Abstract
Glymphatic transport, defined as cerebrospinal fluid (CSF) peri-arterial inflow into brain, and interstitial fluid (ISF) clearance, is reduced in the aging brain. However, it is unclear whether glymphatic transport affects the distribution of soluble Aβ in Alzheimer’s disease (AD). In wild type mice, we show that Aβ40 (fluorescently labeled Aβ40 or unlabeled Aβ40), was distributed from CSF to brain, via the peri-arterial space, and associated with neurons. In contrast, Aβ42 was mostly restricted to the peri-arterial space due mainly to its greater propensity to oligomerize when compared to Aβ40. Interestingly, pretreatment with Aβ40 in the CSF, but not Aβ42, reduced CSF transport into brain. In APP/PS1 mice, a model of AD, with and without extensive amyloid-β deposits, glymphatic transport was reduced, due to the accumulation of toxic Aβ species, such as soluble oligomers. CSF-derived Aβ40 co-localizes with existing endogenous vascular and parenchymal amyloid-β plaques, and thus, may contribute to the progression of both cerebral amyloid angiopathy and parenchymal Aβ accumulation. Importantly, glymphatic failure preceded significant amyloid-β deposits, and thus, may be an early biomarker of AD. By extension, restoring glymphatic inflow and ISF clearance are potential therapeutic targets to slow the onset and progression of AD.
Keywords: Convective ISF flow, astrocytes, AQP4, CSF, brain ISF clearance, lymphatic system, Glymphatic pathways, clearance, amyloid-β, Alzheimer’s disease
Graphical abstract

Introduction
While the pathogenesis of Alzheimer’s disease (AD), the most common neurodegenerative disease, is still unclear, failure to efficiently eliminate potentially toxic molecules from the interstitium of the aging brain may be a contributing factor (Bateman et al., 2006; Deane et al., 2009; Nedergaard, 2013; Zlokovic, 2008a). At present there is no effective therapy to slow or prevent the biological progression of this devastating disease. Thus, there is no available approach to slow or prevent the ‘AD tsunami’. However, understanding how the brain clears the interstitium of waste products could lead to the discovery of novel targets to help in delaying the onset of AD.
In peripheral organs, the very permeable capillaries and the lymphatic system facilitate rapid clearance of proteins and metabolic products from the interstitial fluid (ISF)(Michel, 1988). However, it is well established that the CNS vascular barriers restrict trafficking of polar molecules into and out of the brain, and the CNS lacks conventional lymphatic vessels (Abbott et al., 2006; Bradbury, 1985; Zlokovic, 2008b). Despite these features there is rapid clearance of metabolic waste products in the normal brain (Abbott et al., 2006; Bradbury, 1985; Deane et al., 2009; Nedergaard, 2013; Zlokovic, 2008b). The glymphatic fluid transporting pathways act as a ‘pseudo–lymphatic system’, and have been described as a brain-wide macroscopic system for the rapid clearance of waste from the interstitium (Iliff et al., 2012; Nedergaard, 2013). The glymphatic transport pathways consist of 1) the flow of cerebrospinal fluid (CSF) into brain via the peri-arterial space, as suggested by earlier observations (Rennels et al., 1985), astrocytic specific aquaporin 4 (AQP4)-mediated convective flow of ISF through the interstitium, and 3) clearance of molecules via the perivascular space surrounding the deep cerebral veins, and via the cervical lymph nodes (Boulton et al., 1996; Bradbury and Westrop, 1983; Brinker et al., 1997; Iliff et al., 2012; Kida et al., 1993; Plog et al., 2015; Yamada et al., 1991). AQP4, water channels, are present at high levels at the astrocytic endfeet lining the interfaces between brain tissue and fluid (Haj-Yasein et al., 2011; Mathiisen et al., 2010). Glymphatic transport and waste removal are suppressed with aging (Kress et al., 2014), which may contribute to the accumulation of amyloid-β (Aβ) in brain. In the aging brain AQP4 delocalization from the endfeet to the soma of astrocytes is, in part, associated with glymphatic failure. While amyloid-β accumulation is known to cause AQP4 delocalization (Yang et al., 2011), it is unclear whether the glymphatic transport is disrupted in mouse models of AD.
Herein, we show that glymphatic transport is indeed suppressed in old APP/PS1 mice with prominent amyloid-β deposits, but most importantly, we also observe that glymphatic clearance is reduced prior to presence of substantial amyloid-β deposits in younger APP/PS1 mice when compared to age-matched control mice. Thus decline of glymphatic fluid transport may serve as an early biomarker of AD, and treatments directed at restoring normal glymphatic transport and waste removal from the brain may become a novel preventive or therapeutic approach for this dreadful disease.
Materials and Methods
Mice
Mice [of either sex, C57BL/6J, Tg(Cspg4-Ds Red.T1)1Akik/J (NG2-DsRed reporter mice), APPswe/PS1dE9 (APP/PS1) and their littermate controls] were purchased from Jackson Laboratory (Bar Harbor, ME, USA). NG2-DsRed reporter mice are on a C57BL/6J genetic background. In mice older than 6–8 weeks, NG2-Ds-red expression is restricted to peri-vascular smooth muscle cells (arteries) and pericytes (Iliff et al., 2012; Zhu et al., 2008). The APP/PS1 mice are double transgenic mice expressing a chimeric mouse/human amyloid precursor protein (Mo/HuAPP695swe) and a mutant human presenilin 1 (PS1-dE9) both directed to CNS neurons. Mice were housed in the vivarium facilities at the University of Rochester, School of Medicine and Dentistry. All animal studies were performed according to the NIH guidelines using protocols approved by the University of Rochester Committee on Animal Resource. Mice were housed depending on the experiment, and maintained on a 12:12 light/dark schedule (6 AM : 6PM) with food and water ad libitum. Mice were anesthetized with a mixture of ketamine (100 mg/kg) and xylazine (10 mg/kg) by intraperitoneal injection (IP).
Materials
Aβ40-HiLyte Fluor 488 and Aβ42-HiLyte Fluor 488 were obtained from AnaSpec (Fremont, CA, USA), and prepared as a 0.05% solution in artificial CSF (aCSF) following the manufacturer’s instructions. Samples were diluted in aCSF, aliquoted, stored at −20°C and used within two days. HFIP (1, 1, 1, 3, 3, 3-hexafluoro-2-propanol) treated lyophilized Aβ40 and Aβ42 were also obtained from AnaSpec, dissolved in ammonium hydroxide (1%) at room temperature for 15 minutes with intermittent mixing, sonicated for 5 minutes, diluted in aCSF to 20 μM, aliquoted and stored at −80°C. Each aliquot was used immediately and only once. Cascade blue (CB) labeled dextran 10 kDa (fixable), fluorescein labeled dextran 40 kDa (fixable) and Texas Red labeled dextran 3 kDa (fixable) were obtained from Molecular Probe (Eugene, OR, USA) and dissolved in aCSF (2% solution). Lectin was obtained from Vector Laboratory (Lycopersicon esculentum (tomato); Burlingame CA, USA). 125I-Aβ40 and 14C-inulin were obtained from PerkinElmer (Waltham, MA, USA). We used 125I-Aβ40 without added aprotinin, a potential inhibitor of LRP1-mediated transport. ELISA kits for Aβ40 (KHB 3481) and Aβ42 (KHB 3441) were obtained from Invitrogen (Camarilla, CA, USA) and Aβ oligomer ELISA kit (BEK-2215-1P) from Biosensis (Termecula, CA, USA).
Intracisternal injections
Mice were anesthetized as indicated above, fixed to a stereotactic frame, cisterna magna exposed and cannulated with a 30G needle (Iliff et al., 2012; Kress et al., 2014). Fluorescent tracers (Aβ40 or 42 HiLyte 488, 5 μL of a 10 μM solution in aCSF, containing CB tagged dextran (10 kDa at 1%) were injected at 1μl/min using a Hamilton syringe connected to a Micro Syringe pump controller (Micro 4; World Precision Instruments, Inc., Sarasota, FL, USA). We used 10 μM Aβ as this was the concentration used in earlier studies on the effect of Aβ on cerebrovascular reactivity (Niwa et al., 2001). In a separate series of experiments, unlabeled Aβ (2 μM) was also intracisternally injected (5 μL at 1 μL/min). To quantify the glymphatic inflow, 125I-Aβ40, at a very low concentration (10 nM, due to the greater resolution of radioactivity analysis), and 14C-inulin (6 kDa, 1.0 μCi) were intracisternally injected at 1 μL/min for 5 minutes. We used a small volume and a slow rate of injection (5 μL at 1 μL/min), which does not significantly increase the intracranial pressure (Yang et al., 2013), thereby, minimizing CSF reflux into the cerebral ventricles from the cisterna magna, as indicated by the lack of injected fluorescent molecules in the ependymal layer (Iliff et al., 2012).
Aβ diffusion from arteries
A custom ImageJ plugin program was written to analyze Aβ and CB-dextran transport by convective fluid flow as a function of distance from the vessel wall. In these studies, arteries were identified by DsRed expression in the NG2 DsRed reporter mice, and veins were DsRed-negative. The digital images of photomicrographs were analyzed using ImageJ. The plugin plotted the intensity value of the fluorescence with respect to distance when a line is drawn across the blood vessel identified by the NG2-DsRed. The person analyzing the images and data was blinded to the experimental condition.
Immunohistochemistry
Immunohistochemistry was performed as reported (Rangroo Thrane et al., 2013b; Wang et al., 2012). Mice were transcardially perfused with ice-cold phosphate-buffered saline (PBS, pH 7.4, Sigma-Aldrich, St. Louis, MO, USA) followed by 4% paraformaldehyde (PFA; Sigma-Aldrich). Free floating brain sections (100 μm) were immunostained for astrocytes (GFAP), AQP4, neurons (NeuN), human Aβ (6E10) and Aβ oligomer (A11). The tissue was blocked with 7% donkey serum for 1 hour and the primary antibodies incubated overnight. The primary antibodies were rabbit human specific anti-Aβ (6E10; 1:200, BioLegend, San Diego, CA, USA), goat anti-aquaporin 4 (1:200, cs9888; Santa Cruz Technology, Inc., Dallas, Texas, USA), mouse anti-GFAP (1:500, MAB360; Millipore, Billerica, MA, USA), chicken anti-NeuN (1:500, Abcam 134014. Abcam Inc., Cambridge, MA. USA), anti-Aβ oligomer (A11, 5 μg/ml; Rockland, Limerick, PA, USA). Then, Fluor-conjugated secondary antibodies (Life Technologies, Carlsbad, CA, USA 1:500) were added and incubated for 2 hours at room temperature (RT). Immunofluorescence was visualized using an epifluorescence microscope (Olympus BX51) with CellSens software, and a Bio-Rad MRC500 confocal scanning head attached to an inverted microscope (IX81, Olympus, Tokyo, Japan) controlled by Olympus Fluoview 500 software. The person analyzing the images and data was blinded to the experimental conditions.
Effect of Aβ on glymphatic CSF inflow into brain
We used 10 μM Aβ40 or Aβ42 as reported in brain superfusion studies (Niwa et al., 2001). In the glymphatic distribution of Aβ experiments (first series), 5 μL of aCSF, containing Aβ (10 μM) and dextran (1%; fluorescein 40 kDa and Texas Red, 3 kDa, both lysine fixable; Molecular Probe), were injected, intracisternally, at 1 μl/min and after 30 minutes the brain fixed by PFA perfusion and 100 μm brain sections analyzed. In a second series of experiments, the Aβ (5 μL of the 10 μM solution) was pre-infused over 40 minutes prior to the infusion of the Aβ and fluorescence dextran solution, as above. The degree of tracer permeation throughout the brain was evaluated ex vivo by an epifluorescence microscopy. Multichannel whole-slice montages were acquired via the CellSens software (version 1.12, Olympus CellSens Software) in the green and red emission channels, at a magnification of 4x. Exposure and gain were chosen based on the control brain slices and fixed for all experimental groups. To quantify the extent of tracer permeation throughout the brain the whole-slice montages were analyzed using ImageJ (National Institutes of Health, imagej.nih.gov/ij/). For each slice, fluorescence emission channels were split and a whole-brain ROI was defined. The pixel area of positive fluorescence (threshold pixel intensity >80 A.U) for both green and red channel) was calculated for 6 slices from each brain and the average expressed as a percentage of the brain. The person analyzing the images and data was blinded to the experimental condition.
Dot blot assay
Aβ40 and Aβ42 samples were prepared as described above, and diluted to 10 μM. Each sample was separated into two: one before centrifugation (before) and the other for centrifugation (after). For centrifugation, samples were spun at 100,000 g at 4°C for 1 hour to separate fibrils. Supernatants and the un-centrifuged samples were blotted onto nitrocellulose membranes (Bio-rad; 10μL per dot). The membranes were allowed to dry for 1 hour at RT and then blocked in 5% nonfat dry milk, containing 50 mM Tris-HCl, 0.5 M NaCl, 0.05% and Tween-20, at pH 7.4 (TBS-T) for 1 hour at RT. The membranes were then incubated with one of the following primary antibodies: mouse anti-amyloid-β 1–16 from clone 6E10 (BioLegend, 830003, 1:1000), rabbit anti-amyloid-β oligomers (A11, Rockland, 200-401-E88, 1:1000) or rabbit anti-amyloid-β fibrils (clone M87, Millipore, MABN638, 0.1 μg/mL) for 1 h at RT in TBS-T. Membranes were then washed three times for 10 min each in TBS-T prior to incubating with either a goat anti-mouse horseradish peroxidase (HRP; Santa Cruz, sc-2031, 1:300)- or goat anti-rabbit HRP- conjugated (Santa Cruz, sc-2030, 1:300) secondary antibody for 1 h at RT. After washing, the blot was developed using Western Lightning Plus-ECL (Perkin Elmer) for 2–5 min and the film was exposed for 30 seconds. Dots were scanned and analyzed with the Dot Blot Analyzer plugin for ImageJ (NIH). The percent of Aβ species in each sample was estimated (since different antibodies were used for each amyloid-β form) by using the following formula: Total Aβ (6E10) = monomeric Aβ + oligomeric Aβ (A11) + fibrillar Aβ (M87).
Brain clearance
To quantify solute inflow into brain from CSF and clearance from the brain, radio-labeled tracers were used since the data generated for Aβ clearance were similar using radio-labeled molecules and by using ELISA (Bell et al., 2007). 125I-Aβ40 (10 nM) and 14C-inulin (6 kDa, 1.0 μCi) were stereotaxtically microinjected into the right frontal cortex, as we recently reported (Xie et al., 2013). Briefly, a stainless steel guide cannula (Plastic One, Roanoke, VA, USA) was implanted stereotaxically into the left frontal cortex of anesthetized mice (2% isoflurane) with the coordinates of the cannula tip at 1.0 mm anterior and 3.5 mm lateral to the bregma, and 1.5 mm below the surface of the brain. Animals were allowed to recover after surgery and the experiments performed 18–24 hrs after the guide tube cannulation, as reported (Deane et al., 2004). In each mouse, a small volume of aCSF (0.5 μL), containing 125I-Aβ40 (10 nM) and 14C-inulin (1.0 μCi), was simultaneously injected (33 GA cannula, Plastic One) into the brain ISF over 5 minutes. At the end of the experiments (30 min) the brain was removed and prepared for radioactivity analysis. Samples were first counted for gamma radioactivity (125I-Aβ40) using a Wizard Automatic Gamma Counter (Wallac 1471) and counts corrected for trichloroacetic acid (TCA) precipitability. All samples were solubilized in 0.5 ml tissue solubilizer (Solvable, Perkin Elmer) overnight followed by the addition of 5 ml of scintillation cocktail (Ultima Gold, PerkinElmer). Samples were then analyzed in a liquid scintillation counter for 14C- radioactivity (LS6500 Multi-purpose Scintillation Counter (Beckman Coulter, GA, USA). Calculations: The percentage of radioactivity remaining in the brain (% recovery) after microinjection was determined as % recovery in brain = 100 x (Nb/Ni), where, Nb is the radioactivity remaining in the brain at the end of the experiment and Ni is the radioactivity injected into the brain ISF, i.e., counts per minute (cpm) for 125I- (TCA-precipitable) and the disintegration per minute (dpm) for 14C-. The TCA precipitable 125I-Aβ40 in brain was unchanged compared to the injectate (Deane et al., 2008; Deane et al., 2004). Total clearance was determined as 100-% recovery. 14C-inulin was used as an inert polar molecule which is neither transported across the BBB nor retained by the brain, and thus, its clearance provides a measure of the ISF bulk flow only.
Lectin administration
Lectin (Lycopersicon esculentum (tomato); Vector Laboratory Burlingame CA, USA) was administered during the cardio-perfusion stage of the experiment. The mice were cardio-perfused with cold PBS containing the lectin (0.02 mg/ml) at 2 ml/min for 10 min. This was then followed by perfusion with PFA (4% in PBS), as described above.
Methoxy-X04 administration
Methoxy-X04 (Tocris Bioscience, Bristol, UK) was administered intraperitoneally (IP; 10 mg/kg) and after 60 minutes Aβ40 was intracisternally injected. The brain was analyzed after an addition 30 minutes.
Brain extraction
Frozen cerebral cortex (150 mg) was homogenized in ice-cold TBS buffer (1 ml; 20 mM Tris-HCl, 150 mM NaCl, pH 7.4), containing complete protease inhibitor (Roche Applied Sciences), and centrifuged at 16,000 g for 30 minutes at 4°C. The supernatant was used to determine levels of soluble Aβ. This pellet was immediately re-suspended in 5 M guanidine-HCl, sonicated, mixed for 3 hrs, centrifuged at 10,000 g for 30 minutes at 4°C and the supernatant collected and used to determine levels of insoluble Aβ. For soluble Aβ oligomers, frozen cerebral cortex was homogenized in TBS containing 1% Triton-X and complete protease inhibitor and centrifuged at 16,000 g for 30 minutes at 4°C. The supernatant was used to determine levels of soluble Aβ oligomers.
Aβ ELISA
Levels (pmol/g brain tissue) of human Aβ40, Aβ42 and soluble Aβ oligomers were determined using ELISA kits [Aβ40 (KHB 3481) and Aβ42 (KHB 3441); Invitrogen (Camarilla, CA, USA] and following the manufacturers’ instructions. Levels of Aβ were determined from the standard curves. Since soluble Aβ oligomers vary in size and number of oligomers the unit is arbitrary and is indicated as equivalent to Aβ. Brains from wild type mice were used as controls and the signal subtracted from that obtained from the APP/PS1 mice.
Statistical analysis
Data were analyzed by analysis of variance (ANOVA) followed by post hoc Tukey test, or Student’s t test. The differences were considered to be significant at p < 0.05. All values were expressed as mean ± SEM.
Results
Earlier reports have shown that intracisternal injection of tagged molecules, such as inulin, dextran and ovalbumin, enter the brain via the peri-arterial space and are cleared by convective driven glymphatic transport through the interstitium (Iliff et al., 2012; Kress et al., 2014). However, it is unclear whether glymphatic transport characteristics of Aβ40 and Aβ42 are similar. This is important to establish because, in contrast to Aβ40, Aβ42 is a CSF biomarker of AD and a toxic molecule (Blennow, 2004; Fagan et al., 2006; Fagan et al., 2014; Zetterberg et al., 2007). We simultaneously injected, intracisternally, Aβ40 HiLyte Fluor 488 (Aβ40-488) or Aβ42 HiLyte Fluor 488 (Aβ42-488) and an inert reference molecule, labeled dextran 10 kDa (CB-dextran), and mapped their distribution in brain sections (Figure 1A). NG2-dsRed reporter mice were used to clearly identify arteries, as reported (Iliff et al., 2012). Interestingly, the distribution of CSF-derived Aβ42-488 within brain was mostly confined to the arteries when compared to Aβ40-488, while CB-dextran distribution was similar in both experiments (Figure 1B–J). The diffusion of Aβ40 from the brain surface and glymphatic transport along the penetrating arteries into brain was more efficient than Aβ42 while CD-dextran transport was similar in both Aβ40 and Aβ42 experiments (Figure 1B–C). Accordingly, the CB-dextran distribution areas were grouped together and compared to that of Aβ40 and Aβ42 (Figure 1E–F). While Aβ40-488, Aβ42-488 and CB-dextran were all present along the penetrating arteries (Figure 1G–H), glymphatic transport of Aβ42 within the parencyhma was restricted (Figure 1I–J). When compared to Aβ40, the reduced Aβ42 glymphatic inflow may reflect its greater propensity to oligomerize into larger fibrils (Supplementary Figure 1), which would reduce Aβ42 inflow, since molecular weight is known to limit the brain-wide glymphatic transport (Iliff et al., 2012). In addition, Aβ/receptor interactions may also interfere with Aβ glymphatic transport compared to the inert reference dextran molecule. To confirm that the Alexa 488 tagging of Aβ was not altering the glymphatic transport of Aβ, we injected untagged Aβ40 or Aβ42 (2 μM), intracisternally, and mapped its distribution pattern by immunolabeling with a human specific anti-Aβ antibody (6E10), which does not reacts with mouse Aβ (Supplementary Figure 2). In this setting, Aβ40 and Aβ42 were also present along the penetrating arteries, within the parenchyma, and associate with brain cells (Aβ40>Aβ42), such as neurons (Figure 2 A–P). Again, Aβ42 distribution was almost totally restricted to the penetrating artery when compared to Aβ40 (Figure 2 E/H and M/P), similar to that shown in Figure 1 H–J). The association of CSF-derived Aβ40 with cells within the parenchyma may reflect their expression of Aβ receptors (Deane et al., 2004; Herz, 2009; Holtzman et al., 2012; Kanekiyo et al., 2012).
Figure 1. Fluorescently-tagged Aβ40 and Aβ42 enter brain along the peri-arterial space and the pial membrane after intracisternal delivery.
A) Schematic diagram showing the injection site. Representative images of Aβ40 and Aβ42 HiLyte Alexa 488 (B); cascade blue labeled dextran (CD-Dextran, a reference molecule; 10 kDa; C) and NG2-DsRed labeling of smooth muscle cells (D). E) Quantification of the areas of Aβ40, Aβ42 and CB-Dextran distribution. F) Standardization of Aβ distribution area as a percentage of CB-Dextran area. Peri-arterial inflow of CB-dextran and Aβ (G–J). Panel H shows the white boxed areas in G. White arrow head in panel H indicate the vessel magnified in panel I. J) Distribution of Aβ40 or Aβ42 (green) and CB-Dextran (blue) from an artery (red) shown in panel I. Vessel lumen in gray. Values are mean ± SEM, N=5. Scale bars: B, 2 mm; H, 500 μm; I, 100 μm. Aβ40-488 or Aβ42-488 and CD-dextran were intracisternally injected in NG2-DsRed reporter mice (2–3 months old) and the animals fixed with PFA 15 minutes later followed by preparation of vibratome sectioning and analysis of tracer distribution in brain sections.
Figure 2. Unlabeled human Aβ binds to cells after intracisternal delivery.
A–D). Immunolabeling of CSF injected Aβ40 in brain (A), GFAP+-astrocytes (B),NeuN+-neurons (C) and merged images (D). E–H) Boxed area in panel A showing Aβ40 association with cells, mainly neurons (white arrows). I–L) Immunolabeling of Aβ42 (I), GFAP+-astrocytes (J), NeuN+-neurons (K) and merged images (L). M–P) Boxed area in panel I showing CSF Aβ42 mainly restricted to vessels. Representative images from 3 wild type mice (2–3 months old). Scale bars: A and I, 0.8 mm; E and M, 25 μm. Aβ40 or Aβ42 was intracisternally injected and after 30 minutes, to allow for cellular uptake, the brains were fixed and analyzed by immunolabeling.
Topical application of Aβ (μM range) is known to reduce cerebral blood flow (CBF)(Niwa et al., 2001), and this could alter glymphatic peri-arterial inflow of CSF. However, in our studies neither Aβ40 nor Aβ42 (10 μM; 5μL) appeared to affect glymphatic transport of dextran into brain (Figure 3 A–F). Nevertheless, we tested the effect of Aβ pre-infusion, as reported (Niwa et al., 2001), on glymphatic transport (Figure 3G). For these experiments, Aβ was pre-infused for 40 minutes, and Aβ40, but not Aβ42, reduced the glymphatic transport of the dextran (3 kDa and 40 kDa) into brain (Figure 3 H–L). Specifically, Aβ40 reduced CSF inflow into the cortex but not into the hippocampus due, perhaps, to the low but detectable levels present in this region (Supplementary Figure 3). Collectively, our data show that soluble Aβ in CSF enters brain via the penetrating arteries and, thus, may contribute to the accumulation and toxicity of amyloid-β, in the long-term. We evaluated glymphatic transport in the APP/PS1 mice with and without prominent amyloid-β deposits in comparison to control littermates.
Figure 3. Effect of Aβ on glymphatic inflow of CSF into brain.
A) Schematic diagram of the experimental design. B) Representative images of vehicle (Veh) treated mice and mice treated with Aβ40 (C) and Aβ42 (D). Quantification of the distribution areas of Texas Red Dextran 3 kDa (E) and FITC-Dextran 40 kDa (F) from images as in panels B–D. CSF containing Aβ40 or Aβ42 (10 μM;5 μL), FITC-Dextran 40 kDa (1%) and Texas Red Dextran 3 kDa (1%) were intracisternally injected and after 30 min the PFA fixed brain sections were analyzed. Values are mean ± SEM, N=5–6 mice per group. G) Schematic diagram of the experimental design. Representative images of vehicle treated mice (H) and mice treated with Aβ40 (I) and Aβ42 (J). Quantification of the distribution areas of Texas Red Dextran 3 kDa (K) and FITC-Dextran40 kDa (L). First, 5 μL of CSF containing Aβ40 or Aβ42 (10 μM) was pre-infused intracisternally over 40 min, followed by CSF containing Aβ40 or Aβ42 (10 μM), FITC-Dextran40 kDa and Texas Red Dextran 3 kDa (1%), and the PFA fixed brain sections were analyzed. Values are mean ± SEM, N=6–9 mice (2–3 months old) per group.
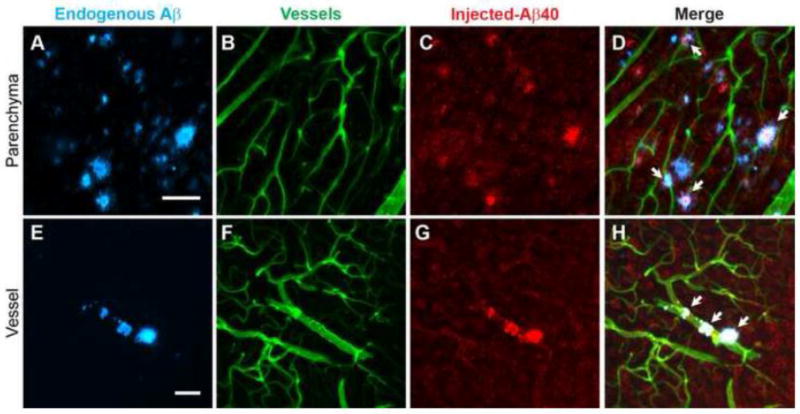
We quantified the glymphatic inflow of Aβ and inulin from CSF into brain using a radiolabeled approach, which allows the use of tracer levels of Aβ (Deane et al., 2012; Iliff et al., 2012; Xie et al., 2013; Zlokovic, 2008a). Artificial CSF, containing 125I-Aβ40 (monomeric, 10 nM) (Deane et al., 2004) and 14C-inulin (1.0 μCi; the reference molecule), was injected intracisternally and after 30 minutes the brain collected and analyzed for radioactivity. First, the data showed that the inflow of Aβ and inulin was reduced with aging, as reported for other molecules (Kress et al., 2014). Second, inflow of Aβ40 and inulin was reduced in the APP/PS1 mice when compared to their controls but more so in the mice with prominent amyloid-β deposits i.e., old mice (2-fold) compared to young mice (1.5-fold; Figure 4A–C). We also quantified Aβ clearance from the frontal cortex using the same radiolabeled approach (Deane et al., 2012; Iliff et al., 2012; Kress et al., 2014; Singh et al., 2013; Xie et al., 2013). CSF (0.5 μL) containing 125I-Aβ40 (monomeric, 10 nM) and 14C-inulin (1.0 μCi) was microinjected into the frontal cortex and after 30 minutes the brains were collected for analysis of the remaining radioactivity (Figure 4D). While the clearance of both Aβ and inulin was reduced in the APP/PS1 mice when compared to their controls, the effect was greater for inulin (2-fold) compared to Aβ (1.2-fold; Figure 4E–F), suggesting that reduced ISF convective flow may promote greater Aβ/receptor interactions, due to slower transit time, and possibly influencing its clearance. Aβ clearance from brain is due to receptor mediated transport at the blood-brain barrier (BBB) and bulk flow of ISF, which are reduced with aging (Banks et al., 2003; Deane et al., 2004; Jaeger et al., 2009; Kress et al., 2014; Zlokovic, 2008a). Since CSF-derived Aβ may interact with the amyloid-β deposits, Aβ40-488 was injected intracisternally and its association with endogenous amyloid-β mapped in the older APP/PS1 mice (Figure 5). A clear interaction between the CSF-derived Aβ40 and the endogenous Aβ plaques within the parenchyma and vasculature was observed (Figure 5A–H), suggesting that Aβ40 adhering to existing amyloid-β plaques may promote further amyloid-β accumulation. Collectively, by extension, the data show that glymphatic activities are disrupted in AD, and before the presence of significant amyloid-β deposits as seen in the younger APP/PS1 mice compared to their controls (Figure 4).
Figure 4. Reduced CSF inflow and ISF clearance in APP/PS1 mice.
A) Schematic diagram showing the injection site of the radiolabeled molecules. B–C) Inflow of 125I-Aβ40 and 14C-inulin from CSF into brain in young (3–4 months old; B) and old (C; 12–13 months old) APP/PS1mice (APP) compared to their controls (C). 125I-Aβ40 (10 nM) and 14C-inulin were injected intracisternally (1 μL/min for 5 min) and after 30 minutes the brain analyzed for radioactivity. N=4. D) Schematic diagram showing the cortical injection site. E–F) Clearance of 125I-Aβ40 and 14C-inulin in young (E; 6–8 months old) and old (F; 12–18 months old) APP/PS1mice compared to their controls. 125I-Aβ40 (10 nM) and 14C-inulin were microinjected intracortically (0.5 μL; 0.1 μL/min for 5 min) and after 30 minutes the brain analyzed for radioactivity. N=3–6. Values are mean ± SEM.
Figure 5. Intracisternal injected fluorescently labeled Aβ40 is associated with amyloid-β plaques in APP/PS1 mice.
A–H) Endogenous Aβ detected with methoxy-X04 (A, E), cerebrovasculature delineated with intravascular injected lectin (B,F), CSF Aβ40-488 is present in brain (colored red) (C,G) and merged images (D,H) showing co-localization of the intracisternal injected Aβ40 with endogenous amyloid-β (white areas) in brain parenchyma and vessels. Representative images from 3 mice (7–9 months old). White areas show the co-localization of methoxy-X04 labeled endogenous amyloid-β plaques and injected Aβ40-488. Scale bars: A and E= 50 μm. Methoxy-X04 was administered intraperitoneally (IP; 10 mg/kg) and after 60 minutes Aβ40 was intracisternally injected, and the brain analyzed after an addition 30 minutes.
It was shown that amyloid-β accumulation is linked to the loss of AQP4 polarization at the astrocytic endfeet in the TgArcSwe mouse model of AD (Yang et al., 2011). Our recent studies also found that the loss of AQP4 polarization at the endfeet processes of reactive astrocytes suppresses glymphatic transport (Iliff et al., 2014; Kress et al., 2014). Here, we show that astrocytes were in a reactive state in the vicinity of amyloid-β deposits in APP/PS1 mice (Supplementary Figure 4A–F). This condition is associated with loss of AQP4 polarity and suppression of glymphatic transport (Iliff et al., 2014; Kress et al., 2014). Interestingly, soluble Aβ oligomers, a more toxic form of Aβ than amyloid-β plaques (Selkoe, 2001), were also observed in the vicinity of AQP4/GFAP-positive astrocytes along vessels but not in the parenchyma (Supplementary Figure 5A–H). Since Aβ species may disrupt glymphatic transport their levels were also determined. Compared to the younger mice, the levels of soluble and insoluble Aβ40 and Aβ42 were increased by about 2-fold in the old mice (Figure 6A–D), while the levels of soluble Aβ oligomers were considerably increased (6-fold) in the older mice (Figure 6E), as reported (Bruggink et al., 2013). Thus soluble Aβ oligomers may also contribute to the progressive failure in glymphatic transport in the aging brain. Figure 7 shows a schematic diagram of our working model of glymphatic fluid of Aβ in AD brains.
Figure 6. Levels of Aβ40, Aβ42 and oligomers are increased with age in the APP/PS1 mice.
Levels of soluble Aβ40 (A) and Aβ42 (B), insoluble Aβ40 (C) and Aβ42 (D) and soluble Aβ oligomers (E) in cortical brain tissues of young (3–4 months old) and old (12–13 months old) mice. N=4–6 mice per group. Values are mean ± SEM.
Figure 7. Working model for the glymphatic distribution of Aβ.
Aβ from the CSF enters brain (Aβ40>Aβ42) via the peri-arterial space and associated with parenchymal cells and amyloid β deposits. Aβ42 is mainly confined to the peri-arterial space due to, possibly, to the formation of fibrils, which is larger in size.
Discussion
Aβ is released during neuronal activity and is known to accumulate in the aging brain due to inefficient clearance from the ISF in animals and in human subjects (Banks et al., 2003; Cirrito et al., 2005b; Deane et al., 2009; Deane et al., 2004; Kress et al., 2014; Mawuenyega et al., 2010; Shibata et al., 2000; Zlokovic, 2008a). While accumulation of amyloid-β as extracellular deposit is one of the hallmarks of AD, the role of amyloid-β in the pathogenesis of AD is still unclear since amyloid-β is also present in the brain of non-AD subjects (Herrup, 2015; Musiek and Holtzman, 2015). Nevertheless, it is undisputed that continuous removal of Aβ from the aging brain is important to prevent the accumulation of Aβ neurotoxins.
It is believed that Aβ in CSF is derived mainly from brain (Fagan and Holtzman, 2010). Thus, CSF levels of Aβ42 are used as a biomarker of AD, which is reduced as AD progresses presumably due to its accumulation within brain (Blennow, 2004; Fagan et al., 2014; Zetterberg et al., 2007). However, the fate of CSF Aβ is unclear. The ultimate drainage of CSF into blood facilitates the clearance of soluble molecules, including Aβ (Abbott et al., 2006; Bradbury, 1985; Iliff et al., 2012; Johanson et al., 2008; Pollay, 2010). However, our data demonstrate that CSF may serve as a medium for the delivery of Aβ to brain via the glymphatic inflow pathway, the peri-arterial space, in the same direction as blood flow, as reported for other solutes, such as dextran and ovalbumin (Iliff et al., 2012), lipophilic molecules (Rangroo Thrane et al., 2013a), tau (Iliff et al., 2014) and apoE (Deane et al., 2015). Earlier reports suggested that Aβ transport along the peri-vascular space occurs in the opposite direction to blood flow (Hawkes et al., 2011; Rolyan et al., 2011; Thal et al., 2007; Weller et al., 1992). While it is possible that Aβ trafficking in the perivascular space may be bidirectional, more investigation will be needed to fully clarify this. We speculate, that circulation of Aβ via the glymphatic fluid transporting system may contribute to the formation of amyloid-β plaques in the long-term, particularly to cerebral amyloid angiopathy (CAA) and ultimately to lower CSF Aβ42 levels observed in progressing AD. Intriguingly, the greater inflow of Aβ40 compared to Aβ42 along the arteries could also explain the Aβ40 and Aβ42 composition (Aβ40>Aβ42) in cerebral amyloid CAA (Kumar-Singh, 2008; Van Dorpe et al., 2000). The concentration of Aβ40 in CSF is greater than that of Aβ42 in both normal and AD subjects with an Aβ42/Aβ40 ratio of 0.3 and 0.17, respectively (Oe et al., 2006; Schoonenboom et al., 2005). Since glymphatic fluid transport from CSF into brain depends on bulk flow the amount of Aβ entering brain would reflect the concentration of soluble Aβ species in CSF.
Interestingly, the differences in glymphatic distribution of Aβ in brain (Aβ40>Aβ42) corroborate earlier reports on their respective ISF clearance pattern, which demonstrated that Aβ40 elimination is greater than that of Aβ42 (Banks et al., 2003; Deane et al., 2004). The restricted distribution of Aβ within brain when compared to dextran (cascade blue) may reflect Aβ/Aβ receptor interactions, which promote cellular interaction, retention within brain or clearance (Deane et al., 2004; Kanekiyo and Bu, 2014; Kanekiyo et al., 2013). Multiple cell types (e.g., vascular smooth muscle cells, endothelial cells, astrocytes, microglia and neurons) express several types of Aβ receptors, such as low-density lipoprotein receptor-related protein-1 (LRP1), receptor for advanced glycation end products (RAGE) and α7 nicotinic acetylcholine receptor (Clifford et al., 2008; Deane et al., 2005; Kanekiyo and Bu, 2014; Kanekiyo et al., 2013). As Aβ flows along the perivascular space, capillaries and veins interaction with LRP1, for example, may lead to cellular uptake and transcytosis into blood (Cirrito et al., 2005a; Deane et al., 2005; Deane et al., 2004; Kanekiyo et al., 2012). Aβ40 clearance is rapid since 60% of the injected dose is eliminated within 30 minutes (Deane et al., 2004; Xie et al., 2013). This may explain the low levels of Aβ40 present in brain after intracisternal injection. Other cells within the brain parenchyma also express receptors, which could interact with Aβ and promote uptake and retention (Kanekiyo and Bu, 2014; Kanekiyo et al., 2013). The unexpected reduced levels of Aβ42 entering brain from the CSF is likely due to higher levels of fibrils since its more prone to oligomerization when compared to Aβ40 (Selkoe, 2001). Thus, the lower levels of monomeric Aβ42 compared to Aβ40 may explain its reduced brain-wide distribution. Earlier studies have documented that molecular weight inversely controls glymphatic transport (Iliff et al., 2012). Interestingly, pre-treatment with Aβ40, but not Aβ42, caused suppression of glymphatic CSF inflow, which may contribute to the long-term effect of Aβ on the suppression of glymphatic transport with aging (Kress et al., 2014) and in AD. Aβ40 in CSF or blood reduces CBF by inducing vasoconstriction (Niwa et al., 2001; Paris et al., 2000; Suo et al., 1998) due to interaction with receptors, such as RAGE (Deane et al., 2003), generation of free radicals (Niwa et al., 2001) and proinflammatory molecules (Paris et al., 2000). Therefore, the increase in Aβ40 levels prior to substantial amyloid-β deposits may contribute to the reduction of glymphatic fluid transport. Thus, the distribution of CSF-derived Aβ into brain may depend on their species and on the duration of Aβ40 exposure.
The interaction of CSF-derived Aβ with existing amyloid-β deposits would also prevent efficient Aβ clearance by promoting further oligomerization and the formation of toxic Aβ species. The oligomerization of Aβ is a progressive process in which the monomeric Aβ (Aβ42≫Aβ40) oligomerize into various soluble oligomers (low but varying molecular weights), which eventually progress to fibrils and plaques (Selkoe, 2001). The soluble oligomers are believed to be the more toxic Aβ species since they correlate better with neurotoxicity in vivo, and are more toxic than fibrils to neurons in culture (Kayed et al., 2010; Kayed et al., 2003; Lue et al., 1999; McLean et al., 1999; Walsh et al., 2002). Aβ42 present in brain ISF may, accordingly, result in formation of more neurotoxic Aβ oligomers (Selkoe, 2001). While many Aβ species may cause glymphatic dysfunction (i.e.,CSF inflow and ISF clearance) in the aging brain, our data suggest that the soluble Aβ oligomers, which also co-localized with AQP4/GFAP-positive astrocytes, may contribute to this process by, possibly, causing delocalization of AQP4 from the endfeet to the soma of astrocytes. In addition, amyloid-β deposits markedly suppressed ISF clearance of inulin. Since the size of the interstitial space may influence ISF convective flow (Xie et al., 2013), extracellular amyloid-β aggregation may impede this solute transport due to reduced extracellular volume and/or increased tortuosity.
Recently, it was shown that glymphatic activity is profoundly suppressed in the aging brain of wild type mice mainly due to delocalization of AQP4 from the endfeet processes of astrocytes (Kress et al., 2014). AQP4, the predominant water channel in brain, is selective expressed in astrocytic endfeet (10-fold greater than that in the rest of the astrocyte (Yang et al., 2011). Earlier studies have shown that this polarized distribution of AQP4 is altered in ArcSwe (Arctic (E693G) and Swedish (K670N, M671L) mutations) mouse model of AD (Yang et al., 2011). In these mice there was less AQP4 at the endfeet that were associated with amyloid-β plaques. Interestingly, Aqp4 gene deletion reduced Aβ42-induced astrocyte activation, in vitro (Yang et al., 2012). Recently, it was shown that deletion of Aqp4 in the APP/PS1 mice exacerbates Aβ accumulation in brain and contributes to memory deficits (Xu et al., 2015). Our data are congruent with these studies, but also indicated that soluble Aβ oligomers, a more toxic form of Aβ, may contribute to the suppression of glymphatic activities in aging APP/PS1 mice.
The implications of these findings are that reduced glymphatic inflow into the aging brain could restrict distribution of CSF to brain, reduce ISF convective flow and thus, clearance of Aβ and other neurotoxic waste products. The reduced flow of CSF along the penetrating arteries may lead to the development of CAA due to the slower transit time that promotes greater cellular binding and uptake. In addition, reduced ISF clearance would exacerbate the oligomerization and accumulation of brain Aβ, and consequently its toxicity due the generation of soluble oligomers. Reduced CSF inflow into brain and impeded downstream ISF clearance appear to be critical stages, whereby the CSF-derived Aβ aggravates vascular and parenchymal Aβ accumulation and the generation of toxic species. Thus, therapeutic targets that maintain glymphatic function and the continuous removal of Aβ monomeric molecules from the aging CNS hold promise for preventing or delaying the accumulation of potentially neurotoxic molecules. In addition, since glymphatic activity is reduced before significant amyloid-β deposits this could be an important early indicator of the disease.
Conclusions
We show for the first time that 1) glymphatic transport, i.e., CSF inflow into brain and ISF clearance, is suppressed in a mouse model of AD (APP/PS1) 2) glymphatic transport is suppressed prior to significant accumulation of amyloid-β, 3) both Aβ40 and Aβ42 (a CSF biomarker of AD), circulate by glymphatic pathways but oligomerization may limit their distribution, especially for Aβ42, due to increasing molecular size, 4) CSF-derived Aβ is taken up by cells within the parenchyma 5) CSF-derived Aβ40 delivered to the parenchyma via the glymphatic transport co-localized with amyloid-β plaque, suggesting that this source of Aβ40 may also contribute to plaque formation, 6) soluble Aβ oligomers, a more toxic forms of Aβ, may also contribute to glymphatic dysfunction, and 7) long-term exposure to Aβ40 in the subarachnoid CSF may suppress glymphatic transport prior to the presence of substantial amyloid-β deposits.
Supplementary Material
Highlights.
Glymphatic transport is suppressed prior to significant accumulation of amyloid-β.
CSF Aβ40 and Aβ42 circulate through the brain parenchyma.
CSF-derived Aβ is taken up by cells within the parenchyma, e.g., neurons,
CSF-derived Aβ may contribute to amyloid-β plaque formation.
Long-term exposure to Aβ40 in CSF suppresses glymphatic transport.
Acknowledgments
Sources of support: This work was supported by the National Institutes of Health-National Institute of Neurological Disorders and Stroke and National Institute of Aging (1R56NS086924 and R21AG050212 to RD; R01NS078167 and R01NS078304 to MN and R01AG048769 to HB).
Footnotes
Disclosure/Conflict of Interest. The authors declare no conflict of interest
Contribution of authors:
WP, MT, BL, YL, HM, SR and TK performed experiments.
WP, MT, BL, HM, EM, TK, SP and FD analyzed data.
MN and RD designed experiments.
RD wrote the paper.
MT, HM, HB and MN provided critical comments and edited the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott NJ, et al. Astrocyte-endothelial interactions at the blood-brain barrier. Nat Rev Neurosci. 2006;7:41–53. doi: 10.1038/nrn1824. [DOI] [PubMed] [Google Scholar]
- Banks WA, et al. Efflux of human and mouse amyloid beta proteins 1-40 and 1-42 from brain: impairment in a mouse model of Alzheimer’s disease. Neuroscience. 2003;121:487–92. doi: 10.1016/s0306-4522(03)00474-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bateman RJ, et al. Human amyloid-beta synthesis and clearance rates as measured in cerebrospinal fluid in vivo. Nat Med. 2006;12:856–61. doi: 10.1038/nm1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell RD, et al. Transport pathways for clearance of human Alzheimer’s amyloid beta-peptide and apolipoproteins E and J in the mouse central nervous system. J Cereb Blood Flow Metab. 2007;27:909–18. doi: 10.1038/sj.jcbfm.9600419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer’s disease. NeuroRx. 2004;1:213–25. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boulton M, et al. Drainage of CSF through lymphatic pathways and arachnoid villi in sheep: measurement of 125I-albumin clearance. Neuropathol Appl Neurobiol. 1996;22:325–33. doi: 10.1111/j.1365-2990.1996.tb01111.x. [DOI] [PubMed] [Google Scholar]
- Bradbury MW. The blood-brain barrier. Transport across the cerebral endothelium. Circ Res. 1985;57:213–22. doi: 10.1161/01.res.57.2.213. [DOI] [PubMed] [Google Scholar]
- Bradbury MW, Westrop RJ. Factors influencing exit of substances from cerebrospinal fluid into deep cervical lymph of the rabbit. J Physiol. 1983;339:519–34. doi: 10.1113/jphysiol.1983.sp014731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinker T, et al. Dynamic properties of lymphatic pathways for the absorption of cerebrospinal fluid. Acta Neuropathol. 1997;94:493–8. doi: 10.1007/s004010050738. [DOI] [PubMed] [Google Scholar]
- Bruggink KA, et al. Amyloid-beta oligomer detection by ELISA in cerebrospinal fluid and brain tissue. Anal Biochem. 2013;433:112–20. doi: 10.1016/j.ab.2012.09.014. [DOI] [PubMed] [Google Scholar]
- Cirrito JR, et al. P-glycoprotein deficiency at the blood-brain barrier increases amyloid-beta deposition in an Alzheimer disease mouse model. J Clin Invest. 2005a;115:3285–90. doi: 10.1172/JCI25247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, et al. Synaptic activity regulates interstitial fluid amyloid-beta levels in vivo. Neuron. 2005b;48:913–22. doi: 10.1016/j.neuron.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Clifford PM, et al. Alpha7 nicotinic acetylcholine receptor expression by vascular smooth muscle cells facilitates the deposition of Abeta peptides and promotes cerebrovascular amyloid angiopathy. Brain Res. 2008;1234:158–71. doi: 10.1016/j.brainres.2008.07.092. [DOI] [PubMed] [Google Scholar]
- Deane R, et al. Clearance of amyloid-beta peptide across the blood-brain barrier: implication for therapies in Alzheimer’s disease. CNS Neurol Disord Drug Targets. 2009;8:16–30. doi: 10.2174/187152709787601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, et al. RAGE mediates amyloid-beta peptide transport across the blood-brain barrier and accumulation in brain. Nat Med. 2003;9:907–13. doi: 10.1038/nm890. [DOI] [PubMed] [Google Scholar]
- Deane R, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest. 2008;118:4002–13. doi: 10.1172/JCI36663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, et al. IgG-assisted age-dependent clearance of Alzheimer’s amyloid beta peptide by the blood-brain barrier neonatal Fc receptor. J Neurosci. 2005;25:11495–503. doi: 10.1523/JNEUROSCI.3697-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, et al. A multimodal RAGE-specific inhibitor reduces amyloid beta-mediated brain disorder in a mouse model of Alzheimer disease. J Clin Invest. 2012;122:1377–92. doi: 10.1172/JCI58642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deane R, et al. Neuroscience. Vol. 461. Chicago: 2015. Sleep deprivation disrupts apoE isoform specific radial diffusion from the penetrating arteries into brain by the glymphatic system: Implications for CAA and Alzheimer’s disease. [Google Scholar]
- Deane R, et al. LRP/amyloid beta-peptide interaction mediates differential brain efflux of Abeta isoforms. Neuron. 2004;43:333–44. doi: 10.1016/j.neuron.2004.07.017. [DOI] [PubMed] [Google Scholar]
- Fagan AM, Holtzman DM. Cerebrospinal fluid biomarkers of Alzheimer’s disease. Biomark Med. 2010;4:51–63. doi: 10.2217/BMM.09.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagan AM, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol. 2006;59:512–9. doi: 10.1002/ana.20730. [DOI] [PubMed] [Google Scholar]
- Fagan AM, et al. Longitudinal change in CSF biomarkers in autosomal-dominant Alzheimer’s disease. Sci Transl Med. 2014;6:226ra30. doi: 10.1126/scitranslmed.3007901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haj-Yasein NN, et al. Glial-conditional deletion of aquaporin-4 (Aqp4) reduces blood-brain water uptake and confers barrier function on perivascular astrocyte endfeet. Proc Natl Acad Sci U S A. 2011;108:17815–20. doi: 10.1073/pnas.1110655108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes CA, et al. Perivascular drainage of solutes is impaired in the ageing mouse brain and in the presence of cerebral amyloid angiopathy. Acta Neuropathol. 2011;121:431–43. doi: 10.1007/s00401-011-0801-7. [DOI] [PubMed] [Google Scholar]
- Herrup K. The case for rejecting the amyloid cascade hypothesis. Nat Neurosci. 2015;18:794–9. doi: 10.1038/nn.4017. [DOI] [PubMed] [Google Scholar]
- Herz J. Apolipoprotein E receptors in the nervous system. Curr Opin Lipidol. 2009;20:190–6. doi: 10.1097/MOL.0b013e32832d3a10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtzman DM, et al. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2:a006312. doi: 10.1101/cshperspect.a006312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, et al. Impairment of glymphatic pathway function promotes tau pathology after traumatic brain injury. J Neurosci. 2014;34:16180–93. doi: 10.1523/JNEUROSCI.3020-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Transl Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger LB, et al. Testing the neurovascular hypothesis of Alzheimer’s disease: LRP-1 antisense reduces blood-brain barrier clearance, increases brain levels of amyloid-beta protein, and impairs cognition. J Alzheimers Dis. 2009;17:553–70. doi: 10.3233/JAD-2009-1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, et al. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, Bu G. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Front Aging Neurosci. 2014;6:93. doi: 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, et al. Neuronal clearance of amyloid-beta by endocytic receptor LRP1. J Neurosci. 2013;33:19276–83. doi: 10.1523/JNEUROSCI.3487-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanekiyo T, et al. LRP1 in brain vascular smooth muscle cells mediates local clearance of Alzheimer’s amyloid-beta. J Neurosci. 2012;32:16458–65. doi: 10.1523/JNEUROSCI.3987-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, et al. Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Abeta oligomers. Mol Neurodegener. 2010;5:57. doi: 10.1186/1750-1326-5-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayed R, et al. Common structure of soluble amyloid oligomers implies common mechanism of pathogenesis. Science. 2003;300:486–9. doi: 10.1126/science.1079469. [DOI] [PubMed] [Google Scholar]
- Kida S, et al. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480–8. doi: 10.1111/j.1365-2990.1993.tb00476.x. [DOI] [PubMed] [Google Scholar]
- Kress BT, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014 doi: 10.1002/ana.24271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar-Singh S. Cerebral amyloid angiopathy: pathogenetic mechanisms and link to dense amyloid plaques. Genes Brain Behav. 2008;7(Suppl 1):67–82. doi: 10.1111/j.1601-183X.2007.00380.x. [DOI] [PubMed] [Google Scholar]
- Lue LF, et al. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer’s disease. Am J Pathol. 1999;155:853–62. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiisen TM, et al. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094–103. doi: 10.1002/glia.20990. [DOI] [PubMed] [Google Scholar]
- Mawuenyega KG, et al. Decreased clearance of CNS beta-amyloid in Alzheimer’s disease. Science. 2010;330:1774. doi: 10.1126/science.1197623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CA, et al. Soluble pool of Abeta amyloid as a determinant of severity of neurodegeneration in Alzheimer’s disease. Ann Neurol. 1999;46:860–6. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Michel CC. Capillary permeability and how it may change. J Physiol. 1988;404:1–29. doi: 10.1113/jphysiol.1988.sp017275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and ‘wingmen’. Nat Neurosci. 2015;18:800–6. doi: 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nedergaard M. Neuroscience. Garbage truck of the brain. Science. 2013;340:1529–30. doi: 10.1126/science.1240514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa K, et al. A beta-peptides enhance vasoconstriction in cerebral circulation. Am J Physiol Heart Circ Physiol. 2001;281:H2417–24. doi: 10.1152/ajpheart.2001.281.6.H2417. [DOI] [PubMed] [Google Scholar]
- Oe T, et al. Quantitative analysis of amyloid beta peptides in cerebrospinal fluid of Alzheimer’s disease patients by immunoaffinity purification and stable isotope dilution liquid chromatography/negative electrospray ionization tandem mass spectrometry. Rapid Commun Mass Spectrom. 2006;20:3723–35. doi: 10.1002/rcm.2787. [DOI] [PubMed] [Google Scholar]
- Paris D, et al. Soluble beta-amyloid peptides mediate vasoactivity via activation of a pro-inflammatory pathway. Neurobiol Aging. 2000;21:183–97. doi: 10.1016/s0197-4580(99)00111-6. [DOI] [PubMed] [Google Scholar]
- Plog BA, et al. Biomarkers of traumatic injury are transported from brain to blood via the glymphatic system. J Neurosci. 2015;35:518–26. doi: 10.1523/JNEUROSCI.3742-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollay M. The function and structure of the cerebrospinal fluid outflow system. Cerebrospinal Fluid Res. 2010;7:9. doi: 10.1186/1743-8454-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangroo Thrane V, et al. Paravascular microcirculation facilitates rapid lipid transport and astrocyte signaling in the brain. Sci Rep. 2013a;3:2582. doi: 10.1038/srep02582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rangroo Thrane V, et al. Ammonia triggers neuronal disinhibition and seizures by impairing astrocyte potassium buffering. Nat Med. 2013b;19:1643–8. doi: 10.1038/nm.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennels ML, et al. Evidence for a ‘paravascular’ fluid circulation in the mammalian central nervous system, provided by the rapid distribution of tracer protein throughout the brain from the subarachnoid space. Brain Res. 1985;326:47–63. doi: 10.1016/0006-8993(85)91383-6. [DOI] [PubMed] [Google Scholar]
- Rolyan H, et al. Amyloid-beta protein modulates the perivascular clearance of neuronal apolipoprotein E in mouse models of Alzheimer’s disease. J Neural Transm. 2011;118:699–712. doi: 10.1007/s00702-010-0572-7. [DOI] [PubMed] [Google Scholar]
- Schoonenboom NS, et al. Amyloid beta 38, 40, and 42 species in cerebrospinal fluid: more of the same? Ann Neurol. 2005;58:139–42. doi: 10.1002/ana.20508. [DOI] [PubMed] [Google Scholar]
- Selkoe DJ. Clearing the brain’s amyloid cobwebs. Neuron. 2001;32:177–80. doi: 10.1016/s0896-6273(01)00475-5. [DOI] [PubMed] [Google Scholar]
- Shibata M, et al. Clearance of Alzheimer’s amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest. 2000;106:1489–99. doi: 10.1172/JCI10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I, et al. Low levels of copper disrupt brain amyloid-beta homeostasis by altering its production and clearance. Proc Natl Acad Sci U S A. 2013;110:14771–6. doi: 10.1073/pnas.1302212110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suo Z, et al. Soluble Alzheimers beta-amyloid constricts the cerebral vasculature in vivo. Neurosci Lett. 1998;257:77–80. doi: 10.1016/s0304-3940(98)00814-3. [DOI] [PubMed] [Google Scholar]
- Thal DR, et al. Occurrence and co-localization of amyloid beta-protein and apolipoprotein E in perivascular drainage channels of wild-type and APP-transgenic mice. Neurobiol Aging. 2007;28:1221–30. doi: 10.1016/j.neurobiolaging.2006.05.029. [DOI] [PubMed] [Google Scholar]
- Van Dorpe J, et al. Prominent cerebral amyloid angiopathy in transgenic mice overexpressing the london mutant of human APP in neurons. Am J Pathol. 2000;157:1283–98. doi: 10.1016/S0002-9440(10)64644-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh DM, et al. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–9. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- Wang M, et al. Cognitive deficits and delayed neuronal loss in a mouse model of multiple microinfarcts. J Neurosci. 2012;32:17948–60. doi: 10.1523/JNEUROSCI.1860-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller RO, et al. Pathways of fluid drainage from the brain--morphological aspects and immunological significance in rat and man. Brain Pathol. 1992;2:277–84. doi: 10.1111/j.1750-3639.1992.tb00704.x. [DOI] [PubMed] [Google Scholar]
- Xie L, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342:373–7. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Abeta accumulation and memory deficits. Mol Neurodegener. 2015;10:58. doi: 10.1186/s13024-015-0056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, et al. Albumin outflow into deep cervical lymph from different regions of rabbit brain. Am J Physiol. 1991;261:H1197–204. doi: 10.1152/ajpheart.1991.261.4.H1197. [DOI] [PubMed] [Google Scholar]
- Yang J, et al. Loss of astrocyte polarization in the tg-ArcSwe mouse model of Alzheimer’s disease. J Alzheimers Dis. 2011;27:711–22. doi: 10.3233/JAD-2011-110725. [DOI] [PubMed] [Google Scholar]
- Yang L, et al. Evaluating glymphatic pathway function utilizing clinically relevant intrathecal infusion of CSF tracer. J Transl Med. 2013;11:107. doi: 10.1186/1479-5876-11-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, et al. Aquaporin-4 mediates astrocyte response to beta-amyloid. Mol Cell Neurosci. 2012;49:406–14. doi: 10.1016/j.mcn.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Zetterberg H, et al. Intra-individual stability of CSF biomarkers for Alzheimer’s disease over two years. J Alzheimers Dis. 2007;12:255–60. doi: 10.3233/jad-2007-12307. [DOI] [PubMed] [Google Scholar]
- Zhu X, et al. NG2 cells generate both oligodendrocytes and gray matter astrocytes. Development. 2008;135:145–57. doi: 10.1242/dev.004895. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. The blood-brain barrier in health and chronic neurodegenerative disorders. Neuron. 2008a;57:178–201. doi: 10.1016/j.neuron.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. New therapeutic targets in the neurovascular pathway in Alzheimer’s disease. Neurotherapeutics. 2008b;5:409–14. doi: 10.1016/j.nurt.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.