Abstract
Objective:
The aim of this study is to investigate the possible protective effects of melatonin and caffeic acid phenethyl ester (CAPE) on potassium dichromate (K2 Cr2O7)-induced nephrotoxicity and genotoxicity.
Methods:
A total of 40 Wistar albino rats were divided into five groups: control, K2Cr2O7(K2Cr2O715 mg/kg, one dose, i.p.), K2Cr2O7 + melatonin, K2Cr2O7 + CAPE, and K2Cr2O7 + melatonin + CAPE. Urine and blood samples were collected from rats before scarification. One kidney was collected for histopathological studies, and the other was stored at −80°C for further determination of catalase (CAT), superoxide dismutase (SOD), malondialdehyde (MDA), glutathione (GSH), glutathione S-transferase (GST), and glutathione reductase (GR) levels with spectrophotometric method. Comet assay was used to evaluate the genotoxicity.
Results:
We observed a significant amelioration in genotoxicity by melatonin and simultaneous melatonin + CAPE treatment compared to K2Cr2O7 group (p1, p2< 0.05). SOD, CAT, GSH, GST, and MDA levels did not change when compared with controls. When K2Cr2O7 applied group was treated with melatonin and CAPE, neither melatonin nor CAPE made any changes in kidney GSH, GST, SOD, and MDA levels (P > 0.05). We noted that treatment with CAPE and melatonin + CAPE together caused a significant decrease in renal tissue damage, an upregulation in the kidney CAT levels (P < 0.05) and a slight healing at GR levels when compared with the K2Cr2O7 group.
Conclusion:
Our results revealed, CAPE and melatonin may have protective effects on K2Cr2O7 induced nephrotoxicity and cellular damage in rats.
Keywords: Caffeic acid phenethyl ester, melatonin, nephrotoxicity, oxidative stress, potassium dichromate
Chromium is the sixth element seen on the earth. It is used in the production of stainless steel, painting, welding, metallurgy industry, and leather industry.[1] Occupational exposure to Cr (VI) is found in nearly half a million industrial workers in the developing countries and several million worldwide.[2] Hexavalent chromium, Cr (VI), has been identified as extremely toxic and carcinogenic.[3] The major adverse effect of the chromium toxicity has been reported as nephrotoxicity.[4] Some studies performed on mammalians showed that exposure to Cr (IV) is related with cancer progression.[3,5] In vivo and in vitro reactions mediated by Cr (VI) have shown that Cr (VI) may cause the activation of nuclear transcription factor-KB, single and double DNA chain fractionation, formation of 8-hydroxy-deoxyguanosine included cellular damage.[6] The reduction of Cr (VI) by cellular reductants to its lower oxidation states, Cr (V), Cr (IV), and Cr (III), has been considered an important step in cellular mechanisms. Data show that these reactive chromium intermediates are capable of generating reactive oxygen species (ROS) that induce the oxidative stress.[7] The increased ROS production has been suggested to be an important agent of cell death in different renal diseases and genotoxicity.[6]
Caffeic acid phenethyl ester (CAPE), produced from the bee propolis, may protect organisms against oxidative stress-induced damage. A study has demonstrated the healing effects of CAPE in the treatment of oxidative stress.[8] Melatonin (N-acetyl-5-methoxytryptamine) is a neurohormone which is secreted rhythmically and has physiological functions such as regulation of immune system and circadian rhythms.[9] Recently, it has been shown that melatonin has an oxidative stress healing effect.[10] It performs this activity by scavenging free radicals.
In light of the above data, this study was undertaken to assess possible protective effects of melatonin and CAPE on potassium dichromate (K2Cr2O7)-induced nephrotoxicity and genotoxicity.
Materials and Methods
Ethics
All experimental procedures were approved by the Committee for Animal Experiments and the Ethics Committee of Istanbul University (approval number: 2011/89).
Wistar albino rats weighing about 250–300 g housed under 12 h light/12 h dark, and fed standard diet and water. Rats were divided into five groups (n = 8/group). (1) Control received single-dose isotonic saline intraperitonally (i.p.), (2) K2Cr2O7, rats received one dose injection of K2Cr2O7,15 mg/kg i.p., (3) K2Cr2O7 + melatonin, which was given single-dose K2Cr2O7i.p. and 4 mg/kg melatonin injected per day for 2 days, (4) K2Cr2O7 + CAPE, which were given single dose K2Cr2O7i.p. and 10 μmol/kg CAPE injected per day i.p. for 2 days, (5) K2Cr2O7 + melatonin + CAPE given i.p. single dose per day for 2 days. Rats were sacrified 24 h later.
Sample Collection and Biochemical Assays
After scarification of rats under ketamine (100 mg/kg) and xylazine (10 mg/kg) anesthesia, both kidneys were taken, and one of them was fixed in 10% formalin for further histopathological assays. The other kidney was stored at −80°C for biochemical analysis. Kidney samples were then thawed and homogenized in 0.15 M KCI (10 w/v) and centrifuged at 10.000 g for 45 min (+4°C) for the biochemical analysis. Blood samples were taken before scarification and used for both genotoxicity and ELISA assays.
Comet Assay for DNA Damage Detection
Genotoxic effects were analyzed by electrophoresis using alkaline single cell gel electrophoresis (Comet assay).[11] This method depends on the detection of single chain fractionation, double chain fractionation, and miss nucleotide excision repair of DNA damage in a single cell and distribution in the cell population. Damaged DNA is shown as a tail in the gel electrophoresis.
Histopathological Examination of the Kidney
The kidney tissues were removed and fixed in 10% neutral phosphate-buffered formaldehyde, dehydrated with graded alcohol series, cleared with toluene and embedded into paraffin. These kidney tissues were cut into five micrometer thick sections and were stained with hematoxylin and eosin. Slides were examined under the light microscope at ×400 (LEICA DM2500). A semi-quantitative evaluation of kidney tissues was accomplished by scoring the degree of severity according to previously published criteria.[12] For each renal section, the whole slide was examined for tubular necrosis, tubular vacuolization, and hyaline casts (hcs). In brief, minimum of fifty proximal tubules associated with fifty glomeruli were examined for each slide and an average score was obtained. Slides were examined and assigned for severity of changes using scores on a scale of none (0), mild (1), moderate (2), and severe damage (3), in which (0) denotes no change; grade (1) changes affecting <25% of tubular damage (mild); grade (2) changes affecting 25–50% of the tubules (moderate); grade (3) changes affecting >50% of the tubules (severe).[13]
Determination of Glutathione Reductase, Superoxide Dismutase, Catalase, Glutathione, Glutathione S-transferase and Malondialdehyde Levels
Supernatant was used for glutathione (GSH), catalase (CAT), superoxide dismutase (SOD), glutathione reductase (GR), Glutathione S-transferase (GST), and malondialdehyde (MDA) experiments. GSH amounts were determined according to the method of Beutler et al.[14] CAT activity has been determined by measuring the decomposition of hydrogen peroxide.[15] Total SOD activity measured inhibition of nitro blue tetrazolium reduction by xantine oxidase used as a superoxide generator.[16] Color concentration was measured at 560 nm. GST levels were evaluated by the method of Habig et al.[17] GR levels were measured by described elsewhere.[18] GR assay depends on reduction of oxidized GSSG by NADPH GR. MDA levels were determined by measurement of thiobarbituric acid (TBA) reactivity.[19]
ELISA Assay
Serum melatonin levels were measured with ELISA method[20] (Cusabio Biotech, Wuhen, China) according to the manufacturer's instructions.
Results
Comet Assay Scores
The K2Cr2O7 group of lymphocytes showed 2nd and 3rd degree damage as compared to control group. When the K2Cr2O7 group was compared with the K2Cr2O7 + melatonin group and K2Cr2O7 + CAPE group, it was found that DNA breaks were significantly decreased (P < 0.05). When the K2Cr2O7 group was compared with the K2Cr2O7 + CAPE group, tail factor did not change [Figure 1].
Figure 1.
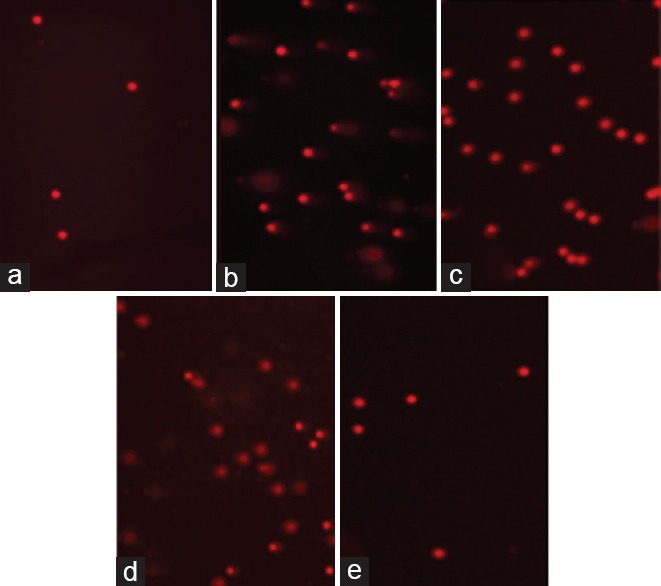
Semi-quantitative analysis of renal injury in different treatment groupsComet assay. Control group (a). Potassium dichromate group (b). Melatonin-treated potassium dichromate group (c), caffeic acid phenethyl ester-treated potassium dichromate group (d), melatonin and caffeic acid phenethyl ester treated potassium dichromate group (e)
Blood Urea Nitrogen and Serum Creatinine Levels
Urea and serum creatinine (SCr) levels were 21.51 ± 1.43 mg/dl and 0.36 ± 0.02 mg/dl in the control group, 125 ± 4.09 mg/dl and 3.08 ± 0.10 mg/dl in K2Cr2O7 group and 103.02 ± 3.70 mg/dl and 0.72 ± 0.15 mg/dl in K2Cr2O7+ Melatonin + CAPE group, respectively (P < 0.001).
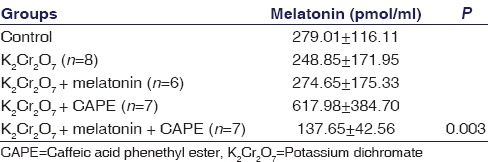
Serum Melatonin Levels
Serum melatonin levels for different treatment groups are shown in Table 1. Serum levels of melatonin in K2Cr2O7+ CAPE-treated group were significantly higher when all groups were compared with each other (P = 0.003) [Table 1].
Table 1.
Effect of different treatments on serum melatonin levels in rats
Histopathological Results
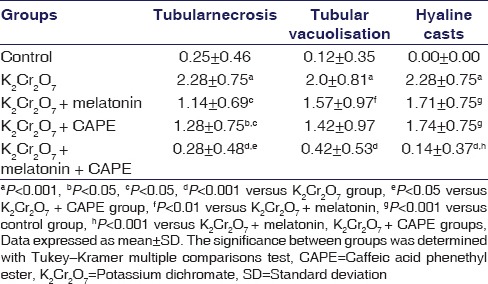
The kidney sections from the control group showed normal structural integrity [Figure 2a]. Necrosis in the proximal and distal tubules and hcs were observed in the rats given K2Cr2O7 [Figure 2b]. Although the administration of melatonin reduced histopathological damage in the nephrotoxicity caused by potassium dichromate, histological damage was not completely recovered as compared with controls. In the renal cortex, tubular necrosis and hc depositions were decreased [Figure 2c]. In the CAPE group, renal damage was significantly decreased as compared with K2Cr2O7 group [Figure 2d]. The CAPE and melatonin coadministered group, showed better histopathologic findings compared with all three treatment groups [Figure 2e and Table 2].
Figure 2.

H and E, staining of kidney sections. In the control group, normal histological appearance of renal cortex, glomerulus, and tubular epithelial cells (a). In the potassium dichromate, group glomeruli appear normal, damage to tubular epithelial cells (→) and presence of hyaline casts in the tubular lumen (b). In the melatonin-treated potassium dichromate group (c) and caffeic acid phenethyl ester-treated potassium dichromate (d), the presence of hyaline casts in tubular lumen. Glomeruli appear normal. Melatonin and caffeic acid phenethyl ester treated potassium dichromate group, the generally intact tubular epithelial cells and a cast free tubular lumen. Glomeruli appear normal (e) (p: Proximal tubule, d: Distal tubule, g: Glomeruli, hc: Hyaline cast [Bar 20 μm])
Table 2.
Semi-quantitative analysis of renal injury in different treatment groups
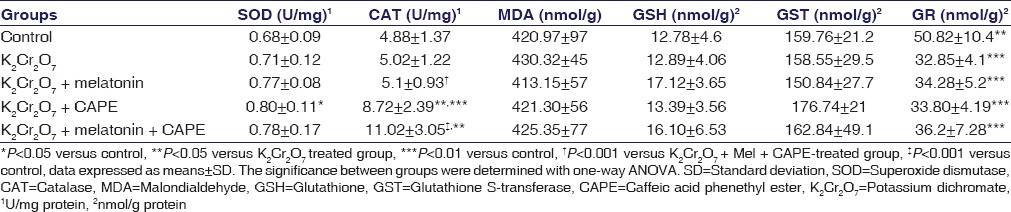
Glutathione Reductase, Superoxide Dismutase, Catalase, Glutathione, Glutathione S-transferase, and Malondialdehyde Levels
GR levels were decreased by K2Cr2O7 administration when compared with control group as shown in Table 3 (P < 0.05). This decrease is tend to be slightly increased by melatonin and CAPE administration together. However, this increase could not reach to statistically significant levels. SOD, CAT, GSH, GST, and MDA levels have not changed in K2Cr2O7-treated rats when compared with controls, but CAT activities were increased by administration of CAPE and CAPE + melatonin (P < 0.05), [Table 3].
Table 3.
Effects of melatonin and caffeic acid phenethyl ester on rat renal injury parameters
Discussion
The K2Cr2O7 is widely used in industry and exposure to K2Cr2O7 has been involved in the pathophysiology of cytotoxicity, genotoxicity, neurotoxicity, immunotoxicity, and nephrotoxicity. Chromium exposure has been reported to cause acute renal failure in mammals. It was also reported that chronic exposure to chromium, caused damage in renal proximal tubular epithelial cells, and this leads to proximal tubules failure which was also reported to cause lipid peroxidation.[3,8] In our study, we observed that there was severe histopathological changes such as necrosis, vacuolation and hcs in single dose K2Cr2O7-treated group of rat kidney tissue proximal tubules. The work carried out to date of antioxidants consisting of chromium has been reported to show a protective effect on oxidative damage and nephrotoxicity. Therefore, it is considered antioxidants may have a protective effect in the treatment of chromium exposure.
It has been shown that incubation of primary cultures of rat hepatocytes with K2Cr2O7 plus the pineal hormone melatonin resulted a decrease in DNA single-strand breaks caused by K2Cr2O7, suppressed dichromate-induced cytotoxicity, lipid peroxidation, and the inhibition of CAT activity.[4,5] The CAPE has many biological functions including anti-inflammatory, antiviral, immunomodulatory, antiangiogenic, anti-invasive, antimetastatic, carcinostatic, and antioxidant.[8]
To the best of our knowledge, there is no study in the literature exploring short-term protective effects of both melatonin and CAPE together in K2Cr2O7 induced nephrotoxicity in rats. However, it has been determined that melatonin has a protective effect on methotrexate, FK506, gentamicin, cisplatin, uranium, acetaminophen, and radiation-induced renal damage and CAPE has a protective effect on acetylsalicylic acid, gentamicin, cyclosporin A, methotrexate, and cadmium-induced nephrotoxicity in rats in some studies.[21,22] This in turn suggests that melatonin and CAPE may be effective in improving the K2Cr2O7 induced nephrotoxicity and has helped us to establish our hypothesis.
A study, reported that melatonin reduced the level of CAT enzyme inhibition, however, has no alteration in cellular GSH, GR, GPx, and SOD.[4] In another study, they investigated the effects of the K2Cr2O7 induced nephrotoxicity on antioxidant enzymes, and they reported that GR and CAT enzyme levels were decreased, however, SOD enzyme levels were not significantly changed in K2Cr2O7 induced rats.[5] In our study, we detected that K2Cr2O7 treatment did not change CAT enzyme activity, however, the treatment of both melatonin and CAPE have significantly augmented CAT enzyme activity. The augmentation in CAT activity may reduce the free radicals formed by K2Cr2O7 and may ameliorate nephrotoxicity suggesting the synergistic roles of melatonin and CAPE. Our results are consistent with Soudani et al.'s and Susa et al.'s studies in which increased activity of CAT enzyme with the antioxidant applications were observed.[4,5,23] A significant difference was observed between all groups when compared with one-way ANOVA, however, when the two groups were compared with each other protective applications did not have a significant role. This result is consistent with Soudani et al.'s findings in which they observed increased MDA levels with antioxidant treatment.[23] Pedraza-Chaverri et al. reported that GR levels were decreased with K2Cr2O7 treatment, and they observed no significant effect of the antioxidant treatment on enzyme levels.[5] When all the groups were examined, there was no significant difference between the GSH levels and the GST, and SOD enzyme activities. Our results were parallel with the findings of Pedraza-Chaverri et al. and Susa et al., however, were not consistent with Soudani et al.[4,5]
Besides this, in our study, melatonin and CAPE together, inhibited the increase in urea and SCr levels induced by K2Cr2O7. Our results were consistent with the previously reported study in which melatonin significantly reduced methotrexate-induced elevation in plasma creatinine in rats.[21]
There are some reports investigating the genotoxicity of K2Cr2O7.[24,25,26] In a study which is performed in Cyprinus carpio, it has been noted that genotoxicity induced by K2Cr2O7 indicated a concentration-dependent increase in DNA damage.[24] Kaya et al. reported that ascorbic acid was effective in reducing the genotoxicity of K2Cr2O7 according to the control level in their study.[25] Fahmy et al. showed the genotoxic effect of K2Cr2O7 in mice. The results of the same study also confirmed the protective role of thiola and soybean seeds against the genotoxicity of K2Cr2O7.[26] CAPE and melatonin are known to have oxidative stress healing features.[12,14] The cause of the genotoxicity may be oxidative stress,[25] and these compounds may have healing effects.
In our study, we observed that CAPE-treated group has lower tail factors (%) than that of K2Cr2O7 group, however, our results were not statistically significant. Besides, in the melatonin-treated group, we observed a repair in the DNA damage and a statistically significant reduction in the % of the tail factor. Our study was in parallel with Susa et al.'s study.[4]
In standard drug therapy, management is focused on life-threatening features. Fluid balance, treatment of acidosis, using diuretics, dopamine, and coping with obstruction are the issues in the further management. Furthermore, standard drug therapy may ameliorate increasing SCr levels however renal failure may remain in acute chromium poisoning. That is why novel therapies are preferred in addition to standard drug therapy. These therapies involve more specific experimental treatments including some oxygen free radical scavengers designed to reduce renal damage. In our study, melatonin and CAPE-treated groups exhibited lower renal damage, and melatonin and CAPE may serve as free radical scavengers in conjunction with each other.
We also have found that serum melatonin levels increased significantly in the group treated with CAPE. These results may show that CAPE may contribute to endogenous melatonin synthesis and may increase the levels of this molecule. On the other hand, our histopathological results showed that the improvement of the histopathological changes in renal tubular epithelial cells was found to be more prominent in melatonin + CAPE group than that of K2Cr2O7. Thus, we suggest that treatment of melatonin together with CAPE may have renoprotective effect against K2Cr2O7 nephrotoxicity. There are also some limitations in our study. Some of the antioxidant enzyme levels we studied, did not have a statistically significant increase since we examined the acute effects of K2Cr2O7 on nephrotoxicity. It is concluded that although melatonin + CAPE supplement has a very powerful nephroprotective effect, the amelioration of the antioxidant parameters take a long time to be significant enough. Long-term drug treatment may be more effective for this condition. Further studies may enlighten the synergistic effects of melatonin together with CAPE on the chronic effects of K2Cr2O7 nephrotoxicity.
Financial Support and Sponsorship
This study was supported by the Scientific Research Projects Coordination Unit of Istanbul University, Project number 17077.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
This study was supported by the Scientific Research Projects Coordination Unit of Istanbul University, Project number 17077. The preliminary findings of this study were presented at the 13th National Medical Biology and Genetics Congress, October 27–30, 2013, Aydin, Turkey.
References
- 1.Kamerud KL, Hobbie KA, Anderson KA. Stainless steel leaches nickel and chromium into foods during cooking. J Agric Food Chem. 2013;61:9495–501. doi: 10.1021/jf402400v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Crump C, Crump K, Hack E, Luippold R, Mundt K, Liebig E, et al. Dose-response and risk assessment of airborne hexavalent chromium and lung cancer mortality. Risk Anal. 2003;23:1147–63. doi: 10.1111/j.0272-4332.2003.00388.x. [DOI] [PubMed] [Google Scholar]
- 3.Fatima S, Mahmood R. Vitamin C attenuates potassium dichromate-induced nephrotoxicity and alterations in renal brush border membrane enzymes and phosphate transport in rats. Clin Chim Acta. 2007;386:94–9. doi: 10.1016/j.cca.2007.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Susa N, Ueno S, Furukawa Y, Ueda J, Sugiyama M. Potent protective effect of melatonin on chromium (VI)-induced DNA single-strand breaks, cytotoxicity, and lipid peroxidation in primary cultures of rat hepatocytes. Toxicol Appl Pharmacol. 1997;144:377–84. doi: 10.1006/taap.1997.8151. [DOI] [PubMed] [Google Scholar]
- 5.Pedraza-Chaverrí J, Barrera D, Medina-Campos ON, Carvajal RC, Hernández-Pando R, Macías-Ruvalcaba NA, et al. Time course study of oxidative and nitrosative stress and antioxidant enzymes in K2Cr2O7-induced nephrotoxicity. BMC Nephrol. 2005;6:4. doi: 10.1186/1471-2369-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sughis M, Nawrot TS, Haufroid V, Nemery B. Adverse health effects of child labor: High exposure to chromium and oxidative DNA damage in children manufacturing surgical instruments. Environ Health Perspect. 2012;120:1469–74. doi: 10.1289/ehp.1104678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shi X, Chiu A, Chen CT, Halliwell B, Castranova V, Vallyathan V. Reduction of chromium (VI) and its relationship to carcinogenesis. J Toxicol Environ Health B Crit Rev. 1999;2:87–104. doi: 10.1080/109374099281241. [DOI] [PubMed] [Google Scholar]
- 8.Song JJ, Lim HW, Kim K, Kim KM, Cho S, Chae SW. Effect of caffeic acid phenethyl ester (CAPE) on H2O2 induced oxidative and inflammatory responses in human middle ear epithelial cells. Int J Pediatr Otorhinolaryngol. 2012;76:675–9. doi: 10.1016/j.ijporl.2012.01.041. [DOI] [PubMed] [Google Scholar]
- 9.Reiter RJ. Pineal melatonin: Cell biology of its synthesis and of its physiological interactions. Endocr Rev. 1991;12:151–80. doi: 10.1210/edrv-12-2-151. [DOI] [PubMed] [Google Scholar]
- 10.Álvarez-Diduk R, Galano A, Tan DX, Reiter RJ. N-acetylserotonin and 6-hydroxymelatonin against oxidative stress: Implications for the overall protection exerted by melatonin. J Phys Chem B. 2015;119:8535–43. doi: 10.1021/acs.jpcb.5b04920. [DOI] [PubMed] [Google Scholar]
- 11.Olive PL, Banáth JP, Durand RE. Heterogeneity in radiation-induced DNA damage and repair in tumor and normal cells measured using the comet assay. Radiat Res. 1990;122:86–94. [PubMed] [Google Scholar]
- 12.Silan C, Uzun O, Comunoglu NU, Gokçen S, Bedirhan S, Cengiz M. Gentamicin-induced nephrotoxicity in rats ameliorated and healing effects of resveratrol. Biol Pharm Bull. 2007;30:79–83. doi: 10.1248/bpb.30.79. [DOI] [PubMed] [Google Scholar]
- 13.Tunçdemir M, Ozturk M. The effects of ACE inhibitor and angiotensin receptor blocker on clusterin and apoptosis in the kidney tissue of streptozotocin-diabetic rats. J Mol Histol. 2008;39:605–16. doi: 10.1007/s10735-008-9201-2. [DOI] [PubMed] [Google Scholar]
- 14.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 15.Aebi HE. Catalase. In: Bergmeyer HU, editor. Methods in enzymatic analysis. New York, NY, USA: Academic Press; 1974. pp. 673–77. [Google Scholar]
- 16.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 17.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 18.Mohandas J, Marshall JJ, Duggin GG, Horvath JS, Tiller DJ. Low activities of glutathione-related enzymes as factors in the genesis of urinary bladder cancer. Cancer Res. 1984;44:5086–91. [PubMed] [Google Scholar]
- 19.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 20.Cevik C, Aslan R. Effects of photoperiod variations and alpha-lipoic acid treatment on melatonin, cortisol, and oxidative stress levels in the blood of rats. Turk J Biol. 2015;39:941–9. [Google Scholar]
- 21.Abraham P, Kolli VK, Rabi S. Melatonin attenuates methotrexate-induced oxidative stress and renal damage in rats. Cell Biochem Funct. 2010;28:426–33. doi: 10.1002/cbf.1676. [DOI] [PubMed] [Google Scholar]
- 22.Ilbey YO, Ozbek E, Cekmen M, Somay A, Ozcan L, Otünctemur A, et al. Melatonin prevents acetaminophen-induced nephrotoxicity in rats. Int Urol Nephrol. 2009;41:695–702. doi: 10.1007/s11255-008-9503-z. [DOI] [PubMed] [Google Scholar]
- 23.Soudani N, Sefi M, Bouaziz H, Chtourou Y, Boudawara T, Zeghal N. Nephrotoxicity induced by chromium (VI) in adults rats and their progeny. Hum Exp Toxicol. 2011;30:1233–45. doi: 10.1177/0960327110387454. [DOI] [PubMed] [Google Scholar]
- 24.Kumar P, Kumar R, Nagpure NS, Nautiyal P, Kushwaha B, Dabas A. Genotoxicity and antioxidant enzyme activity induced by hexavalent chromium in cyprinus carpio after in vivo exposure. Drug Chem Toxicol. 2013;36:451–60. doi: 10.3109/01480545.2013.776581. [DOI] [PubMed] [Google Scholar]
- 25.Kaya B, Creus A, Velázquez A, Yanikoglu A, Marcos R. Genotoxicity is modulated by ascorbic acid. Studies using the wing spot test in Drosophila. Mutat Res. 2002;520:93–101. doi: 10.1016/s1383-5718(02)00173-0. [DOI] [PubMed] [Google Scholar]
- 26.Fahmy MA, Shoman HM, Hassan EE. The protective role of thiola and soybean seeds against the genotoxicity induced by potassium dichromate in mice. Mutat Res. 2002;517:1–12. doi: 10.1016/s1383-5718(02)00013-x. [DOI] [PubMed] [Google Scholar]


