu L. Exisulind, a novel proapoptotic drug, inhibits rat urinary bladder tumorigenesis. Cancer Res. 2001;61:3961–8. [PubMed] [Google Scholar]
- 5.Whitehead CM, Earle KA, Fetter J, Xu S, Hartman T, Chan DC, et al. Exisulind-induced apoptosis in a non-small cell lung cancer orthotopic lung tumor model augments docetaxel treatment and contributes to increased survival. Mol Cancer Ther. 2003;2:479–88. [PubMed] [Google Scholar]
- 6.D’Andrea MR, Qiu Y, Haynes-Johnson D, Bahattacharjee S, Kraft P, Lundeen S. Expression of PDE11A in normal and malignant human tissues. J Histochem Cytochem. 2005;53:895–903. doi: 10.1369/jhc.5A6625.2005. [DOI] [PubMed] [Google Scholar]
- 7.Zhu B, Strada SJ. The novel functions of cGMP-specific phosphodiesterase 5 and its inhibitors in carcinoma cells and pulmonary/cardiovascular vessels. Curr Top Med Chem. 2007;7:437–54. doi: 10.2174/156802607779941198. [DOI] [PubMed] [Google Scholar]
- 8.Li H, Pamukcu R, Thompson WJ. beta-Catenin signaling: therapeutic strategies in oncology. Cancer Biol Ther. 2002;1:621–5. doi: 10.4161/cbt.309. [DOI] [PubMed] [Google Scholar]
- 9.Soh JW, Mao Y, Kim M, Pamucku R, Li H, Piazza GA, et al. Cyclic GMP mediates apoptosis induced by sulindac derivatives via activation of c-Jun NH2 terminal kinase 1. Clin Cancer Res. 2001;6:4136–41. [PubMed] [Google Scholar]
- 10.Dondoni A, Perrone D. Synthesis of 1,1,-Dimethylethyl(S)-4-formyl-2,2-dimethyl-3-oxazolidinecarboxylate by oxidation of the alcohol. Org Syn. 2000;77:64. [Google Scholar]
- 11.Lopez-Rodriguez ML, Morcillo MJ, Garrido M, Garrido M, Benhamu B, Perez V, et al. Stereospecificity in the reaction of tetrahydro-β-carboline-3-carboxylic acids with isocyanates and isothiocyanates. kinetic vs thermodynamic Control. J Org Chem. 1994;59:1583–5. [Google Scholar]
- 12.Sunder-Plassmann N, Sarli V, Gartner M, Utz M, Seiler J, Huemmer S, et al. Synthesis and biological evaluation of new tetrahydro-beta-carbolines as inhibtors of the mitotic kinesin Eg5. Bioorg Med Chem. 2005;13:6094–111. doi: 10.1016/j.bmc.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 13.Ungemach F, Soerens D, Weber R, DiPierro M, Campos M, Mokry P, et al. General method for the assignment of stereochemistry of 1,3-disubstituted-1,2,3,4-tetrahydro-β-carbolines by carbon-13-spectroscopy. J Am Chem Soc. 1980;102:6976–84. [Google Scholar]
- 14.Sandrin J, Soerens D, Cook JM. 13C-NMR of 1,3-disubstituted-1,2,3,4-tetrahydro-β-carbolines. Heterocycles. 1976;4:1249–55. [Google Scholar]
- 15.Huang W, Zhang Y, Sportsman JR. A fluorescence polarization assay for cyclic nucleotide phosphodiesterases. J Biomol Screen. 2002;7:215–22. doi: 10.1177/108705710200700305. [DOI] [PubMed] [Google Scholar]
- 16.Liu L, Li H, Underwood T, Lloyd M, David M, Sperl G, et al. Cyclic GMP-dependent protein kinase activation and induction by exisulind and CP461 in colon tumor cells. J Pharm Exp Ther. 2001;299:583–92. [PubMed] [Google Scholar]