Abstract
Objectives:
The aim of the present work was to study the anti-inflammatory and anti-arthritic activities of petroleum ether extract of fenugreek seeds.
Materials and Methods:
Fenugreek seed powder was extracted in petroleum ether by cold maceration. This fenugreek seed petroleum ether extract (FSPEE) was analyzed by gas–liquid chromatography (GLC) and tested on rats against carrageenan and formaldehyde-induced paw edema, complete Freund's adjuvant (CFA)-induced arthritis and cotton pellet-induced granuloma. Changes in serum glutamic oxaloacetic tansaminase (SGOT), serum glutamate-pyruvate transaminase (SGPT), and alkaline phosphatase (ALP) activities in liver and serum were also studied in cotton pellet-induced arthritic rats. Data were analyzed by Student's t-test. P <0.05 was considered statistically significant.
Results:
GLC of FSPEE showed oleic (33.61%), linoleic (40.37%), and linolenic (12.51%) acids. With 0.5 mL/kg FSPEE treatment, there was 37% (P < 0.05) and 85% (P < 0.05) reduction in inflammation of the paw in carrageenan and formaldehyde-induced paw edema. In CFA-induced arthritis, a biphasic increase in paw volume followed by decrease was seen. There was 42.5% (P < 0.01) reduction in the weight of cotton pellets and significant (P < 0.01) reductions in the elevated SGPT and ALP activities in serum and liver of FSPEE (0.5 mL/kg) treated rats.
Conclusion:
Thus, petroleum ether extract of fenugreek seeds has significant anti-inflammatory and anti-arthritic activities which are due to the presence of linolenic and linoleic acids.
Keywords: Anti-inflammatory activity, complete Freund's adjuvant, cotton pellet, formaldehyde, Trigonella foenum-graecum
Fenugreek seeds (Trigonella foenum-graecum Linn. Fabaceae), are used as a spice, in colic, flatulence, dysentery, diarrhea, diabetes, and lipid disorders in India.[1] Mishkinsky et al.[2] reported that ethanol extract of fenugreek seed powder and an alkaloid trigonelline isolated from it, when given for 3 weeks, reduced hyperglycemia in diabetic rats. Riyad et al.[3] found a reduction in hyperglycemia of diabetic rats when they were pretreated with 20% fenugreek seed in the diet. Khosla et al.[4] observed a lowering of blood glucose in diabetic rats treated with 2–8 g/kg fenugreek powder. Ribes et al.[5] treated nondiabetic and diabetic dogs with defatted, fiber-rich fraction of fenugreek seeds for 8 days. They observed a lowering of basal blood glucose level, plasma glucagon, and somatostatin in diabetic dogs. The fiber rich defatted fenugreek powder was also found to reduce hypercholesterolemia in diabetic dogs.[6] In Iranian traditional medicine, fenugreek, under the name of “Shanbalileh,” is used to treat rheumatism.[7] Ethanol extract, mucilage, and flavonoids of fenugreek seeds were found to have anti-inflammatory, anti-arthritic, and anti-oxidant activities.[8,9,10] During preliminary screening experiments, we found good anti-inflammatory activity in fenugreek seed petroleum ether extract (FSPEE). A 38–40% of reduction in inflammation in carrageenan-induced rat paw was noted when FSPEE was given at a dose of 0.5 mL/kg. Hence, we studied the fatty acid composition of this extract and its anti-inflammatory activity employing inflammatory and arthritic models in rats.
Materials and Methods
Seeds
Trigonella foenum-graecum seeds were procured from the local market and authenticated by a trained botanist.
Chemicals
The chemicals procured are indicated in the parenthesis. Petroleum ether (boiling range 60–80°C) (S. D. Fine Chemicals Ltd., India); κ Carrageenan Type IV and Formaldehyde (Sigma-Aldrich, USA); complete Freund's adjuvant (CFA) (Difco Laboratories, USA.); Liver enzyme standard kits (Star Diagnostics and Crest Bio-systems, Ahmedabad, India); phenylbutazone (PBZ) (Zydus Cadila Healthcare, Ahmedabad, India); standard rat feed (Lipton's India, Bombay, India).
Instruments
Gas-liquid chromatography (GLC) instrument (Perkin-Elmer, U.S.A., model: F-11); colorimeter (Systronics Ltd., India).
Animals
The experiments were carried out after obtaining necessary clearances from the Institutional Animal Ethics Committee (CPCSEA registration No. 197/2000/CPCSEA). Albino rats (Wistar strain) of either sex (200–250 g) were used in the experiments. Animals were procured from the animal breeding house, Zydus Cadila Healthcare Laboratories. The animals were maintained under standard laboratory conditions of 12 h day-night cycle, 25 ± 3°C, 45–50% RH, and had free access to food and water.
Extraction of Seeds
Seed powder of fenugreek (500 g) was extracted with 2 × 2.5 L petroleum ether (60–80°C) at room temperature for 4 days. The extract was evaporated to get a viscous oily extract (FSPEE).
Gas–Liquid Chromatographic Analysis of Fenugreek Seed Petroleum Ether Extract
FSPEE was analyzed by GLC after converting the fatty acids into methyl esters. FSPEE (1.0 mL) was dissolved in 18 mL of petroleum ether in a stoppered test tube and to this 2 mL 2M solution of potassium hydroxide in methanol was added.
After thorough mixing, the upper petroleum ether layer was taken for GLC analysis under the following conditions:
Column: 1.52 m glass column with 10% DEGS on chromosorb WHP; Oven temperature: 180°C.
Carrier gas: Nitrogen; injection volume: 0.4 μL; amplification: 5 × 102; Chart speed: 10 mm/min. Some available reference standards of fatty acids were used for comparison and identification of the fatty acids.
Dosing and Drug Administration
FSPEE, ground nut oil (as vehicle control) and PBZ (PBZ-as positive control) were administered p.o. in all the experiments. FSPEE (1.0 mL) was mixed with 1.5 mL ground nut oil. PBZ was dissolved in 1% of normal saline with two drops of tween-80. In the carrageenan paw edema experiment, five groups of five animals in each group were employed which were treated as under:
Group I (0.5 mL/kg groundnut oil); Groups II, III, and IV (0.25 mL/kg, 0.5 mL/kg and 0.75 mL/kg FSPEE, respectively); Group V (100 mg/kg PBZ).
In all other experimental models, three groups of five animals each were used. Animals in Groups I, II, and III were administered groundnut oil (0.5 mL/kg), FSPEE (0.5 mL/kg), and PBZ (100 mg/kg), respectively.
Carrageenan-Induced Paw Edema
The method used was that suggested by Singh and Ghosh, which employed a plethysmometer.[11] Vehicle, FSPEE, and PBZ were administered to animals and after 1 h, 0.1 mL carrageenan was injected in the subplantar region of the right hind paw. Volume of the paw was measured at 0 h and after 3 h with a plethysmometer.
Anti-inflammatory activity was calculated as follows:
% Inhibition of inflammation = 1−(VT/VC) ×100
Where VT is the change in the paw volume in the treatment group and VC is the change in the paw volume in the control group.
Formaldehyde-Induced Paw Edema
The method described by Brownlee[12] was used. Paw volume was measured at 0 and 48 h after formaldehyde challenge. Anti-inflammatory activity was calculated as described above.
Complete Freund's Adjuvant-Induced Arthritis
CFA was made into a fine emulsion with a glass syringe. Vehicle/FSPEE/PBZ was administered orally 30 min before the injection of CFA (day 1) and once daily until the end of the experiment.[13] CFA was injected beneath the plantar aponeurosis in the right hind paw (0.1 mL) and into the root of the tail (0.05 mL) subcutaneously. The volumes of both the hind paws were measured just before adjuvant injection and on the 2nd, 3rd, 5th, 7th, 10th, 14th, 17th, 22nd, and 26th days after injection.
Cotton Pellet-Induced Granuloma
The method employed was that suggested by Goldstein et al.[14] Vehicle, FSPEE, and PBZ were given for 7 days to the respective groups. On the 8th day, the cotton pellets were removed from the rats after light ether anesthesia, cleared of adherent blood, and dried to a constant weight in a hot air oven at 80°C. Reduction in the granuloma formation was calculated as under:
% Reduction in granuloma formation = 1−(Wt/WC) ×100
Where Wt is the change in the weight of cotton pellet in the treatment group and WC is the change in the weight of the cotton pellet in the control group.
Blood Sampling and Serum Analysis
After removal of the cotton pellets, 4–5 mL blood was collected for serum analysis from the coronary artery of animals by decapitation under light ether anesthesia. Livers were carefully excised immediately after the collection of blood and homogenized separately in cold water at 4–6°C. Serum and the liver homogenates were analyzed for serum glutamate-pyruvate transaminase (SGPT), serum glutamic oxaloacetic transaminase (SGOT), and alkaline phosphatase (ALP) activities using standard kits.
Statistical Analysis
Data are expressed as mean ± standard error of mean. Data of control groups and treatment groups were compared by Student's t-test using GraphPad Prism 5.0 software (Graph Pad Software Inc., La Jolla, CA, U.S.A.). P <</i> 0.05 was considered statistically significant.
Results
Fenugreek seeds yielded 7% of w/w FSPEE, which mainly consisted stearic (1.72%), palmitic (9.58%), oleic (33.61%), linoleic (40.37%), and linolenic (12.51%) acids.
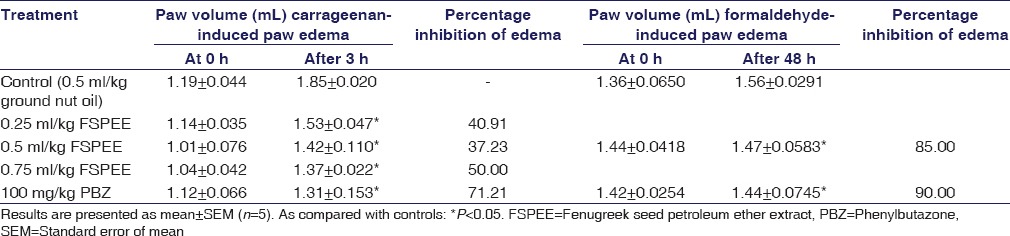
FSPEE exhibited significant (P < 0.05) anti-inflammatory activity against carrageenan-induced paw edema at all the three doses tested. At 0.5 mL/kg dose, formaldehyde-induced inflammation was significantly inhibited (P < 0.05) in 48 h [Table 1]. It showed 85% of inhibition while a 90% of inhibition was noted in the PBZ-treated group.
Table 1.
Effect of oral administration of different doses of fenugreek seed petroleum ether extract on carrageenan-induced paw edema and formaldehyde-induced paw edema in rats
In FSPEE-treated CFA arthritic rats, the right paw showed a sudden inflammatory response which reduced to near half on day 5. A biphasic phenomenon of increase in paw volume followed by decrease was observed in all the groups [Figure 1].
Figure 1.
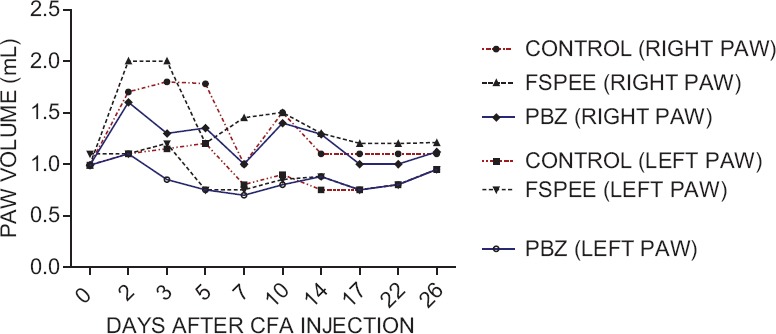
Effect of Fenugreek seed petroleum ether extract on complete Freund's adjuvant-induced granuloma in rats. (FSPEE: Fenugreek seed petroleum ether extract; PBZ: Phenylbutazone; CFA: Complete Freund's adjuvant)
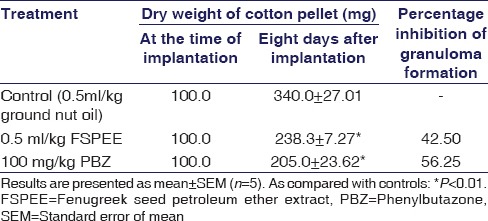
There was a 45% (P < 0.05) reduction in the weights of cotton pellets due to the formation of granuloma in animals treated with FSPEE [Table 2]. This reduction is comparable to that of animals treated with 100 mg/kg PBZ (56%).
Table 2.
Effect of oral administration of fenugreek seed petroleum ether extract on cotton pellet-induced granuloma in rats
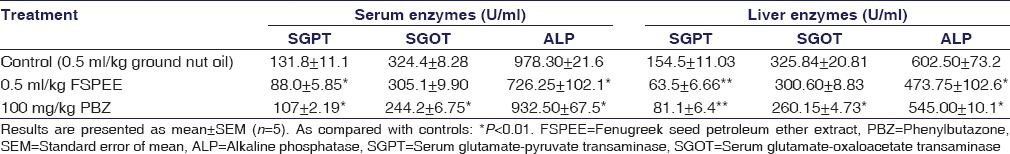
The activities of various liver enzymes are higher in untreated animals than in rats treated with either PBZ or FSPEE. The activity of SGPT in serum was reduced from the elevated values of 131.8 ± 11.1 to 107 ± 2.19 in PBZ-treated rats (P < 0.01). FSPEE treatment lowered SGPT activity to 88 ± 5.85 U/mL (P < 0.01). The reduction in serum SGOT activity was lower in FSPEE-treated rats than that found in PBZ-treated rats. FSPEE significantly (P < 0.01) reduced serum ALP activity. Elevated SGPT and ALP activities were reduced significantly (P < 0.01; P < 0.01) in liver of rats treated with FSPEE or PBZ [Table 3]. Two phenomena are conspicuous in arthritic conditions. They are marked increase in the serum and liver peroxide enzyme activities in the arthritic animals and anti-arthritic activities of FSPEE and PBZ, which also resulted in the inhibition of these enzyme activities.
Table 3.
Effect of oral administration of fenugreek seed petroleum ether extract on serum and liver enzymes in rats with cotton pellet - induced granuloma
Discussion
FSPEE contained saturated and unsaturated fatty acids. The amounts of linolenic and linolenic acids obtained in our analysis are more or less similar to those reported by Skakovskii et al.[15] Significant anti-inflammatory activity was noted with linolenic acid in various acute models involving carrageenan, prostaglandin E2, leukotrienes, and arachidonic acid-induced inflammation[16] suggesting its ability to inhibit both cyclooxygenase and lipoxygenase pathways. FSPEE, rich in linolenic acid, and linolenic acids may also be acting in a similar way in carrageenan and formaldehyde-induced inflammation. The reduction in the weights of cotton pellets in FSPEE-treated animals indicates protection against production, migration, and cellular infiltration of chemoattractant factors. This may be due to anti-oxidant activities of FSPEE which help in the maintenance of cell membrane integrity and stability. There was a significant reduction in activities of SGPT and ALP suggesting a strong anti-oxidant role of FSPEE. Chronic inflammation has been attributed to hepatomegaly due to hypertrophy of hepatocytes.[17] Inflammatory diseases result in the release of lysosomal enzymes which, in turn, stimulate prostaglandin synthesis.[18] Various marker enzymes in the liver are enhanced in inflammatory conditions reflecting overall changes in metabolism.[19] Omega fatty acids have an unequivocal bearing on total lipid metabolism and recent findings point toward involvement of impaired lipid metabolism which enhances the production of proinflammatory mediators.[20] CFA-induced arthritis is akin to a number of clinical and immunological symptoms of human arthritis. Most of the studies with polar fractions of fenugreek seeds point toward a strong anti-inflammatory and anti-arthritic activities mediated through anti-oxidant mechanisms.[8,9,10]
FSPEE, rich in anti-oxidant fatty acids might be acting in a similar way.
Conclusion
Our studies show petroleum ether extract of fenugreek seeds (FSPEE) to be rich in linolenic and linolenic acids with significant anti-inflammatory and anti-arthritic activities in all the models tested. The results also indicate that the activity resides not only in the mucilage and polar fractions of seeds as reported by other workers but also in the nonpolar fixed oil. Thus, the entire seed may be more beneficial in inflammation and arthritis.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
The authors wish to thank Royal Castor Oil Pvt., Ltd., Unja for giving facilities to carry out GLC; and Zydus Cadila Health Care for the supply of animals and for the gift of phenylbutazone.
References
- 1.Kirtikar KR, Basu BD. New Delhi (India): Bishen Singh Mahendra Pal Singh; 1980. Indian Medicinal Plants; p. 700. [Google Scholar]
- 2.Mishkinsky JS, Goldschmied A, Joseph B, Ahronson Z, Sulman FG. Hypoglycaemic effect of Trigonella foenum graecum and Lupinus termis (leguminosae) seeds and their major alkaloids in alloxan-diabetic and normal rats. Arch Int Pharmacodyn Ther. 1974;210:27–37. [PubMed] [Google Scholar]
- 3.Riyad MA, Abdul-Salam SA, Mohammad SS. Effect of fenugreek and lupine seeds on the development of experimental diabetes in rats. Planta Med. 1988;54:286–90. doi: 10.1055/s-2006-962434. [DOI] [PubMed] [Google Scholar]
- 4.Khosla P, Gupta DD, Nagpal RK. Effect of Trigonella foenum graecum (Fenugreek) on blood glucose in normal and diabetic rats. Indian J Physiol Pharmacol. 1995;39:173–4. [PubMed] [Google Scholar]
- 5.Ribes G, Sauvaire Y, Baccou JC, Valette G, Chenon D, Trimble ER, et al. Effects of fenugreek seeds on endocrine pancreatic secretions in dogs. Ann Nutr Metab. 1984;28:37–43. doi: 10.1159/000176780. [DOI] [PubMed] [Google Scholar]
- 6.Valette G, Sauvaire Y, Baccou JC, Ribes G. Hypocholesterolaemic effect of fenugreek seeds in dogs. Atherosclerosis. 1984;50:105–11. doi: 10.1016/0021-9150(84)90012-1. [DOI] [PubMed] [Google Scholar]
- 7.Zargari A. Tehran (Iran): Tehran University Press; 1996. Medicinal Plants; p. 362. [Google Scholar]
- 8.Suresh P, Kavita CH, Babu SM, Reddy VP, Latha AK. Effect of ethanol extract of Trigonella foenum-graecum (Fenugreek) seeds on Freund's adjuvant induced arthritis in albino rats. Inflammation. 2012;35:1314–21. doi: 10.1007/s10753-012-9444-7. [DOI] [PubMed] [Google Scholar]
- 9.Sindhu G, Ratheesh M, Shyni GL, Nambisan B, Helen A. Anti-inflammatory and antioxidative effects of mucilage of Trigonella foenum graecum (Fenugreek) on adjuvant induced arthritic rats. Int Immunopharmacol. 2012;12:205–11. doi: 10.1016/j.intimp.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Liu Y, Kakani R, Nair MG. Compounds in functional food fenugreek spice exhibit anti-inflammatory and antioxidant activities. Food Chem. 2012;131:1187–92. [Google Scholar]
- 11.Singh H, Ghosh MN. Modified plethysmometer for measuring foot volume of unanesthetized rats. J Pharm Pharmacol. 1968;20:316–7. doi: 10.1111/j.2042-7158.1968.tb09747.x. [DOI] [PubMed] [Google Scholar]
- 12.Brownlee G. Effect of deoxycortone and ascorbic acid on formaldehyde-induced arthritis in normal and adrenalectomized rats. Lancet. 1950;1:157–9. doi: 10.1016/s0140-6736(50)90259-5. [DOI] [PubMed] [Google Scholar]
- 13.Newbould BB. Chemotherapy of arthritis induced in rats by mycobacterial adjuvant. Br J Pharmacol Chemother. 1963;21:127–36. doi: 10.1111/j.1476-5381.1963.tb01508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldstein S, Shemano I, Demer R, Beiler JM. Cotton pellet granuloma method for evaluation of anti-inflammatory activity. Arch Int Pharmacodyn Ther. 1967;165:294–301. [PubMed] [Google Scholar]
- 15.Skakovskii ED, Yu TL, Mauchanava VA, Karankevich EG, Lamotkin SA, Ahabalayeva AD, et al. Combining NMR spectroscopy and gas liquid chromatography for analysis of the fatty acid composition of fenugreek seed oil (Trigonella foenum-graecum L.) J Appl Spectrosc. 2013;80:779–82. [Google Scholar]
- 16.Singh S, Majumdar DK. Evaluation of antiinflammatory activity of fatty acids of Ocimum sanctum fixed oil. Indian J Exp Biol. 1997;35:380–3. [PubMed] [Google Scholar]
- 17.Bendele AM. Animal models of rheumatoid arthritis. Neuronal Interact. 2001;1:377–85. [PubMed] [Google Scholar]
- 18.Gupta OP, Sharma N, Chand D. A sensitive and relevant model for evaluating anti-inflammatory activity-papaya latex-induced rat paw inflammation. J Pharmacol Toxicol Methods. 1992;28:15–9. doi: 10.1016/1056-8719(92)90060-e. [DOI] [PubMed] [Google Scholar]
- 19.Rainsford KD. Adjuvant polyarthritis in rats: Is this a satisfactory model for screening anti-arthritic drugs? Agents Actions. 1982;12:452–8. doi: 10.1007/BF01965926. [DOI] [PubMed] [Google Scholar]
- 20.Gonzalez-Gay MA, Gonzalez-Juanatey C, Martin J. Rheumatoid arthritis: A disease associated with accelerated atherogenesis. Semin Arthritis Rheum. 2005;35:8–17. doi: 10.1016/j.semarthrit.2005.03.004. [DOI] [PubMed] [Google Scholar]


