Abstract
New derivatives with the tetrahydro-β-carboline-imidazolidinedione and tetrahydro-β-carboline-piperazinedione scaffolds and a pendant bromothienyl moiety at C-5/C-6 were synthesized and tested for their ability to inhibit PDE5 in vitro. The following SAR can be concluded: The tetracyclic scaffold is essential for PDE5 inhibition; the ethyl group is the most suitable among the adopted N-substituents on the terminal ring (hydantoin/piperazinedione); the appropriate stereochemistry of C-5/C-6 derived from the aldehyde rather than C-11a/C-12a derived from tryptophan appears crucial for inhibition of PDE5; surprisingly, derivatives with the hydantoin terminal ring are more active than their analogs with the piperazinedione ring; the selectivity versus PDE5 relative to PDE11 with cGMP as a substrate is mainly a function of the substitution and stereochemistry pattern of the external ring, in other words of the interaction with the H-loop residues of the isozymes. Thirteen derivatives showed PDE5 inhibitory activity with IC50 values in the range of 0.16–5.4 μm. Compound 8 was the most potent PDE5 inhibitor and showed selectivity towards PDE5 versus other PDEs, with a selectivity index of 49 towards PDE5 rather than PDE11 with cGMP as the substrate.
Keywords: β-Carboline, Growth inhibition, Phosphodiesterase-5 inhibitors, Stereochemistry
Introduction
Cyclic adenosine monophosphate (cAMP) and cyclic guanosine monophosphate (cGMP) are critical intracellular second messengers, which through activation of protein kinase A (PKA) and protein kinase G (PKG), respectively, mediate biological responses to a variety of cellular stimuli. Catabolism of cAMP and cGMP is directed by phosphodiesterases (PDEs), a complex superfamily containing 11 highly related gene families and over 100 distinct isoforms. PDE isoforms differ in amino acid sequence, tissue distribution, substrate specificity, and mode of regulation [1–5]. PDE5 is a cGMP-specific phosphodiesterase found in vascular and tracheal smooth muscles, platelets, gastrointestinal epithelial cells, spleen, kidney, prostate gland, and in the Purkinje cells of the cerebellum [1, 6, 7]. Three PDE5 inhibitors, sildenafil, vardenafil, and tadalafil, have been marketed for the treatment of male erectile dysfunction (MED) [8], and were recently approved for treatment of pulmonary arterial hypertension (PAH) [9]. In addition, PDE5 inhibitors have gained attention as potential therapeutic agents for cardiac hypertrophy associated with heart failure [10, 11], Raynaud’s disease [12], stroke [13], Alzheimer’s disease [14, 15], anticancer agents [16, 17], and reversal of multiple drug resistance [18].
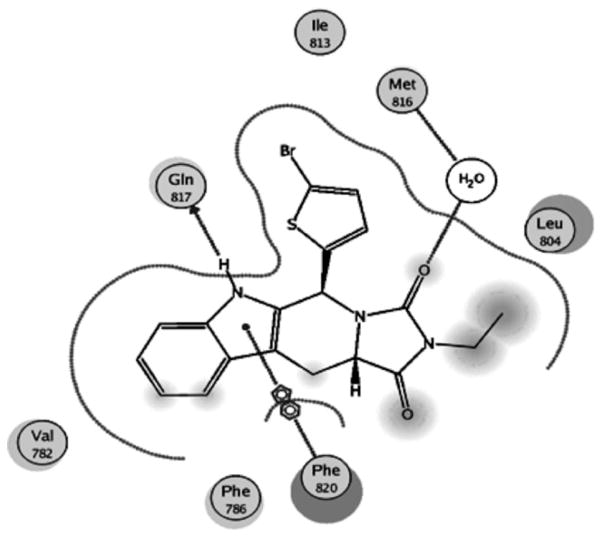
The superposition of all reported PDE5A inhibitors has revealed a conserved binding mode with the enzyme that compromise two major interaction types: one or two hydrogen bond interactions with Gln 817 and hydrophobic π–π stacking interactions between the planar ring portion of the inhibitors and a hydrophobic P clamp that anchors these inhibitors in the active site (PDB: 1UDU, PDB: 1XOZ, PDB: 2H44; Fig. 1) [19–21]. Recently, reported tadalafil analogs have revealed two further hydrophobic interactions: interaction between the alkyl side chain on the nitrogen of the terminal ring and Ile665 and hydrophobic interaction of the pendant aryl with Met816 in the Q2 pocket [22].
Figure 1.
Detailed mode view showing the docking and interaction of tadalafil with human PDE5.
Based on previously reported interactions, our laboratory designed novel tadalafil analogs based upon the tetrahydro-β-carboline-imidazolidinedione and tetrahydro-β-carboline-piperazinedione scaffolds with 5-bromo-2-thienyl substituent as the pendant aryl to increase the potential hydrophobic interaction with residues lining the Q2 pocket. The effects of size, branching, and unsaturation of substituents on the nitrogen of the terminal ring, and the configuration of the two chiral carbons upon activity were studied.
Results and discussion
Chemistry
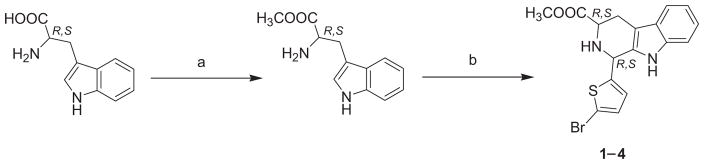
The synthesis of the 1,3-disubstituted tetrahydro-β-carbolines (Scheme 1), tetrahydro-β-carboline-hydantoin derivatives (Scheme 2) and tetrahydro-β-carboline-piperazinedione derivatives (Scheme 3) are shown. Both D-tryptophan and L-tryptophan methyl esters were synthesized by a general synthetic procedure for amino acid esters [23]. The respective ester and 5-bromo-2-thiophenecarbaldehyde were subjected to a Pictet–Spengler reaction. The reaction was carried out at room temperature under non-stereoselective conditions, since both the cis-and trans-isomers (1–4) were required. The trans-isomer precipitated throughout the reaction and was obtained by filtration. The filtrate contained cis- and trans-diastereomers of the 1,3-disubstituted tetrahydro-β-carboline (1–4) that were separated by flash column chromatography using CH2Cl2 as an eluent.
Scheme 1.
Synthesis of the 1,3-disubstituted tetrahydro-betacarbolines. Reagents and conditions: (a) CH3COCl, methanol, reflux, 5 h, basify with dilute NH4OH and extract with CH2Cl2; (b) 5-bromothiophene-2-carboxaldehyde, TFA, RT, 4 days, 11–50.3%. For exact individual assignment, see Table 1.
Scheme 2.
Synthesis of tetrahydro-betacarboline-hydantoin derivatives. Reagents and conditions: (a) ethyl or butyl or t-butyl or allyl isocyanate, 2-butanone, reflux, under N2 atmosphere, 16–70 h, 25–87.2%; R = –C2H5 or –(CH2)3CH3 or –C–(CH3)3 or –CH2–CH=CH2. For exact individual assignment, see Table 2.
Scheme 3.
Synthesis of tetrahydro-betacarboline-piperazinedione derivatives. Reagents and conditions: (a) Cl–CO–CH2–Cl, chloroform, NaHCO3, RT, 2 h; (b) ethylamine, methanol, reflux, 16 h, 12–52.1%. For exact individual assignment, see Table 3.
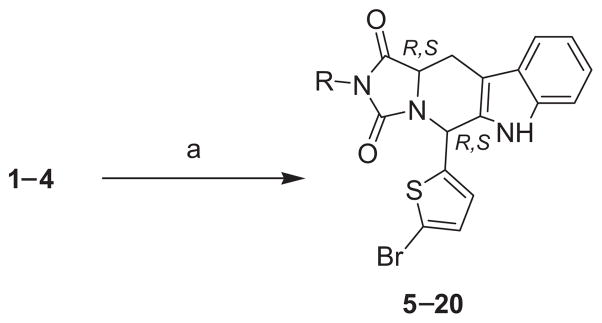
The pure cis- and trans-diastereomers were reacted with commercially available ethyl, butyl, tert-butyl and allyl iso-cyanates to yield the desired cis- and trans-hydantoin derivatives (5–20). The cis-tetrahydro-β-carbolines needed 70 h for reaction completion compared to the respective trans-isomers which needed only 16 h. This can be attributed to the fact that cis- and trans-isomers are diastereomers that show different rates in chemical reactions.
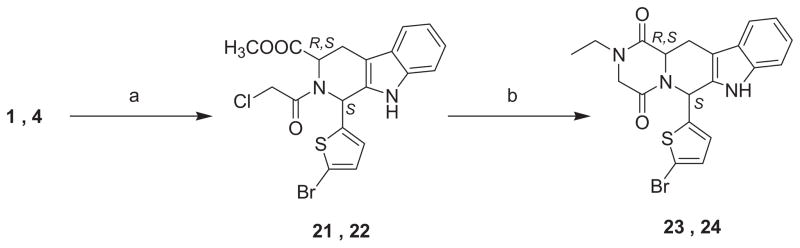
Treatment of the tetrahydro-β-carbolines (1, 4), having the S configuration at C-1, with chloroacetyl chloride provided the corresponding chloroethanone derivatives (21, 22). The piperazinedione derivatives with the respective N-ethyl substituent were obtained by ring closure of the chloroethanone derivative in the presence of ethyl amine in refluxing methanol (23, 24).
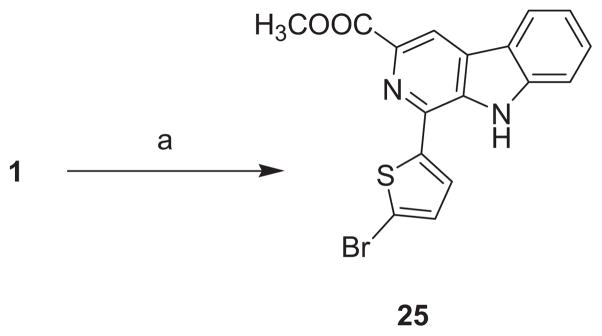
Refluxing the cis-tetrahydro-β-carboline 1 in butanone under oxygen atmosphere led to its full aromatization into the β-carboline 25 (Scheme 4). Analogous conditions failed to oxidize the trans-tetrahydro-β-carboline 2 suggesting that the cis-isomers are more susceptible to oxidation than the trans-isomers.
Scheme 4.
Synthesis of fully aromatic betacarboline. Reagents and conditions: (a) 2-butanone, reflux, under O2 atmosphere, 16 h, 72.8%.
The assignment of cis/trans configuration for the tetrahydro-β-carbolines (1–4) was based on a detailed study of 13C NMR spectroscopy data well-established in previous literature. The 13C NMR signals for C1 and C3 are more shielded in the trans-isomer compared to the cis-isomer with Δδ ~ 4 ppm, which may be due to 13C γ-gauche effect. The tetrahydropyridine ring exists in half-chair conformation where the C-3 ester is equatorially located, leading to 1,3-diaxial spatial interactions in the trans-isomer [24]. Moreover, a correlation exists between the Rf value on TLC and the configuration of the 1,3-disubstituted tetrahydro-β-carbolines (1–4). The cis-isomer appears to be less polar than the trans-isomer. However, in the hydantoin series, the polarity is reversed; thus, the cis-isomer becomes more polar than the trans-isomer. For example, the Rf values of the 1S, 3R and 1R, 3R isomers 1, 2 and their corresponding hydantoin analogs 5, 6 using CH2Cl2 as the mobile phase were 0.53, 0.22, and 0.26, 0.49, respectively.
Interpretation of 13C NMR signals was based on both the chemical shifts in 13C NMR spectra and DEPT-135 spectra (distortionless enhancement by polarization transfer). All carbons that have an attached proton provided a signal in DEPT spectra but the phase of the signal differed based on whether the number of attached hydrogens is an odd or an even number. Signals arising from –CH or –CH3 groups gave positive peaks, while signals arising from –CH2 and –C groups gave negative peaks.
Concerning the 1H NMR signals for the proton at C-5 of hydantoin and C-6 of piperazinedione derivatives, they appeared at δ 6.16–7.00 which is greatly downshifted from the same proton in the respective tetrahydro-β-carbolines (1–4) that appeared at δ 5.51–5.54. This can be attributed to the electron-withdrawing effect of carbonyl functional groups in hydantoin and piperazinedione rings.
Mass spectrometry of all derivatives showed a molecular ion peak at M+ and M++2 with relative ratio of approximately 1:1 due to the isotopic nature of the bromine atom.
The infrared spectra of all derivatives showed bands at ~3300 cm−1 for the N–H stretching. All the tetrahydro-β-carbolines (1–4) showed peaks at ~1740–1700 cm−1 for the carbonyl stretching (ester carbonyl). On the other hand the hydantoins showed two carbonyl stretching peaks at ~1760 and 1700 cm−1, as one of the carbonyls is flanked between a N and a C, meanwhile the other is flanked between two nitrogen atoms leading to higher single bond character of the carbonyl group. The piperazinediones showed two carbonyl stretching peaks at ~1670 and 1640 cm−1. The relatively lower stretching values of the carbonyls of the six-membered derivatives relative to the five-membered derivatives may be explained by the higher ring strain of the hydantoin ring, leading to higher double bond characters of the carbonyl groups.
Biology
All the final compounds and tetrahydro-β-carboline intermediates synthesized were tested for in vitro inhibition of recombinant human PDE5 at screening doses of 10 μM; for compounds displaying a percentage of inhibition >60%, the IC50 was then determined by testing a range of 10 concentrations, each with double replicates. The results are shown in Tables 1–3. Moreover, the two most active compounds 8, 9 were tested versus an array of other PDEs (PDE1A, PDE2A, PDE3A, PDE9A, PDE10A, and PDE11A) to decide about their selectivity profile using tadalafil as a positive control; results are shown in Table 4.
Table 1.
PDE5 inhibitory activity of 1, 3-disubstituted tetrahydro-β-carbolines (1–4) and the fully aromatic β-carboline 25.
| Compound | Chirality | PDE5 % inhibition at 10 μma) | PDE5 inhibition IC50 (μm) |
|---|---|---|---|
| 1 | 1S, 3R | <60 | ND |
| 2 | 1R, 3R | <60 | ND |
| 3 | 1R, 3S | <60 | ND |
| 4 | 1S, 3S | <60 | ND |
| 25 | NA | <60 | ND |
Calculated from triplicate values.
Table 3.
PDE5 inhibitory activity of tetrahydro-β-carboline-piperazinediones (23, 24).
| Compound | R | Chirality | PDE5 % inhibition at 10 μma) | PDE5 inhibition IC50 (μm)b) |
|---|---|---|---|---|
| 23 | C2H5 | 6S, 12aR | 81 | 1.71 |
| 24 | C2H5 | 6S, 12aS | 87 | 1 |
Calculated from triplicate values.
Calculated from 10 concentrations, each with double replicates.
Table 4.
IC50 (μm)a) of the most active tetrahydro-β-carboline-hydantoins and tadalafil against an array of PDEs.
| Compound | PDE1A cGMP | PDE2A cGMP | PDE3A cGMP | PDE5A cGMP | PDE9A cGMP | PDE10A cGMP | PDE11A cGMP | PDE11A cAMP | Selectivity index IC50 (PDE11A/PDE5A) cGMP/cGMP |
|---|---|---|---|---|---|---|---|---|---|
| 8 | >50 | >50 | >50 | 0.16 | >50 | >50 | 7.8 | 11.3 | 49 |
| 9 | >50 | >50 | >50 | 0.17 | >50 | >50 | 2.3 | 4 | 14 |
| Tadalafil | >50 | >50 | >50 | 0.004 | >50 | >50 | 0.05 | 0.3 | 13 |
Calculated from 10 concentrations, each with double replicates.
Based on the introduced structural modifications, the following SAR for PDE5 inhibitors can be concluded:
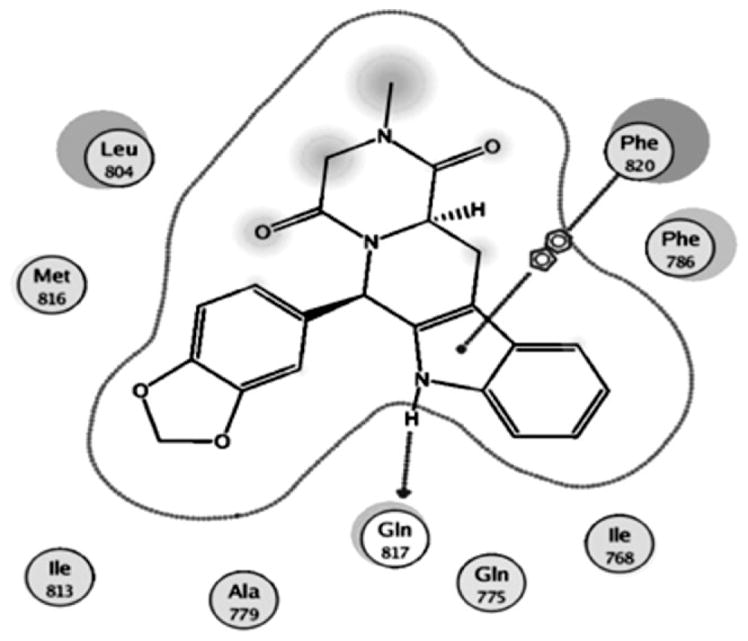
The tetrahydro-β-carboline derivatives 1–4 and the fully aromatic β-carboline 25 showed no PDE5 inhibition, suggesting that the tetracyclic scaffold is an essential feature for PDE5 inhibition. Moreover, lacking of an N-alkyl substituent and thus the lack of possible hydrophobic interaction with Ile665 of the H-loop could be another factor contributing to their lack of activity. The importance of this type of interaction has been reported before. Moreover, a docking experiment showed the involvement of one of the amide carbonyls in indirect interaction through a water molecule with Met816 (Fig. 2). This confirms the importance of the external ring in modulating the inhibition of PDE5.
Figure 2.
Detailed mode view showing the docking and interaction of 8 with human PDE5.
Replacing the 1,3-benzodioxole group of tadalafil with a 5-bromo-2-thienyl group still produces active candidates with appreciable PDE5 inhibitory activity, compound 8 showed the highest activity with an IC50 of 0.16 μm. The relatively smaller size of the 5-bromo-2-thienyl group seems to allow its fitting in the relatively narrow Q2 pocket of the PDE5 enzyme whose size is believed to govern the binding of the ligands to the enzyme [19, 25]. In addition, the lipophilicity of the 5-bromo-2-thienyl group was calculated to be higher than that of the 1,3-benzodioxole group of tadalafil (clog p = 2.693 and 2.107, respectively), which may improve the possible hydrophobic interaction with Met816 in the Q2 pocket of the enzyme. However, looking for the thiophene ring as an isosteric replacement of pendant phenyl led to pronounced reduction in PDE5 inhibitory activity. The most potent compound 8 showed four times lower potency than a previously reported classical isostere, shown in Fig. 3 [26]. The two isosteres, thiophene, and benzene rings were compared. The thiophene ring has higher electron-rich properties, compared to benzene, which arises from the fact that the lone pair of electrons in the p orbital of the sulfur atom contributes to the aromatic sextet and pushes high electron density toward the ring carbons that accordingly acquire partial negative charge. Accordingly, it was suggested that the large atomic polarizability of the sulfur atom would provide higher dispersion forces compared to benzene, which may lead to better π–π stacking and/or Van der Waals forces with hydrophobic residues lining the Q2 hydrophobic pocket [27]. This suggestion was further encouraged by a quantum mechanics-based study which proved that benzene-thiophene heterodimers are more stable π–π complexes than benzene–benzene homodimers with interaction energies of −2.774 and −0.38 kcal/mol, respectively [28]. On the other hand, the lipophilicity of the 5-bromo-2-thienyl group was calculated to be relatively lower than that of the 2-bromo-phenyl group (clog p = 2.693 and 3.005, respectively), which may have caused lower hydrophobic interaction with residues in the Q2 pocket and thus the unexpected marked decrease in potency. This may imply that the relatively lower lipophilic properties of thiophene compared to benzene had more influence on interaction and potency than its polarizability. Accordingly, in future studies, it would be beneficial to use lipophilic carboaryl substituents rather than the polarizable thienyl in order to maximize possible hydrophobic interaction with the enzyme.
Figure 3.
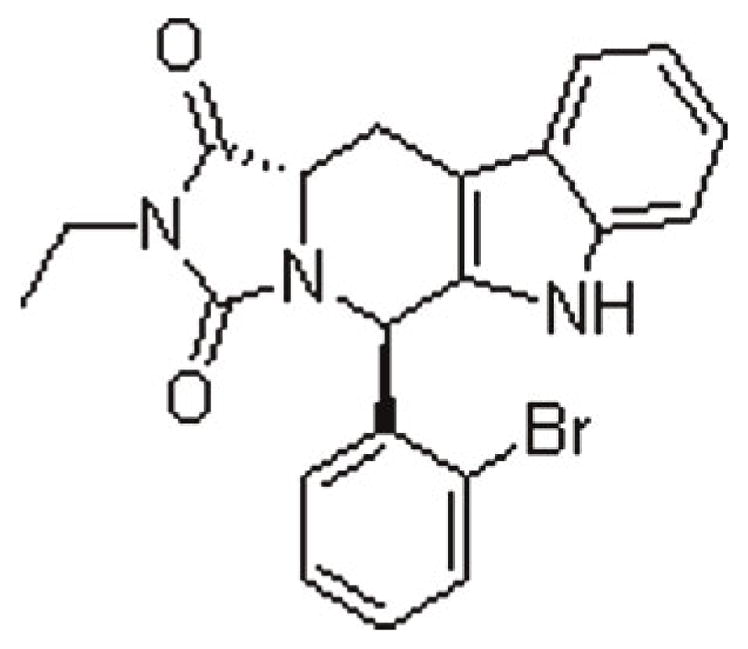
Chemical structure of the bromophenyl isostere of compound 8, PDE5 inhibition IC50 = 0.038 μm [26].
Regarding the hydantoin analogues (5–20), the substituent on the nitrogen of the terminal ring was modified from N-methyl of tadalafil to N-ethyl, butyl, tert-butyl, and allyl in hydantoin derivatives with 11 compounds showing PDE5 inhibitory activity. N-Substitution seems to have slight influence on activity, with the ethyl substituent being the most active. All N-ethyl derivatives were active as PDE5 inhibitors with IC50 ranging from 0.16 to 4.84 μm where the most active compound 8 had an N-ethyl substituent. This may encourage the use of less bulky groups in the future. However, none of the other bulkier or unsaturated side chains had deleterious effect on activity and they possessed comparable PDE5 inhibitory IC50 values, indicating the ability of the nitrogen of the hydantoin ring to tolerate various types of substituents. These N-alkyl derivatives are expected to have hydrophobic interaction with residue Ile665 of the H-loop.
Expansion of the terminal ring from hydantoin in compounds 5, 8 to piperazinedione in compounds 23, 24 led to active candidates but, surprisingly, showed a six- to ninefold decrease in IC50 of PDE5 inhibitory activity compared to the respective hydantoins. This implies that the increase in the size of the fused ring has an impact on PDE5 inhibitory activity in this series of compounds.
Regarding the absolute stereochemical requirement for PDE5 inhibition, the stereochemistry of C5 of the hydantoins and C6 of the piperazinediones appeared to be critical for activity. The results showed that all potent compounds with IC50 < 1 μM possess an absolute S configuration at C-5 or C-6 regardless of the absolute configuration at C-11a or C-12a. This suggests that activity is mainly set by the stereochemistry of the chiral carbon derived from the aldehyde rather than that derived from the amino acid. This may double the chemical space from which PDE5 inhibitors might be discovered and open a new horizon towards economical synthesis of active compounds using the cheaper L-tryptophan.
Compounds 8, 9 were the most potent PDE5 inhibitors. Therefore, we determined their isozyme selectivity by comparing their potency for PDE5 inhibition to their potency for inhibition of PDE1A, PDE2A, PDE3A, PDE9A, PDE10A using cGMP as a substrate and inhibition of PDE11A using both cAMP and cGMP as substrates; the results are summarized in Table 4. Interestingly, our most potent compound 8 showed 49 times selectivity towards PDE5 rather than PDE11 with cGMP as substrate. Meanwhile, tadalafil was only 13 times as selective. Moreover, compound 9 showed 14 times selectivity towards PDE5 rather than PDE11 which is slightly higher compared to tadalafil. This indicates that the selectivity to PDE5 versus PDE11 is mainly a function of the size of the terminal ring and its terminal nitrogen substituent, the smaller is the better. This indicates that there are potential differences between PDE5 and PDE11 in the H-loop region. Moreover, compounds 8, 9 as well as the reference compound tadalafil lacked the ability to inhibit cGMP hydrolysis by PDE1A, PDE2A, PDE3A, PDE9A, PDE10A at concentrations up to 50 μM, showing that PDE5 and PDE11 are of the highest catalytic pocket similarity among different PDEs. This indicates that compounds 8, 9 might be free from those side effects attributed to cross-reactivity with other PDEs, particularly PDE11.
In silico binding mode
To further investigate the binding mode of our compounds compared to tadalafil, in silico docking experiment was implemented to dock tadalafil and the most active compound 8 to the PDE5 crystal structure (1XOZ) using the MOE 2009.10 software. A 2D view of the interaction between tadalafil and the human PDE5 binding pocket was compared to the interaction of 8 with the residues lining the binding pocket of PDE5 as shown in Figs. 1 and 2. The results showed that 8 interacts with human PDE5 in a similar manner to tadalafil, where both can form a single H-bond with Gln817, the protein residue involved in nucleotide recognition, through the NH of the indole ring of the tetrahydro-β-carboline moiety. The indole ring in both compounds showed π–π stacking with the hydrophobic residues lining the cavity of the active sites, namely Phe820. Moreover, compound 8 showed a water-mediated interaction with Met816. Finally, the bromine atom seems to occupy the relatively narrow Q2 pocket, occupied by the 1,3-benzodioxole group of tadalafil, allowing some kind of hydrophobic interaction with Met816.
Experimental
Chemistry
Mass spectrometric analysis (HPLC–ESI-MS) was performed on a TSQ quantum (Thermo Electron Corporation) instrument equipped with an ESI source and a triple quadrupole mass detector (Thermo Finnigan, San Jose, CA). The MS detection was carried out at a spray voltage of 4.2 kV, a nitrogen sheath gas pressure of 4.0 × 105 Pa, an auxiliary gas pressure of 1.0 × 105 Pa, a capillary temperature of 400°C, capillary voltage of 35 V, and source CID of 10 V. All samples were injected by autosampler (Surveyor, Thermo Finnigan) with an injection volume of 10 μL. A RP C18 NUCLEODUR 100-3 (125 mm × 3 mm) column (Macherey-Nagel) was used as stationary phase. The solvent system consisted of water containing 0.1% TFA in acetonitrile. HPLC method: flow rate 400 μL/min. The percentage of the solvent system started at an initial of 5%, was increased up to 100% during 16 min, kept at 100% for 2 min, and flushed back to the 5% in 2 min. Melting points were determined on a Mettler FP1 melting point apparatus and are uncorrected. FTIR spectra were recorded on a Nicolet Avatar 380 spectrometer. 1H NMR spectra were recorded at 500 MHz while 13C NMR and DEPT spectra were recorded at 125 Hz using a Bruker DRX-500 spectrometer. 1H shifts are referenced to the residual protonated solvent signal (δ 2.50 for DMSO-d6 and δ 7.26 for CDCl3) and 13C shifts are referenced to the deuterated solvent signal (δ 39.5 for DMSO-d6 and δ 77.2 for CDCl3). Chemical shifts are given in parts per million (ppm), and all coupling constants (J) are given in Hz. The following abbreviations are used for multiplicity of NMR signals: s, singlet; d, doublet; t, triplet; q, quartret; m, multiplet; dd, doublet of doublet; dt, doublet of triplet; qd, quartet of doublet; ddd, doublet of doublet of doublet. Flash column chromatography was carried out using silica gel 60 (40–63 μM). The reaction progress was determined by TLC analysis on Alugram SIL G/UV254 (Macherey-Nagel). Visualization was accomplished with UV light. All reactions were carried out under nitrogen or argon. All chemicals and solvents were obtained from Sigma–Aldrich and were used without further purification. All the described compounds had >95% purity as determined by HPLC/MS and the areas under the peaks.
Procedure for the preparation of D- and L-tryptophan methyl ester [23]
A 250 mL round bottom flask containing methanol (150 mL) was cooled in an ice-water bath, then acetyl chloride (10 mL) was added dropwise. Solid D- or L-tryptophan (15 g, 73.45 mmol) was added to the well-stirred solution. The solution was then heated to reflux for 5 h, the solvent was removed under reduced pressure to give tryptophan methyl ester hydrochloride. The free base was obtained by adding dilute NH4OH (50 mL) and extraction with CH2Cl2 (5 × 50 mL). The organic layer was dried over anhydrous Na2SO4, evaporated under reduced pressure to give a yellow oil which solidifies on cooling. The product was used without further purification.
General procedure for the preparation of methyl 1-(5-bromo-2-thienyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylates 1–4
The appropriate tryptophan methyl ester (10 g, 45.82 mmol) was added to 5-bromothiophene-2-carboxaldehyde (9.5 g, 49.73 mmol) and dissolved in CH2Cl2 (20 mL). The solution was cooled to 0°C in an ice bath. To this solution trifluoroacetic acid (2 mL) was added dropwise, and the mixture was stirred at room temperature for 4 days under nitrogen atmosphere. The reaction mixture was then basified with dilute NH4OH solution. The trans-isomer was precipitated in the organic layer and obtained by vacuum filtration, washed with CH2Cl2 and ether. The filtrate, containing a mixture of cis- and trans-isomers, was then extracted with CH2Cl2 (3 × 50 mL). The organic layer was washed with water, dried over anhydrous Na2SO4, filtered, and evaporated under reduced pressure. The residue was purified and the isomers were separated by flash column chromatography on silica gel eluting with CH2Cl2, to give first the appropriate cis-isomer followed by the trans-isomer.
(1S,3R) Methyl 1-(5-bromo-2-thienyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (1)
Yellow powder (2.17 g, 12.1%): Rf = 0.53 (CH2Cl2); mp: 157–159°C; 1H NMR (500 MHz, DMSO-d6): δ 10.49 (s, 1H, NH), 7.41 (d, J = 7.8 Hz, 1H, Ar), 7.25–7.22 (m, 1H, Ar), 7.14–7.08 (m, 2H, Ar), 7.04–6.98 (m, 1H, Ar), 6.97–6.92 (m, 1H, Ar), 5.54 (s, 1H, NHCHCS), 3.84 (dd, J = 11.1, 4.0 Hz, 1H, CHCOOCH3), 3.70 (s, 3H, OCH3), 3.02–2.97 (m, 1H, CHaHb), 2.79–2.71 ppm (m, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 172.40 (COOCH3), 148.23, 136.30, 134.15, 129.59, 126.66, 126.33, 121.01, 118.57, 117.79, 111.28, 110.95, 106.29, 55.97 (C1), 53.38 (C3), 51.85 (CH3), 24.94 ppm (CHaHb). IR: 3394.39, 3328.32 (–NH–), 1737.67 cm−1 (–CO–). HPLC/MS (ESI): Purity 98.37%, for C17H15BrN2O2S: m/z (%): 392 [M++2], 390 [M+], 305 (100%).
(1R,3R) Methyl 1-(5-bromo-2-thienyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (2)
Pale yellow powder (9.02 g, 50.3%): Rf = 0.22 (CH2Cl2); mp: 205–208°C; 1H NMR (500 MHz, DMSO-d6): δ 10.81 (s, 1H, NH), 7.41 (d, J = 7.8 Hz, 1H, Ar), 7.27 (d, J = 8.0 Hz, 1H, Ar), 7.06–7.02 (m, 2H, Ar), 6.96–6.93 (m, 1H, Ar), 6.85 (dd, J = 3.8, 0.9 Hz, 1H, Ar), 5.51 (s, 1H, NHCHCS), 3.83 (dd, J = 7.7, 5.2 Hz, 1H, CHCOOCH3), 3.63 (s, 3H, OCH3), 2.98 (dd, J = 15.2, 5.1 Hz, 1H, CHaHb), 2.83 ppm (dd, J = 14.7, 7.3 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 173.56 (COOCH3), 149.93, 135.94, 133.46, 129.88, 126.23, 125.88, 121.16, 118.56, 117.93, 111.17, 110.48, 105.97, 52.17 (C1), 51.72 (C3), 50.08 (CH3), 24.61 ppm (CHaHb). IR: 3317.89 (–NH–), 1697.84 cm−1 (–CO–). HPLC/MS (ESI): Purity 98%, for C17H15BrN2O2S: m/z (%): 392 [M++2], 390 [M+], 303 (100%).
(1R,3S) Methyl 1-(5-bromo-2-thienyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (3)
Yellow powder (1.97 g, 11%): Rf = 0.53 (CH2Cl2); mp: 157–158°C; 1H NMR (500 MHz, DMSO-d6): δ 10.49 (s, 1H, NH), 7.41 (d, J = 7.8 Hz, 1H, Ar), 7.25–7.23 (m, 1H, Ar), 7.14–7.08 (m, 2H, Ar), 7.04–6.99 (m, 1H, Ar), 6.94 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar), 5.53 (s, 1H, NHCHCS), 3.84 (dd, J = 11.1, 4.0 Hz, 1H, CHCOOCH3), 3.70 (s, 3H, OCH3), 2.99 (ddd, J = 14.7, 4.0, 1.6 Hz, 1H, CHaHb), 2.75 ppm (ddd, J = 14.7, 11.1, 2.3 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 172.49 (COOCH3), 148.31, 136.29, 134.21, 129.58, 126.61, 126.34, 121.00, 118.56, 117.79, 111.27, 110.91, 106.29, 55.97 (C1), 53.38 (C3), 51.84 (CH3), 24.97 ppm (CHaHb). IR: 3393.8, 3328.06 (–NH–), 1737.5 cm−1 (–CO–). HPLC/ MS (ESI): Purity 100%, for C17H15BrN2O2S: m/z (%) 392 [M++2], 390 [M+], 305 (100%).
(1S,3S) Methyl 1-(5-bromo-2-thienyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylate (4)
Pale yellow powder (7.3 g, 40.7%): Rf = 0.22 (CH2Cl2); mp: 205–207°C; 1H NMR (500 MHz, DMSO-d6): δ 10.81 (s, 1H, NH), 7.41 (d, J = 7.8 Hz, 1H, Ar), 7.27 (d, J = 8.0 Hz, 1H, Ar), 7.06–7.01 (m, 2H, Ar), 6.95 (td, J = 7.5, 1.0 Hz, 1H, Ar), 6.85 (dd, J = 3.8, 1.0 Hz, 1H, Ar), 5.51 (s, 1H, NHCHCS), 3.83 (dd, J = 7.7, 5.2 Hz, 1H, CHCOOCH3), 3.63 (s, 3H, OCH3), 2.98 (dd, J = 15.3, 4.8 Hz, 1H, CHaHb), 2.83 ppm (ddd, J = 15.2, 7.8, 1.2 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 173.61 (COOCH3), 149.93, 136.00, 133.33, 129.82, 126.29, 125.88, 121.12, 118.57, 117.84, 111.17, 110.42, 106.00, 52.12 (C1), 51.72 (C3), 50.08 (CH3), 24.67 ppm (CHaHb). IR: 3319.24 (–NH–), 1699.41 cm−1 (–CO–). HPLC/MS (ESI): Purity 98.5%, for C17H15BrN2O2S: m/z (%) 392 [M++2], 390 [M+], 303 (100%).
General procedure for the preparation of 5-(5-bromo-2-thienyl)-2-alkyl-5,6,11,11a-tetrahydro-1H-imidazo-[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-diones 5–20
Excess ethyl or butyl or tert-butyl or allyl isocyanate (1.6 mmol) was added to a well-stirred solution of the appropriate tetra-hydro-β-carbolines 1–4 (0.39 g, 1 mmol) in methyl ethyl ketone (10 mL). The mixture was then refluxed under nitrogen atmosphere for 70 h in case of cis-tetrahydro-β-carbolines 1, 3 while the trans-tetrahydro-β-carbolines 2, 4 were refluxed for 16 h. The solvent was evaporated to dryness under reduced pressure and the product was then purified using flash column chromatography on silica gel, eluting with CH2Cl2.
(5S,11aR) 5-(5-Bromo-2-thienyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (5)
Yellow powder (0.13 g, 30%): Rf = 0.26 (CH2Cl2); mp: 189–191°C; 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H, NH), 7.57–7.54 (m, 1H, Ar), 7.29 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.12–7.07 (m, 3H, Ar), 7.02 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.23 (s, 1H, NCHCS), 4.50 (dd, J = 11.5, 4.3 Hz, 1H, CHC(O)N), 3.42 (qd, J = 7.3, 2.9 Hz, 2H, CH2CH3), 3.29 (dd, J = 14.5, 4.3 Hz, 1H, NCHCHaHb), 2.85 (ddd, J = 14.6, 11.6, 1.8 Hz, 1H, NCHCHaHb), 1.10 ppm (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 171.03, 154.20 (NCO), 146.13, 136.69, 133.52, 129.53, 127.46, 125.69, 121.78, 118.95, 118.40, 111.50, 111.12, 105.43, 57.76 (C5), 51.26 (C11a), 32.78 (CH2CH3), 21.67 (CHaHb), 13.28 ppm (CH2CH3). IR: 3275.82 (–NH–), 1764.63, 1698.05 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C19H16BrN3O2S: m/z (%):431 [M++2], 429 [M+], 242 (100%).
(5R,11aR) 5-(5-Bromo-2-thienyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (6)
White powder (0.11 g, 25%): Rf = 0.49 (CH2Cl2); mp: 132–135°C; 1H NMR (500 MHz, DMSO-d6): δ 11.10 (s, 1H, NH), 7.54 (d, J = 7.8, 1H, Ar), 7.34 (dt, J = 8.2, 0.9 Hz, 1H, Ar), 7.17–7.07 (m, 2H, Ar), 7.03 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.92 (dd, J = 3.8, 0.7 Hz, 1H, Ar), 6.45 (s, 1H, NCHCS), 4.48 (dd, J = 10.9, 5.6 Hz, 1H, CHC(O)N), 3.46 (q, J = 7.1 Hz, 2H, CH2CH3), 3.38–3.30 (dd, J = 15.0, 5.5 Hz, 1H, NCHCHaHb), 2.80 (ddd, J = 15.1, 11.0, 1.7 Hz, 1H, NCHCHaHb), 1.12 ppm (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 171.99, 153.94 (NCO), 144.65, 136.58, 130.16, 130.05, 127.54, 125.54, 122.01, 118.95, 118.41, 111.58, 111.48, 106.25, 52.43 (C5), 46.70 (C11a), 33.14 (CH2CH3), 22.31 (CHaHb), 13.24 ppm (CH2CH3). IR: 3323.84 (–NH–), 1765.72, 1697.64 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C19H16BrN3O2S: m/z (%): 431 [M++2], 429 [M+], 242 (100%).
(5R,11aS) 5-(5-Bromo-2-thienyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (7)
Dark yellow powder (0.14 g, 32.9%): Rf = 0.26 (CH2Cl2); mp: 193–196°C; 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H, NH), 7.57–7.54 (m,1H, Ar), 7.29 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.13–7.07 (m, 3H, Ar), 7.02 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.23 (s, 1H, NCHCS), 4.50 (dd, J = 11.5, 4.3 Hz, 1H, CHC(O)N), 3.42 (qd, J = 7.2, 3.3 Hz, 2H, CH2CH3), 3.32–3.28 (m, 1H, NCHCHaHb), 2.85 (ddd, J = 14.8, 11.6, 1.8 Hz, 1H, NCHCHaHb), 1.11 ppm (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 171.05, 154.23 (NCO), 146.08, 136.63, 133.51, 129.52, 127.45, 125.72, 121.77, 118.94, 118.39, 111.43, 111.11, 105.19, 57.57 (C5), 51.47 (C11a), 32.67 (CH2CH3), 21.68 (CHaHb), 13.28 ppm (CH2CH3). IR: 3328.56 (–NH–), 1764.73, 1697.84 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C19H16BrN3O2S: m/z (%) 431 [M++2], 429 [M+], 242 (100%).
(5S,11aS) 5-(5-Bromo-2-thienyl)-2-ethyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (8)
Yellow powder (0.25 g, 58.4%): Rf = 0.48 (CH2Cl2); mp: 135–138°C; 1H NMR (500 MHz, DMSO-d6): δ 11.10 (s, 1H, NH), 7.54 (m, 1H, Ar), 7.34 (dt, J = 8.2, 0.9 Hz, 1H, Ar), 7.15–7.10 (m, 2H, Ar), 7.03 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.92 (dd, J = 3.8, 0.7 Hz, 1H, Ar), 6.45 (s, 1H, NCHCS), 4.48 (dd, J = 10.9, 5.7 Hz, 1H, CHC(O)N), 3.46 (q, J = 7.2, 2H, CH2CH3), 3.37–3.32 (dd, J = 15.0, 5.5 Hz, 1H, NCHCHaHb), 2.80 (ddd, J = 15.1, 10.9, 1.7 Hz, 1H, NCHCHaHb), 1.12 ppm (t, J = 7.2 Hz, 3H, CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 171.98, 153.94 (NCO), 144.65, 136.62, 130.16, 130.05, 127.50, 125.54, 122.00, 118.94, 118.41, 111.60, 111.54, 106.25, 52.43 (C5), 46.61 (C11a), 33.05 (CH2CH3), 22.31 (CHaHb), 13.24 ppm (CH2CH3). IR: 3316.6 (–NH–), 1765.74, 1695.22 cm−1 (–CO–). HPLC/MS (ESI): Purity 97.62%, for C19H16BrN3O2S: m/z (%): 431 [M++2], 429 [M+] (100%).
(5S,11aR) 5-(5-Bromo-2-thienyl)-2-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (9)
Yellow powder (0.2 g, 43.4%): Rf = 0.35 (CH2Cl2); mp: 178–181°C; 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H, NH), 7.57–7.54 (m, 1H, Ar), 7.29 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.13–7.07 (m, 3H, Ar), 7.02 (ddd, J = 8.0, 7.1, 1.1 Hz, 1H, Ar), 6.23 (s, 1H, NCHCS), 4.52 (dd, J = 11.5, 4.3 Hz, 1H, CHC(O)N), 3.39 (t, J = 7.1, 2H, CH2 CH2CH2CH3), 3.34–3.28 (m, 1H, NCHCHaHb), 2.84 (ddd, J = 14.8, 11.6, 1.8 Hz, 1H, NCHCHaHb), 1.52–1.46 (m, 2H, CH2 CH2CH2CH3), 1.29–1.24 (m, 2H, CH2 CH2CH2CH3), 0.88 ppm (t, J = 7.4 Hz, 3H, CH2CH2CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 171.28, 154.48 (NCO), 146.11, 136.74, 133.46, 129.49, 127.51, 125.75, 121.77, 119.05, 118.39, 111.43, 111.10, 105.42, 57.71 (C5), 51.31 (C11a), 37.45, 29.64, 21.82, 19.31, 13.45 ppm (CH3). IR: 3304.49 (–NH–), 1755.60, 1698.53 cm−1 (–CO–). HPLC/MS (ESI): Purity 99.5%, for C21H20BrN3O2S: m/z (%): 459 [M++2], 457 [M+] (100%).
(5R,11aR) 5-(5-Bromo-2-thienyl)-2-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (10)
Yellow powder (0.26 g, 57.6%): Rf = 0.62 (CH2Cl2); mp: 100–102°C; 1H NMR (500 MHz, DMSO-d6): δ 11.11 (s, 1H, NH), 7.56–7.53 (m, 1H, Ar), 7.36–7.31 (m, 1H, Ar), 7.15–7.11 (m, 2H, Ar), 7.06–7.01 (m, 1H, Ar), 6.92 (dt, J = 2.3, 1.4 Hz, 1H, Ar), 6.45 (s, 1H, NCHCS), 4.49 (dd, J = 11.0, 5.5 Hz, 1H, CHC(O)N), 3.42 (t, J = 7.1, 2H, CH2 CH2CH2CH3), 3.35 (dd, J = 15.0, 5.5 Hz, 1H, NCHCHaHb), 2.79 (ddd, J = 15.1, 11.0, 1.8 Hz, 1H, NCHCHaHb), 1.56–1.48 (m, 2H, CH2 CH2CH2CH3), 1.29–1.23 (m, 2H, CH2 CH2CH2CH3), 0.87 ppm (t, J = 7.5 Hz, 3H, CH2CH2CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 172.23, 154.12 (NCO), 144.65, 136.62, 130.17, 130.05, 127.46, 125.54, 122.01, 118.95, 118.42, 111.56, 111.48, 106.25, 52.40 (C5), 46.64 (C11a), 37.71, 29.59, 22.46, 19.33, 13.46 ppm (CH3). IR: 3317.76 (–NH–), 1764.58, 1695.60 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C21H20BrN3O2S: m/z (%): 459 [M++2], 457 [M+] (100%).
(5R,11aS) 5-(5-Bromo-2-thienyl)-2-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (11)
Yellow powder (0.27 g, 58.7%): Rf = 0.35 (CH2Cl2); mp: 181–183°C; 1H NMR (500 MHz, DMSO-d6): δ 10.90 (s, 1H, NH), 7.56–7.53 (m, 1H, Ar), 7.29 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.13–7.07 (m, 3H, Ar), 7.02 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.23 (s, 1H, NCHCS), 4.52 (dd, J = 11.5, 4.3 Hz, 1H, CHC(O)N), 3.39 (t, J = 7.1, 2H, CH2 CH2CH2CH3), 3.34–3.29 (m, 1H, NCHCHaHb), 2.84 (ddd, J = 14.8, 11.6, 1.8 Hz, 1H, NCHCHaHb), 1.52–1.46 (m, 2H, CH2 CH2CH2CH3), 1.29–1.24 (m, 2H, CH2 CH2CH2CH3), 0.88 ppm (t, J = 7.3 Hz, 3H, CH2CH2CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 171.28, 154.34 (NCO), 146.13, 136.62, 133.52, 129.52, 127.40, 125.69, 121.77, 118.94, 118.39, 111.43, 111.08, 105.36, 57.65 (C5), 51.26 (C11a), 37.45, 29.58, 21.78, 19.28, 13.45 ppm (CH3). IR: 3303.53 (–NH–), 1755.54, 1697.13 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C21H20BrN3O2S: m/z (%): 459 [M++2], 457 [M+] (100%).
(5S,11aS) 5-(5-Bromo-2-thienyl)-2-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (12)
Yellow powder (0.31 g, 67.4%): Rf = 0.62 (CH2Cl2); mp: 98–101°C; 1H NMR (500 MHz, DMSO-d6): δ 11.11 (s, 1H, NH), 7.56–7.53 (m, 1H, Ar), 7.34 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.15–7.10 (m, 2H, Ar), 7.03 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.91 (dd, J = 3.8, 0.8 Hz, 1H, Ar), 6.45 (s, 1H, NCHCS), 4.49 (dd, J = 11.0, 5.6 Hz, 1H, CHC(O)N), 3.42 (t, J = 7.1, 2H, CH2 CH2CH2CH3), 3.36 (dd, J = 15.0, 5.5 Hz, 1H, NCHCHaHb), 2.79 (ddd, J = 15.1, 11.0, 1.8 Hz, 1H, NCHCHaHb), 1.56–1.49 (m, 2H, CH2 CH2CH2CH3), 1.29–1.24 (m, 2H, CH2 CH2CH2CH3), 0.88 ppm (t, J = 7.4 Hz, 3H, CH2CH2CH2CH3). 13C NMR (125 MHz, DMSO-d6): δ 172.23, 154.12 (NCO), 144.65, 136.62, 130.17, 130.05, 127.46, 125.54, 122.01, 118.95, 118.42, 111.56, 111.48, 106.24, 52.40 (C5), 46.63 (C11a), 37.80, 29.67, 22.46, 19.33, 13.46 ppm (CH3). IR: 3321.19 (–NH–), 1765.08, 1696.47 cm−1 (–CO–). HPLC/MS (ESI): Purity 97.62%, for C21H20BrN3O2S: m/z (%): 459 [M++2], 457 [M+], 242 (100%).
(5S,11aR) 5-(5-Bromo-2-thienyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (13)
Yellow powder (0.23 g, 50%): Rf = 0.42 (CH2Cl2); mp: 196–199°C; 1H NMR (500 MHz, DMSO-d6): δ 10.88 (s, 1H, NH), 7.56–7.53 (m, 1H, Ar), 7.29 (dt, J = 8.2, 1.0 Hz, 1H, Ar), 7.12–7.06 (m, 3H, Ar), 7.01 (ddd, J = 8.0, 7.1, 1.1 Hz, 1H, Ar), 6.17 (s, 1H, NCHCS), 4.36 (dd, J = 11.4, 4.2 Hz, 1H, CHC(O)N), 3.23 (dd, J = 14.9, 4.3 Hz, 1H, CHaHb), 2.77 (ddd, J = 14.8, 11.6, 1.8 Hz, 1H, CHaHb), 1.53 ppm (s, 9H, CH3). 13C NMR (125 MHz, DMSO-d6): δ 172.09, 155.06 (NCO), 146.78, 136.60, 133.65, 129.58, 126.84, 125.72, 121.72, 118.92, 118.37, 111.41, 110.74, 105.39, 57.00 (C5), 56.86 (C(CH3)3), 51.21 (C11a), 28.41 ((CH3)3), 21.99 ppm (CHaHb). IR: 3336.41 (–NH–), 1762.7, 1701.88 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C21H20BrN3O2S: m/z (%): 459 [M++2], 457 [M+], 242 (100%).
(5R,11aR) 5-(5-Bromo-2-thienyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (14)
Dark yellow powder (0.16 g, 34.6%): Rf = 0.67 (CH2Cl2); mp: 206–207°C; 1H NMR (500 MHz, DMSO-d6): δ 11.09 (s, 1H, NH), 7.56–7.53 (m, 1H, Ar), 7.33 (dt, J = 8.2, 0.9 Hz, 1H, Ar), 7.15–7.10 (m, 2H, Ar), 7.03 (ddd, J = 7.9, 7.0, 1.0 Hz, 1H, Ar), 6.90 (dd, J = 3.8, 0.7 Hz, 1H, Ar), 6.41 (s, 1H, NCHCS), 4.33 (dd, J = 10.9, 5.7 Hz, 1H, CHC(O)N), 3.3 (dd, J = 15.2, 5.7 Hz, 1H, CHaHb), 2.75 (ddd, J = 15.1, 10.8, 1.8 Hz, 1H, CHaHb), 1.56 ppm (s, 9H, CH3). 13C NMR (125 MHz, DMSO-d6): δ 173.06, 154.66 (NCO), 144.60, 136.67, 130.17, 130.13, 127.48, 125.58, 121.99, 118.95, 118.40, 111.57, 111.47, 106.11, 56.92 (C(CH3)3), 51.46 (C5), 46.37 (C11a), 28.13 ((CH3)3), 22.70 ppm (CHaHb). IR: 3333.69 (–NH–), 1761.99, 1693.57 cm−1 (–CO–). HPLC/MS (ESI): Purity 99%, for C21H20BrN3O2S: m/z (%): 459 [M++2] (100%), 457 [M+].
(5R,11aS) 5-(5-Bromo-2-thienyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6] pyrido[3,4-b]indole-1,3(2H)-dione (15)
Pale yellow powder (0.25 g, 54.8%): Rf = 0.42 (CH2Cl2); mp: 200–203°C; 1H NMR (500 MHz, DMSO-d6): δ 10.88 (s, 1H, NH), 7.55–7.53 (m, 1H, Ar), 7.2 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.11–7.06 (m, 3H, Ar), 7.01 (ddd, J = 8.0, 7.1, 1.1 Hz, 1H, Ar), 6.16 (s, 1H, NCHCS), 4.36 (dd, J = 11.4, 4.2 Hz, 1H, CHC(O)N), 3.25 (ddd, J = 14.7, 4.3, 1.3 Hz, 1H, CHaHb), 2.77 (ddd, J = 14.8, 11.5, 1.8 Hz, 1H, CHaHb), 1.53 ppm (s, 9H, CH3). 13C NMR (125 MHz, DMSO-d6): δ 172.01, 155.03 (NCO), 146.83, 136.57, 133.64, 129.58, 126.84, 125.72, 121.72, 118.92, 118.37, 111.41, 110.74, 105.39, 57.00 (C5), 56.86 (C(CH3)3), 51.21 (C11a), 28.41 ((CH3)3), 21.90 ppm (CHaHb). IR: 3336.12 (–NH–), 1762.37, 1702.56 cm−1 (–CO–). HPLC/MS (ESI): Purity 96.34%, for C21H20BrN3O2S: m/z (%): 459 [M++2], 457 [M+], 242 (100%).
(5S,11aS) 5-(5-Bromo-2-thienyl)-2-tert-butyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (16)
Pale yellow powder (0.17 g, 37%): Rf = 0.67 (CH2Cl2); mp: 206–207°C; 1H NMR (500 MHz, DMSO-d6): δ 11.09 (s, 1H, NH), 7.55–7.52 (m, 1H, Ar), 7.33 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.15–7.10 (m, 2H, Ar), 7.03 (ddd, J = 7.9, 7.1, 1.0 Hz, 1H, Ar), 6.90 (dd, J = 3.8, 0.7 Hz, 1H, Ar), 6.40 (s, 1H, NCHCS), 4.33 (dd, J = 10.5, 5.5 Hz, 1H, CHC(O)N), 3.30 (dd, J = 15.2, 5.7 Hz, 1H, CHaHb), 2.75 (ddd, J = 15.2, 10.7, 1.8 Hz, 1H, CHaHb), 1.56 ppm (s, 9H, CH3). 13C NMR (125 MHz, DMSO-d6): δ 173.05, 154.60 (NCO), 144.63, 136.59, 130.16, 130.12, 127.47, 125.57, 121.98, 118.94, 118.39, 111.56, 111.46, 106.21, 56.98 (C(CH3)3), 51.56 (C5), 46.37 (C11a), 28.31 ((CH3)3), 22.74 ppm (CHaHb). IR: 3333.69 (–NH–), 1761.99, 1693.57 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C21H20BrN3O2S: m/z (%): 459 [M++2] (100%), 457 [M+].
(5S,11aR) 5-(5-Bromo-2-thienyl)-2-allyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (17)
Yellow powder (0.24 g, 53.2%): Rf = 0.27 (CH2Cl2); mp: 116–119°C; 1H NMR (500 MHz, DMSO-d6): δ 10.91 (s, 1H, NH), 7.56 (d, J = 8 Hz, 1H, Ar), 7.29 (ddd, J = 8.1, 1.8, 0.8 Hz, 1H, Ar), 7.13–7.07 (m, 3H, Ar), 7.04–7.00 (m, 1H, Ar), 6.25 (s, 1H, NCHCS), 5.83–5.77 (m, 1H, CH=CHaHb), 5.15–5.12 (m, 1H, CH=CHaHb), 5.11–5.08 (m, 1H, CH=CHaHb), 4.58 (dd, J = 11.5, 4.3 Hz, 1H, CHC(O)N), 4.00 (dt, J = 5.0, 1.5 Hz, 2H, NCH2), 3.36–3.30 (m, 1H, CHaHb), 2.87 ppm (ddd, J = 13.4, 11.6, 1.8 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 170.90, 153.98 (NCO), 145.99, 136.63, 133.48, 132.05, 129.57, 127.54, 125.68, 121.79, 118.96, 118.44, 116.30 (CH=CHaHb), 111.50, 111.13, 105.39, 57.80 (C5), 51.37 (C11a), 39.85 (NCH2), 21.85 ppm (CHaHb). IR: 3303.75 (–NH–), 1766.58, 1701.92 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C20H16BrN3O2S: m/z (%): 443 [M++2], 441 [M+], 383 (100%).
(5R,11aR) 5-(5-Bromo-2-thienyl)-2-allyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (18)
Yellow powder (0.39 g, 87.2%): Rf = 0.53 (CH2Cl2); mp: 80–83°C; 1H NMR (500 MHz, DMSO-d6): δ 11.11 (s, 1H, NH), 7.55 (dd, J = 7.9, 2.7 Hz, 1H, Ar), 7.36–7.33 (m, 1H, Ar), 7.16–7.11 (m, 2H, Ar), 7.04 (ddt, J = 7.9, 7.0, 0.9 Hz, 1H, Ar), 6.92 (dd, J = 3.8, 0.7 Hz, 1H, Ar), 6.47 (s, 1H, NCHCS), 5.87–5.79 (m, 1H, CH=CHaHb), 5.14–5.12 (m, 1H, CH=CHaHb), 5.11–5.08 (m, 1H, CH=CHaHb), 4.54 (dd, J = 10.8, 5.3 Hz, 1H, CHC(O)N), 4.04 (dt, J = 5.1, 1.6 Hz, 2H, NCH2), 3.36 (dd, J= 15,5.5 Hz, 1H, CHaHb), 2.82 ppm (ddd, J = 15.1, 11.0, 1.7 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 171.85, 153.71 (NCO), 144.58, 136.63, 132.09, 130.17, 130.02, 127.50, 125.54, 122.02, 118.96, 118.43, 116.45 (CH=CHaHb), 111.58, 111.49, 106.24, 52.55 (C5), 46.71 (C11a), 40.05 (NCH2), 22.44 (CHaHb). IR: 3322.87 (–NH–), 1767.37, 1698.06 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C20H16BrN3O2S: m/z (%): 443 [M++2], 441 [M+] (100%).
(5R,11aS) 5-(5-Bromo-2-thienyl)-2-allyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (19)
Yellow powder (0.37 g, 83.6%): Rf = 0.28 (CH2Cl2); mp: 121–123°C; 1H NMR (500 MHz, DMSO-d6): δ 10.91 (s, 1H, NH), 7.56 (d, J = 8 Hz, 1H, Ar), 7.29 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.13–7.07 (m, 3H, Ar), 7.02 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.25 (s, 1H, NCHCS), 5.83–5.77 (m, 1H, CH=CHaHb), 5.16–5.14 (m, 1H, CH=CHaHb), 5.13–5.10 (m, 1H, CH=CHaHb), 4.58 (dd, J = 11.5, 4.3 Hz, 1H, CHC(O)N), 4.00 (dt, J = 5.0, 1.6 Hz, 2H, NCH2), 3.36–3.30 (m, 1H, CHaHb), 2.88 ppm (ddd, J = 14.7, 11.6, 1.8 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 170.90, 153.93 (NCO), 146.04, 136.64, 133.49, 132.01, 129.53, 127.48, 125.68, 121.79, 118.96, 118.40, 116.31 (CH=CHaHb), 111.43, 111.18, 105.36, 57.80 (C5), 51.30 (C11a), 39.76 (NCH2), 21.77 ppm (CHaHb). IR: 3286.42 (–NH–), 1764.54, 1700.95 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C20H16BrN3O2S: m/z (%): 443 [M++2], 441 [M+], 383 (100%).
(5S,11aS) 5-(5-Bromo-2-thienyl)-2-allyl-5,6,11,11a-tetrahydro-1H-imidazo[1′,5 ′:1,6]pyrido[3,4-b]indole-1,3(2H)-dione (20)
Yellow powder (0.36 g, 82.5%); Rf = 0.52 (CH2Cl2); mp 75–78°C; 1H NMR (500 MHz, DMSO-d6): δ 11.11 (s, 1H, NH), 7.55 (d, J = 8 Hz, 1H, Ar), 7.34 (dt, J = 8.2, 0.9 Hz, 1H, Ar), 7.15–7.11 (m, 2H, Ar), 7.04 (ddd, J = 7.9, 7.0, 1.0 Hz, 1H, Ar), 6.92 (dd, J = 3.8, 0.7 Hz, 1H, Ar), 6.47 (s, 1H, NCHCS), 5.87–5.79 (m, 1H, CH=CHaHb), 5.14–5.12 (m, 1H, CH=CHaHb), 5.11–5.09 (m, 1H, CH=CHaHb), 4.55 (dd, J = 11.1, 5.6 Hz, 1H, CHC(O)N), 4.04 (dt, J = 5.3, 1.7 Hz, 2H, NCH2), 3.37 (dd, J = 15, 5.5 Hz, 1H, CHaHb), 2.83 ppm (ddd, J = 15.1, 11.0, 1.7 Hz, 1H, CHaHb). 13C NMR (125 MHz, DMSO-d6): δ 171.85, 153.76 (NCO), 144.58, 136.62, 132.10, 130.17, 130.05, 127.50, 125.58, 122.03, 118.96, 118.43, 116.44 (CH=CHaHb), 111.62, 111.49, 106.24, 52.55 (C5), 46.71 (C11a), 40.5 (NCH2), 22.44 ppm (CHaHb). IR: 3319.27 (–NH–), 1766.89, 1697.98 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C20H16BrN3O2S: m/z (%): 443 [M++2], 441 [M+], 242 (100%).
General procedure for the preparation of methyl 1-(5-bromo-2-thienyl)-2-(2-chloroacetyl)-2,3,4,9-tetrahydro-1H-β-carboline-3-carboxylates 21, 22
Chloroacetyl chloride (1 mL, 12.5 mmol) was added dropwise to a well-stirred solution of the appropriate β-carboline 1, 4 (2 g, 5.13 mmol) and NaHCO3 (0.52 g, 6.19 mmol) in CHCl3 (40 mL) under ice cooling. The mixture was then stirred at room temperature under a nitrogen atmosphere for 2 h. The mixture was diluted with CH2Cl2, washed with a solution of NaHCO3, dried over Na2SO4, and evaporated under reduced pressure. The residue was then crystallized from diethyl ether. The product was then used without further purification.
General procedure for the preparation of 2-ethyl-6-(5-bromo-2-thienyl)-2,3,6,7,12,12a-hexahydropyrazino-[1′,2 ′:1,6]pyrido[3,4-b]indole-1,4-diones 23, 24
A solution of the appropriate chloroethanone derivative (1.4 mmol, 1 equiv, 0.65 g) and ethylamine (2.8 mmol, 2 equiv) in methanol (25 mL) was heated to reflux under nitrogen atmosphere for 16 h. The reaction mixture was cooled to room temperature and evaporated to dryness under reduced pressure. The residue was dissolved in CH2Cl2, and the organic layer was washed with water, dried over Na2SO4, filtered, and concentrated to dryness. The crude product was then purified using flash column chromatography eluting with CH2Cl2/MeOH (99.5:0.5).
(6S,12aR) 6-(5-Bromo-2-thienyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2 ′:1,6]pyrido[3,4-b]indole-1,4-dione (23)
White powder (75 mg, 12%): Rf = 0.27 (CH2Cl2/MeOH 99:1); mp: 173–175°C; 1H NMR (500 MHz, CDCl3): δ 11.09 (s, 1H, NH), 7.51 (d, J = 7.9 Hz, 1H, Ar), 7.30 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.19 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar), 7.13 (ddd, J = 8.0, 7.2, 1.0 Hz, 1H, Ar), 7.00 (s, 1H, NCHCS), 6.84 (d, J = 3.8, 1H, Ar), 6.61 (dd, J = 3.8, 0.8 Hz, 1H, Ar), 4.36 (dd, J = 11.8, 4.2 Hz, 1H, CHC(O)N), 4.11 (d, J = 17.8 Hz, 1H, NC(O)CHaHb), 3.99 (d, J = 17.8 Hz, 1H, NC(O)CHaHb), 3.58–3.50 (m, 2H, NCH2CH3), 3.43–3.36 (m, 1H, NCHCHaHb), 2.91 (ddd, J = 15.6, 11.8, 1.6 Hz, 1H, NCHCHaHb), 1.18 ppm (t, J = 7.2 Hz, 3H, NCH2CH3). 13C NMR (125 MHz, CDCl3): δ 164.48, 161.84 (NCO), 142.24, 136.36, 129.47, 128.45, 128.31, 126.04, 123.06, 120.19, 118.64, 113.89, 111.27, 109.24, 57.60 (C6), 51.30 (C12a), 48.78 (NC(O)CHaHb), 41.05 (NCH2CH3), 27.45 (NCHCHaHb), 11.59 ppm (NCH2CH3). IR: 3245.67 (–NH–), 1663.53, 1646.86 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C20H18BrN3O2S: m/z (%): 445 [M++2], 443 [M+], 242 (100%).
(6S,12aS) 6-(5-Bromo-2-thienyl)-2-ethyl-2,3,6,7,12,12a-hexahydropyrazino[1′,2 ′:1,6]pyrido[3,4-b]indole-1,4-dione (24)
Yellow powder (0.32 g, 52.1%): Rf = 0.26 (CH2Cl2/MeOH 99:1); mp: 191–194°C; 1H NMR (500 MHz, CDCl3): δ 11.15 (s, 1H, NH), 7.54–7.51 (m, 1H, Ar), 7.34 (dt, J = 8.1, 0.9 Hz, 1H, Ar), 7.15–7.09 (m, 2H, Ar), 7.03 (ddd, J = 8.0, 7.0, 1.0 Hz, 1H, Ar), 6.93 (s, 1H, NCHCS) 6.70 (dd, J = 3.8, 0.9 Hz, 1H, Ar), 4.28 (d, J = 17.8 Hz, 1H, NC(O)CHaHb), 4.18 (dd, J = 11.8, 4.3 Hz, 1H, CHC(O)N), 4.06 (d, J = 17.8 Hz, 1H, NC(O)CHaHb), 3.46–3.39 (m, 1H, NCHCHaHb), 3.33–3.26 (m, 2H, NCH2CH3), 2.95 (ddd, J = 15.4, 11.8, 1.5 Hz, 1H, NCHCHaHb), 1.08 ppm (t, J = 7.1 Hz, 3H, NCH2CH3). 13C NMR (125 MHz, CDCl3): δ 163.84, 162.46 (NCO), 143.23, 136.27, 129.98, 129.20, 128.21, 125.66, 121.93, 118.96, 118.30, 111.88, 111.42, 107.52, 52.27 (C6), 48.20 (NC(O)CHaHb), 47.04 (C12a), 40.14 (NCH2CH3), 26.53 (NCHCHaHb), 11.40 ppm (NCH2CH3). IR: 3261.47 (–NH–), 1673.06, 1644.96 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C20H18BrN3O2S: m/z (%) 445 [M++2], 443 [M+], 282 (100%).
Methyl 1-(5-bromo-2-thienyl)-β-carboline-3-carboxylate (25)
A well-stirred solution of the tetrahydro-β-carboline 1 (0.39 g, 1 mmol) in methyl ethyl ketone (10 mL) was refluxed under oxygen atmosphere for 20 h. The solvent was evaporated to dryness under reduced pressure and the product was then purified using flash column chromatography on silica gel, eluting with CH2Cl2.
Yellow powder (0.28 g, 72.8%): Rf = 0.65 (CH2Cl2); mp: 223–225°C; 1H NMR (500 MHz, DMSO-d6): δ 11.91 (s, 1H, NH), 8.86 (s, 1H, CHCN), 8.41 (dt, J = 8.0, 1.0 Hz, 1H, Ar), 7.98 (d, J = 4.0 Hz, 1H, Ar), 7.75 (dt, J = 8.2, 0.9 Hz, 1H, Ar), 7.64 (ddd, J = 8.2, 7.1, 1.2 Hz, 1H, Ar), 7.47 (d, J = 4.0 Hz, 1H, Ar), 7.35 (ddd, J = 8.0, 7.1, 1.0 Hz, 1H, Ar), 3.94 ppm (s, 3H, OCH3). 13C NMR (125 MHz, DMSO-d6): δ 165.35 (COOCH3), 144.55, 141.61, 136.13, 135.11, 131.90, 131.79, 130.14, 129.13, 127.15, 122.00, 120.92, 120.81, 116.75, 115.09, 112.91, 52.16 ppm (CH3). IR: 3326.68 (–NH–), 1739.34 cm−1 (–CO–). HPLC/MS (ESI): Purity 100%, for C17H11BrN2O2S: m/z (%): 388 [M++2] (100%), 386 [M+].
Biology
Phosphodiesterase assay
Five units per milliliter of purified PDE5 (BPS Biosciences) was added to the wells of black 96-well non-binding plates. Immediately, the protein was treated with compound or vehicle control and 50 nM TAMRA-cGMP and 50 nM fluorescein-cAMP (Molecular Devices) were added to each assay well. The plates were incubated for 1.5 h at 30°C. After incubation, IMAP FP phosphodiesterase evaluation assay (Molecular Devices) binding reagent was added to each well and the plates were incubated for an additional 30 min at 30°C. FP was measured according to the manufacturer’s specifications using a Biotek Synergy 4 plate reader.
Experimental design and data analysis
The IC50 value was determined by testing a range of ten concentrations with at least two replicates per concentration. Dose response curves were analyzed using PrismTM 4 software (GraphPad) to calculate IC50 values using a four parameter logistic equation. All in vitro experiments involved dose–response analysis, which was repeated at least twice to confirm reproducibility of IC50 values.
Molecular modeling
To reveal the mode of interaction of 8 with the residues lining the binding pocket of PDE5, a docking experiment was implemented to dock compound 8 into the active site of PDE5 with the program MOE version 2009.10. 8 was constructed into the MOE window, the energy of the compound was minimized and the minimized conformer was saved. The Protein Data Bank crystal structure of PDE5 co-crystallized with tadalafil (1XOZ) was imported into MOE. The structure was protonated and the binding pocket was selected and extended 4.5 Å around the pocket. The tadalafil molecule was then removed and the ligand was loaded into the MOE, where docking into the pocket was launched. The poses from the ligand conformation were generated using alpha triangle, the scoring function used was London dG with no refinement. To ensure more accurate docking procedures, tadalafil was redocked to the binding pocket using the same MOE settings as for compound 8.
Table 2.
PDE5 inhibitory activity of tetrahydro-β-carboline-hydantoins (5–20).
| Compd | R | Chirality | PDE5 % inhibition at 10 μma) | PDE5 inhibition IC50 (μm)b) |
|---|---|---|---|---|
| 5 | –C2H5 | 5S, 11aR | 99 | 0.2 |
| 6 | –C2H5 | 5R, 11aR | 66 | 4.8 |
| 7 | –C2H5 | 5R, 11aS | 78 | 2 |
| 8 | –C2H5 | 5S, 11aS | 99 | 0.16 |
| 9 | –(CH2)3CH3 | 5S, 11aR | 99 | 0.17 |
| 10 | –(CH2)3CH3 | 5R, 11aR | <60 | ND |
| 11 | –(CH2)3CH3 | 5R, 11aS | <60 | ND |
| 12 | –(CH2)3CH3 | 5S, 11aS | 92 | 0.47 |
| 13 | –C–(CH3)3 | 5S, 11aR | 97 | 0.39 |
| 14 | –C–(CH3)3 | 5R, 11aR | <60 | ND |
| 15 | –C–(CH3)3 | 5R, 11aS | <60 | ND |
| 16 | –C–(CH3)3 | 5S, 11aS | 90 | 1 |
| 17 | –CH2–CH=CH2 | 5S, 11aR | 96 | 0.29 |
| 18 | –CH2–CH=CH2 | 5R, 11aR | 67 | 5.4 |
| 19 | –CH2–CH=CH2 | 5R, 11aS | <60 | ND |
| 20 | –CH2–CH=CH2 | 5S, 11aS | 97 | 0.32 |
Calculated from triplicate values.
Calculated from 10 concentrations, each with double replicates.
Footnotes
The authors have declared no conflict of interest.
References
- 1.Francis SH, Turko IV, Corbin JD. Prog Nucleic Acid Res Mol Biol. 2001;65:1–52. doi: 10.1016/s0079-6603(00)65001-8. [DOI] [PubMed] [Google Scholar]
- 2.Mehats C, Andersen CB, Filopanti M, Jin SL, Conti M. Trends Endocrinol Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 3.Conti M, Jin SL. Prog Nucleic Acid Res Mol Biol. 1999;63:1–38. doi: 10.1016/s0079-6603(08)60718-7. [DOI] [PubMed] [Google Scholar]
- 4.Soderling SH, Beavo JA. Curr Opin Cell Biol. 2000;12:174–179. doi: 10.1016/s0955-0674(99)00073-3. [DOI] [PubMed] [Google Scholar]
- 5.Conti M, Beavo J. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 6.Kotera J, Fujishige K, Akatsuka H, Imai Y, Yanaka N, Omori K. J Biol Chem. 1998;273:26982–26990. doi: 10.1074/jbc.273.41.26982. [DOI] [PubMed] [Google Scholar]
- 7.Bender AT, Beavo JA. Neuochem Int. 2004;45:853–857. doi: 10.1016/j.neuint.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 8.Briganti A, Salonia A, Gallina A, Sacca A, Montorsi P, Rigatti P, Montorsi F. Nat Clin Pract Urol. 2005;2:239–247. doi: 10.1038/ncpuro0186. [DOI] [PubMed] [Google Scholar]
- 9.Sebkhi A, Strange JW, Phillips SC, Wharton J, Wilkins MR. Circulation. 2003;107:3230–3235. doi: 10.1161/01.CIR.0000074226.20466.B1. [DOI] [PubMed] [Google Scholar]
- 10.Shin JT, Semigran MJ. Heart Fail Clin. 2010;6:215–222. doi: 10.1016/j.hfc.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takimoto E, Champion HC, Li M, Belardi D, Ren S, Rodriguez ER, Bedja D, Gabrielson KL, Wang Y, Kass DA. Nat Med. 2005;11:214–222. doi: 10.1038/nm1175. [DOI] [PubMed] [Google Scholar]
- 12.Freedman RR, Girgis R, Mayes MD. Lancet. 1999;354:739. doi: 10.1016/S0140-6736(99)03557-6. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Zhang RL, Wang Y, Zhang C, Zhang ZG, Meng H, Chopp M. Stroke. 2005;36:847–852. doi: 10.1161/01.STR.0000158923.19956.73. [DOI] [PubMed] [Google Scholar]
- 14.Puzzo D, Staniszewski A, Deng SX, Privitera L, Leznik E, Liu S, Zhang H, Feng Y, Palmeri A, Landry DW, Arancio O. J Neurosci. 2009;29:8075–8086. doi: 10.1523/JNEUROSCI.0864-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ko IG, Shin MS, Kim BK, Kim SE, Sung YH, Kim TS, Shin MC, Cho HJ, Kim SC, Kim SH, Kim KH, Shin KHDH, Kim CJ. Pharmacol Biochem Behav. 2009;91:629–635. doi: 10.1016/j.pbb.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 16.Han K, Arber N, Yamamoto H, Lim JT, Delohery T, Pamukcu R, Piazza GA, Xing WQ, Weinstein IB. Breast Cancer Res Treat. 1998;48:195–203. doi: 10.1023/a:1005924730450. [DOI] [PubMed] [Google Scholar]
- 17.Whitt JD, Li N, Tinsley HN, Chen X, Zhang W, Li Y, Gary BD, Keeton AB, Xi Y, Abadi AH, Grizzle WE, Piazza GA. Cancer Prev Res (Phila) 2012;5:822–833. doi: 10.1158/1940-6207.CAPR-11-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ding PR, Tiwari AK, Ohnuma S, Lee JW, An X, Dai CL, Lu QS, Singh S, Yang DH, Talele TT, Ambudkar SV, Chen ZS. PLoS ONE. 2011;6:e19329. doi: 10.1371/journal.pone.0019329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Card GL, England BP, Suzuki Y, Fong D, Powell B, Lee B, Luu C, Tabrizizad M, Gillette S, Ibrahim PN, Artis DR, Bollag G, Milburn MV, Kim SH, Schlessinger J, Zhang KY. Structure. 2004;12:2233–2247. doi: 10.1016/j.str.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 20.Zoraghi R, Corbin JD, Francis SH. J Biol Chem. 2006;281:5553–5558. doi: 10.1074/jbc.M510372200. [DOI] [PubMed] [Google Scholar]
- 21.Zoraghi R, Francis SH, Corbin JD. Biochemistry. 2007;46:13554–13563. doi: 10.1021/bi7010702. [DOI] [PubMed] [Google Scholar]
- 22.Mohamed HA, Girgis NM, Wilcken R, Bauer MR, Tinsley HN, Gary BD, Piazza GA, Boeckler FM, Abadi AH. J Med Chem. 2011;54:495–509. doi: 10.1021/jm100842v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dondoni A, Perrone D. Org Syn Coll. 2000;10:140. [Google Scholar]
- 24.Ungemach F, Soerens D, Weber R, DiPierro M, Campos O, Mokry P, Cook JM, Silverton JV. J Am Chem Soc. 1980;102:6976–6984. [Google Scholar]
- 25.Sung J, Hwang KY, Jeon YH, Lee JI, Heo YS, Kim JH, Moon J, Yoon JM, Hyun YL, Kim E, Eum SJ, Park SY, Lee JO, Lee TG, Ro S, Cho JM. Nature. 2003;425:98–102. doi: 10.1038/nature01914. [DOI] [PubMed] [Google Scholar]
- 26.Abadi AH, Gary BD, Tinsley HN, Piazza GA, Abdel-Halim M. Eur J Med Chem. 2010;45:1278–1286. doi: 10.1016/j.ejmech.2009.10.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuzuki S, Honda K, Azumi R. J Am Chem Soc. 2002;124:12200–12209. doi: 10.1021/ja0204877. [DOI] [PubMed] [Google Scholar]
- 28.Castellano O, Gimon R, Soscun H. Energy Fuels. 2011;25:2526–2541. [Google Scholar]