Abstract
Objectives:
People with epilepsy have greater cognitive and behavioral dysfunction than the general population. There is no specific treatment available for cognitive impairment of these patients. We aimed to evaluate the effects of memantine, an N-methyl-D-aspartate-type glutamate receptor noncompetitive antagonist, on improving cognition and memory functions in epileptic patients with cognitive and memory impairment, who received anti-epileptic drugs (AEDs).
Methods:
We did a randomized, double-blind, placebo-controlled parallel group trial, in SRM Medical College Hospital and Research Centre, Kattankulathur, Kancheepuram, Tamil Nadu, India between April 2013 and September 2013. Fifty-nine epileptic patients taking AEDs with subjective memory complaints were recruited and randomized to either Group 1 to receive 16 weeks of once-daily memantine, (5 mg for first 8 weeks, followed by memantine 10 mg for next 8 weeks) or Group 2 to receive once daily placebo. This trial is registered with Clinical Trial Registry of India CTRI/2013/04/003573.
Results:
Of 59 randomized patients, 55 patients completed the study (26 memantine and 29 placebo). Memantine group showed statistically significant improvement in total mini mental state examination score from baseline (P = 0.765) to 16th week (P < 0.001) in comparison with the placebo. The Weshler's Memory Scale total score in memantine group improved significantly after 8 weeks (P = 0.002) compared with baseline (P = 0.873) and highly significant at the end of 16th week (P < 0.001). The self-rated quality of life and memory in memantine group also significantly improved at the study end.
Conclusion:
We conclude that once-daily memantine (10 mg) treatment significantly improved cognition, memory and quality of life in epileptic patients with mild to moderate cognitive impairment and was found to have a favorable safety profile.
Keywords: Cognitive functions, epilepsy, Memantine
Introduction
Epilepsy is one of the most common neurological disorders that affect people in every country throughout the world. Today an estimated 50 million people live with epilepsy, 80% of whom reside in developing countries. The prevalence of epilepsy is estimated to be around 10 million in Indian population. People with epilepsy have greater cognitive and behavioral dysfunction than the general population. The incidence of cognitive impairment among epileptics is 40%.[1] Their worries include learning disabilities, academic difficulties, poor attention and concentration, and impoverished memory.[2,3] In a survey by the International Bureau of Epilepsy, 44% of patients with epilepsy complained of difficulty in learning, and 45% of slowness in thinking.[4] It has also been demonstrated that cognitive function is a significant predictor of self-evaluation of the quality of life among adults with epilepsy.[5]
Cognitive disturbances in epilepsy are multifactorial in origin including underlying neuropathology, anti-epileptic drugs (AEDs) and psychosocial issues of the patient due to the disease.[6] The incidence of AED-induced cognitive dysfunction is about 28%.[7] Older AEDs have higher cognitive disturbances than newer AEDs.[8,9] There is no specific treatment available for the cognitive impairment of these patients.[10] Excitotoxicity, mediated by glutamate acting on N-methyl-D-aspartate (NMDA) receptors in the hippocampus, can cause memory dysfunction in epilepsy.[11,12] Alteration of this excitotoxic pathway can be a novel, potentially safer and more effective, approach to the treatment of memory loss.[13,14]
Memantine is a noncompetitive antagonist of the NMDA-type glutamate receptor. It interacts with the Mg2+ binding site of the channel to prevent excessive activation while sparing normal function.[15] Memantine significantly reduces the rate of clinical deterioration in patients with moderate to severe Alzheimer's dementia by reducing excitotoxicity and preventing cell damage.[16] This drug exerts its therapeutic effect in various conditions mainly by blocking glutamate excitotoxicity. It also has antioxidant property and increases production of brain-derived neurotropic factor (BDNF).[13,17] Memantine is approved drug by Food and Drug Administration (2004) for the treatment of cognitive impairment in Alzheimer's dementia. This drug is also commonly used to treat other neurodegenerative dementias.
Memantine available as memantine hydrochloride is administered orally at an initial dose of 5 mg once daily. The maximum tolerable dose is 20 mg.[14] Memantine is well tolerated and safe as shown in all reported trials with hardly few side effects a headache or dizziness, but are usually mild and reversible.[12,18,19]
Hence, we aimed to evaluate primarily the efficacy of memantine on improving cognition and memory in epileptic patients taking AEDs, with mild to moderate cognitive and memory impairment.
Methods
Study design and eligibility criteria
This study was a randomized, double-blind, placebo-controlled, parallel design trial done for 16 weeks. Epilepsy patients taking AEDs with subjective memory complaints visiting Neurology Outpatient Department of SRM. Medical College Hospitals and Research Centre, Potheri, Kattankulathur, Kancheepuram, Tamil Nadu, India were recruited for the study. Patients on AEDs for more than 6 months with mild to moderate cognitive impairment of age group between 18 and 55 years with seizure frequency of <4 per month were included in the study. The exclusion criteria were patients with the progressive neurologic disease, psychiatric disorders, mental retardation, severe medical illnesses including renal insufficiency, pregnant or lactating women, patients who are not willing to give consent. Patients in inter-ictal period (2 weeks) and patients with more than four seizure attacks per month also were excluded participation as they might have severe memory impairment.
Trial procedures
The study was approved by the Institutional Ethics Committee of SRM Medical College Hospital and Research Centre and conducted according to International Conference on Harmonization-Good Clinical Practice, guidelines and the principles of the Declaration of Helsinki. The trial was registered prospectively in Clinical Trials Registry of India that participates in WHO International Clinical Trial Registry Platform (CTRI/2013/04/003573).
Recruitment of the patients began in March 2013. Patients were assessed for intelligent quotient (IQ) testing by clinical psychologist using Stanford–Binet Intelligence Scale. After verifying eligibility for normal IQ and signing the consent for participation, patients were included in the study.
Randomization and blinding
We used sequentially numbered, opaque, sealed envelope pattern for allocation concealment.[20] The random code was generated before commencement of the study. After a screening period of up to 4 weeks, eligible participants were randomly assigned to one of the two groups, Group 1 memantine and Group 2 placebo. Randomization was done by random number generator software. Patients, investigators and outcome assessors were blinded. The identity of the treatment was concealed by the use of study drugs that were identical in appearance, packaging, labelling and in the schedule of administration.[20]
Evaluation of patients and treatment
Participants underwent a screening neurological history and examination; documentation of a seizure diary and case record forms were maintained for each patient. The baseline assessment of cognition was done using Folstein mini-mental state examination (MMSE) scale, and memory was done using Weshler Memory Scale (WMS) for adults, quality of life in epilepsy (QOLIE) was by QOLIE-10-P questionnaire.[21,22] Interventional drug memantine hydrochloride tablet 5 mg, 10 mg was obtained from Sun Pharma Pvt. Ltd. Treatment and placebo drugs were concealed as prelabelled kits.
Memantine hydrochloride tablet was started at the dose of 5 mg once daily orally, and the same dose was given for 8 weeks and dose is titrated to 10 mg once daily orally cautiously for the remaining 8 weeks. Duration of intervention was 4 months. Placebo was given once daily orally for 16 weeks. Patients were instructed to take the study medication at the same time every day. Duration of intervention was 4 months. Follow-up evaluation for cognitive and memory assessment using MMSE and WMS, questionnaire were done at the end of 4th, 8th, 12th and 16th week respectively and quality of life using QOLIE-10 was also assessed at the same time. Patients were asked to bring the dispensed drug pack at each visit to check for drug accountability record. Patients were asked for any adverse reactions during each visit, the data was recorded, and seizure diary was also monitored.
Statistical analysis
We expressed descriptive data as mean ± standard deviation. Continuous data post-treatment changes compared to baseline by paired Student's t-test and between groups by unpaired t-test. We used Wilcoxon signed ranks test to compare the WMS subset assessment between baseline and their respective follow-up weeks. Mann–Whitney U-test was the statistical method used to compare the mean WMS subset assessment scores between the groups at their respective follow-up weeks. Pearson Chi-square continuity ratio and Fisher's exact test were used for WMS and MMSE scale interpretation. Levene's test for equality of variances was used for quality of life assessment and significance was given by t-test for equality of means between baseline and their respective follow-up weeks. We used Statistical Package for Social Sciences (SPSS Statistics for Windows Version 17.0. Chicago: SPSS Inc.) P ≤ 0.05 was considered statistically significant.
Results
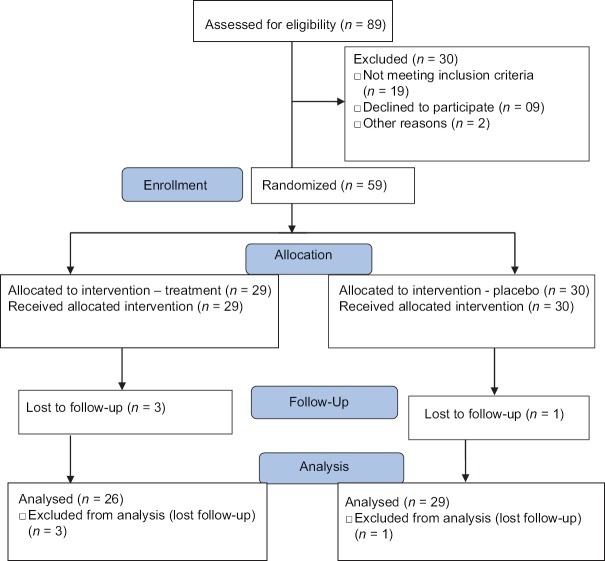
We screened 89 epileptic patients with the subjective memory complaints for eligibility to enrol in the study. A total of 59 patients were included in the study. The reasons for exclusion of other screened cases were severity of seizure, age > 55 years, severe memory impairment, alcohol withdrawal seizures and not willing for consent. Between April 2013 and May 2013, the eligible patients were enrolled, allocated into two groups, randomly assigned to one of two groups, which was shown in Figure 1.
Figure 1.
Trial profile
Group 1 with 29 patients received memantine and Group 2 with 30 patients received placebo treatment. Out of 29, three patients of memantine group and out of 30, one patient of placebo group lost follow-up. Fifty-five patients were taken for follow-up analysis, with 26 patients in memantine group, and 29 patients in placebo group.
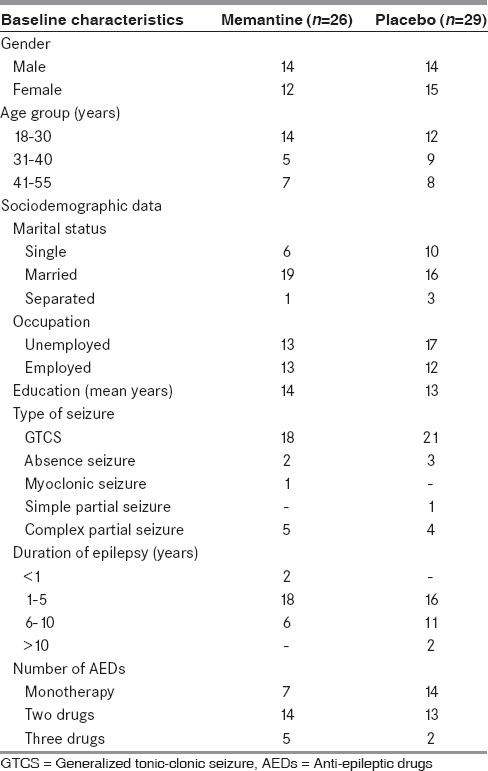
The demographic data were discussed in Table 1. Baseline characteristics were similar in all the treatment groups.
Table 1.
Baseline characteristics of the study groups
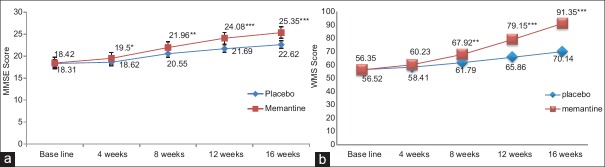
MMSE score increased in memantine group, baseline (P = 0.765) to 16th week (P = 0.000***) (CI = 95%) in comparison with the placebo as shown in Figure 2a.
Figure 2.
(a) Mini mental state examination score of memantine versus placebo group. In memantine group, mini mental state examination score increased, which is statistically significant at the 4th and 8th week follow-up, and highly significant at the 12th and 16th follow-up week in comparison with the placebo *P<0.05, **P<0.01, ***P<0.001. (b) Weshler's Memory Scale score of memantine versus placebo group. In memantine group, Weshler's Memory Scale score increased, which is statistically significant at the 8th week follow-up, and highly significant at the 12th and 16th follow-up week in comparison with the placebo. **P<0.01, ***P<0.001
WMS score increased in memantine group, improved significantly after 8 weeks (P = 0.002**) compared with baseline (P = 0.873) and highly significant at the end of 16th week (P = 0.000***) (CI = 95%) in comparison with the placebo as shown in Figure 2b. The within-group comparison of memantine for 5 mg and 10 mg, 10 mg dose titration showed a highly significant improvement in MMSE and WMS score (P = 0.000***) at the end of 16th week of treatment when compared with 5 mg dose of memantine (P ≤ 0.05*) at the end of 8th week.
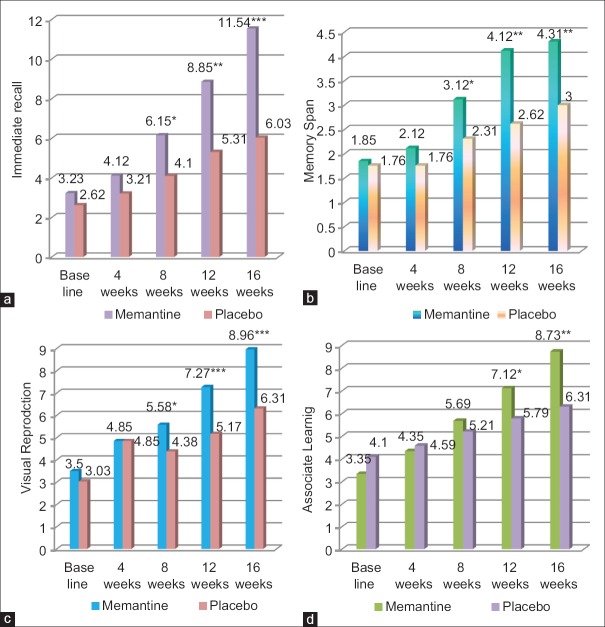
Memantine group showed improved logical memory from baseline (P = 0.247), which is statistically significant at the 8th week follow-up (P = 0.002**), and highly significant at the 12th (P = 0.000***) and 16th follow-up week (P = 0.000***) in comparison with the placebo [Figure 3a]. Memory span is increased in memantine group from baseline (P = 0.661) which is statistically significant at the 8th week follow-up (P = 0.003**) week, and highly significant at the 12th and 16th follow-up week (P = 0.000***) in comparison with the placebo [Figure 3b]. There is increase visual reproduction memory in memantine group, which is statistically significant at the 8th week follow-up (P = 0.008**), and highly significant at the 12th and 16th follow-up week (P = 0.000***) as shown in Figure 3c, in comparison with the placebo. In Memantine group, associate learning is improved, which is statistically significant at the 12th week follow up (*P < 0.05), and highly significant at 16th week follow up (** P < 0.001) as shown in Figure 3d in comparison with the placebo.
Figure 3.
Weshler's Memory Scale subset assessment of memantine and placebo group. *P<0.05, **P<0.01, ***P<0.001. (a) Immediate recall (logical memory) subset of memantine and placebo group. (b) Memory span (delayed recall) subset of memantine and placebo group. (c) Visual reproduction subset of memantine and placebo group. (d) Associate learning subset of memantine and placebo group
Memantine treatment showed statistically significant improvement in memory at the end of 16th week (P = 0.000***) in comparison with the placebo which is shown in Table 2a.
Table 2.
Quality of life assessment of memantine and placebo group
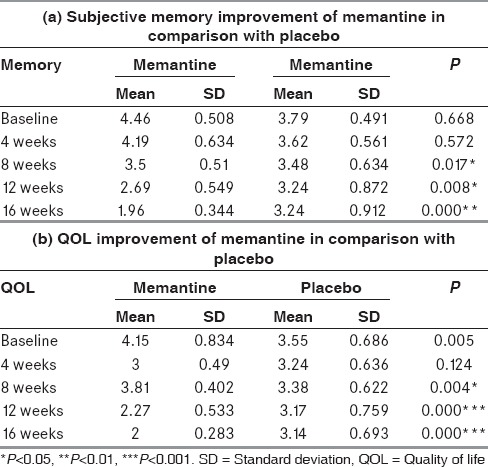
The self-rated quality of life questionnaire showed statistically (P = 0.000***) significant improvement in memory and quality of life in patients receiving memantine treatment at the end of the study as given in Table 2b.
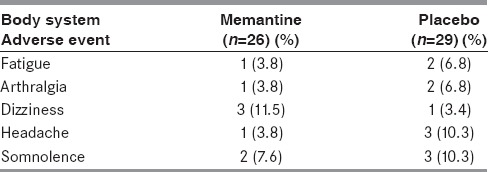
Table 3 gives the list of adverse drug events noted during the study period.
Table 3.
Adverse events reported in study patients
Discussion
Cognitive and memory impairment are very common in epileptic patients due to multifactorial etiology.[23,24] AED treatment itself can cause cognitive dysfunction as side effect.[25,26] To our knowledge, this trial is the first clinical study to evaluate the effect of memantine on improving cognition and memory in epileptic patients.
In our study, there is a significant improvement in cognition in memantine group assessed by MMSE score compared with the placebo. This effect started at the 4th week of memantine therapy and highly significant improvement in cognitive score was seen at the 12th and 16th week of follow-up (P = 0.000), which corresponds to 10 mg once daily dosage of memantine. Our study also showed statistically significant improvement in memory assessed by WMS in memantine group compared with the placebo. This significant effect was observed from the 9th week of memantine therapy and highly significant improvement was seen at the 16th week of follow-up (P = 0.000).
The molecular mechanism behind cognitive and memory impairment in epileptic patients receiving AEDs is due to excitotoxic damage caused to the brain by the excitatory neurotransmitter glutamate through NMDA receptor. This glutamate excess affects long term potentiation and learning under pathological conditions which lead to neuronal cell death and free radical formation.[12,27]
The interventional drug in our study, memantine is an NMDA receptor antagonist (noncompetitive). It can prevent the excitotoxic damage to the brain by blocking the NMDA receptor channel and by preventing glutamate action, which contribute to cognitive and memory improvement as shown by our study. In addition, memantine reduces oxidative stress and increases the level of BDNF, which in turn would add to memory improvement. The transient blockade of activated NMDA receptors by low-affinity agents, inhibits their pathological functions only, leaving physiological functions unaltered.[14] Memantine is a fast-binding antagonist which binds to the channel in a pseudo- first order manner. It also dissociates from the receptor quickly and in a concentration independent manner which allows the binding of memantine without affecting its removal from the site of action and increased potency with fewer side effects.[28] In comparison with other antagonists (dextromethorphan, ketamine, amantadine), memantine is fast acting and has less effect on physiologic mechanisms.[29]
Subset assessment of memory showed improvement in mental control in memantine group (P < 0.05) at 10 mg dose. Logical memory showed statistically significant improvement in the memantine group (P = 0.000) at 12th and 16th week which corresponds to the dose of 10 mg once daily. Memory span subset of WMS also showed highly significant improvement in the memantine group at the 12th and 16th week in comparison with the placebo. Visual reproduction subset also showed highly significant improvement in the memantine group at the 12th and 16th week. Associate learning subset of memantine group showed statistically significant improvement 16th follow-up week in comparison with the placebo.
Based on our results, there is significant improvement in cognitive and memory dysfunction for epileptic patients at 10 mg once daily oral dose.
As the efficacy of memantine is investigated in epilepsy, which is one of a critical disease, we cautiously started with an initial therapeutic dose of 5 mg for the first 8 weeks followed by 10 mg for the next 8 weeks.
Memantine also improved the self-reported memory function and quality of life in epileptic patients as assessed by QOLIE-10-P questionnaire with minimal side effects. In our study, the memantine group had low seizure frequency in comparison with baseline as observed from the seizure dairy of patients. The seizure reducing ability of memantine can be evaluated in detail for control of seizures in future.
This study also has some limitations. The study period was relatively short. Long-term assessment for the efficacy and safety of memantine can be investigated. We recruited adult patients of age between 18 and 55 years; however the incidence of cognitive impairment in adolescent age group epileptic patients is around 50%, which would certainly impair the cognitive and academic performances of these patients. The possible effect of memantine to improve memory in children and adolescent age group epileptic patients can also be done.
Conclusion
From this randomized, double-blind, placebo-controlled, parallel design study to evaluate the efficacy of memantine for mild to moderate cognitive impairment in epileptic patients, we conclude that memantine 10 mg oral, once daily significantly improves mild to moderate cognitive and memory impairment in epileptic patients receiving AEDs when compared with placebo group. Self-reported memory and quality of life significantly improved with 10 mg once-daily memantine at the end of 16th week of treatment.
Financial support and sponsorship
SRM Medical College Hospital and Research Centre, Kattankulathur - 603 203, Kancheepuram district, Tamil Nadu, India.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
We would like to thank the study participants for their cooperation and commitment. We thank all members of the trial team for their active contribution. We express our sincere gratitude to Prof. Dr. James Pandian, M.S., M.Ch. (Plastic), Dean, and Prof. Dr. Thangaraju, Pro-Vice Chancellor, SRM Medical College Hospital and Research Centre for their administrative support. We thank Dr. Prabavathi, Clinician at Nandhivaram Health Centre for her support in patient recruitment. We thank Mr. Sivakumar and Mr. Rathinavel, Clinical Pharmacists for their support in arranging clinical supplies of the study drugs. We thank Prof. Dr. Balakrishnan, Professor and Head, Department of Clinical Psychology, Madras Medical College, Chennai for giving his contribution in Psychological Assessment Resources.
References
- 1.Kwan P, Brodie MJ. Neuropsychological effects of epilepsy and antiepileptic drugs. Lancet. 2001;357:216–22. doi: 10.1016/S0140-6736(00)03600-X. [DOI] [PubMed] [Google Scholar]
- 2.Dodrill CB. Neuropsychological effects of seizures. Epilepsy Behav. 2004;5(Suppl 1):S21–4. doi: 10.1016/j.yebeh.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 3.Helmstaedter C, Kurthen M, Lux S, Reuber M, Elger CE. Chronic epilepsy and cognition: A longitudinal study in temporal lobe epilepsy. Ann Neurol. 2003;54:425–32. doi: 10.1002/ana.10692. [DOI] [PubMed] [Google Scholar]
- 4.Cognitive Function Survey. International Bureau for Epilepsy. 2004. [Last accessed on 2012 Nov 23]. Available from: http://www.ibe-epilepsy.org/whatsnewdet.asp .
- 5.Perrine K, Hermann BP, Meador KJ, Vickrey BG, Cramer JA, Hays RD, et al. The relationship of neuropsychological functioning to quality of life in epilepsy. Arch Neurol. 1995;52:997–1003. doi: 10.1001/archneur.1995.00540340089017. [DOI] [PubMed] [Google Scholar]
- 6.Vermeulen J, Aldenkamp AP, Alpherts WC. Memory complaints in epilepsy: Correlations with cognitive performance and neuroticism. Epilepsy Res. 1993;15:157–70. doi: 10.1016/0920-1211(93)90096-p. [DOI] [PubMed] [Google Scholar]
- 7.Devinsky O. Cognitive and behavioral effects of antiepileptic drugs. Epilepsia. 1995;36(Suppl 2):S46–65. doi: 10.1111/j.1528-1157.1995.tb05999.x. [DOI] [PubMed] [Google Scholar]
- 8.Motamedi GK, Meador KJ. Antiepileptic drugs and memory. Epilepsy Behav. 2004;5:435–9. doi: 10.1016/j.yebeh.2004.03.006. [DOI] [PubMed] [Google Scholar]
- 9.Meador KJ, Gilliam FG, Kanner AM, Pellock JM. Cognitive and behavioral effects of antiepileptic drugs. Epilepsy Behav. 2001;2:SS1–17. doi: 10.1006/ebeh.2001.0235. [DOI] [PubMed] [Google Scholar]
- 10.Motamedi G, Meador K. Epilepsy and cognition. Epilepsy Behav. 2003;4(Suppl 2):S25–38. doi: 10.1016/j.yebeh.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 11.Packer L, Tritschler HJ, Wessel K. Neuroprotection by the metabolic antioxidant alpha-lipoic acid. Free Radic Biol Med. 1997;22:359–78. doi: 10.1016/s0891-5849(96)00269-9. [DOI] [PubMed] [Google Scholar]
- 12.Brunton LL, Chabner BA, Knollmann BC. Treatment of central nervous system degenerative disorders. Goodman & Gilman's the Pharmacological Basis of Therapeutics. 12th ed. Ch 22. New York: McGraw-Hill; 2011. [Google Scholar]
- 13.Gardoni F, Di Luca M. New targets for pharmacological intervention in the glutamatergic synapse. Eur J Pharmacol. 2006;545:2–10. doi: 10.1016/j.ejphar.2006.06.022. [DOI] [PubMed] [Google Scholar]
- 14.Sonkusare SK, Kaul CL, Ramarao P. Dementia of Alzheimer's disease and other neurodegenerative disorders – Memantine, a new hope. Pharmacol Res. 2005;51:1–17. doi: 10.1016/j.phrs.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Lipton SA. Pathologically activated therapeutics for neuroprotection. Nat Rev Neurosci. 2007;8:803–8. doi: 10.1038/nrn2229. [DOI] [PubMed] [Google Scholar]
- 16.Raina P, Santaguida P, Ismaila A, Patterson C, Cowan D, Levine M, et al. Effectiveness of cholinesterase inhibitors and memantine for treating dementia: Evidence review for a clinical practice guideline. Ann Intern Med. 2008;148:379–97. doi: 10.7326/0003-4819-148-5-200803040-00009. [DOI] [PubMed] [Google Scholar]
- 17.Kumar S. Memantine: Pharmacological properties and clinical uses. Neurol India. 2004;52:307–9. [PubMed] [Google Scholar]
- 18.Maidment ID, Fox CG, Boustani M, Rodriguez J, Brown RC, Katona CL. Efficacy of memantine on behavioral and psychological symptoms related to dementia: A systematic meta-analysis. Ann Pharmacother. 2008;42:32–8. doi: 10.1345/aph.1K372. [DOI] [PubMed] [Google Scholar]
- 19.Kavirajan H, Schneider LS. Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: A meta-analysis of randomised controlled trials. Lancet Neurol. 2007;6:782–92. doi: 10.1016/S1474-4422(07)70195-3. [DOI] [PubMed] [Google Scholar]
- 20.Schulz KF, Grimes DA. Allocation concealment in randomised trials: Defending against deciphering. Lancet. 2002;359:614–8. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- 21.Wechsler, D A standardized memory scale for clinical use. The Journal of Psychology: Interdisciplinary and Applied. 1945;19:87–95. [Google Scholar]
- 22.Cramer JA. A brief questionnaire to screen for quality of life in epilepsy the QOLIE-10. Eur J Neurol. 2009;352:116–22. doi: 10.1111/j.1528-1157.1996.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 23.Giovagnoli AR. Awareness, overestimation, and underestimation of cognitive functions in epilepsy. Epilepsy Behav. 2013;26:75–80. doi: 10.1016/j.yebeh.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 24.Kudo T, Yagi K. Chronological progression of a language deficit appearing to be postictally reversible in a patient with symptomatic localization-related epilepsy. Psychiatry Clin Neurosci. 2000;54:131–8. doi: 10.1046/j.1440-1819.2000.00648.x. [DOI] [PubMed] [Google Scholar]
- 25.Ortinski P, Meador KJ. Cognitive side effects of antiepileptic drugs. Epilepsy Behav. 2004;5(Suppl 1):S60–5. doi: 10.1016/j.yebeh.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 26.Trimble MR. Antiepileptic drugs, cognitive function, and behavior in children: Evidence from recent studies. Epilepsia. 1990;31(Suppl 4):S30–4. doi: 10.1111/j.1528-1157.1990.tb05867.x. [DOI] [PubMed] [Google Scholar]
- 27.Cattabeni F, Gardoni F, Di Luca M. Pathophysiological implications of the structural organization of the excitatory synapse. Eur J Pharmacol. 1999;375:339–47. doi: 10.1016/s0014-2999(99)00299-x. [DOI] [PubMed] [Google Scholar]
- 28.Kornhuber J, Weller M. Psychotogenicity and N-methyl-D-aspartate receptor antagonism: Implications for neuroprotective pharmacotherapy. Biol Psychiatry. 1997;41:135–44. doi: 10.1016/S0006-3223(96)00047-9. [DOI] [PubMed] [Google Scholar]
- 29.Thomas SJ, Grossberg GT. Memantine: A review of studies into its safety and efficacy in treating Alzheimer's disease and other dementias. Clin Interv Aging. 2009;4:367–77. doi: 10.2147/cia.s6666. [DOI] [PMC free article] [PubMed] [Google Scholar]