Abstract
Aim:
The aim of this study is to investigate whether nitric oxide (NO)-mediated colonic motility was altered in rat irritable bowel syndrome (IBS) model, using different isoforms of NO-synthase (NOS) inhibitors.
Materials and Methods:
The animal model of IBS-like visceral hypersensitivity was induced by intra-colonic infusion of 0.5% acetic acid (AA) in saline once daily from postnatal days 8 to 21. Control animals received saline instead of AA. Experiments were performed at the end of 8 weeks. Distal colon tissues were resected and direct effects of different NOS inhibitors; N-omega-nitro-L-arginine methyl ester hydrochloride, (L-NAME), ARL-17477 dihydrochloride hydrate (ARL 17477), N-[3-(Aminomethyl) phenyl] methyl]-ethanimidamidedihydrochloride (1400 W), and N5-(1-Iminoethyl)-L-ornithine dihydrochloride (L-NIO) were evaluated concentration-dependently in vitro tissue bath. Besides, morphology of both groups was assessed with hematoxylin and eosin (H and E) staining and the impact of NO antibodies was determined using the immunohistochemical method.
Results:
The mean pressure values of spontaneous contractions and KCL (80 mmol/L) responses of distal colonic segments were similar in normal and IBS rats. L-NAME and ARL-17477 significantly increased the mean pressure of spontaneous colonic contractions in normal rats versus own base values (P < 0.05), but this increase did not significantly different when compared to IBS rats. In H and E staining, there was no difference with regard to morphology between two groups. Neuronal NOS (nNOS) immunoreactivity was found to be significantly decreased in IBS when compared to control groups (P < 0.05).
Conclusion:
L-NAME and ARL-17477 mediated mean pressure values were found to be slightly decreased in IBS rats. These findings may be related to a decrease in nNOS level in IBS.
Keywords: Distal colon, immunohistochemistry, irritable bowel syndrome, nitric oxide synthase
The irritable bowel syndrome (IBS) is a chronic functional bowel disorder characterized by the presence of episodic abdominal pain or discomfort in association with altered bowel habits and other GI symptoms such as bloating and flatulence.[1] It has been demonstrated that the bowel disorder in IBS patients results from abnormal motor function of the colon.[2,3] However, the mechanisms underlying the disordered colonic motility in IBS are still not well-understood.
Nitric oxide (NO) is a lipophilic, highly diffusible, and short-lived physiological messenger which plays an essential role in the physiology and pathophysiology of the gastrointestinal tract.[4,5] NO is synthesized from L-arginine catalyzed by NO-synthase (NOS). Three isoforms of NOSs have been identified so far: Neuronal NOS (nNOS) and endothelial NOS (eNOS) are involved in smooth muscle and vascular relaxation,[6] while inducible NOS (iNOS) is induced in response to inflammation.[7] Tjong et al. showed that neonatal maternal separation increased NO production by upregulation of nNOS expression in rat distal colon.[8] NK1R-mediated colonic motor response was also shown to be increased in IBS rats, due to a decrease in the nitrergic inhibitory neural component.[9] Previous clinical studies have revealed an increase in rectal and plasma NO levels in patients with IBS.[10,11] These findings suggest that nitrergic system is probably involved in the development of IBS. However, the role iNOS, eNOS and nNOS in the IBS-induced bowel dysmotility is not well-understood.
We aimed to evaluate the possible role of different isoforms of NOS on colonic motility in a rat IBS model.
Materials and Methods
Animals
Experiments were performed on male Wistar-Albino rats in Experimental Animal Laboratory of Dokuz Eylul University. Rats were housed with ad libitum food and water in standard rodent cages at 22°C ± 2°C in a 12-h light-dark controlled room. All neonates used in the experiment were housed per cage with 1 adult female rat until they were 1-month-old. The study protocol was reviewed and approved by the Animal Ethics Committee of the Dokuz Eylul University.
Induction of Irritable Bowel Syndrome
Neonatal male Wistar-Albino rats were randomly divided into two groups. Group 1 received colonic infusion of 0.9% saline as the control group. Group 2 received 0.5% acetic acid (AA) solution from postnatal days 8–21 (0.3 mL daily for days 8–14 and 0.5 mL daily for days 15–21). The infusion was performed through a coronary arteriography catheter inserted 2 cm from the anus. The sensitivity to colorectal distention were tested on day 43.[12] Experiments were conducted in these rats at the end of 8 weeks.
Evaluation of Visceral Sensitivity
On the 43rd day of our study, it was recorded that the threshold degree induced visually identifiable contraction of the abdominal wall and body arching during rectal distention to evaluate visceral hypersensitivity. After 30 min of adaptation in small box (20 cm × 8 cm × 8 cm), rectal distention was performed using the 6F Fogarty arterial embolectomy catheter (Edwards Lifesciences LLC, USA) in the descending colon (1 cm from the anal verge) Rectal distentions were performed with increasing volumes of saline by adding increments 20 µL, starting at 100 µL. For each measurement, the rats were given rectal distention for 20 s every 2 min. The measurements were repeated three times for accuracy, and the difference between replicate measurements was <20%.
Recording of Colonic Motor Activities
At the end of 8 weeks, rats were sacrificed by cervical dislocation, and a 2 cm distal colonic segment was removed. 0.5 cm thickness rings of distal colon was placed in the circular direction in 20 ml tissue baths, filled with preaerated (95% O2 and 5% CO2) Krebs bicarbonate solution at 37°C. Krebs bicarbonate solution (composition in mM: NaCl, 120; KCl, 4.6; CaCl2, 2.5; MgCl2, 1.2; NaHCO3, 22; NaH2PO4, 1.14 and glucose 11.5). The upper end of the segments was tied to an isometric force displacement transducer (FDT-05, MAY, Commat, Ankara, Turkey) and preloaded with 0.6 g tension. Tissues were allowed to equilibrate for 30 min and washed at every 10 min.
After equilibrium, N-omega-nitro-L-arginine methyl ester hydrochloride, a nonselective inhibitor NOS, (L-NAME, 10−5 and 10−4 mol/L, Sigma, St. Louis, MO, USA); ARL-17477 dihydrochloride hydrate, a selective inhibitor of neuronal-NOS, (ARL 17477, 10−7 and 10−6 mol/L, Sigma, St. Louis, MO, USA); N-[3-(Aminomethyl) phenyl] methyl]-ethanimidamidedihydrochloride, a selective inhibitor of inducible-NOS, (1400 W, 10−6 and 10−5 mol/L, Sigma, St. Louis, MO, USA); and N5-(1-Iminoethyl)-L-ornithine dihydrochloride, a selective inhibitor of eNOS, (L-NIO, 10−5 and 10−4 mol/L, Tocris, Ellisville, MO, USA) were added cumulatively to the tissue bath to investigate the direct effect on distal colon segments of NOS inhibitors. All drugs were prepared freshly on the day of the experiment.
Direct effects of cumulative concentrations of NOS inhibitors on the mean pressure of spontaneous colonic contractions were calculated as a percentage of the mean pressure of the initial (base) spontaneous colonic contraction for 5-min intervals in both control and IBS groups. At the end of all experiments, the tonic contraction by KCl (80 mmol/L) was measured to test the contraction health of distal colon smooth muscle isolated from the control and IBS groups.
Histological Tests
All samples were fixed in 10% formalin for 24 h and processed for embedding in paraffin using routine protocol. Sections 5 µm thick were cut on a rotary microtome (Leica RM2245) and stained with hematoxylin and eosin (H and E).
Immunohistochemistry
Formalin-fixed, paraffin-embedded sections were used for immunohistochemical staining. Tissue samples were stored at 60°C overnight and then were deparaffinized by xylene for 30 min. After dehydration of the sections with ethanol, they were washed with distilled water. The tissues were then treated with 2% trypsin (ab970, Abcam, Cambridge, UK) at 37°C for 15 min and incubated in 3% H2O2 solution for 15 min to inhibit endogenous peroxidase activity. Then, sections were incubated with anti-nNOS primer antibody (sc-55521, Santa Cruz Biotechnology, Inc.), anti-iNOS primer antibody (sc-649, Santa Cruz Biotechnology, Inc.), anti-eNOS antibody (sc-654, Santa Cruz Biotechnology, Inc.) in a 1/100 dilution for 18 h at +4°C. They were then given an additional three 5-washes in PBS, followed by incubation with biotinylated IgG and administration of streptavidin peroxidase (Histostain Plus Kit Cat. No: 85-9043, Invitrogen). After washing the secondary antibody with PBS three times for 5 min, the sections were stained with DAB substrate system containing diaminobenzidine (DAB-plus Substrate Kit, Invitrogen) to detect the immunoreactivity, and then stained with Mayer's hematoxylin (72804E, Microm, Walldorf, Germany) for counterstaining. They were covered with mounting medium (Clear Mount, Mounting Medium Ref: 008110 Invitrogen, USA) and observed with light microscopy (Olympus BX-43, Tokyo, Japan). Two observers blinded to treatment assignment assessed the staining scores independently.
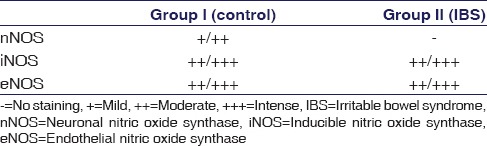
Immunostaining for nNOS, iNOS and eNOS immunostaining intensity was categorized by the following scores: 0 (no staining), 1 (mild staining), 2 (moderate staining) and 3 (intense staining).
Statistical Analysis
Statistical analyses were performed using Prism 5.0 software (GraphPad Software Inc., CA, USA). All data were expressed as mean ± standard error of mean. Statistical comparisons for repeated measurements within groups were evaluated using Friedman test followed by Wilcoxon signed rank tests to determine the significant group. Comparisons between groups were evaluated with Mann–Whitney U-test. Differences were considered statistically significant when P < 0.05.
Results
Response to Visceral Sensitivity
We observed visceral hypersensitivity in adult rats. Rats were tested for sensitivity to colorectal distention on day 43. Threshold of abdominal wall contraction and body arching in response to increasing colorectal distension was significantly lower in the AA (n = 7) group than in the control (n = 5) group (0.21 ± 0.06 vs. 0.42 ± 0.19 mL for abdominal muscle contraction and 0.43 ± 0.13 vs. 0.85 ± 0.24 mL for body arching, P < 0.01, t-test) There was no difference in body weight between Group 1 and Group 2.
Effect of Nitric Oxide Synthase Inhibitors on Mean Pressure of Spontaneous Motor Activities of Isolated Colonic Segments
The isolated colonic segments showed spontaneous motor activities in rest. Spontaneous contractility of the distal colon did not significantly change in IBS rats compared to control rats. The mean pressure of the spontaneous colonic contractions was 320.40 ± 15.43 mmHg and 298.00 ± 14.22 mmHg in normal (n = 5) and IBS rats (n = 7), respectively (P > 0.05).
Muscle segments isolated from control and IBS rats responded to nonselective NOS inhibitor L-NAME (10−5 and 10−4 mol/L) and selective nNOS inhibitor ARL-17477 (10−7 and 10−6 mol/L) with a progressive increase of contractile activity in a concentration-dependent manner [Figure 1a-d]. L-NAME significantly increased the mean pressure in both control and IBS groups at concentration of 10−4 mol/L (P < 0.05). ARL-17477 significantly increased the mean pressure on only in the control group at concentrations of 10−7 and 10−6 mol/L, respectively (P < 0.05). The mean pressure values for both L-NAME and ARL-17477 were higher in the control groups compared with IBS groups; although, these high responses were not found to be statistically significant (P > 0.05) [Figure 2]. Selective iNOS inhibitor 1400 W (10−6, 10−5 mol/L) and selective eNOS inhibitor L-NIO (10−6, 10−5 mol/L) had no significant effect on colonic contractile activity in both control and IBS rats [Figure 2].
Figure 1.

Representative record of effects of L-NAME and ARL-17477 on spontaneous motor contractions of colonic tissues isolated from control and IBS-rats. L-NAME significantly increased the mean pressure in both control (a) and IBS (b) groups at concentration of 10−4 mol/L (P < 0.05). ARL-17477 significantly increased the mean pressure on only in the control (c) group at concentrations of 10−7 and 10−6 mol/L (P < 0.05); did not increase significant in IBS (d) group (P > 0.05). IBS: Irritable bowel syndrome
Figure 2.

Effects of L-NAME, ARL-17477, 1400 W and L-NIO on mean pressure of spontaneous contractions of colonic tissues isolated from control and IBS-rats. The mean pressure value of L-NAME was higher in both control and IBS groups at concentration of 10-4 mol/L. (a) P < 0.05 vs own base values assessed by Friedman test followed by Wilcoxon signed rank tests. The mean pressure value of ARL-17477 was higher in the only control group at concentrations of 10-7 and 10-6 mol/L, but was not in IBS group. (b) P < 0.05 vs own base value assessed by Friedman test followed by Wilcoxon signed rank tests. 1400 W (10-6, 10-5 mol/L) and L-NIO (10-6, 10-5 mol/L) had no significant effect on spontaneous colonic activity in both control and IBS groups (P > 0.05)
The contractile responses to KCl (80 mmol/L), which were tested at the end of each experiment, did not differ significantly between control (n = 5) and IBS (n = 7) groups. KCl contractile responses (mg contractions) were 2692.27 ± 569.87 and 3161.98 ± 671.64 in normal (n = 5) and IBS rat (n = 7) colon, respectively (P > 0.05).
Histopathological Findings
As above, histological examination showed no difference with regard to morphology between two groups in H and E staining. There was no significant structural damage to colonic tissues. Particularly, the thickness of smooth muscles was similar in both two tissues when measured with light microscopy (Olympus BX43, Tokyo, Kanto, Japan) [Figure 3a and b].
Figure 3.
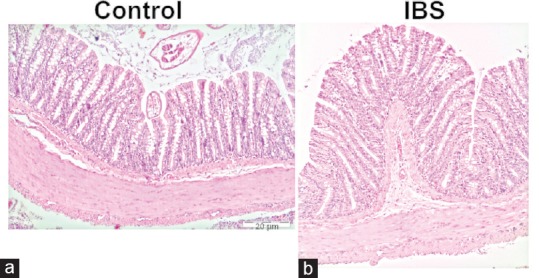
Photomicrographs of hematoxylin and eosin staining within the control and IBS tissues is depicted (×10). There was no difference with regard to morphology between control (a) and IBS (b) tissues in hematoxylin and eosin staining. IBS: Irritable bowel syndrome
Immunohistochemical Analysis
As a result of the immunohistochemical staining using anti-nNOS primary antibody, mild (1) to moderate (2) immunoreactivity was observed in the control groups [Figure 4a] while no staining (0) was detected in the IBS tissues [Figure 4b] (P < 0.05).
Figure 4.

Photomicrographsof nNOS, iNOS and eNOS staining within the control and IBS tissues is depicted (×10). The immunoreactivity of sections stained with (a and b) nNOS (c and d) iNOS and (e and f) eNOS primary antibodies. Intensity of reactivity (score: 0= no staining; 1= mild; 2= moderate, and; 3= intense). nNOS: Neuronal nitric oxide synthase, iNOS: Inducible nitric oxide synthase, eNOS: Endothelial nitric oxide synthase
Although the immunoreactivity of iNOS and eNOS did not show a significant difference between the control and IBS groups; moderate (2) to intense (3) immunoreactivities of the two antibodies were found [Figure 4c-f] (P > 0.05), Immunolabeling intensity of nNOS, iNOS and eNOS is presented in Table 1.
Table 1.
Immunolabeling intensity of neuronal nitric oxide synthase, inducible nitric oxide synthase, and endothelial nitric oxide synthase
Discussion
IBS is a common disorder characterized by recurrent abdominal pain and alterations in the defecation pattern. Based on the alterations in the bowel habit of patients, IBS is often classified into IBS with diarrhea (D-IBS), IBS with constipation (C-IBS) and alternating form.[13] While much work has been carried out on visceral hypersensitivity and gastrointestinal motility of IBS subgroups, little is known about the differences in the myenteric plexus of IBS subgroups.[14,15] The amount of NO in the myenteric plexus of IBS subgroups may be related to the alterations in the bowel habit.
NO is synthesized and released from the myenteric plexus of the distal colon. NO activates soluble guanylyl cyclase[16] and modulates motility in rat distal colon.[17,18] Xu et al. reported an enhanced neurotransmitter NO in the myenteric plexus of the colon in rodent model of IBS compared with the control group.[19] Ragy and Elbassuoni found that sodium nitroprusside, the NO donor, produced significant decrease, whereas nitric oxide synthase inhibitor produced a significant increase in the amplitude of spontaneous contractions of the rabbit ileum. Inhibitory effect on spontaneous activity of NO is mediated by cyclic GMP generation system and Ca (2+)-dependent K(+) channels.[20]
In this study, the animal model of IBS-like visceral hypersensitivity was induced by intra-colonic infusion of 0.5% AA. The direct effects of different NOS inhibitors on distal colonic segments were evaluated in vitro tissue bath. First, the initial spontaneous motor activities of distal colon were analyzed in both the control and IBS rat groups and were not found significantly different between two groups. Thereafter, the direct effects on initial spontaneous colonic contractions of NOS inhibitors were estimated with mean pressure value, because of the amplitude and frequency of spontaneous contractions was found to be extremely frequent and variable in the distal colon. The mean pressure of the initial spontaneous motor contractions, KCl contractile responses, and histopathology of colonic smooth muscle was not significantly different between the two groups. These results indicate that contractile mechanisms of the colonic tissues were intact in both the control and IBS rats. In addition, transient colonic irritation due to 0.5% AA in neonatal rats has no significant effects on the tissue histology of the colon mucosa of adult rats but lead to visceral hypersensitivity. This is thus a suitable model for studies of chronic visceral hypersensitivity and IBS.
Nonselective NOS inhibitor L-NAME significantly increased the mean pressure values in both groups at concentration of 10−4 mol/L versus own base value. The selective nNOS inhibitor ARL-17477 significantly increased the mean pressure values in the only control group at concentration of 10−7 and 10−6 mol/L versus own base value, whereas mean pressure values in IBS groups did not change significantly. Although the mean pressure values of L-NAME and ARL-17477 were higher in the control groups compared to IBS groups, these differences were not statistically significant. In addition, we observed that selective iNOS inhibitor 1400W and selective eNOS inhibitor L-NIO did not significantly change the mean pressure values of spontaneous colonic contractions in both control and IBS groups. The SEM of mean pressure values in each concentration were found to be high in all groups which may possibly resulted from the individual variability of bowel motility in IBS subgroups.
There are no adequate studies in the literature on the relation between NO and IBS. While Dykhuizen et al.[21] reported no significant change in NO levels between IBS patients and control subjects, Yazar et al.[11] reported an increase in patients with C-IBS. Using an immunohistochemical technique, Reinders et al.[10] also revealed an increase in NO concentrations in rectal mucosa of patients with IBS. In our experimental model, D-IBS and/or alternating form may have occurred in rats. In our study, analysis of immunohistochemical staining demonstrated mild/moderate immunoreactivity for nNOS in control group, but no immunoreactivity in IBS group; these differences were statistically significant. Decrease immunoreactivity to ARL may be due to the reduction of nNOS levels in tissues with IBS. On the other hand, we did not find a significant difference in immunoreactivity of iNOS and eNOS between two groups. Tjong et al. showed which elevated NO production and upregulation of nNOS expression in the distal colon of neonatal separation rats. This study provided new information that nNOS was involved in the mechanism of early-life stress in the pathogenesis of IBS.[8] We used that IBS-like visceral hypersensitivity was induced by AA, but not neonatal maternal stress model. Therefore, we claimed that decreased nNOS level may be responsible from changes in colonic response in visceral hypersensitivity model of IBS. However, this finding should be further tested using an NO-enhancer agent such as sildenafil.
A close correlation is found between NO production and gastrointestinal diseases. It is proposed that amount of NO plays an important role in the pathogenesis of these diseases.[22] It has been suggested that constitutive NO exerts a protective effect on intestinal mucosa.[23] However, the mechanisms of such putative protection have not been elucidated. In our study, contractile responses of L-NAME and ARL-17477 decreased in IBS rats compared to control rats, although not statistically significant. Immunoreactivity of nNOS in immunohistochemical staining significantly decreased in IBS rats. Besides, the contractile responses of 1400 W and L-NIO and immunoreactivities of iNOS and eNOS did not significantly change in both control and IBS rats.
Conclusion
These findings suggest that IBS-like visceral hypersensitivity model reduces the nNOS-mediated endogenous NO levels in the distal colon of IBS rats. This data partly explain the diminished mean pressure values to L-NAME and especially ARL-17477 in the distal colon of rats with IBS. Because there is not NO product of nNOS in colonic tissue with IBS and inhibition of nNOS does not increase the mean pressure the value. These results may contribute to the literature for understanding the mechanisms of decreased colonic motility in IBS. Further studies are needed to determine the exact mechanism of alterations in the bowel motility in IBS.
Financial Support and Sponsorship
Izmir Katip Celebi University of Scientific Research Projects Coordination Unit, No. 213-3-TSBP-22.
Conflicts of Interest
There are no conflicts of interest.
References
- 1.Longstreth GF, Thompson WG, Chey WD, Houghton LA, Mearin F, Spiller RC. Functional bowel disorders. Gastroenterology. 2006;130:1480–91. doi: 10.1053/j.gastro.2005.11.061. [DOI] [PubMed] [Google Scholar]
- 2.Chey WY, Jin HO, Lee MH, Sun SW, Lee KY. Colonic motility abnormality in patients with irritable bowel syndrome exhibiting abdominal pain and diarrhea. Am J Gastroenterol. 2001;96:1499–506. doi: 10.1111/j.1572-0241.2001.03804.x. [DOI] [PubMed] [Google Scholar]
- 3.Vassallo MJ, Camilleri M, Phillips SF, Steadman CJ, Talley NJ, Hanson RB, et al. Colonic tone and motility in patients with irritable bowel syndrome. Mayo Clin Proc. 1992;67:725–31. doi: 10.1016/s0025-6196(12)60796-4. [DOI] [PubMed] [Google Scholar]
- 4.Lancaster JR., Jr A tutorial on the diffusibility and reactivity of free nitric oxide. Nitric Oxide. 1997;1:18–30. doi: 10.1006/niox.1996.0112. [DOI] [PubMed] [Google Scholar]
- 5.Shah V, Lyford G, Gores G, Farrugia G. Nitric oxide in gastrointestinal health and disease. Gastroenterology. 2004;126:903–13. doi: 10.1053/j.gastro.2003.11.046. [DOI] [PubMed] [Google Scholar]
- 6.Zhou L, Zhu DY. Neuronal nitric oxide synthase: Structure, subcellular localization, regulation, and clinical implications. Nitric Oxide. 2009;20:223–30. doi: 10.1016/j.niox.2009.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Dijkstra G, van Goor H, Jansen PL, Moshage H. Targeting nitric oxide in the gastrointestinal tract. Curr Opin Investig Drugs. 2004;5:529–36. [PubMed] [Google Scholar]
- 8.Tjong YW, Ip SP, Lao L, Wu J, Fong HH, Sung JJ, et al. Role of neuronal nitric oxide synthase in colonic distension-induced hyperalgesia in distal colon of neonatal maternal separated male rats. Neurogastroenterol Motil. 2011;23:666–e278. doi: 10.1111/j.1365-2982.2011.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.La JH, Kim TW, Sung TS, Kim HJ, Kim JY, Yang IS. Increase in neurokinin-1 receptor-mediated colonic motor response in a rat model of irritable bowel syndrome. World J Gastroenterol. 2005;11:237–41. doi: 10.3748/wjg.v11.i2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reinders CI, Herulf M, Ljung T, Hollenberg J, Weitzberg E, Lundberg JO, et al. Rectal mucosal nitric oxide in differentiation of inflammatory bowel disease and irritable bowel syndrome. Clin Gastroenterol Hepatol. 2005;3:777–83. doi: 10.1016/s1542-3565(05)00182-5. [DOI] [PubMed] [Google Scholar]
- 11.Yazar A, Büyükafpar K, Polat G, Pata C, Kanýk A, Tiftik EN, et al. The urinary 5-hydroxyindole acetic acid and plasma nitric oxide levels in irritable bowel syndrome: A preliminary study. Scott Med J. 2005;50:27–9. doi: 10.1177/003693300505000111. [DOI] [PubMed] [Google Scholar]
- 12.Yan C, Xin-Guang L, Hua-Hong W, Jun-Xia L, Yi-Xuan L. Effect of the 5-HT4 receptor and serotonin transporter on visceral hypersensitivity in rats. Braz J Med Biol Res. 2012;45:948–54. doi: 10.1590/S0100-879X2012007500122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bouin M, Plourde V, Boivin M, Riberdy M, Lupien F, Laganière M, et al. Rectal distention testing in patients with irritable bowel syndrome: Sensitivity, specificity, and predictive values of pain sensory thresholds. Gastroenterology. 2002;122:1771–7. doi: 10.1053/gast.2002.33601. [DOI] [PubMed] [Google Scholar]
- 14.Lee OY. Asian motility studies in irritable bowel syndrome. J Neurogastroenterol Motil. 2010;16:120–30. doi: 10.5056/jnm.2010.16.2.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamshiri H, Paragomi P, Paydar MJ, Moezi L, Bahadori M, Behfar B, et al. Antinociceptive effect of chronic lithium on visceral hypersensitivity in a rat model of diarrhea-predominant irritable bowel syndrome: The role of nitric oxide pathway. J Gastroenterol Hepatol. 2009;24:672–80. doi: 10.1111/j.1440-1746.2008.05652.x. [DOI] [PubMed] [Google Scholar]
- 16.Garthwaite J. New insight into the functioning of nitric oxide-receptive guanylyl cyclase: Physiological and pharmacological implications. Mol Cell Biochem. 2010;334:221–32. doi: 10.1007/s11010-009-0318-8. [DOI] [PubMed] [Google Scholar]
- 17.Mizuta Y, Takahashi T, Owyang C. Nitrergic regulation of colonic transit in rats. Am J Physiol. 1999;277(2 Pt 1):G275–9. doi: 10.1152/ajpgi.1999.277.2.G275. [DOI] [PubMed] [Google Scholar]
- 18.Benabdallah H, Messaoudi D, Gharzouli K. The spontaneous mechanical activity of the circular smooth muscle of the rabbit colon in vitro. Pharmacol Res. 2008;57:132–41. doi: 10.1016/j.phrs.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 19.Xu JR, Luo JY, Shang L, Kong WM. Effect of change in an inhibitory neurotransmitter of the myenteric plexus on the pathogenetic mechanism of irritable bowel syndrome subgroups in rat models. Chin J Dig Dis. 2006;7:89–96. doi: 10.1111/j.1443-9573.2006.00248.x. [DOI] [PubMed] [Google Scholar]
- 20.Ragy M, Elbassuoni E. The role of nitric oxide and L-type calcium channel blocker in the contractility of rabbit ileum in vitro. J Physiol Biochem. 2012;68:521–8. doi: 10.1007/s13105-012-0167-x. [DOI] [PubMed] [Google Scholar]
- 21.Dykhuizen RS, Masson J, McKnight G, Mowat AN, Smith CC, Smith LM, et al. Plasma nitrate concentration in infective gastroenteritis and inflammatory bowel disease. Gut. 1996;39:393–5. doi: 10.1136/gut.39.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rivera LR, Poole DP, Thacker M, Furness JB. The involvement of nitric oxide synthase neurons in enteric neuropathies. Neurogastroenterol Motil. 2011;23:980–8. doi: 10.1111/j.1365-2982.2011.01780.x. [DOI] [PubMed] [Google Scholar]
- 23.Boughton-Smith NK, Hutcheson IR, Deakin AM, Whittle BJ, Moncada S. Protective effect of S-nitroso-N-acetyl-penicillamine in endotoxin-induced acute intestinal damage in the rat. Eur J Pharmacol. 1990;191:485–8. doi: 10.1016/0014-2999(90)94185-z. [DOI] [PubMed] [Google Scholar]


